Abstract
Impaired apoptosis is both critical in cancer development and a major barrier to effective treatment. In response to diverse intracellular damage signals, including those evoked by cancer therapy, the cell’s decision to undergo apoptosis is determined by interactions between three factions of the Bcl-2 protein family. The damage signals are transduced by the diverse ‘BH3-only’ proteins, distinguished by the BH3 domain used to engage their pro-survival relatives: Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1. This interaction ablates pro-survival function and allows activation of Bax and Bak, which commit the cell to apoptosis by permeabilizing the outer membrane of the mitochondrion. Certain BH3-only proteins (e.g. Bim, Puma) can engage all the pro-survival proteins, but others (e.g. Bad, Noxa) engage only subsets. Activation of Bax and Bak appears to require that the BH3-only proteins engage the multiple pro-survival proteins guarding Bax and Bak, rather than binding to the latter. The balance between the pro-survival proteins and their BH3 ligands regulates tissue homeostasis, and either overexpression of a pro-survival family member or loss of a proapoptotic relative can be oncogenic. Better understanding of the Bcl-2 family is clarifying its role in cancer development, revealing how conventional therapy works and stimulating the search for ‘BH3 mimetics’ as a novel class of anticancer drugs.
Keywords: Bcl-2, apoptosis, cancer, oncogenesis, chemotherapy, BH3 mimetic drugs
Introduction
Metazoa eliminate redundant, damaged or infected cells by apoptosis, a stereotypic program of cell suicide during which cells shrink, undergo inter-nucleosomal DNA cleavage and break up into vesicles that are rapidly engulfed by other cells. Apoptosis is vital for embryogenesis, tissue homeostasis and defence against pathogens. Notably, its deregulation can lead to cancer, as well as to autoimmune and degenerative diseases (Cory and Adams, 2002). Elucidation of the underlying regulatory mechanisms is relevant not only to disease etiology, but also to treatment. In particular, as cytotoxic therapies for cancer rely heavily upon inducing apoptosis, a deeper understanding of apoptosis regulation in normal and neoplastic cells offers great promise for developing more selective and efficacious therapeutic agents.
The first apoptotic regulator to be identified was Bcl-2, the oncoprotein activated via chromosome translocation in human follicular lymphoma (Cory and Adams, 2002). Our laboratory discovered that Bcl-2 acts by promoting cell survival rather than by driving cell proliferation and proposed the concept, now widely accepted (Hanahan and Weinberg, 2000; Johnstone et al., 2002), that impairment of apoptosis is a critical step in tumor development (Vaux et al., 1988; Strasser et al., 1990). Presumably, apoptosis normally eliminates most cells whose cell cycle control is disturbed by oncogenic mutations. Impaired apoptosis would preserve not only such preneoplastic cells but also those bearing mutations that ablate their need for trophic factors, allow their breach of normal tissue barriers or facilitate their metastasis to foreign sites. As well as potentially driving malignant progression at multiple steps, a wealth of observations, for example (Sentman et al., 1991; Strasser et al., 1991a; Schmitt et al., 2000), indicates that overexpression of Bcl-2 and its close relatives is a major component of chemo-resistance. For example, with the National Cancer Institute (NCI) panel of 60 diverse cancer cell lines, the expression level of Bcl-xL strongly correlates with resistance to most chemotherapy agents (Amundson et al., 2000).
Here we discuss new insights into how the interactions between three factions of the Bcl-2 protein family determine whether the cell commits to apoptosis. We also sketch how these proteins normally regulate tissue homeostasis, briefly address their role in cancer development and describe recent advances in delineating how different modes of cancer therapy work through this family. Finally, we discuss the exciting progress towards creating novel anticancer agents to target this family, which may represent the Achilles’ heel of the tumor. Some of these issues are discussed more extensively in earlier reviews (Strasser et al., 2000; Cory and Adams, 2002; Adams, 2003; Cory et al., 2003; Danial and Korsmeyer, 2004; Fesik, 2005; Strasser, 2005).
The two major pathways to apoptosis
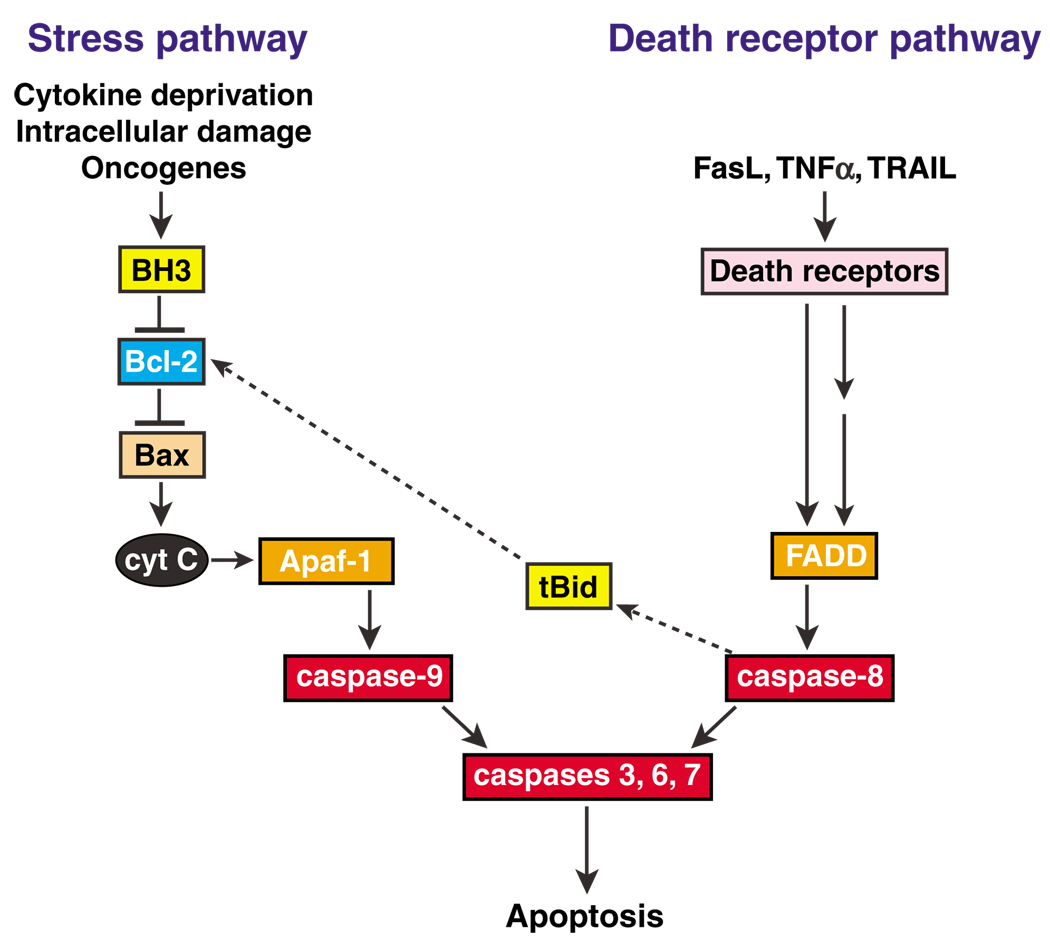
Apoptosis is executed by intracellular cysteine proteases called caspases (Adams, 2003). The dozen or so caspases in mammalian cells are synthesized as largely inactive zymogens, and two principal pathways activate those involved in apoptosis (Figure 1). The more ancient, evolutionarily conserved ‘stress’ pathway, which is triggered by developmental cues and diverse intracellular stresses, activates caspase-9 on a scaffold formed by Apaf-1 in response to cytochrome c released from damaged mitochondria. This pathway, also termed ‘mitochondrial’ or ‘intrinsic’, is primarily regulated by the Bcl-2 family. The ‘extrinsic’ pathway, on the other hand, is induced when the so-called ‘death receptors’ on the cell surface are engaged by cognate ligands of the tumor necrosis factor (TNF) family. This pathway instead activates caspase-8 (and caspase-10 in humans), through adaptor proteins that include Fas-associated death domain protein (FADD). Once activated, caspase-9 or -8 (-10) activates downstream ‘effector caspases’ (i.e. caspases-3, 6 and 7), which provoke cellular destruction by cleaving several hundred cellular proteins. The two pathways are largely independent, as overexpressed Bcl-2 does not protect lymphocytes from apoptosis induced by death receptor ligands (Strasser et al., 1995; Huang et al., 1999). In certain other cell types (e.g. hepatocytes), however, the two pathways intersect, because caspase-8 can process the pro-apoptotic Bid into its active truncated form (tBid) (Figure 1). To prevent catastrophic unscheduled cell death, both pathways are tightly regulated, at multiple steps.
