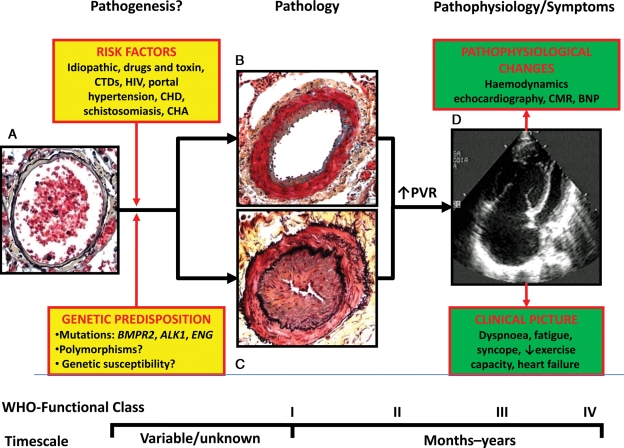
Pulmonary arterial hypertension (PAH) is a rare and severe clinical condition characterized by a progressive increase of pulmonary vascular resistance leading to right ventricular failure and premature death (Figure 1).1 Its prevalence ranges from 15 to 50 patients per million population2,3 and it affects a relatively young patient population (average age of 50 years) when compared with the more common thoracic organ diseases such as coronary artery disease and chronic obstructive lung disease. PAH in adults includes at least nine clinical subgroups with virtually identical obstructive pathologic changes (Figures 1 and 2) in the distal pulmonary arteries: idiopathic, heritable, drug- and toxin-induced, associated with, connective tissue diseases, HIV infection, portal hypertension, congenital heart disease, schistosomiasis, and chronic haemolytic anaemia.1
Figure 1.
Schematic representation of pathogenesis, pathology, pathophysiology, and symptoms in pulmonary arterial hypertension. (A) Normal small distal pulmonary artery: the thin wall is constituted by a single elastic lamina and a thin layer of smooth muscle cells; a large lumen with red blood cells is also shown. (B) Distal pulmonary artery in pulmonary arterial hypertension: increased thickness of the media due to hypertrophy and hyperplasia of smooth muscle cells and moderate lumen reduction are present. This picture may represent an initial phase of the disease and/or the prevalent changes in patients responding to vasoreactivity tests. (C) Distal pulmonary artery in pulmonary arterial hypertension: increased thickness of the media and also of the intima due to proliferation/migration of myofibroblasts and fibrosis are present. Severe lumen reduction is also shown. This picture may represent an advanced phase of the disease and/or the prevalent changes in patients not responding to vasoreactivity tests. (D) Echocardiographic four-chamber view in pulmonary arterial hypertension: Severe dilatation of the right atrium and ventricle and reduction in size of the left ventricle are shown. ALK1, activin-like kinase-type 1 gene; BNP, brain natriuretic peptide; BMPR2, bone morphogenetic protein receptor type 2 gene; CHA, chronic haemolytic anaemia; CHD, congenital heart disease; CTD, connective tissue diseases; ENG, endoglin gene; HIV, human immunodeficiency virus; CMR, cardiac magnetic resonance; PAH, pulmonary arterial hypertension; PVR, pulmonary vascular resistance; WHO, world health organization. The pathological pictures are a courtesy of Dr Carol Farver, Cleveland Clinic.
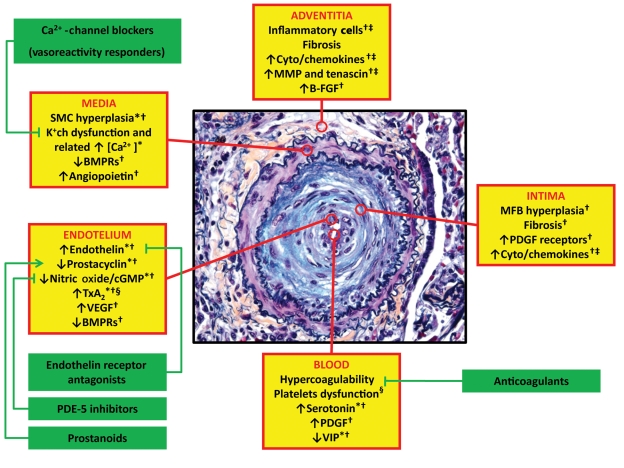
Figure 2.
