Abstract
Aims
Hypertrophic cardiomyopathy (HCM) is an important cause of heart failure-related disability over a wide range of ages. Profiles of severe progressive heart failure symptoms and death, or heart transplantation deserve more complete definition within large patient cohorts.
Methods and results
Clinical and morphological features of heart failure were assessed in 293 consecutive HCM patients over a median follow-up of 6 (inter-quartile range 2–11) years. Gross and histopathological features were analysed in 12 patients for whom the heart was available for inspection. Of the 293 patients, 50 (17%) developed severe progressive heart failure, including 18 who died or were transplanted. Three profiles of heart failure were identified predominantly associated with: (i) end-stage systolic dysfunction (ejection fraction <50%) (15; 30%); (ii) left ventricular (LV) outflow obstruction at rest (11; 22%); and (iii) non-obstructive with preserved systolic function (24; 48%). Overall, atrial fibrillation (AF) contributed to heart failure in 32 patients (64%) among the three profiles. Compared with other patients, those non-obstructive with preserved systolic function had earlier onset of heart failure symptoms mainly due to diastolic dysfunction, and the most accelerated progression to advanced heart failure and adverse outcome (P = 0.04). Thrombi were identified in the left atrial appendage of five gross heart specimens all belonging to patients with AF, including three of which were unrecognized clinically and had previously embolized. Extensive myocardial scarring with LV remodelling was evident in all end-stage patients; no or only focal scars were present in other patients.
Conclusion
Profiles of advanced heart failure in HCM are due to diverse pathophysiological mechanisms, including LV outflow obstruction and diastolic or global systolic ventricular dysfunction. Atrial fibrillation proved to be the most common disease variable associated with progressive heart failure. Recognition of the heterogeneous pathophysiology of heart failure in HCM is relevant, given the targeted management strategies necessary in this disease.
Keywords: Hypertrophic cardiomyopathy, Heart failure, Cardiovascular pathology, Atrial fibrillation, Cardiac transplant
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiac disease1,2 with a prevalence of 1:500 in the general population.2 The clinical presentation and natural history is particularly heterogeneous, ranging from benign asymptomatic forms to more malignant expressions that may result in premature death through several pathways.2–4
Although the risk for sudden cardiac death in young patients has been a highly visible complication, it should be underscored that HCM is also an important cause of heart failure-related disability and death. Moreover, it is now evident that progressive heart failure in HCM does not occur in a single unique clinical setting, but under a variety of circumstances due to diverse pathophysiological mechanisms.1,2 We have longitudinally assessed a large consecutive single-institution cohort of HCM patients, to derive insights into the profiles and pathophysiology of heart failure in this complex disease.
Methods
Patient selection
From January 1980 to November 2001, 293 consecutive patients with HCM were evaluated in Padua University HCM Center and were assessed for the occurrence and clinicopathological profile of progressive, severe heart failure. Median follow-up was 6 years (inter-quartile range 2–11 years). Fifty-one patients with chest pain underwent coronary arteriography and 12 had obstructive coronary artery disease (CAD) (i.e. diameter stenosis >75% of ≥1 major epicardial coronary artery).
Echocardiography
Echocardiographic studies were performed with IREX System III, Hewlett–Packard 77020, and SONOS 1000, 2500, 5500 instruments. Maximal left ventricular (LV) wall thickness was the greatest dimension within the chamber. Left ventricular cavity and left atrial dimensions were obtained from M-mode echocardiograms, derived from two-dimensional images.5 Outflow obstruction at rest, due to mitral valve systolic anterior motion, was identified by peak instantaneous LV outflow gradient ≥30 mmHg.6 Left ventricular ejection fraction was calculated from two-dimensional images with modified Simpson's formula or area–length method.5
Parameters of LV filling were obtained with pulsed Doppler echocardiography:7 peak flow velocity in early diastole (E) and during atrial contraction (A), and E/A ratio; deceleration time (DT) of early diastolic flow velocity (descent of E/F slope).7
Definitions
Hypertrophic cardiomyopathy was diagnosed by a hypertrophied non-dilated LV, with absolute wall thickness ≥13 mm (or the equivalent corrected for body size in children), in the absence of another cardiac or systemic disease capable of producing the magnitude of hypertrophy evident, at some point in the clinical course.1,2
Heart failure death was defined as progressive cardiac decompensation over ≥1 year before death or transplant, which frequently required hospitalization, particularly if complicated by pulmonary oedema. Severe progressive heart failure is defined as symptomatic evolution to New York Heart Association (NYHA) functional class III/IV, characterized by exertional dyspnoea (with or without chest pain), refractory to maximal medical management. Diastolic dysfunction causing symptoms of heart failure requires two conditions to be satisfied: (i) normal LV systolic function (ejection fraction >50%); and (ii) evidence of abnormal LV relaxation and distensibility as defined by pulsed Doppler echocardiography.8,9 Abnormal LV relaxation (mild or grade 1 diastolic dysfunction) was defined as a decrease in early diastolic flow velocity (reduced E-wave amplitude) associated with increase in atrial contraction (greater A-wave amplitude) with E/A <1. Restrictive LV filling pattern was considered present in the setting of sinus rhythm and shortened DT < 150 ms and E/A > 2.8
Genetics
Mutation screening of exon 2–40 of MYH7 gene, exon 1–35 of MYBPC3 gene, and exon 1–8 and exon 8–16 of TNNI3 and TNNT2 genes, respectively, was performed in 18 of 50 patients with progressive heart failure. The method consisted of PCR amplification of each DNA segment, denaturing high-performance liquid chromatography and DNA sequencing of segments showing extra peaks. Novel mutations were surveyed for polymorphism in a large cohort of healthy controls (n = 400).
Pathology
Gross examination addressed heart weight, LV wall thickness (i.e. exclusive papillary muscles and trabeculae), chamber dilatation (0 to 3+), coronary arterial anatomy, and scarring. Full-thickness tissue blocks were obtained from ventricular septum and LV free wall. Tissue specimens were embedded in paraffin, sectioned at 6 µm and stained with haematoxylin–eosin and azan-Heidenhein trichrome.
Blocks were examined microscopically to assess myocyte disarray, interstitial and replacement fibrosis, and intramural small vessel disease.10–13 Fibrosis was defined as interstitial when myocytes were encircled by collagen matrix and replacement-type when myocytes were substituted by connective tissue. Disarray and fibrosis were graded 0 to 3+.
Statistical analysis
Data are expressed as mean ± SD for continuous variables. Differences between means were tested by unpaired Student's t-test. Categorical frequencies were compared by χ2 or Fisher's exact test, where appropriate. Probability values reported are two-sided, and values <0.05 were considered statistically significant. SAS Statistical System 9.1 (SAS Institute Inc., Cary, NC, USA) and SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) were used for analysis.