Figure 1.
Pathways to cell death. The stress pathway is initiated by BH3-only proteins (‘BH3’), which inactivate the Bcl-2-like proteins, keeping them from restraining Bax and Bak. Bax or Bak can permeabilize the mitochondrial outer membrane, releasing cyto-chrome c, which provokes Apaf-1 (apoptotic protease-activating factor 1) to activate caspase-9. The ‘death receptor’ pathway is activated when ligands of the TNF family engage with and aggregate their cognate receptors on the cell surface and activate caspase-8 via adaptor proteins that include FADD. The two pathways are largely independent, but in certain cells the death receptor pathway engages the stress pathway via a cleaved form of the BH3-only protein Bid (tBid), which can engage Bcl-2 homologs and perhaps Bax (see text).
The Bcl-2 family, which includes 17 or more members in mammalian cells (Cory and Adams, 2002), functions as a ‘life/death switch’ that integrates diverse inter- and intracellular cues to determine whether or not the stress apoptosis pathway should be activated. The switch operates through the interactions between the proteins within three subfamilies. Whereas the pro-survival subfamily (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1 and also Bcl-B in humans) protects cells exposed to diverse cytotoxic conditions, two other subfamilies, many members of which were identified as Bcl-2-binding proteins, instead promote cell death. Members of the Bax-like apoptotic sub-family (Bax, Bak and Bok) are very similar to Bcl-2 in sequence, particularly in three conserved ‘Bcl-2 Homology’ regions (BH1, BH2 and BH3), and both Bax and Bak have structures that closely resemble their pro-survival relatives (Suzuki et al., 2000; Moldoveanu et al., 2006). The other proapoptotic subfamily, the ‘BH3-only proteins’, includes at least eight members: Bik, Bad, Bid, Bim, Bmf, Hrk, Noxa and Puma. These proteins are largely unrelated in sequence to either Bcl-2 or each other, apart from the signature BH3 domain, which is essential for their killing function (Huang and Strasser, 2000; Willis and Adams, 2005).
Importantly, stress-induced apoptosis requires both types of pro-death proteins: the BH3-only proteins seem to act as damage sensors and direct antagonists of Bcl-2 and the other pro-survival proteins, whereas the Bax-like proteins, once activated, act further downstream (Figure 1), probably by permeabilizing the mitochondrial outer membrane and perhaps also by perturbing the endoplasmic reticulum (ER)/nuclear envelope. The crucial battle seems to be fought on these membranes and most family members either normally reside on their cytosolic surfaces, or rapidly congregate there after an apoptotic signal. Most bear a C-terminal hydrophobic sequence that targets and/or anchors them to those membranes, but full integration of the multidomain proteins probably involves insertion of additional helices in the molecules (Kim et al., 2004; Annis et al., 2005), and that also might hold for tBid, which is myristoylated (Zha et al., 2000) but lacks a C-terminal anchor. Integration into the outer mitochondrial membrane may require components of the general import machinery such as TOM22 or TOM70 (Bellot et al., 2006; Chou et al., 2006).
As reviewed recently (Fesik, 2005; Hinds and Day, 2005), illuminating structural studies have revealed that the BH1, BH2 and BH3 domains in each pro-survival family member fold into a globular domain having a hydrophobic groove on its surface, to which a BH3 domain, an amphipathic alpha helix of ~24 residues, can bind (Sattler et al., 1997; Liu et al., 2003). This coupling neutralizes the pro-survival family member. In healthy cells, the pro-survival proteins act at least predominantly by preventing Bax or Bak from perturbing the integrity of intracellular membranes, in particular the outer mitochondrial membrane.
Bax and Bak appear to be largely redundant in function. Whereas the loss of either single gene has little effect in most cells and tissues (Bax is required for spermatogenesis and in certain neuronal cells), the absence of both proteins blocks apoptosis in many cell types (Wei et al., 2001) and dramatically impairs developmentally programmed attrition in several tissues (Lindsten et al., 2000), typically resulting in perinatal death. A notable biochemical difference between Bax and Bak is that, in healthy cells, Bax is largely cytosolic or loosely associated with mitochondria, whereas Bak is an integral membrane protein on the cytosolic face of the mitochondrion and ER. In response to cytotoxic signals, Bax translocates to those membranes, and both Bax and Bak change conformation and form membrane-associated homo-oligomers. These oligomers are thought to permeabilize the outer mitochondrial membrane, but the mechanism remains obscure (Newmeyer and Ferguson-Miller, 2003; Green, 2005). Bax and Bak can also influence the shape of the mitochondria via effects on the mitochondrial fission/fusion machinery (Karbowski et al., 2006), but whether that machinery contributes to permeabilization is unclear (Parone et al., 2006).
How the life–death switch operates
Previously, activated BH3-only proteins were generally assumed to bind indiscriminately to all their pro-survival counterparts. Quantitative assessment of the binding of BH3 peptides to all the mammalian Bcl-2-like proteins, however, has revealed that the affinities of different pairs vary enormously (Chen et al., 2005; Kuwana et al., 2005). Whereas Bim, Puma and tBid bind avidly to all the pro-survival proteins, the other BH3-only proteins associate only with subsets (Chen et al., 2005). For example, Noxa engages only Mcl-1 and A1 and Bad only Bcl2, Bcl-xL and Bcl-w (Figure 2a). Importantly, the promiscuous binders (e.g. Bim and Puma) are much more potent killers than those that cannot engage all the pro-survival proteins, but the combination of Noxa and Bad potently induces cell death (Figure 2b; Chen et al., 2005). These findings indicate that efficient apoptosis requires neutralization of multiple pro-survival proteins and suggest that all the pro-survival proteins may not have equivalent function.
Figure 2.
Differing binding profiles and apoptotic potency of BH3-only proteins. (a) The ability of Bim, Puma and tBid to engage all the pro-survival proteins contrasts with the selective binding of others, such as Bad and Noxa (Chen et al., 2005). (b) Cytotoxicity assays in fibroblasts show that Bim and Puma are potent killers, but Bad and Noxa alone are much weaker. Nevertheless, Noxa in conjunction with a Bad BH3 (within an inert backbone) kills potently (Chen et al., 2005).