Obstructive remodelling of a small pulmonary artery in pulmonary arterial hypertension (increased thickness of the three vessel layers and severe lumen reduction are shown) and ongoing pathobiological processes in the different layers of the vessel wall (yellow boxes) and in the blood. Asterisks indicate the potential processes involved. Corrective interactions of the related approved therapeutic interventions are also reported (green boxes). *Vasoconstriction; †Proliferation/migration; ‡Inflammation; §Thrombosis. B-FGF, basic fibroblast growth factor; BMPR, bone morphogenetic protein receptor; [Ca2+], intracellular calcium concentration; K+ch, membrane potassium channels; SMC, smooth muscle cells; MFB, myofibroblasts; MMP, matrix metalloproteases; PDE-5, phosphodiesterase type 5; PDGF, platelet-derived growth factor; TxA2, tromboxane A2; VEGF, vascular endothelial growth factor; VIP, vasoactive intestinal peptide. The pathological picture is a courtesy of Dr Carol Farver, Cleveland Clinic.
Pathogenesis1
The exact processes that initiate the pathological changes seen in PAH are largely unknown. It is hypothesized that the interaction between genetic predisposition and environmental risk factors (Figure 1) may be involved in the initial stages of the disease. However, even the exact role of the auto-antibodies in connective tissue diseases or the viral involvement in HIV infection is unclear. It appears that a specific injury on the vessel wall of distal pulmonary arteries may initiate, in predisposed individuals, a pathobiological cascade of events which leads to a common final pathological obstructive condition (Figures 1B and C and 2). In the initial phases of these processes, the disease is asymptomatic, and detectable pathophysiological changes and symptoms limiting exercise capacity appear usually when the pathological lesions are fully developed (Figure 1).
Pathology4,5
Pathological lesions in PAH patients affect the distal pulmonary arteries (<500 µm), in particular. The lesions are characterized by medial hypertrophy, intimal proliferative and fibrotic changes (concentric and/or eccentric), adventitial thickening with moderate peri-vascular inflammatory infiltrates (Figures 1B and C and 2), complex lesions (plexiform, dilated), and thrombotic lesion.
Pathobiology6–8
The pathobiology of the distal pulmonary arteries in PAH patients is multifactorial and involves various biochemical pathways and cell types (Figure 2). Excessive vasoconstriction has been related to abnormal function or expression of potassium channels in the smooth muscle cells and to endothelial dysfunction. Endothelial dysfunction leads to chronically impaired production of vasodilator and antiproliferative agents such as nitric oxide and prostacyclin, along with over-expression of vasoconstrictor and proliferative substances such as thromboxane A2 and endothelin. Many of these abnormalities both elevate vascular tone and promote vascular remodelling by proliferative changes that involve several cell types, including endothelial and smooth muscle cells as well as fibroblasts. In addition, in the adventitia, there is increased production of extracellular matrix including collagen, elastin, and fibronectin and of matrix-bound smooth muscle cell mitogens, such as basic fibroblast growth factor. Other matrix metalloproteases can stimulate the production of tenascin, a smooth muscle cell mitogenic cofactor. Several additional growth factors including vascular endothelial growth factor, platelet-derived growth factor, insulin-like growth factor-1, and epidermal growth factor have been implicated in the development of remodelling and all have been reported to be increased (the molecule and/or the specific receptors) in the lung and/or in the blood of PAH patients. Reduced plasma levels of other vasodilator and antiproliferative substances such as vasoactive intestinal peptide have also been demonstrated. Angiopoietin-1, an angiogenic factor essential for vascular lung development, seems to be up-regulated in cases of PAH correlating directly with the severity of the disease. Receptors of the bone morphogenetic protein pathway, involved in cellular proliferation and apoptosis, are down-regulated and/or malfunctioning in the lung vasculature of both heritable and acquired PAH. Inflammatory cells, cyto- and chemokines, and platelets (through the serotonin pathway) may also play a significant role in PAH. Prothrombotic abnormalities have been demonstrated in PAH patients and thrombi are present in both the small distal pulmonary arteries and in proximal elastic pulmonary arteries.
Pathophysiology9,10
The increase of pulmonary vascular resistance in PAH patients is therefore related to different mechanisms, including vasoconstriction, proliferative and obstructive remodelling of the pulmonary vessel wall, inflammation, and thrombosis (Figure 2). Vasoconstriction is likely prevalent in the small group of patients responding to the acute vasoreactivity test.1
The increase in pulmonary vascular resistance leads to right ventricular overload, hypertrophy, and dilatation and eventually to right ventricle failure and death (Figure 1). The importance of the progression of right ventricle failure on the symptoms, exercise limitation, and outcome of PAH patients is confirmed by the prognostic impact of right atrial pressure, cardiac index and pulmonary arterial pressure, the three main haemodynamic factors linked to right ventricle pump function. Echocardiography and cardiac magnetic resonance parameters and brain natriuretic peptide plasma levels can also identify non-invasively the presence and extent of right ventricular dysfunction (Figure 1). Afterload mismatch remains the leading determinant of right heart failure in patients with PAH because its removal, as follows lung transplantation, leads almost invariably to sustained recovery of right ventricle function. It is, therefore, conceivable that the drug therapies tested in PAH patients have included compounds which could potentially interfere with the pathobiological mechanisms of the disease trying to achieve a reverse remodelling of the obstructive lesions and a reduction of the right ventricular afterload (Figure 2).