Results
Advanced heart failure
Progressive heart failure leading to NYHA functional classes III and IV occurred in 50 of 293 study patients (17%; Table 1). Among the remaining 243 patients who developed mild or no heart failure, 160 (66%) had NYHA I and 83 (34%) NYHA II. Incidence of advanced heart failure and death was 24/1000 HCM patient-years (95% confidence interval: 18–32). Of the 50 patients, 10 died of heart failure and 8 underwent heart transplantation. Patients with advanced heart failure were 41 ± 20 years old at HCM diagnosis and 48 ± 19 years at HCM-related heart failure symptom onset (interval 7 ± 9 years). Compared with other study patients, those with progressive heart failure were more commonly women and, more frequently, had atrial fibrillation (AF) and moderate-to-severe mitral regurgitation (Table 1). No statistically significant difference in CAD prevalence was found between patients in NYHA III–IV and those in NYHA I–II heart failure classes. Among the 51 patients who underwent coronary arteriography, 12 (24%) were ≤39 years old, 20 (39%) were 40–59 years, and the remaining 19 (37%) ≥60 years; CAD was detectable only in the two older age groups, i.e. in 4 patients 40–59 years of age and in 8 patients aged >60 years.
Table 1.
Baseline characteristics in hypertrophic cardiomyopathy patients with or without progressive heart failure
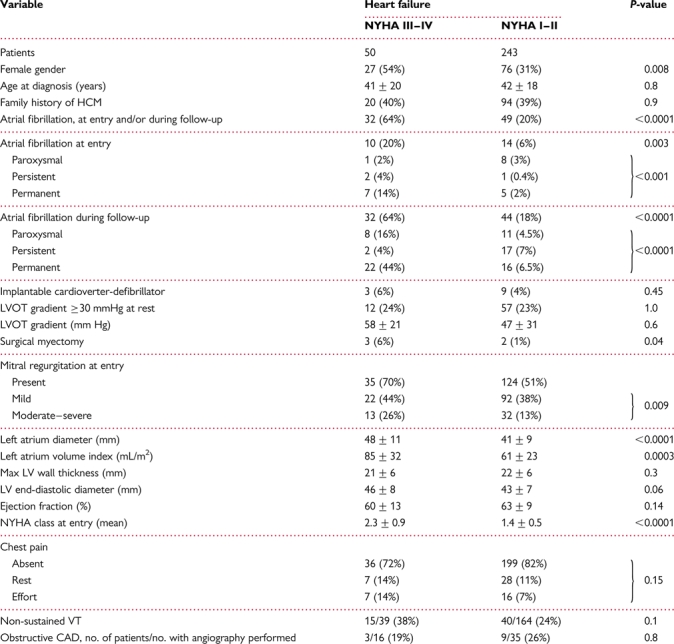 |
CAD, coronary artery disease; HCM, hypertrophic cardiomyopathy; LV, left ventricle; LVOT, left ventricle outflow tract, NYHA, New York Heart Association; VT, ventricular tachycardia.
Profiles of heart failure
Three diverse profiles of severe heart failure were defined, based on the predominant pathophysiological disease component: (i) end-stage systolic dysfunction (n = 15; 30%); (ii) LV outflow obstruction (n = 11; 22%); and (iii) non-obstructive with preserved systolic function (n = 24; 48%) (Tables 2 and 3; Figures 1–5).
Table 2.
Comparison of demographic, clinical, and functional data in three hypertrophic cardiomyopathy heart failure profiles
| End-stage | LVOTO | Non-obstructive (preserved systolic function) |
P-values |
|||||
|---|---|---|---|---|---|---|---|---|
| All | With AF | NSR | End-stage vs. LVOTO | End-stage vs. non-obstructive (preserved systolic function) | LVOTO vs. non-obstructive (preserved systolic function) | |||
| No. of patients | 15 | 11 | 24 | 15 | 9 | |||
| Male | 7 (47%) | 5 (45%) | 11 (46%) | 8 (53%) | 3 (33%) | 0.9 | 0.9 | 0.9 |
| Age at diagnosis (years) | 46 ± 20 | 50 ± 14 | 35 ± 20 | 36 ± 15 | 34 ± 27 | 0.5 | 0.1 | 0.02 |
| Age at onset HF symptoms (years) | 57 ± 14 | 54 ± 15 | 40 ± 21 | 42 ± 15 | 36 ± 29 | 0.6 | 0.004 | 0.03 |
| Age at death/transplant (years) | 62 ± 10 | 67 | 41 ± 22 | 41 ± 16 | 41 ± 26 | 0.6 | 0.01 | 0.2 |
| Interval: symptom onset to death/transplant (years) | 5 ± 6 | 7 | 6 ± 8 | 7 ± 8 | 3 ± 5 | 0.7 | 0.7 | 0.9 |
| Death/transplant | 6 (40%) | 1 (9%) | 11 (46%) | 5 (33%) | 6 (67%) | 0.07 | 0.7 | 0.03 |
| Left atrial dimension | ||||||||
| At entry (mm) | 49 ± 9 | 44 ± 7 | 51 ± 13 | 53 ± 12 | 46 ± 15 | 0.1 | 0.6 | 0.1* |
| Most recent (mm) | 55 ± 7 | 53 ± 8 | 59 ± 23 | 63 ± 24 | 50 ± 17 | 0.5 | 0.6 | 0.4 |
| Atrial fibrillation | ||||||||
| At entry | 4 (27%) | 1 (9%) | 5 (21%) | 5 (33%) | 0 | 0.3 | 0.7 | 0.4 |
| Most recent | 11 (73%) | 6 (54%) | 15 (62%) | 15 (100%) | 0 | 0.3 | 0.6† | 0.5‡ |
| Ejection fraction | ||||||||
| At entry (%) | 51 ± 16 | 69 ± 9 | 62 ± 9 | 63 ± 9 | 61 ± 10 | 0.003 | 0.006 | 0.06 |
| Most recent (%) | 40 ± 6 | 59 ± 7 | 60 ± 7 | 60 ± 7 | 62 ± 9 | <0.001 | <0.001 | 0.6 |
| LV end-diastolic diameter | ||||||||
| At entry (mm) | 50 ± 7 | 46 ± 6 | 43 ± 9 | 44 ± 9 | 40 ± 9 | 0.1 | 0.01 | 0.2 |
| Most recent (mm) | 56 ± 10 | 43 ± 5 | 44 ± 8 | 45 ± 7 | 41 ± 10 | <0.001 | 0.001 | 0.8 |
| LVOT peak systolic gradient | ||||||||
| At entry (mmHg) | 5 ± 17 | 58 ± 21 | 3 ± 7 | 3 ± 7 | 2 ± 5 | <0.001 | 0.6 | <0.001 |
| Most recent (mmHg) | 0 ± 0 | 89 ± 59 | 0 ± 2 | 0 | 1 ± 3 | <0.001 | 0.3 | <0.001 |
| Diastolic dysfunction | ||||||||
| At entry, n/n available | 6/13 (46%) | 1/7 (14%) | 17/21 (81%) | 8/12 (67%) | 9/9 (100%) | 0.1 | 0.04§ | 0.002 |
| Most recent, n/n available | 9/12 (75%) | 1/3 (33%) | 13/13 (100%) | 6/6 (100%) | 7/7 (100%) | 0.2 | 0.05 | 0.002 |
| CAD/coronary angiograms | 2/8 | 0/2 | 1/6 | 1/4 | 0/2 | 0.4 | 0.7 | 0.5 |
| Myectomy/MVR | 0/0 | 2/2 | 0/2 | 0/2 | 0/0 | 0.09 | n.a./0.2 | 0.03/0.4 |
AF, atrial fibrillation; CAD, coronary artery disease; HF, heart failure; LV, left ventricle; LVOTO, left ventricular outflow tract obstruction; MVR, mitral valve replacement; n.a., not available; NSR, normal sinus rhythm.