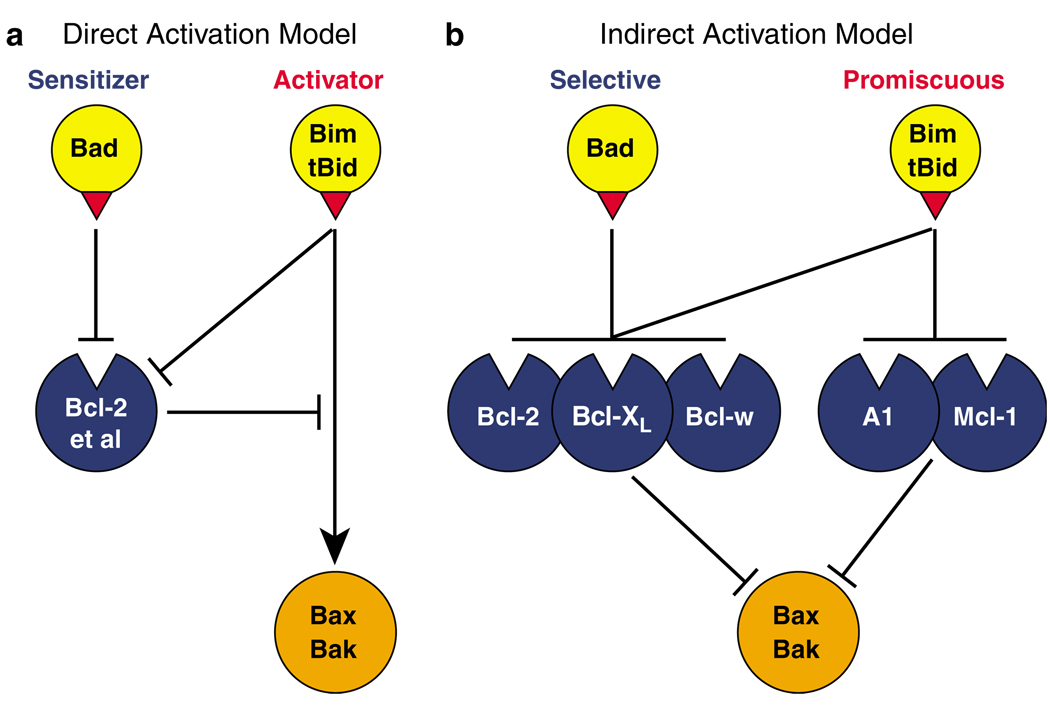
The BH3-only proteins clearly act upstream of Bax and Bak, because they cannot induce apoptosis in cells lacking both Bax and Bak (Cheng et al., 2001; Zong et al., 2001). How they induce activation of Bax and Bak is addressed by two distinct models (Figure 3; Willis and Adams, 2005). The direct activation model (Figure 3a) holds that certain BH3-only proteins, termed activators, namely Bim and tBid, can bind to Bax and Bak directly and promote their activation (Letai et al., 2002; Kuwana et al., 2005; Certo et al., 2006; Oh et al., 2006; Walensky et al., 2006). Puma has also been implicated as an activator (Carton et al., 2004; Kim et al., 2006a). In this model, the remaining BH3-only proteins, termed sensitizers, bind only to the pro-survival proteins and purportedly act by displacing any bound Bim or tBid, allowing them to directly activate Bax and Bak. The indirect activation model (Figure 3b), on the other hand, suggests that all the BH3-only proteins engage only their pro-survival relatives and act by preventing them from countering Bax or Bak activation (Chen et al., 2005; Willis et al., 2005; Willis et al., 2007). In this model, Bim and tBid are potent inducers of apoptosis simply because they can engage all the pro-survival proteins (Chen et al., 2005).
Figure 3.
Contrasting direct and indirect activation models for Bax and Bak. (a) In the direct model (Letai et al., 2002), the putative activators Bim and tBid bind directly to Bax and Bak to drive their activation, whereas the sensitizers only bind to the pro-survival Bcl-2 homologs (‘Bcl-2 et al.’) via the BH3 domain (red triangle). (b) In the indirect activation model (Chen et al., 2005; Willis et al., 2005, 2007), the BH3-only proteins activate Bax and Bak not by binding to them directly, but instead by engaging the multiple pro-survival proteins that guard Bax and Bak. In this model, Bim and tBid are more potent than Bad and other BH3-only proteins owing to the greater range of pro-survival proteins that they can engage and neutralize.
Several recent findings strongly challenge the direct activation model (Willis et al., 2007). Firstly, Bak bound to none of the BH3-only proteins. Secondly, Bax did not bind to either of the two physiologically relevant forms of Bim, namely BimEL and BimL, and no Bax could be co-immunoprecipitated with Bim in dying cells. Thirdly, although tBid and a very minor Bim isoform (BimS) did bind weakly to Bax (albeit only in detergents that alter the Bax conformation), tBid and BimS proteins bearing subtle BH3 mutations that prevent binding to Bax but not to pro-survival proteins killed cells as potently as wild-type tBid and BimS. Finally, and most tellingly, in response to several apoptotic stimuli, including imposed co-expression of Noxa and Bad, cells lacking both Bid and Bim underwent apoptosis as readily as wild-type cells. Although it has been suggested that Puma might also serve as an activator for Bax (Cartron et al., 2004; Kim et al., 2006a), this has been disputed (Kuwana et al., 2005; Certo et al., 2006; Ming et al., 2006) and downregulation of Puma by RNA interefernce, in cells lacking both Bim and Bid, did not impair apoptosis driven by several stimuli (Willis et al., 2007).
Collectively, these findings make it unlikely that direct activation of Bax or Bak by BH3-only proteins (Figure 3a) is the major physiological pathway to apoptosis. Nevertheless, it remains possible that direct activation can occur in some circumstances, to account for the ability of tBid or a Bid BH3 peptide with a chemically stabilized α-helix or one targeted to membranes to cooperate with Bax in the lysis of protein-free liposomes (Kuwana et al., 2002, 2005; Oh et al., 2006; Walensky et al., 2006).
The indirect activation model (Figure 3b) envisages that the pro-survival proteins function mainly by inhibiting Bax/Bak activation. Our recent evidence suggests that, at least for Bak, this occurs by direct interaction (Willis et al., 2005, 2007). In healthy cells, Bak can form a complex with either Mcl-1 or Bcl-xL (although, unexpectedly, not with Bcl-2), and this interaction requires the Bak BH3 domain (Willis et al., 2005; Figure 4a, top panel). Early in apoptosis, BH3-only proteins seem to displace Bak from these pro-survival proteins. Bak can drive cell death if and only if both Mcl-1 and Bcl-xL are inactivated by, for example, the combination of Noxa and Bad (Figure 2). As the Bak BH3 domain is critical for Bak-mediated apoptosis (Willis et al., 2005), one might speculate that this motif not only allows restraint of Bak by its pro-survival counterparts but also, once Bak is released from them, nucleates Bak oligomerization, perhaps by allowing a BH3-exposed conformer (‘primed’ Bak) to dimerize with an ‘unprimed’ receptor-like Bak conformer (Willis et al., 2005; Figure 4a, bottom panel).
Figure 4.
Model for the regulation of (a) Bak and (b) Bax by their pro-survival relatives. (a) In the healthy cell, both Bcl-xL and Mcl-1 can bind to Bak, presumably to a ‘primed’ conformer with its BH3 (red) exposed (Willis et al., 2007). Apoptosis is induced if and only if BH3-only proteins displace Bak from both these pro-survival guards. The free primed Bak may nucleate formation of the Bak oligomers (of unknown structure) that elicit permeabilization of the mitochondrial outer membrane and release of cytochrome c. (b) Bax appears to be regulated analogously (Willis et al., 2007), but most of the ‘unprimed’ Bax is cytosolic, and all the pro-survival family members (‘Bcl-2 et al’) appear to inhibit Bax activation. Whether a small amount of the postulated primed Bak and Bax exists in healthy cells, perhaps on the mitochondrial membrane, or instead is formed early in apoptosis by an unknown signal, is not yet established. The structures of the complexes is not known, and the integration of the proteins into the membrane probably includes regions in addition to their C-terminal regions (see text).
In view of the predominant cytosolic location of Bax in healthy cells (Wolter et al., 1997), its regulation must be more complex than that of Bak. Nevertheless, very recent findings (Willis et al., 2007) suggest that the indirect activation model (Figure 3b) also holds for Bax (Figure 4b). A complication has been that, although all of the pro-survival family members can bind to Bax, these interactions are generally detectable only in nonionic detergents, which change the Bax conformation (Hsu and Youle, 1997). Furthermore, only a small proportion of Bax molecules may be required to launch the apoptotic program (Lakhani et al., 2006; Zhu et al., 2006). Notably, recent functional tests have demonstrated that neutralization of multiple pro-survival proteins by BH3-only proteins is necessary and apparently sufficient to allow Bax-mediated apoptosis (Willis et al., 2007).