Progress of medical treatment in pulmonary arterial hypertension
Two decades ago, patients with idiopathic PAH were defined as the ‘kingdom of the near-dead’11 to outline their dismal median survival rate that, at that time, was 2.8 years from the diagnosis,9 despite any available supportive treatment. Usually, rare and severe conditions are neglected by both the medical community and the pharmaceutical industry due to the intrinsic difficulties of performing experimental and clinical research in these fields. In fact, meaningful randomized controlled trials (RCTs) in these settings require a worldwide collaboration of expert centres operating with a similar standard of medical care and relatively large financial resources with a rather high risk of study failure. The recent history of drug development in PAH contradicts this traditional perspective and it is worthwhile to analyse the details of this phenomenon.
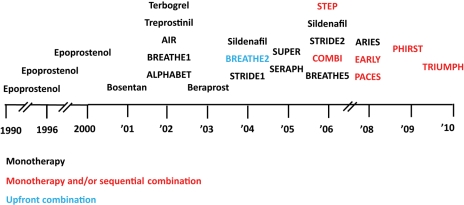
Without a doubt, the progress made in the medical treatment of PAH in the past 15 years is unique, particularly for a rare and severe condition: almost 30 RCTs have been completed and more than 10 are either ongoing or planned. Twenty-five RCTs have been published as of May 2010 (Figure 3). Eight drugs (ambrisentan, bosentan, epoprostenol, iloprost, sildenafil, sitaxentan, tadalafil, treprostinil) belonging to three pharmacological classes (endothelin receptor antagonists, phosphodiesterase type-5 inhibitors, and prostanoids) administered by four different routes (oral, inhaled, subcutaneous, and intravenous) have been currently approved by the Food and Drug Administration (FDA) and/or by the European Medicines Agency (EMA). All three classes of drugs exert both vasodilator and antiproliferative effects and interfere with the endothelial dysfunction abnormalities observed in PAH patients (Figure 2).
Figure 3.
Time-course of 25 published randomized controlled studies (identified by acronyms, if any, or drug and year of publication) in pulmonary arterial hypertension as of May 2010. Colour code identifies the design of the study: monotherapy (black): investigational drug vs. placebo in patients naive for pulmonary arterial hypertension approved drugs. Monotherapy and/or sequential combination (red): investigational drug vs. placebo in patients either naive for, or treated with pulmonary arterial hypertension approved drugs. Upfront combination (light blue): single drug vs. combination of two drugs in patients naive for pulmonary arterial hypertension approved drugs.
Traditional endpoints for clinical trials in pulmonary arterial hypertension
The traditional primary endpoint of the RCTs performed in PAH has been the 6 min walk test (6MWT)12 that assesses the exercise capacity, and secondary endpoints have included haemodynamics and time to clinical worsening (TtCW), a composite endpoint including death, hospitalization, and disease progression.13,14 Based on the primary endpoint, the specific labelling indication of all approved medications for PAH usually includes the wording ‘to improve exercise capacity’ followed by the functional classes of the patient population enrolled in the studies. This reflects the fact that both regulatory agencies and investigators agree that in rare and severe conditions dramatically impairing the functional capacity of relatively young patients, exercise capacity improvement can be considered a worthwhile initial objective. In addition, the usual concordant favourable changes in haemodynamics12 and TtCW13,14 observed in some studies reinforced the results of the 6MWT. The use of this test as a primary endpoint has allowed reasonable study sample sizes (from 100 to 500 patients)12 and randomized study duration (from 2 to 6 months),12 which are favourable feasibility characteristics in a rare and severe disease. On the other hand, these same characteristics have prevented the observation of effects on the mortality rate except for studies enrolling the most severe patient population.15,16
Meta-analyses to assess the effect on mortality
Meta-analysis of multiple studies is a technique that may allow the analysis of parameters or events with a larger sample size and with additional statistical insights. This approach requires the inclusion of homogeneous studies as far as disease aetiology and study designs are concerned. A first meta-analysis on 16 RCTs performed in PAH17 concluded that the treatments ‘produced limited benefits in clinical end-points and failed to support a significant survival advantage’. However, the meta-analysis included both acute and long-term studies and one study on patients with lung fibrosis and did not consider six RCTs published before its submission. We performed a second meta-analysis on 23 RCTs12 in 3199 PAH patients published as of October 2008. This analysis showed a reduction in mortality ranging between 38 and 43% (according to different inclusion criteria) after an average treatment period of 14.3 weeks. A subgroup analysis showed that all three classes of PAH-approved drugs achieved a similar favourable reduction in mortality, although no statistical significance was achieved individually. Updating our meta-analysis with two recently published studies,18,19 the overall reduction in mortality has been confirmed (Figure 4). These data have recently been validated by the authors of the first meta-analysis, who reported a reduction in mortality of 39%, analysing the data of 3363 patients.20 The concordance of these results suggests, therefore, an improvement of survival in patients treated with the targeted therapies currently approved for PAH. The limitations of this conclusion include the short duration of the RCTs whose primary endpoint was not mortality and the lack of evidence of a favourable long-term effect.