*P= 0.04 LVOTO vs. non-obstructive (preserved systolic function) with AF.
†P= 0.03 end-stage vs. non-obstructive (preserved systolic function) with AF.
‡P= 0.004 LVOTO vs. non-obstructive (preserved systolic function) with AF.
§P= 0.007 end-stage vs. non-obstructive (preserved systolic function) with NSR.
Table 3.
Clinical data of hypertrophic cardiomyopathy patients with advanced heart failure
| Patient no. | Gender | Gene mutation | Age at Dx, years | Age at symptom onset, years | CAD | LA entry, mm | LA FU, mm | AF entry | AF FU | LVOT peak gradient entry, mmHg | LVOT peak gradient FU, mm Hg | EF entry, % | EF FU, % | Max- LV, mm | LVED entry, mm | LVED FU, mm | PW LV filling pattern entry | PW LV filling pattern FU | MR entry | MR FU | NYHA class entry | NYHA class FU | Surgery | SE | Outcome (age) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| End-stage | |||||||||||||||||||||||||
| 1 | M | 0 | 5 | 26 | 0 | 30 | 49 | 0 | parox | 0 | 0 | 62 | 38 | 25 | 45 | 60 | E/A > 1 | E/A > 1 | 0 | 1 | I | III | 0 | 0 | Alive |
| 2 | F | 0 | 11 | 40 | — | 51 | 50 | 0 | 0 | 15 | 0 | 58 | 40 | 32 | 43 | 62 | E/A > 1 | E/A > 1 | 0 | 1 | I | III | 0 | 0 | List for TP |
| 3 | F | 0 | 17 | 47 | 0 | 46 | 54 | 0 | perm | 0 | 0 | 61 | 46 | 20 | 48 | 57 | E/A > 1 | RES | 0 | 1 | II | III | 0 | 0 | Alive, PM |
| 4 | M | 0 | 38 | 38 | — | 65 | 68 | 0 | perm | 0 | 0 | 44 | 45 | 21 | 56 | 56 | RES | RES | 1 | 0 | II | IV | 0 | 0 | TP (50) |
| 5 | M | TNNT2 | 39 | 56 | + | 60 | 70 | perm | perm | 0 | 0 | 26 | 30 | 13 | 58 | 62 | RES | RES | 1 | 1 | II | III | 0 | 0 | TP (59) |
| EX 9 | |||||||||||||||||||||||||
| Arg94Leu | |||||||||||||||||||||||||
| 6 | M | — | 39 | 59 | — | 48 | 56 | 0 | perm | 0 | 0 | 60 | 46 | 22 | 48 | 57 | E/A > 1 | RES | 0 | 1 | II | III | 0 | 0 | Alive, ablation |
| 7 | M | — | 48 | 74 | — | 53 | 56 | 0 | parox | 0 | 0 | 74 | 46 | 25 | 51 | 47 | E/A > 1 | E/A > 1 | 1 | — | I | III | 0 | 0 | Alive, PM |
| 8 | F | — | 54 | 54 | 0 | 44 | 45 | 0 | parox | 0 | 0 | 47 | 46 | 25 | 45 | 31 | RES | RES | 2 | 2 | III | III | 0 | + | HF death (55) |
| 9 | F | — | 55 | 55 | 0 | 58 | 59 | pers | perm | 0 | 0 | 25 | 47 | 19 | 60 | 61 | — | — | 1 | 2 | III | III | 0 | 0 | Alive, PM, ICDa |
| 10 | F | 0 | 59 | 66 | — | 50 | 48 | perm | perm | 0 | 0 | 32 | 36 | 18 | 40 | 38 | — | RES | 1 | 1 | II | IV | 0 | 0 | HF death (68) |
| 11 | F | MYBPC3 | 59 | 63 | 0 | 43 | 51 | perm | perm | 0 | 0 | 34 | 31 | 16 | 55 | 58 | RES | RES | 2 | 2 | II | III | 0 | 0 | TP (63) |
| EX 13 | |||||||||||||||||||||||||
| Gln366X | |||||||||||||||||||||||||
| 12 | M | — | 64 | 65 | 0 | 42 | 60 | 0 | 0 | 0 | 0 | 40 | 32 | 18 | 35 | 68 | E/A > 1 | RES | 2 | 3 | I | III | 0 | 0 | HF death (77) |
| 13 | F | — | 65 | 65 | — | 50 | 52 | 0 | 0 | 64 | 0 | 70 | 31 | 25 | 52 | 64 | E/A < 1 | — | 2 | 0 | III | III | 0 | 0 | Alive |
| 14 | M | — | 65 | 80 | + | 52 | 58 | 0 | perm | 0 | 0 | 71 | 43 | 23 | 53 | 60 | E/A > 1 | — | 1 | 0 | I | III | CABG | 0 | Alive |
| 15 | F | — | 66 | 66 | — | 38 | 55 | 0 | 0 | 0 | 0 | 54 | 41 | 17 | 60 | 60 | E/A > 1 | E/A > 1 | 0 | 0 | IV | III | 0 | 0 | Alive |
| LV outflow obstruction | |||||||||||||||||||||||||
| 16 | M | MYH7 | 16 | 16 | — | 45 | 51 | 0 | 0 | 80 | 50 | 83 | 58 | 35 | 50 | 40 | E/A > 1 | E/A > 1 | 2 | 1 | III | III | 0 | 0 | Alive |
| EX 22 | |||||||||||||||||||||||||
| Met877Ile | |||||||||||||||||||||||||
| 17 | M | — | 41 | 41 | — | 41 | 45 | 0 | parox | 40 | 190b | 75 | 66 | 21 | 41 | 40 | E/A > 1 | E/A < 1 | 1 | 1 | III | III | 0 | 0 | Alive |
| 18 | M | — | 45 | 64 | 0 | 48 | 64 | 0 | perm | 55 | 40 | 75 | 50 | 25 | 52 | 55 | E/A > 1 | — | 1 | 0 | II | III | 0 | 0 | Alive |
| 19 | F | — | 47 | 47 | — | 60 | 65 | 0 | 0 | 50 | 80 | 68 | 57 | 25 | 50 | 42 | E/A > 1 | E/A > 1 | 1 | 2 | IV | III | 0 | 0 | Alive |
| 20 | M | — | 51 | 61 | — | 35 | 38 | 0 | 0 | 60 | 60 | 50 | 50 | 18 | 53 | 47 | E/A > 1 | — | 0 | 1 | III | III | 0 | 0 | Alive, PM |
| 21 | F | — | 51 | 54 | — | 46 | 55 | perm | perm | 50 | 60 | 62 | 66 | 28 | 38 | 40 | — | — | 2 | 2 | III | III | 0 | 0 | Alive |
| 22 | M | 0 | 52 | 58 | 0 | 38 | 58 | 0 | perm | 45 | 75 (0) | 68 | 63 | 27 | 42 | 42 | E/A > 1 | — | 2 | 2 | III | III (I) | Myectomy + MVR | 0 | Alive |
| 23 | F | — | 54 | 62 | — | 36 | 56 | 0 | parox | 40 | 40 (0) | 77 | 67 | 29 | 40 | 41 | E/A < 1 | — | 1 | 2 | IV | III (I) | Myectomy + MVR | 0 | Alive |
| 24 | F | — | 60 | 60 | — | 50 | 48 | 0 | 0 | 110 | 200 | 71 | 59 | 23 | 41 | 46 | E/A > 1 | — | 2 | 2 | III | IV | 0 | 0 | Alive, PM |
| 25 | F | — | 60 | 60 | — | 40 | 54 | 0 | perm | 65 | 95 | 62 | 53 | 25 | 46 | 38 | E/A > 1 | — | 1 | 1 | II | IV | 0 | 0 | HF death (67) |