One interpretation of these results is that, in healthy cells, a small proportion of the Bax molecules exists in a ‘primed’ state, presumably a conformer with the BH3 domain exposed but complexed with various pro-survival relatives (Figure 4b, top panel). The putative primed Bax is likely to be located on the mitochondrial membrane (Figure 4b), because the cytosolic form is monomeric (Hsu and Youle, 1998) and has its membrane anchor buried in a groove on its surface (Suzuki et al., 2000). Alternatively, the primed Bax and Bak may form early in apoptotic signalling, either spontaneously or in response to an independent cytotoxic signal. For example, Bax may be phosphorylated on one site (Kim et al., 2006b) or dephosphorylated on another (Gardai et al., 2004) to induce its translocation to the membrane.
In any case, the functional studies (Willis et al., 2007) suggest that engagement of multiple pro-survival proteins by BH3-only proteins is sufficient to drive apoptosis via activation of either Bax or Bak (Figure 4). Studies with the BH3 mimetic drug ABT-737 (see below) are also consistent with this model (Konopleva et al., 2006; van Delft et al., 2006; Willis et al., 2007). However, the pro-survival proteins may be able to control Bax and Bak without binding to them, because mutants of Bax and Bak have been identified that do not bind the pro-survival proteins but are not constitutively active (Kim et al., 2006a; Fletcher J, DCS Huang and JMA, unpublished results).
For Bax, diverse other proteins or peptides have been proposed to contribute to its regulation (Lucken-Ardjomande and Martinou, 2005), either by restraining it in the cytosol (14-3-3 isoforms, the peptide humanin, Ku70, apoptosis repressor with caspase recruitment domain (ARC), Hsp-70 forms, crystallins) or by promoting its translocation to membranes or activity (Bif-1, p53, apoptosis-associated speck-like protein with caspase-recruitment domain (ASC), MAP-1 modulator of apoptosis-1 (MOAP-1)), but the evidence for a physiological role remains very limited. Similarly, a minor isoform of the voltage-dependent anion channel-2 (VDAC2) has been proposed to restrain Bak (Cheng et al., 2003).
Thus, important questions about the apoptotic switch remain unanswered. What is the critical signal that drives Bax to the mitochondria? Is a portion of Bax and Bak molecules primed for death duty in healthy cells? What is the nature of the putative complexes between the pro-survival family members and Bax or Bak? Can the pro-survival proteins prevent cell death in a fashion independent of binding Bax or Bak? Do non-family members have any role? Finally, what is the nature of the homo-oligomers of Bax and Bak and how do they permeabilize the outer mitochondrial membrane?
Physiological regulation and roles of family members
It is likely that the absence of all pro-survival family members in almost any mammalian cell would impose ‘death by default’. Most cell types express several pro-survival proteins, until trophic factors become limiting. Owing to their partially redundant function and complex expression patterns, the consequences of disrupting individual pro-survival genes vary considerably. For two, the impact appears very specific: loss of Bcl-w precludes adult spermatogenesis (Print et al., 1998; Ross et al., 1998), while A1 loss causes neutrophil attrition (Hamasaki et al., 1998). The others, however, have more diverse non-redundant roles. Bcl-2 is essential for the survival of kidney and melanocyte stem cells, as well as mature B and T lymphoid cells (Nakayama et al., 1993, 1994; Veis et al., 1993) and Bcl-xL is needed to sustain erythroid and neuronal cells (Motoyama et al., 1995, 1999; Wagner et al., 2000). Remarkably, Mcl-1 is obligatory for the survival and implantation of the zygote (Rinkenberger et al., 2000), and its conditional elimination has revealed critical roles in B and T lymphopoiesis and early hematopoiesis (Opferman et al., 2003, 2005), as well as in mature neutrophils but not macrophages (Dzhagalov et al., 2006). The particularly severe defects produced by the loss of Mcl-1 or Bcl-xL may reflect their key roles as guards on Bak (see above) (Willis et al., 2005).
The levels and activity of pro-survival proteins are regulated by diverse mechanisms, including transcriptional control and protein modification and turnover. The levels of Bcl-xL, A1 and Mcl-1, for example, are closely coupled to the supply of cytokines, which affects both their production and stability. Bcl-2 levels may also be controlled, in part, by micro-RNAs (Cimmino et al., 2005) and its activity is affected in complex ways by phosphorylation (Deng et al., 2004). In cells with DNA damage, Bcl-xL becomes deaminated on two asparagines in its flexible loop region (Deverman et al., 2002), but the claim that this blocks Bcl-xL pro-survival activity may well be erroneous (Deverman et al., 2003). The Mcl-1 protein is particularly labile and is rapidly lost by proteasomal degradation early in response to several cytotoxic signals (Cuconati et al., 2003; Nijhawan et al., 2003). Proteins implicated in regulation of its degradation include Noxa (Willis et al., 2005) and a BH3-containing E3 ubiquitin ligase called Mule (Zhong et al., 2005).
The multiplicity of mammalian BH3-only proteins presumably allows more precise control over apoptosis, and indeed the regulation of these proteins is complex, involving both transcriptional and diverse post-translational mechanisms (Puthalakath and Strasser, 2002; Cory et al., 2003). Puma, Noxa and Hrk seem to be regulated largely at the transcriptional level. Whereas bim can also be induced downstream of Akt by a Forkhead transcription factor (Dijkers et al., 2000), the Bim protein is subject to several types of post-translational regulation. In some cells, its binding via the dynein motor complex to microtubules (Puthalakath et al., 1999) may couple its control to the cytoskeleton (Puthalakath et al., 2001), perhaps accounting for its important role in the apoptotic response elicited by paclitaxel, which affects the microtubules (see below). In some cell types, however, Bim is instead bound predominantly to pro-survival relatives on the mitochondria (Zhu et al., 2004; Certo et al., 2006). The level of Bim can be regulated by phosphorylation by Erk, which triggers its degradation by the proteasome (Akiyama et al., 2003; Ley et al., 2003; Luciano et al., 2003), whereas its phosphorylation by c-Jun N-terminal kinase may potentiate its pro-apoptotic activity (Putcha et al., 2003). Bad is also regulated by phosphorylation, which leads to its sequestration by 14-3-3 scaffold proteins (Zha et al., 1996). Bid requires proteolytic processing, typically by a caspase (Figure 1) or granzyme B.
Mice lacking individual BH3-only proteins have provided considerable insight into their physiological roles (Strasser, 2005; Willis and Adams, 2005). BH3-only proteins trigger apoptosis not only in response to physiological stimuli such as cytokine deprivation, detachment from the cell matrix (anoikis) or antigen receptor signalling, but also in response to signals elicited by activated oncogenes, DNA damage, chemotherapy drugs and irradiation with UV light or γ-rays. Given the similar biochemical roles and wide-spread expression of many of these proteins, it is not surprising that most have at least partially redundant biological functions. Bim and Puma, however, have particularly notable roles, probably because they can engage all the pro-survival proteins (see above).
As reviewed recently (Strasser, 2005), Bim is a principal regulator of hematopoietic homeostasis, particularly in the lymphoid compartment (Bouillet et al., 1999). It provokes apoptosis of lymphocytes following certain cytotoxic stimuli, such as cytokine deprivation or Ca2+ flux, but not others such as phorbol 12-myristate 13-acetate (PMA) or etoposide (Bouillet et al., 1999). Bim is also critical for shaping the immune repertoire by eliminating thymocytes that recognize self-antigens (Bouillet et al., 2002) and in terminating T-cell immune responses (Hildeman et al., 2002; Pellegrini et al., 2003). In the differentiation of mammary epithelial cells into 3D structures, Bim appears to contribute to lumen formation, in part, by mediating anoikis of centrally localized cells (Reginato et al., 2003, 2005).
It has long been debated how, in many cell types subjected to DNA damage, the wild-type p53 tumor suppressor provokes apoptosis (Vousden and Lu, 2002). Multiple targets of this transcription factor have been implicated, and it has even been proposed that p53 has a direct apoptogenic role at the mitochondrion by engaging either Bcl-xL and Bcl-2 (Mihara et al., 2003) or Bax (Chipuk et al., 2004). Gene knockout studies, however, have established that the p53 transcriptional target Puma is the critical mediator of this response, particularly in the lymphoid system (Jeffers et al., 2003; Villunger et al., 2003). Noxa, the other BH3-only gene upregulated by p53, acts together with Puma in transformed fibroblasts, but has only a very minor role in lymphocytes. Notably, in several cell types, the protection from genotoxic damage conveyed by the loss of Puma is comparable to that conveyed by loss of p53 itself (Erlacher et al., 2005). At least in these cell types, this finding strongly questions the physiological significance of any Puma-independent pathway from p53 to apoptosis, such as the postulated direct role of cytoplasmic p53 at the mitochondria.
As well as its dominant role in p53-mediated responses, Puma also has a central role in several p53-independent responses (Jeffers et al., 2003; Villunger et al., 2003). It mediates, for example, the cytotoxic response to phorbol 12-myristate β-acetate (PMA) and contributes to that initiated by glucocorticoids.
Tissue homeostasis requires an appropriate balance between the levels of pro-survival family members and their BH3 ligands. Overexpression of Bcl-2 or its homologs provokes an abnormal accumulation of non-cycling cells (McDonnell et al., 1989; Strasser et al., 1991b; Ogilvy et al., 1999). Conversely, the tissue degeneration brought about by inadequate levels of these proteins can be prevented by a reduction in that of their BH3-only adversaries. Remarkably, loss of even a single allele of Bim sufficed to preclude the renal failure and immune collapse that otherwise ensues in mice lacking Bcl-2 (Bouillet et al., 2001). In contrast to the BH3-only proteins, the levels of Bax and Bak do not seem to be modulated significantly. Indeed, loss of single alleles of either, or even three of their (combined) four alleles, has little physiological impact (Lindsten et al., 2000).
Deregulation of the Bcl-2 life/death switch in oncogenesis
As indicated above, BCL-2 was first implicated in cancer through its involvement in the t14;18 chromosome translocation that hallmarks follicular lymphoma. B-lymphoid- specific transgenes (Eµ-bcl-2) that mimicked the translocation confirmed that deregulated Bcl-2 expression was indeed oncogenic, but the tumor incidence was low, latency long and most tumors were plasmacytomas rather than follicular lymphomas (McDonnell and Korsmeyer, 1991; Strasser et al., 1993). More recently, we have found that a transgene with pan-hematopoietic Bcl-2 expression (VavP-bcl-2) preferentially provokes follicular lymphoma, preceded by florid germinal center hyperplasia (Egle et al., 2004a). As the germinal center hyperplasia was driven by an expanded helper T-cell compartment, we have suggested that autoreactivity and hypermutation in the germinal center conspire with overexpressed Bcl-2 to generate human follicular lymphoma (Egle et al., 2004a).
Certain aggressive human follicular lymphomas and most tumors arising in Eµ-bcl-2 transgenic mice bear a translocated myc gene, suggesting that Bcl-2 might synergize with Myc. Evidence of collaboration first emerged from in vitro studies (Vaux et al., 1988). Direct proof came from bitransgenic Eµ-bc-2/Eµ-myc mice, which developed tumors much more rapidly than mice expressing either transgene alone (Strasser et al., 1990). The bcl-2/myc combination has subsequently been shown to promote malignant transformation of diverse mouse cell types (see Cory and Adams, 2002).
Myc is a transcription factor that regulates cell size, proliferation and differentiation. Under stress conditions, such as limiting cytokines, it also sensitizes cells to apoptosis; this function is thought to have evolved to eliminate cells with deregulated, and hence potentially tumorigenic, Myc expression. The marked synergy in oncogenesis between Bcl-2 and Myc therefore probably mainly reflects inhibition of Myc-induced apoptosis by Bcl-2 and its homologs. Many other oncogenes that perturb the cell cycle (e.g. Ras, adenovirus E1A) may also promote apoptosis unless countered by proteins such as Bcl-2.
In view of the oncogenic potential of antiapoptotic Bcl-2 family members, we postulated that their antagonists might be tumor suppressors (Cory and Adams, 2002). Indeed, we demonstrated that Bim is a potent tumor suppressor by showing that in Eµ-myc mice loss of even one bim allele accelerated tumorigenesis, primarily owing to early onset of acute B-cell-leukemia (Egle et al., 2004b). The reduced level of Bim was shown to decrease Myc-imposed apoptosis. Furthermore, although Bim does not appear to be a direct transcriptional target of Myc, Bim levels were higher in premalignant B lymphoid cells from Eµ-myc than non-transgenic mice. Conversely, Myc overexpression lowers expression of Bcl-xL and Bcl-2 (Eischen et al., 2001; Egle et al., 2004b), perhaps by blocking the activity of the MIZ transcription factor, which seems to regulate bcl-2 expression (Patel and McMahon, 2006). We hypothesize that Bim is activated, and Bcl-xL/Bcl-2 suppressed, when Myc-driven proliferation exceeds a physiologic check-point, such as cytokine availability. Loss of Bim would then permit cycling B cells to survive with the attendant risk of acquiring additional mutations (Egle et al., 2004b). Bim is also implicated as a tumor suppressor in mouse epithelial cancer (Tan et al., 2005).
In support of a tumor suppressor role for human BIM, examination of human lymphomas has revealed that the BIM gene has undergone biallelic deletion in a substantial proportion of mantle cell lymphomas (Tagawa et al., 2005; Mestre-Escorihuela et al., 2006) and has been silenced by promoter methylation in some other B-cell lymphomas, including the majority of Burkitt lymphomas (Mestre-Escorihuela et al., 2006), which are hallmarked by Myc translocation. Pertinently, the translocated MYC gene in some Burkitt lymphomas has undergone point mutations that preclude its activation of BIM (Hemann et al., 2005). A few other human lymphomas have a mutated NOXA gene (Mestre-Escorihuela et al., 2006).
The ability of wild-type p53 to elicit apoptosis is thought to be a major component of its tumor suppressor function (Vousden and Lu, 2002). Given that Puma is a major mediator of the p53 apoptotic response (Jeffers et al., 2003; Villunger et al., 2003), it is noteworthy that, unlike p53-deficient animals, Puma-null mice are not susceptible to spontaneous tumorigenesis (E Michalak, A Strasser and JMA, unpublished results). This may indicate that functions of p53 in addition to apoptosis (e.g. cell cycle arrest, senescence) are required for efficient tumor suppression. On the other hand, downregulation of Puma or its deletion does accelerate Myc-induced lymphomagenesis (Hemann et al., 2004; E Michalak, A Strasser and JM Adams, unpublished results). Hence, the tumor suppressor role of Puma, and probably also Bim, may arise mainly in the context where cell cycle control has been disrupted, such as by enforced Myc expression. In keeping with that notion, the tumor suppressor role of p53 appears to be linked much more tightly to signals from activated oncogenes via p19ARF than to those from DNA damage (Christophorou et al., 2006).
The redundancy of Bax and Bak would be expected to limit their roles as tumor suppressors. Indeed, Bax-deficient mice are not inherently tumor-prone (Knudson et al., 2001), and development of epithelial tumors in one study appeared to require loss of both Bax and Bak (Degenhardt et al., 2002). On the other hand, loss of Bax alone did accelerate development of mouse brain tumors driven by SV40-T antigen (Yin et al., 1997) and Myc-induced lymphomas (Eischen et al., 2001), and loss of even one allele accelerated mammary tumor development (Shibata et al., 1999). Another indication that its tumor suppressor role may be greater in certain cell types than others is that Bax loss shifted the tumor spectrum in ARF-deficient mice from lymphomas towards sarcomas and carcinomas (Eischen et al., 2002). Furthermore, some human colorectal carcinomas and hematopoietic tumors exhibit mutated Bax (Rampino et al., 1997; Meijerink et al., 1998) or Bak (Kondo et al., 2000), and these alterations convey a survival advantage (Ionov et al., 2000). Interestingly, several of these studies show that Bax loss is affecting a p53-independent pathway to tumorigenesis, while others suggest that it is acting downstream of p53. However, Bax is unlikely to be a direct p53 target gene, because the putative p53 binding sites identified in human BAX (Miyashita and Reed, 1995) are not used in the mouse (Schmidt et al., 1999).