| 26 | F | — | 72 | 72 | — | 45 | — | 0 | 0 | 45 | — | 68 | — | 19 | 51 | — | E/A > 1 | — | 2 | — | IV | — | 0 | 0 | Lost |
| Non-obstructive with preserved systolic function + atrial fibrillation | |||||||||||||||||||||||||
| 27 | F | — | 14 | 34 | — | 57 | 60 | 0 | parox | 0 | 0 | 50 | 50 | 18 | 46 | 45 | E/A > 1c | RES | 3 | 3 | II | IV | 0 | + | HF death (39) |
| 28 | M | — | 15 | 18 | — | 70 | 70 | 0 | perm | 0 | 0 | 72 | 65 | 14 | 40 | 40 | RES | — | 1 | 0 | III | IV | 0 | 0 | TP (20) |
| 29 | M | — | 20 | 20 | — | 56 | 58 | 0 | parox | 0 | 0 | 70 | 64 | 18 | 57 | 38 | RES | — | 0 | 3 (0) | III | III (II) | MVR | 0 | Alive |
| 30 | M | — | 22 | 25 | — | 38 | 40 | parox | parox | 0 | 0 | 46 | 56 | 25 | 60 | 60 | E/A > 1 | — | 1 | 1 | II | III | 0 | + | Alive, ICDa |
| 31 | F | — | 30 | 31 | — | 70 | 70 | pers | perm | 0 | 0 | 63 | 60 | 20 | 25 | 36 | RES | RES | 0 | 0 | III | IV | 0 | 0 | HF death (34) |
| 32 | M | — | 32 | 47 | 0 | 40 | 43 | 0 | pers | 0 | 0 | 63 | 70 | 23 | 45 | 46 | E/A < 1 | E/A < 1 | 1 | 1 | I | III | 0 | 0 | Alive |
| 33 | F | 0 | 32 | 32 | + | 47 | 48 | 0 | perm | 0 | 0 | 68 | 67 | 25 | 40 | 38 | E/A < 1 | — | 0 | 1 | II | IV | 0 | + | PM, HF death (53) |
| 34 | F | — | 35 | 43 | 0 | 38 | 61 | 0 | perm | 12 | 0 | 64 | 50 | 26 | 35 | 45 | E/A < 1 | — | 1 | 2 | II | III | 0 | 0 | Alive, PM, list for TP |
| 35 | M | 0 | 36 | 36 | — | 44 | 127 | 0 | perm | 20 | — | 75 | 55 | 22 | 50 | 48 | E/A > 1 | — | 2 | 3 (0) | II | III (II) | MVR | 0 | Alive, |
| 36 | M | — | 42 | 44 | — | 70 | — | perm | perm | 0 | — | 58 | — | 15 | 40 | — | — | — | 2 | — | III | — | 0 | 0 | Lost |
| 37 | M | 0 | 48 | 55 | — | 47 | 50 | perm | perm | 0 | 0 | 57 | 65 | 32 | 40 | 42 | — | — | 1 | 1 | III | III | 0 | 0 | Alive, PM |
| 38 | F | MYH7, EX 36 Thr1760Met | 48 | 58 | — | 60 | 56 | 0 | pers | 0 | 0 | 76 | 51 | 18 | 38 | 44 | E/A > 1 | E/A < 1 | 1 | 1 | II | III | 0 | 0 | Alive |
| 39 | M | MYH7, EX 27 Ile1207Met | 49 | 58 | — | 62 | 90 | perm | perm | 20 | 0 | 57 | 57 | 18 | 52 | 55 | — | RES | 1 | 2 | III | IV | 0 | 0 | TP (60) |
| 40 | F | — | 57 | 57 | — | 40 | 44 | 0 | perm | 0 | 0 | 67 | 65 | 16 | 50 | 48 | E/A > 1 | RES | 0 | 0 | I | III | 0 | 0 | Alive, PM |
| 41 | F | — | 63 | 65 | 0 | 55 | — | 0 | perm | 0 | 0 | 62 | — | 15 | 47 | — | RES | — | 2 | — | II | III | 0 | 0 | Alive, ablation + PM |
| Non-obstructive with preserved systolic function + normal sinus rhythm | |||||||||||||||||||||||||
| 42 | F | MYBPC3 | 1 | 1 | 0 | 30 | 33 | 0 | 0 | 15 | 10 | 84 | 72 | 30 | 32 | 36 | E/A < 1 | E/A > 1c | 1 | 0 | IV | IV | 0 | 0 | TP (12) |
| EX 12 | |||||||||||||||||||||||||
| Ala364Thr | |||||||||||||||||||||||||
| 43 | M | 0 | 4 | 4 | — | 60 | 61 | 0 | 0 | 0 | 0 | 52 | 53 | 15 | 30 | 29 | RES | RES | 0 | 2 | III | III | 0 | 0 | TP (9) |
| 44 | F | — | 5 | 5 | 0 | 25 | 25 | 0 | 0 | 0 | 0 | 68 | 72 | 20 | 32 | 32 | E/A > 1c | E/A > 1c | 0 | 0 | III | III | 0 | 0 | Alive |
| 45 | F | TNNI3 | 28 | 28 | — | 53 | — | 0 | 0 | 0 | 0 | 59 | 58 | 17 | 38 | — | RES | RES | 0 | 0 | III | — | 0 | 0 | TP (28) |
| EX 8 | |||||||||||||||||||||||||
| Lys207Thr | |||||||||||||||||||||||||
| 46 | F | — | 37 | 41 | — | 60 | — | 0 | — | 0 | 0 | 55 | — | 18 | 55 | — | RES | — | 0 | 0 | IV | — | 0 | 0 | HF death (41) |
| 47 | M | — | 45 | 56 | — | 39 | 46 | 0 | 0 | 0 | 0 | 60 | 68 | 24 | 49 | 48 | E/A < 1 | RES | 1 | 1 | II | IV | 0 | 0 | ICDd, HF death (56) |
| 48 | F | 0 | 45 | 45 | — | 70 | 55 | 0 | 0 | 0 | 0 | 56 | 51 | 16 | 49 | 48 | RES | RES | 1 | 1 | II | IV | 0 | 0 | PM, HF death (66) |
| 49 | F | — | 65 | 70 | — | 46 | 75 | 0 | 0 | 0 | 0 | 64 | 57 | 15 | 40 | 53 | RES | RES | 0 | 0 | I | IV | 0 | 0 | Alive |
| 50 | M | — | 76 | 76 | — | 35 | — | 0 | 0 | 0 | 0 | 53 | — | 25 | 37 | — | E/A > 1c | — | 1 | — | II | IV | 0 | 0 | Non-HCM-related death (76) |
AF, atrial fibrillation classified as paroxysmal (parox), persistent (pers) or permanent (perm); CABG, coronary artery bypass graft; CAD, coronary artery disease; Dx, diagnosis; EF, ejection fraction; FU, follow-up; HCM, hypertrophic cardiomyopathy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LA, left atrium; LVED, left ventricle end-diastolic diameter; LV, left ventricle; LVOT, left ventricular outflow tract; MR, mitral regurgitation; MVR, mitral valve replacement; NYHA, New York Heart Association; PM, pacemaker; RES, restrictive (defined in setting of early LV diastolic filling deceleration time <150 ms and E/A ratio >2 at sinus rhythm); SE, systemic embolism; TP, transplant; —, data non available; +, present; 0, absent; () data after surgery.
aImplanted for secondary prevention.
bZero gradient at rest, and 190 mmHg with exercise.
cPseudo-normal LV filling pattern [defined in the setting of E/A ratio = 0.9–1.5, early LV diastolic filling deceleration time = 160–240 ms, Valsalva positive (= E/A ratio decrease by ≥0.5 and increase in A velocity during Valsalva manoeuvre)].8
dImplanted for primary prevention.
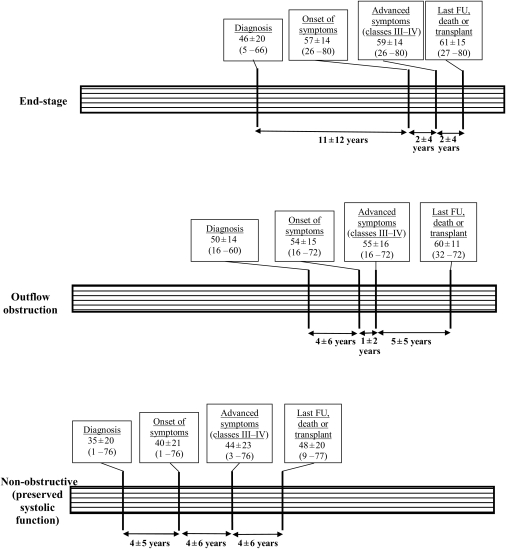
Figure 1.
Time lines shown for the three hypertrophic cardiomyopathy patient subgroups and disease profiles associated with severe, progressive heart failure. Patient ages at intervals describe clinical evolution. FU, follow-up.
Figure 2.
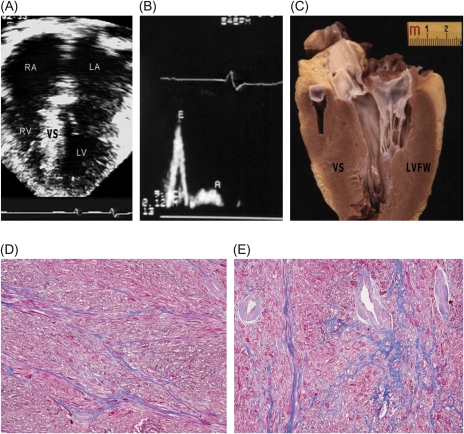
End-stage systolic dysfunction. A 59-year-old transplanted male patient with a troponin T mutation [#5 (Table 3); #3 (Table 4)]. (A) Four-chamber view at end-diastole showing dilatation of both atria [transverse left atrial (LV) dimension = 70 mm], left ventricular (LV) enlargement (i.e. 62 mm), and mild hypertrophy [septal (VS) thickness; 13 mm]; ejection fraction was 30%. (B) Heart removed at transplantation. Note thinning of basal and mid-ventricular septum (12 mm) compared with distal LV. (C) High power of boxed area in (B). Greyish areas (arrows) are indicative of septal scarring. (D) Area of septum shown in (C). Extensive replacement fibrosis is associated with abnormal intramural arterioles. Trichrome stain ×60. RA, right atrium; RV, right ventricle.
Figure 3.
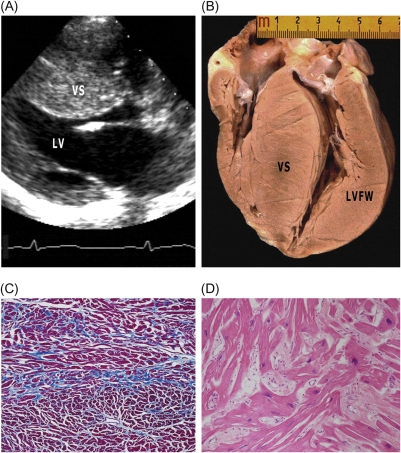
Non-obstructive hypertrophic cardiomyopathy with preserved systolic function (and atrial fibrillation). A 60-year-old male patient with a β-myosin heavy chain mutation [#39 (Table 3); #9 (Table 4)]. (A) Four-chamber view at end-diastole showing severe dilatation of both atria [transverse dimension of left atrium (LA), 90 mm], normal-sized left ventricle (LV) and right ventricle (RV), and mild LV hypertrophy (ventricular septum, VS; 18 mm) as well as preserved systolic function. (B) Heart removed at transplantation. (C) Histological section of left ventricular free wall (LVFW) showing the absence of replacement fibrosis. Trichrome stain ×3. (D) High-power view of area in box in (C) showing increased interstitial fibrosis. Trichrome stain ×40. (E) Thrombus within LA appendage (arrow). RA, right atrium.
Figure 4.
Non-obstructive hypertrophic cardiomyopathy with preserved systolic function. Restrictive form of heart failure due to diastolic dysfunction in sinus rhythm. A 28-year-old woman with troponin I mutation [#45 (Table 3); #12 (Table 4)]. (A) Four-chamber view in end-diastole showing dilatation of both atria (left atrium, LA = 53 mm), normal-sized ventricles, and mild ventricular septal (VS) thickening (17 mm). (B) Pulsed Doppler waveform with evidence of restrictive filling: E/A >2; deceleration time < 150 ms. (C) long-axis left ventricular (LV) plane with mild VS hypertrophy (17 mm); atria missing due to transplantation. (D and E) LV free wall (D) and septum (E) showing diffuse myocardial disarray, mild interstitial fibrosis, and intramural small vessel disease. Trichrome stain ×40. LVFW, left ventricular free wall; RA, right atrium; RV, right ventricle.
Figure 5.
Non-obstructive hypertrophic cardiomyopathy with preserved systolic function. A 12-year-old girl with massive septal hypertrophy in sinus rhythm with a myosin-binding protein C mutation [#42 (Table 3); #11 (Table 4)]. (A) Parasternal long-axis view at end-diastole, showing marked septal hypertrophy (30 mm) and normal-sized left ventricular (LV) cavity. (B) Explanted heart, showing massive asymmetric hypertrophy of ventricular septum (VS), and absence of grossly visible scars. (C and D) Sections of left ventricular free wall (LVFW) (C) and VS (D) showing myocyte hypertrophy and disarray, and increased interstitial fibrosis. Trichrome stain, ×20 and ×40.