Towards a more rational cancer therapy
As reviewed previously (Cory et al., 2003; Willis and Adams, 2005), increasing evidence encourages us to think that most of the cytotoxic stresses imposed on a cell lead to the activation of one or more of the BH3-only proteins as essential transducers of that stress signal. Pertinently, this holds for many, if not all, of the cytotoxic agents currently used in cancer therapy (Table 1). For example, the glucocorticoids that are often successful in acute lymphocytic leukemia (ALL) rely upon Bim and Puma (Bouillet et al., 1999; Villunger et al., 2003), whereas the kinase inhibitor imatinib (Gleevec), which has transformed treatment of chronic myelogenous leukemia (CML), elicits apoptosis mainly through Bim and Bad (Kuribara et al., 2004; Kuroda et al., 2006). Similarly, paclitaxel (Taxol) requires Bim in both lymphocytes (Bouillet et al., 1999) and epithelial tumor cells (Tan et al., 2005). In cells with wild-type p53, the genotoxic damage elicited by etoposide or γ-irradiation proceeds via induction of Puma (alone or with Noxa), but Bim, which is not induced by p53, also plays a minor role (Erlacher et al., 2005). The promising histone deacetylase (HDAC) inhibitors can also work through Bim (Zhao et al., 2005), with Bmf having some role (Zhang et al., 2005).
Table 1.
Anticancer treatments that act via BH3-only proteins
| Treatment | Likely mode of action | Tumor or cell type | BH3-only protein(s) implicated |
References |
|---|---|---|---|---|
| Glucocorti-coid | Activation of transcription | ALL | Bim and Puma | Bouillet et al. (1999), Villunger et al. (2003) |
| Imatinib | Tyrosine kinase inhibitor | CML | Bim and Bad | Kuribara et al. (2004), Kuroda et al. (2006) |
| Paclitaxel | Microtubule stabilizer | Lymphocytes, epithelial cancers | Bim | Bouillet et al. (1999), Tan et al. (2005) |
| Genotoxic damage (via wt p53) | p53-induced transcription | Multiple cell types | Puma and/or Noxa | Jeffers et al. (2003), Villunger et al. (2003) |
| Bortezomib | Proteasome inhibitor | Various cancers | Bim and/or Bik | Nikrad et al. (2005), Tan et al. (2005) |
| Bortezomib | Melanoma, myeloma | Noxa | Fernandez et al. (2005), Qin et al. (2005) | |
| Bortezomib | Squamous cell carcinoma | Noxa | Fribley et al. (2006) | |
| Epoxomicin | Proteasome inhibitor | Tumor with wt p53 | Puma | Concannon et al. (2006) |
Abbreviations: ALL, acute lymphocytic leukemia; CML, chronic myelogenous leukemia.
The critical mediators of the action of proteasome inhibitors seem to vary with cell type and p53 status (Table 1). In certain cancer cells, the Food and Drug Administration (FDA)-approved proteasome inhibitor bortezomib can work through Bim and/or Bik, and collaborate with TRAIL (Nikrad et al., 2005; Tan et al., 2005), but in melanomas and myelomas and squamous cell carcinoma, it works instead through Noxa in a p53-independent manner (Fernandez et al., 2005; Qin et al., 2005; Fribley et al., 2006). In tumor cells with wild-type p53, however, proteasome inhibitors can instead act by driving p53-dependent expression of Puma (Concannon et al., 2006).
Identification of these pathways stimulates the hope that the highly empirical nature of current cancer therapy, particularly combination chemotherapy, can be put on a more rational footing. That prospect is illustrated by a study concerning the role of Bim in the in vivo response of epithelial tumor cells to paclitaxel (Tan et al., 2005). Paclitaxel induced Bim accumulation and Bim-dependent apoptosis. However, if the Ras pathway was activated in the tumor, no apoptosis ensued, because Bim was phosphorylated and targeted for degradation by the proteasome (Akiyama et al., 2003). Nevertheless, addition of bortezomib restored the Bim levels and promoted Bim-dependent tumor regression (Tan et al., 2005). Hence, combination chemotherapy of bortezomib plus paclitaxel has promise, particularly in tumors with an activating Ras mutation.
Therapeutic potential of BH3 mimetics
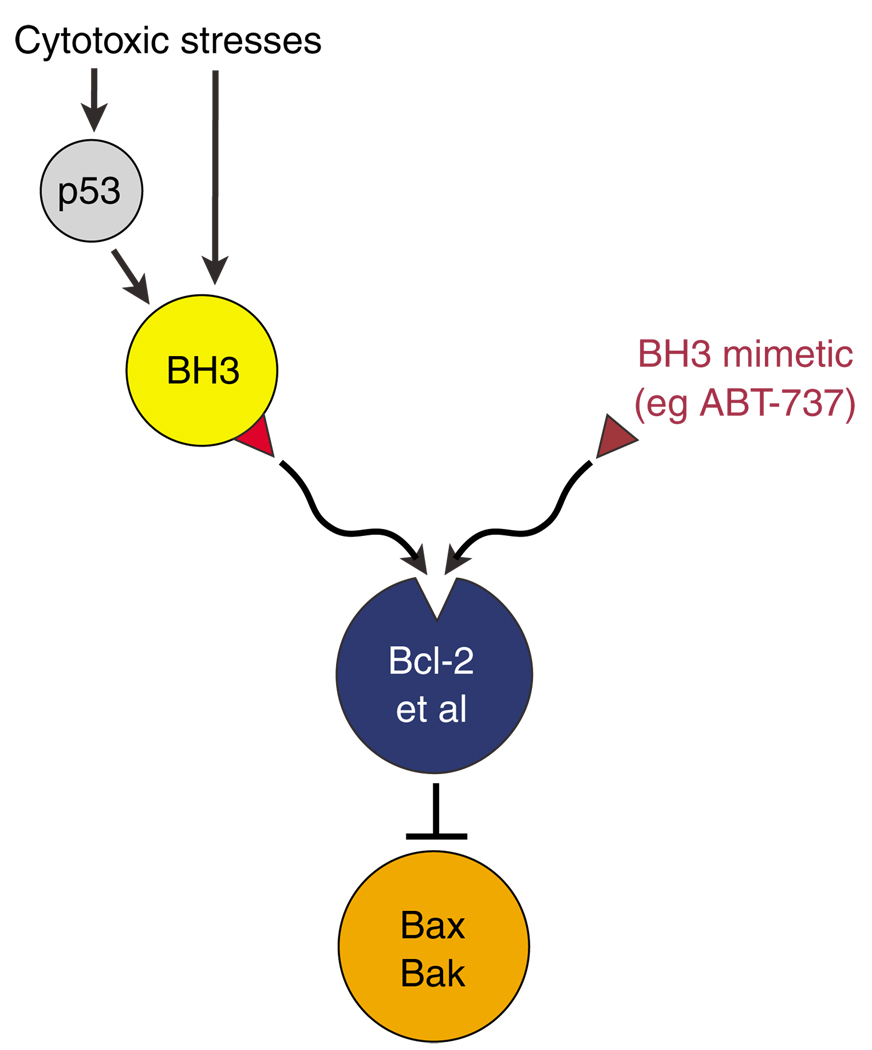
There is now great interest in the prospect of developing drugs that mimic the action of the BH3 domain by binding to one or more of the Bcl-2-like proteins and triggering the apoptotic program (Cory et al., 2003; Fesik, 2005). The appeal of such ‘BH3 mimetics’ is that the majority of tumors have defects in the p53 pathway and many overexpress Bcl-2 or a close relative, such as Bcl-xL (Amundson et al., 2000), but the core apoptotic machinery remains intact (Figure 5). Hence, a drug that could bind to the groove on the pro-survival proteins and inactivate them could be a more effective antitumor agent than drugs that act far upstream. Furthermore, the finding that particular BH3 domains preferentially bind particular pro-survival proteins (Chen et al., 2005) enhances the likelihood that it will be feasible to target specifically the Bcl-2-like protein(s) most important for maintaining a particular tumor type. That specificity offers the prospect of drugs that efficiently kill tumor cells, but have reduced cytotoxicity for normal cells.
Figure 5.
Potential of BH3 mimetic anticancer drugs. Because the upstream signals that induce apoptosis (particularly the p53 pathway) often are compromised in cancer cells, a drug that behaves like a BH3-only protein by binding to one or more of the Bcl-2 homologs to free Bax or Bak should be a more effective way of killing cancer cells.
In principle, the BH3 mimetic might be a BH3 peptide modified to have more suitable pharmacological properties, although the development of peptides as drugs has often failed. Nevertheless, BH3 peptides constrained to maintain an α helix by a chemical staple (Walensky et al., 2004, 2006), and peptides where the α-helical conformation is mimicked by unnatural amino-acid residues (Sadowsky et al., 2005, 2006), which should be more stable in vivo, are showing promise. Furthermore, the ability of a stapled Bid BH3 peptide to activate Bax, at least in vitro (Walensky et al., 2006), raises the possibility that Bax (or Bak) could be targeted, although that should promote more apoptosis in normal cells than a compound engaging selected pro-survival proteins.
At present, small organic molecules that bind to the groove, identified through high throughput screening and/or molecular design seem to have more potential. Although interference with a protein/protein interface is challenging, Abbott Laboratories have recently used a structure-based approach to develop a very promising BH3 mimetic, ABT-737 (Oltersdorf et al., 2005). This compound binds strongly (low nm affinity) to Bcl-2, Bcl-xL and Bcl-w but not to Mcl-1 or A1. It proved cytotoxic on its own with many B lymphoid tumor cell lines, primary cells from human follicular lymphoma, most chronic lymphocytic leukemia samples and even many cell lines from small cell lung cancers. Notably, ABT-737 induced stable regression of human lung cancer in a mouse xenograft model, with minimal side effects (Oltersdorf et al., 2005). The drug also effectively killed acute myeloid leukemia cells but not normal hematopoietic progenitor cells in vitro and also reduced leukemia growth in a xenograft model without overt effects on normal mouse tissues (Konopleva et al., 2006).
To evaluate how a BH3 mimetic might best be used, the mechanism of action of ABT-737 and six other putative BH3 mimetics has been explored (van Delft et al., 2006). Of the seven, only ABT-737 failed to kill cells lacking both Bax and Bak and therefore only it behaved as an authentic BH3 mimetic. Notably, cells expressing Mcl-1, which the drug does not bind, proved resistant to ABT-737, whereas those with little Mcl-1 were highly sensitive (Konopleva et al., 2006; van Delft et al., 2006). Down regulation of Mcl-1 by several strategies, however, conferred sensitivity to ABT-737, and the drug was effective even in cells with a high level of Bcl-2 or Bcl-xL (Konopleva et al., 2006; van Delft et al., 2006). A1, which ABT-737 also fails to bind, also promoted some resistance (van Delft et al., 2006). Hence ABT-737 should prove effective on its own in tumors with low Mcl-1 and A1, and may prove more widely efficacious when combined with agents that prevent Mcl-1 synthesis, promote its degradation or induce BH3-only proteins that can inactivate it.
BH3 mimetics may also prove to have an important adjuvant role in conjunction with conventional therapy. ABT-737 markedly sensitizes cells to diverse chemotherapy agents (Oltersdorf et al., 2005; Chauhan et al., 2006; Konopleva et al., 2006; van Delft et al., 2006), as does another compound (A-385358) that predominately interacts with Bcl-xL (Shoemaker et al., 2006). Conceivably, inclusion of the BH3 mimetic might allow lower doses of the conventional agent or more durable responses, unless substantial adverse effects arise. Another potential application is in imatinib-resistant CML. Bim and Bad are important mediators of imatinib action, but their activation was reduced in cells from some patients who were refractory to the drug (Kuroda et al., 2006). Interestingly, however, the resistance to apoptosis caused by loss of Bim could be overcome by co-treatment with ABT-737, which effectively supplanted its role. Thus, ABT-737 might render imatinib even more effective in some patients.
Collectively, all these preclinical findings with ABT-737 provide strong proof of principle that targeting Bcl-2 pro-survival proteins will have significant benefit in cancer therapy, and the results of the clinical trials just initiated will be eagerly awaited. Indeed, the Bcl-2-guarded life/death switch may well prove to be the Achilles’ heel of many tumors.
Acknowledgements
The issues addressed here have benefited greatly from discussions with many colleagues, including in particular our senior colleagues Drs David Huang and Andreas Strasser, as well as Drs Simon Willis and Jamie Fletcher. This research is supported by the National Health and Medical Research Council (Program Grant 257502), the Leukmia and Lymphoma Society (SCOR Grant 7015-02) and the US National Cancer Institute (CA80188, CA43540).
References
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–6110. [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellot G, Cartron PF, Er E, Oliver L, Juin P, Armstrong LC, et al. TOM22, a core component of the mitochondria outer membrane protein translocation pore, is a mitochondrial receptor for the proapoptotic protein Bax. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4402055. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte home-ostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Certo M, Moore Vdel G, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Velankar M, Brahmandam M, Hideshima T, Podar K, Richardson P, et al. A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene. 2006 doi: 10.1038/sj.onc.1210028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, et al. BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX-and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Chou CH, Lee RS, Yang-Yen HF. An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway. Mol Biol Cell. 2006;17:3952–3963. doi: 10.1091/mbc.E06-04-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Koehler BF, Reimertz C, Murphy BM, Bonner C, Thurow N, et al. Apoptosis induced by proteasome inhibition in cancer cells: predominant role of the p53/PUMA pathway. Oncogene. 2006 doi: 10.1038/sj.onc.1209974. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DCS, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Degenhardt K, Chen G, Lindsten T, White E. BAX and BAK mediate p53-independent suppression of tumorigenesis. Cancer Cell. 2002;2:193–203. doi: 10.1016/s1535-6108(02)00126-5. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, Flagg T, May WS., Jr Mono-and multisite phosphorylation enhances Bcl2’s antiapoptotic function and inhibition of cell cycle entry functions. Proc Natl Acad Sci USA. 2004;101:153–158. doi: 10.1073/pnas.2533920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosová I, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111:51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosová I, et al. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2003;115:503. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JJ, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Dzhagalov I, St John A, He YW. The anti-apoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2006 doi: 10.1182/blood-2006-03-013771. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bath ML, O’Reilly L, Cory S. VavP-Bcl2 transgenic mice develop follicular lymphoma preceded by germinal center hyperplasia. Blood. 2004a;103:2276–2283. doi: 10.1182/blood-2003-07-2469. [DOI] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004b;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eischen CM, Rehg JE, Korsmeyer SJ, Cleveland JL. Loss of Bax alters tumor spectrum and tumor numbers in ARF-deficient mice. Cancer Res. 2002;62:2184–2191. [PubMed] [Google Scholar]
- Eischen CM, Woo D, Roussel MF, Cleveland JL. Apoptosis triggered by myc-induced suppression of Bcl-XL or Bcl-2 Is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–5070. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlacher M, Michalak EM, Kelly PN, Labi V, Niederegger H, Coultas L, et al. BH3-only proteins Puma and Bim are rate-limiting for {gamma}-radiation and glucocorticoid-induced apoptosis of lymphoid cells in vivo. Blood. 2005;106:4131–4138. doi: 10.1182/blood-2005-04-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, et al. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, et al. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–31447. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Hamasaki A, Sendo F, Nakayama K, Ishida N, Negishi I, Nakayama K-I, et al. Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the bcl-2-related A1 gene. J Exp Med. 1998;188:1985–1992. doi: 10.1084/jem.188.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hemann MT, Bric A, Teruya-Feldstein J, Herbst A, Nilsson JA, Cordon-Cardo C, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, et al. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Hinds MG, Day CL. Regulation of apoptosis: uncovering the binding determinants. Curr Opin Struct Biol. 2005;15:690–699. doi: 10.1016/j.sbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Huang DC, Hahne M, Schroeter M, Frei K, Fontana A, Villunger A, et al. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-xL. Proc Natl Acad Sci USA. 1999;96:14871–14876. doi: 10.1073/pnas.96.26.14871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DCS, Strasser A. BH3-only proteins – essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- Ionov Y, Yamamoto H, Krajewski S, Reed JC, Perucho M. Mutational inactivation of the proapoptotic gene BAX confers selective advantage during tumor clonal evolution. Proc Natl Acad Sci USA. 2000;97:10872–10877. doi: 10.1073/pnas.190210897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006a;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Ryu SW, Song BJ. JNK-and p38 kinase-mediated phosphorylation of Bax leads to its activation, mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem. 2006b;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- Kim PK, Annis MG, Dlugosz PJ, Leber B, Andrews DW. During apoptosis bcl-2 changes membrane topology at both the endoplasmic reticulum and mitochondria. Mol Cell. 2004;14:523–529. doi: 10.1016/s1097-2765(04)00263-1. [DOI] [PubMed] [Google Scholar]