End-stage systolic dysfunction
In 15 patients (30%), heart failure symptoms progressed due to the end-stage phase with systolic dysfunction (ejection fraction <50%; mean 40 ± 6%)14 (Tables 2 and 3). Age at diagnosis was 46 ± 20 years with a 11 ± 12-year interval between diagnosis and heart failure symptom onset. Left ventricular end-diastolic dimension at most recent follow-up was greatest in this subgroup (56 ± 10 vs. 44 ± 7 for others, P = 0.001).
Of the 15 end-stage patients, 11 had AF, either at study entry (n = 4) or during follow-up with progression of heart failure (n = 7). Among the 15 patients, 3 died of heart failure (one with a prior renal embolic event (Patient #8), 3 were transplanted, and 1 is awaiting a donor heart.
Left ventricular outflow obstruction
Progressive heart failure was the consequence of outflow obstruction in 11 patients (22%).15 Six of these 11 patients had the combination of outflow obstruction and paroxysmal or permanent AF (Tables 3). Initial peak resting systolic subaortic gradients were 58 ± 20 mmHg (range 40–110). Advanced heart failure symptoms (NYHA classes III–IV) due to outflow obstruction were present at entry in nine patients and developed during follow-up in two. Two patients underwent mitral valve replacement (associated with myectomy), resulting in reduction of outflow gradient, three refused surgery including one lost to follow-up and one who died of heart failure. Patients with outflow obstruction were older at diagnosis (50 ± 14 years) and had greater LV wall thickness (25 ± 5 mm) than other heart failure patients (39 ± 20 years; P = 0.06 and 21 ± 5 mm; P = 0.02).
Non-obstructive with preserved systolic function
The remaining 24 patients (48%) showed advanced heart failure without systolic dysfunction (ejection fraction ≥50%) or LV outflow obstruction. Diastolic dysfunction was commonly present in 17 patients at study entry and in all 13 for whom these data were available at last follow-up (Tables 2 and 3). Moreover, a restrictive filling pattern was present in 13 patients at entry and/or at last follow-up (Table 3).
Of the 24 patients in this subgroup, AF adversely impacted clinical course in 15 (62%), either recurrent and paroxysmal (n = 3) or persistent and permanent (n = 12) (Tables 2 and 3). In AF patients, marked left atrial enlargement was present at study entry (53 ± 12 mm) and increased 11 ± 23 mm over follow-up. Initial left atrial dimension in this subgroup was greater than in patients with LV outflow obstruction (44 ± 7 mm; P = 0.04), but similar to that in end-stage patients.
Six patients died of heart failure, five were transplanted (one awaiting donor heart), and three experienced embolic stroke (#27, #30, and #33; Table 3). Of note, three patients were diagnosed at particularly young ages of ≤5 years, subsequently developed progressive heart failure due to diastolic dysfunction at sinus rhythm and two underwent heart transplantation at ages 9 and 12 years (#43 and #42; Table 3). Compared with the other two heart failure profiles combined, patients who were non-obstructive with preserved systolic function became symptomatic earlier in life (40 ± 21 vs. 56 ± 11 years; P = 0.002) and showed the most accelerated progression to advanced heart failure (NYHA classes III–IV at 44 ± 23 vs. 57 ± 15 years; P = 0.01) and adverse outcome (41 ± 21 vs. 63 ± 9 years at death or transplant; P = 0.02). Progression of heart failure was most delayed in patients with LV outflow obstruction and end-stage (Figure 1).
Pathological features
Heart specimens from 12 patients were available for gross visual and histopathological examination, including 4 from autopsy and 8 from transplantation (Table 4 and Figures 2–5). Heart weights were similar among heart failure subgroups; P = 0.2.
Table 4.
Pathological findings in 12 patients with hypertrophic cardiomyopathy and advanced heart failure
| No. | Patient # | Age at death/ transplant (years) | Source | Heart weight (g) | Atrial thrombi | Wall thickness (mm) |
LV chamber dilatationa | MB | CAD | Replacement fibrosisb |
SVD | Myocyte disarrayb | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VS | LVFW | RV | VS | LVFW | |||||||||||
| End-stage | |||||||||||||||
| 1 | #4 | 50 | Transplant | 520c | n/a | 17 | 17 | 7 | 2+ | 0 | 0 | 3+ | 3+ | 0 | 1+ |
| 2 | #8 | 55 | Autopsy | 700 | + | 23 | 16 | 6 | 1+ | + | 0 | 3+ | 3+ | + | 3+ |
| 3 | #5 | 59 | Transplant | 500c | n/a | 17 | 13 | 6 | 3+ | + | + | 3+ | 1+ | + | 1+ |
| 4 | #11 | 63 | Transplant | 350c | n/a | 15 | 13 | 4 | 2+ | 0 | 0 | 3+ | 3+ | 0 | 1+ |
| 517 ± 143 | |||||||||||||||
| Non-obstructive with preserved systolic function | |||||||||||||||
| 5 | #28 | 20 | Transplant | 340c | n/a | 15 | 14 | 6 | 1+ | 0 | 0 | 0 | 0 | + | 1+ |
| 6 | #31 | 34 | Autopsy | 380 | + | 20 | 18 | 4 | 1+ | + | 0 | 1+ | 0 | + | 1+ |
| 7 | #27 | 39 | Autopsy | 440 | + | 15 | 12 | 7 | 0 | 0 | 0 | 0 | 0 | + | 3+ |
| 8 | #33 | 53 | Autopsy | 420 | + | 16 | 14 | 6 | 0 | + | 0 | 1+ | 0 | + | 3+ |
| 9 | #39 | 60 | Transplant | 530c | + | 17 | 17 | 7 | 0 | + | 0 | 0 | 0 | 0 | 1+ |
| 10 | #43 | 9 | Transplant | 120c | n/a | 8 | 8 | 4 | 0 | + | 0 | 0 | 0 | 0 | 1+ |
| 11 | #42 | 12 | Transplant | 470c | n/a | 30 | 13 | 7 | 0 | 0 | 0 | 1+ | 0 | + | 3+ |
| 12 | #45 | 28 | Transplant | 370c | n/a | 16 | 12 | 6 | 0 | + | 0 | 1+ | 0 | + | 3+ |
| 384 ± 122 | |||||||||||||||
CAD, (atherosclerotic) coronary artery disease; LV, left ventricle; LVFW, left ventricular free wall; MB, myocardial bridge; n/a, not available; RV, right ventricle; SVD, small vessel disease; VS, ventricular septum.
aLV chamber dilatation semi-quantitatively graded on visual inspection from 0 to 3+ (0, absent; 1+, mild, 2+, moderate; 3+, severe).
bMyocyte disarray and myocardial replacement fibrosis graded on histological sections semi-quantitatively from 0 to 3+ (0, absent; 1+, mild; 2+, moderate; 3+, severe); +, present; 0, absent.
cAtria partially or totally missing.
All end-stage patients showed LV chamber dilatation with diffuse, often transmural, fibrous replacement in ventricular septum and LV free wall (Figure 2). In contrast, only isolated or patchy focal areas of scarring in the absence of LV chamber remodelling were present in four of the eight hearts with the non-obstructive and preserved systolic function clinical profile (Figure 3). Two gross morphological patterns were evident with diastolic dysfunction (in the absence of significant scarring): (i) small ventricles and mild hypertrophy (associated with restrictive physiology) (Figure 4); and (ii) massive hypertrophy and particularly small ventricular cavities (Figure 5).