- Knudson CM, Johnson GM, Lin Y, Korsmeyer SJ. Bax accelerates tumorigenesis in p53-deficient mice. Cancer Res. 2001;61:659–665. [PubMed] [Google Scholar]
- Kondo S, Shinomura Y, Miyazaki Y, Kiyohara T, Tsutsui S, Kitamura S, et al. Mutations of the bak gene in human gastric and colorectal cancers. Cancer Res. 2000;60:4328–4330. [PubMed] [Google Scholar]
- Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Kuribara R, Honda H, Matsui H, Shinjyo T, Inukai T, Sugita K, et al. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitor. Mol Cell Biol. 2004;24:6172–6183. doi: 10.1128/MCB.24.14.6172-6183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda J, Puthalakath H, Cragg MS, Kelly PN, Bouillet P, Huang DC, et al. Bim and Bad mediate imatinib-induced killing of Bcr/Abl+ leukemic cells, and resistance due to their loss is overcome by a BH3 mimetic. Proc Natl Acad Sci USA. 2006;103:14907–14912. doi: 10.1073/pnas.0606176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Lakhani SA, Masud A, Kuida K, Porter GA, Jr, Booth CJ, Mehal WZ, et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, et al. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Martinou JC. Newcomers in the process of mitochondrial permeabilization. J Cell Sci. 2005;118:473–483. doi: 10.1242/jcs.01654. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Deane N, Platt FM, Nuñez G, Jaeger U, McKearn JP, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14;18) Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- Meijerink JPP, Mensink EJBM, Wang K, Sedlak TW, Slöetjes AW, de Witte T, et al. Hematopoietic malignancies demonstrate loss-of-function mutations of BAX. Blood. 1998;91:2991–2997. [PubMed] [Google Scholar]
- Mestre-Escorihuela C, Rubio-Moscardo F, Richter JA, Siebert R, Climent J, Fresquet V, et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood. 2006 doi: 10.1182/blood-2006-06-026500. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and Bcl-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a Bak homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Motoyama N, Kimura T, Takahashi T, Watanabe T, Nakano T. bcl-x prevents apoptotic cell death of both primitive and definitive erythrocytes at the end of maturation. J Exp Med. 1999;189:1691–1698. doi: 10.1084/jem.189.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang FP, Roth KA, Sawa H, Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Nakayama K-I, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K-I, Nakayama K, Izumi N, Kulda K, Shinkai Y, Louie MC, et al. Disappearance of the lymphoid system in Bcl-2 homozygous mutant chimeric mice. Science. 1993;261:1584–1588. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–490. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Barbuto S, Pitter K, Morash J, Walensky LD, Korsmeyer SJ. A membrane-targeted BID BH3 peptide is sufficient for high potency activation of BAX in vitro. J Biol Chem. 2006;281:36999–37008. doi: 10.1074/jbc.M602341200. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Opferman J, Iwasaki H, Ong CC, Suh H, Mizuno S, Akashi K, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, et al. Inhibiting the mitochondrial fission machinery does not prevent bax/bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JH, McMahon SB. BCL2 is a downstream effector of MIZ-1 essential for blocking c-MYC induced apoptosis. J Biol Chem. 2006 doi: 10.1074/jbc.M609138200. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Belz G, Bouillet P, Strasser A. Shut down of an acute T cell immune response to viral infection is mediated by the pro-apoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, et al. Apoptosis regulator Bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Huang DCS, O’Reilly LA, King SM, Strasser A. The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- Rampino N, Yamamosto H, Ionov Y, Li Y, Sawai H, Reed JC, et al. Somatic frameshift mutations in the bax gene in colon cancers of the microsatellite mutator phenotype. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, Muthuswamy SK, et al. Bim regulation of lumen formation in cultured mammary epithelial acini is targeted by oncogenes. Mol Cell Biol. 2005;25:4591–4601. doi: 10.1128/MCB.25.11.4591-4601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, et al. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Waymire KG, Moss JE, Parlow AF, Skinner MK, Russell LD, et al. Testicular degeneration in Bclw-deficient mice. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- Sadowsky JD, Fairlie WD, Hadley EB, Lee H-S, Umezawa N, Nikolovska-Coleska Z, et al. Characterization of (a/b+a)-peptide antagonists of BH3 domain/Bcl-XLrecognition: toward general strategies for developing foldamer-based inhibitors of protein–protein interactions. J Am Chem Soc. 2006 doi: 10.1021/ja0662523. in press. [DOI] [PubMed] [Google Scholar]
- Sadowsky JD, Schmitt MA, Lee HS, Umezawa N, Wang S, Tomita Y, et al. Chimeric (alpha/beta+alpha)-peptide ligands for the BH3-recognition cleft of Bcl-XL: critical role of the molecular scaffold in protein surface recognition. J Am Chem Soc. 2005;127:11966–11968. doi: 10.1021/ja053678t. [DOI] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Schmidt T, Korner K, Karsunky H, Korsmeyer S, Muller R, Moroy T. The activity of the murine bax promoter is regulated by Sp1/3 and E-box binding proteins but not by p53. Cell Death Differ. 1999;6:873–882. doi: 10.1038/sj.cdd.4400562. [DOI] [PubMed] [Google Scholar]
- Schmitt CA, Rosenthal CT, Lowe SW. Genetic analysis of chemoresistance in primary murine lymphomas. Nat Med. 2000;6:1029–1035. doi: 10.1038/79542. [DOI] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Shibata MA, Liu ML, Knudson MC, Shibata E, Yoshidome K, Bandey T, et al. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J. 1999;18:2692–2701. doi: 10.1093/emboj/18.10.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker AR, Oleksijew A, Bauch J, Belli BA, Borre T, Bruncko M, et al. A small-molecule inhibitor of Bcl-XL potentiates the activity of cytotoxic drugs in vitro and in vivo. Cancer Res. 2006;66:8731–8739. doi: 10.1158/0008-5472.CAN-06-0367. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S. Bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991a;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S. Eµ-bcl-2 transgene facilitates spontaneous transformation of early pre-B and immunoglobulin-secreting cells but not T cells. Oncogene. 1993;8:1–9. [PubMed] [Google Scholar]
- Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Ann Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci USA. 1991b;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, et al. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–1358. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, et al. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Claudio E, Rucker EB, III, Riedlinger G, Broussard C, Schwartzberg PL, et al. Conditional deletion of the Bcl-x gene from erythroid cells results in hemolytic anemia and profound splenomegaly. Development. 2000;127:4949–4958. doi: 10.1242/dev.127.22.4949. [DOI] [PubMed] [Google Scholar]
- Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Pro-apoptotic Bak is sequestered by Mc1-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007 doi: 10.1126/science.1133289. in press. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin CY, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not Bcl-xL. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adachi M, Kawamura R, Imai K. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 2005;13:129–140. doi: 10.1038/sj.cdd.4401686. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci USA. 2005;102:16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-Only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Liu X, Hildeman D, Peyerl FW, White J, Kushnir E, et al. Bax does not have to adopt its final form to drive T cell death. J Exp Med. 2006;203:1147–1152. doi: 10.1084/jem.20051736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]