Atria were available for inspection from five hearts, i.e. four non-obstructive with preserved systolic function and one end-stage (all with AF), including four at autopsy and one explanted. Each had left atrial enlargement, 45–90 mm by echocardiography, and contained a small (2–4 mm diameter) mobile and weakly attached thrombus within the intertrabecular spaces of the pectinate muscles of the left atrial appendage (Figure 3E). In each of these patients, the thrombus was unsuspected clinically, but had apparently embolized leading to stroke or renal infarct in three (#8, #27, and#33), all without anticoagulation. The remaining two patients with atrial thrombi (#31 and #39) were treated with prophylactic warfarin or aspirin and experienced no embolic events.
Histopathological features of HCM, such as myocyte disarray10 and increased interstitial fibrosis,13 were present in each of the 12 hearts; and abnormal intramural arterioles with thickened walls and narrowed lumen were evident in 8.
Genetics
Disease-causing sarcomeric protein mutations were identified in 7 of 18 unrelated patients. β-Myosin heavy chain mutations were present in two non-obstructive with preserved systolic function and AF patients (#38 and #39) and one with obstruction (#16). Myosin-binding protein C mutations were present in one patient with end-stage systolic dysfunction (#11) and one non-obstructive with preserved systolic function, diastolic dysfunction, and massive LV hypertrophy in normal sinus rhythm (without restrictive physiology) (#42). Troponin I mutation was present in a patient with predominant diastolic dysfunction and restrictive physiology (#45) in the setting of non-obstructive with preserved systolic function; troponin T was identified in one end-stage patient (#5).
Discussion
Despite the substantial information available regarding the pathophysiology and natural history of HCM, certain aspects of this heterogeneous disease remain incompletely understood.2 Although the risks of sudden cardiac death have generally dominated the HCM literature,3,4,16 progressive disability and heart failure is also an important complication of the disease.2 In the Padua tertiary HCM Center, 17% of patients enrolled in this study developed advanced heart failure. These patients were predominantly women (i.e. 54%), consistent with a prior report.17 Furthermore, we found heart failure symptoms progressed along three predominant pathways, i.e. LV systolic dysfunction, outflow obstruction, and the absence of obstruction with preserved systolic function.
It should be underscored that we assigned individual patients to one of these three categories based on the predominant disease variable, that is, that regarded clinically to be most responsible for heart failure. Since HCM is a heterogeneous disease entity, often with multiple pathophysiological components operating,2 it is an unrealistic aspiration to assemble pure clinical profiles limited to single predictors of heart failure. Therefore, ultimately the designation of a predominant pathway for those HCM patients with mixed profiles was necessarily based on the clinical judgement of the investigators, taking into account the available data and personal knowledge of the individual patient.
About 30% of our patients with severe heart failure had systolic dysfunction (ejection fraction <50%), designated as the ‘end-stage’ phase.14 This evolution, with or without LV chamber remodelling,14,18,19 has been reported in 3–5% of HCM cohorts.14,18 End-stage progression in our patients was generally slow, unpredictable, and frequently associated with AF.14,20 On average, 11 years elapsed between HCM diagnosis and onset of heart failure symptoms, but only 4 additional years until death or heart transplantation. We analysed four hearts from these end-stage patients and found LV chamber dilatation and wall thinning due to extensive replacement fibrosis involving both ventricular septum and free wall.14 In such HCM patients, recurrent and silent myocardial ischaemia may lead to extensive myocardial scarring and loss of contractile function, associated with LV chamber remodelling.11,14
Severe heart failure was due largely to LV outflow obstruction in 20% of our cases. This observation is consistent with larger cohort studies in which obstruction proved to be a strong determinant of progressive heart failure and death.15 Notably, of the 11 patients in this subgroup, 6 also had AF, a combination of abnormalities which are particularly adverse in HCM.20
In many cases (nearly 50%), heart failure occurred in the clinical setting of non-obstructive disease with preserved systolic function, either with or without AF, presenting a particularly malignant prognosis. In fact, when compared with the other two progressive heart failure subgroups, these non-obstructive patients became symptomatic and experienced advanced heart failure and adverse outcome earlier. Non-obstructive patients in sinus rhythm deteriorated to NYHA classes III–IV associated with diastolic dysfunction (with or without restrictive LV filling patterns physiology) or AF.21–23 Phenotypic expression in this latter restrictive subgroup consisted of small ventricular cavities, markedly enlarged atria and mild LV hypertrophy (with restrictive LV filling pattern), or alternatively with massive LV hypertrophy and diminutive ventricular cavities.
Noteworthy, in two-thirds of the study patients, AF contributed to a variety of adverse clinical profiles,20,24 proving be the single most important factor in heart failure evolution overall. There have been few pathological studies reported in patients with HCM and AF. We identified mobile thrombi in the left atrial appendage of five AF patients in whom the gross heart could be examined (including three with peripheral embolization in the absence of therapeutic anticoagulation).25 These thrombi were unrecognized by transthoracic echocardiography in each case. Such observations underscore the value of transoesophageal echocardiography in patients with HCM and AF.
Genetic screening was feasible only in a small proportion of our cohort patients (about one-third), prohibiting definitive genotype–phenotype correlations. However, our data do underscore that heart failure in HCM is associated with heterogeneous genetic substrates. Seventy per cent of the gene-positive patients identified had a variety of mutations in either the β-myosin heavy chain or myosin-binding protein C genes, the predominant disease-causing mutant genes in any HCM population.26 The remaining patients had either troponin T or I mutations. Since such sarcomere protein mutations can cause both HCM and dilated cardiomyopathy,27 the present findings in HCM with systolic dysfunction suggest a final common pathway for these two primary myocardial diseases.
In conclusion, we have distinguished three diverse pathways of progressive heart failure in a large HCM referral cohort. Atrial fibrillation was the most common pathophysiological variable associated with progressive heart failure. Extensive transmural myocardial scarring influences progression to end-stage systolic dysfunction. However, no consistent relationship was evident between genetic substrate and heart failure profile, underscoring the broad heterogeneity of HCM from both molecular and clinical perspectives. Recognition of the diverse pathophysiology underlying progressive heart failure in HCM offers a measure of clarity to the selection of targeted management strategies, e.g. antiarrhythmic and other drug therapies (or radiofrequency ablation) for AF; surgical myectomy (or, selectively, alcohol ablation) for LV outflow obstruction, or timely heart transplantation for end-stage systolic or refractory diastolic dysfunction in the absence of LV outflow obstruction.
Funding
This work was supported by Italian Ministry for Scientific and Technologic Research [MURST-COFIN 2008, Number 20083EWHYR_002]; Registry for Cardio-cerebro-vascular Mortality, Veneto Region, Venice, Italy, and Hearst Foundations, San Francisco, CA, USA. Funding to pay the Open Access publication charges for this article was provided by a grant to P.M.
Conflict of interest: none declared.
References
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention. Contemporary definitions and classification of the cardiomyopathies. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. doi:10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. doi: 10.1001/jama.287.10.1308. doi:10.1001/jama.287.10.1308. [DOI] [PubMed] [Google Scholar]
- 3.Elliott PM, Poloniecki J, Dickie S, Sharma S, Monserrat L, Varnava A, Mahon NG, McKenna WJ. Sudden death in hypertrophic cardiomyopathy: identification of high risk patients. J Am Coll Cardiol. 2000;36:2212–2218. doi: 10.1016/s0735-1097(00)01003-2. doi:10.1016/S0735-1097(00)01003-2. [DOI] [PubMed] [Google Scholar]
- 4.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403. doi:10.1056/NEJM200006153422403. [DOI] [PubMed] [Google Scholar]
- 5.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I. Recommendations for quantitation of left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 6.Panza JA, Petrone RK, Fananapazir L, Maron BJ. Utility of continuous wave Doppler echocardiography in the noninvasive assessment of left ventricular outflow tract pressure gradient in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1992;19:91–99. doi: 10.1016/0735-1097(92)90057-t. [DOI] [PubMed] [Google Scholar]
- 7.Maron BJ, Spirito P, Green KJ, Wesley YE, Bonow RO, Arce J. Noninvasive assessment of left ventricular diastolic function by pulsed Doppler echocardiography in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1987;10:733–742. doi: 10.1016/s0735-1097(87)80264-4. [DOI] [PubMed] [Google Scholar]
- 8.Kirkpatrick JN, Vannan MA, Narula J, Lang RM. Echocardiography in heart failure. J Am Coll Cardiol. 2007;50:381–396. doi: 10.1016/j.jacc.2007.03.048. doi:10.1016/j.jacc.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 9.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. doi:10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 10.Maron BJ, Roberts WC. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum of patients with hypertrophic cardiomyopathy. Circulation. 1979;59:689–706. doi: 10.1161/01.cir.59.4.689. [DOI] [PubMed] [Google Scholar]
- 11.Basso C, Thiene G, Corrado D, Buja G, Melacini P, Nava A. Hypertrophic cardiomyopathy and sudden death in the young; pathologic evidence of myocardial ischemia. Hum Pathol. 2000;31:988–998. doi: 10.1053/hupa.2000.16659. doi:10.1053/hupa.2000.16659. [DOI] [PubMed] [Google Scholar]
- 12.Angelini A, Calzolari V, Thiene G, Boffa GM, Valente M, Daliento L, Basso C, Calabrese F, Razzolini R, Livi U, Chioin R. Morphologic spectrum of primary restrictive cardiomyopathy. Am J Cardiol. 1997;80:1046–1050. doi: 10.1016/s0002-9149(97)00601-2. doi:10.1016/S0002-9149(97)00601-2. [DOI] [PubMed] [Google Scholar]
- 13.Shirani J, Pick R, Roberts WC, Maron BJ. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J Am Coll Cardiol. 2000;35:36–44. doi: 10.1016/s0735-1097(99)00492-1. doi:10.1016/S0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- 14.Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. doi: 10.1161/CIRCULATIONAHA.105.583500. doi:10.1161/CIRCULATIONAHA.105.583500. [DOI] [PubMed] [Google Scholar]
- 15.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332. doi:10.1056/NEJMoa021332. [DOI] [PubMed] [Google Scholar]
- 16.Maron BJ, Spirito P, Shen WK, Haas TS, Formisano F, Link MS, Epstein AE, Almquist AK, Daubert JP, Lawrenz T, Boriani G, Estes NA, III, Favale S, Piccininno M, Winters SL, Santini M, Betocchi S, Arribas F, Sherrid MV, Buja G, Semsarian C, Bruzzi P. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298:405–412. doi: 10.1001/jama.298.4.405. doi:10.1001/jama.298.4.405. [DOI] [PubMed] [Google Scholar]
- 17.Olivotto I, Maron MS, Adabag AS, Casey SA, Vargiu D, Link MS, Udelson JE, Cecchi F, Maron BJ. Gender-related differences in the clinical presentation and outcome of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:480–487. doi: 10.1016/j.jacc.2005.04.043. doi:10.1016/j.jacc.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Biagini E, Coccolo F, Ferlito M, Perugini E, Rocchi G, Bacchi-Reggiani L, Lofiego C, Boriani G, Prandstraller D, Picchio FM, Branzi A, Rapezzi C. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol. 2005;46:1543–1550. doi: 10.1016/j.jacc.2005.04.062. doi:10.1016/j.jacc.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 19.Yacoub MH, Olivotto I, Cecchi F. ‘End-stage’ hypertrophic cardiomyopathy: from mystery to model. Nat Clin Pract Cardiovasc Med. 2007;4:232–233. doi: 10.1038/ncpcardio0859. doi:10.1038/ncpcardio0859. [DOI] [PubMed] [Google Scholar]
- 20.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104:2517–2524. doi: 10.1161/hc4601.097997. doi:10.1161/hc4601.097997. [DOI] [PubMed] [Google Scholar]
- 21.Carasso S, Yang H, Woo A, Vannan MA, Jamorski M, Wigle ED, Rakowski H. Systolic myocardial mechanics in hypertrophic cardiomyopathy: novel concepts and implications for clinical status. J Am Soc Echocardiogr. 2008;21:675–683. doi: 10.1016/j.echo.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Geske JB, Sorajja P, Nishimura RA, Ommen SR. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation. 2007;16:2702–2708. doi: 10.1161/CIRCULATIONAHA.107.698985. doi:10.1161/CIRCULATIONAHA.107.698985. [DOI] [PubMed] [Google Scholar]
- 23.Rakowski H, Carasso S. Quantifying diastolic function in hypertrophic cardiomyopathy: the ongoing search for the holy grail. Circulation. 2008;116:2262–2265. doi: 10.1161/CIRCULATIONAHA.107.742395. doi:10.1161/CIRCULATIONAHA.107.742395. [DOI] [PubMed] [Google Scholar]
- 24.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. American College of Cardiology; American Heart Association Task Force; European Society of Cardiology Committee for Practice Guidelines; European Heart Rhythm Association; Heart Rhythm Society. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 25.Maron BJ, Olivotto I, Bellone P, Conte MR, Cecchi F, Flygenring BP, Casey SA, Gohman TE, Bongioanni S, Spirito P. Clinical profile of stroke in 900 patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002;39:301–307. doi: 10.1016/s0735-1097(01)01727-2. doi:10.1016/S0735-1097(01)01727-2. [DOI] [PubMed] [Google Scholar]
- 26.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M for the EUROGENE Heart Failure Project. Hypertrophic cardiomyopathy. Distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation. 2003;107:2227–2232. doi: 10.1161/01.CIR.0000066323.15244.54. doi:10.1161/01.CIR.0000066323.15244.54. [DOI] [PubMed] [Google Scholar]
- 27.Ashrafian H, Watkins H. Reviews of translational medicine and genomics in cardiovascular disease: new disease taxonomy and therapeutic implications. Cardiomyopathies: therapeutics based on molecular phenotype. J Am Coll Cardiol. 2007;49:1251–1264. doi: 10.1016/j.jacc.2006.10.073. doi:10.1016/j.jacc.2006.10.073. [DOI] [PubMed] [Google Scholar]