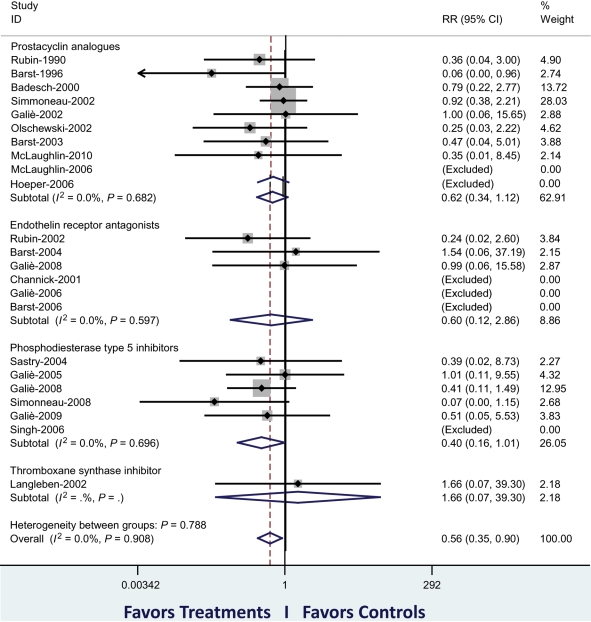
Figure 4.
Meta-analysis of published randomized controlled studies (identified by first author and year of publication) in pulmonary arterial hypertension as of May 2010. The primary analysis has included 3780 patients of 23 trials. The figure shows the cumulative RR estimate of death in active treatment groups when compared with control groups stratified according to treatment class (inverse variance method). Studies with no events in both groups were excluded. An overall reduction of mortality of 44% (P = 0.016) is shown. The sensitivity analysis, including two additional studies (59 patients) in which two treatment strategies were compared, confirmed a reduction in mortality of 39% (P = 0.041). The subgroup analysis of the three classes of approved drugs achieved a similar favourable reduction in mortality, although no statistical significance was achieved individually. RR, relative risk. Modified from Galiè et al.12
Nevertheless, we disagree with the opinion that the above limitations are the key factors which should induce us to adopt new trial designs, new endpoints, and longer durations of the RCTs.20 Given the higher mortality observed in the placebo groups of the more recent meta-analyses12,20 on PAH trials and the higher rate of clinical deterioration observed in placebo-treated groups of individual PAH studies,15,16,18,21–25 it is not ethical, in our view, to repeat RCTs in naïve PAH patients in order to satisfy the scientific curiosity of ‘desk trialists’.
The way forward
The true issues PAH patients and physicians face in the clinical practice is the insufficient efficacy of the present therapeutic resources, despite clear progress and the escape from ‘the kingdom of the near-dead’. The current treatment strategy, optimized in recent guidelines,1 remains inadequate because the mortality rate continues to be high and the functional and haemodynamic impairments are still extensive in many patients. The specific drugs approved for PAH are able to slow the progression of the disease but cannot be considered a cure for the majority of patients.
Current and future plans devoted to increasing our ability to treat PAH are facing new challenges which require scientific creativity and new research strategies. Possible working hypotheses include the drug combination approach and new candidate classes of drugs.
Combination therapy
The rationale for combining approved PAH compounds is related to the different pathobiological pathways targeted by the three classes of approved PAH drugs (Figure 2). This combined approach has successfully been employed in the treatment of other serious and chronic diseases such as congestive heart failure, HIV infection, and cancer. Combination therapy is currently recommended in PAH patients with suboptimal response to the initial monotherapy as an add-on with a compound of an alternative drug class (sequential combination therapy).1 Different RCTs have currently shown the efficacy of this strategy on the improvement of exercise capacity16,18,19,21,26 and the reduction of TtCW.16,18,26
An emerging concept relates to the use of first-line combination therapy with two drugs in PAH patients when compared with the initial monotherapy. This hypothesis was tested in the BREATHE-2 trial, but the small sample size of the study did not allow for a definitive conclusion.27 The appropriate design to assess the efficacy of this strategy appears to be a three-arm study, comparing combination therapy with two arms of monotherapy, using the single compounds.
New candidate classes of drugs
Paradoxically, there is no shortage of novel candidate therapies for PAH, including drugs, gene, and/or stem-cell treatments. These approaches are intended to address alternative pathobiological pathways (Figure 2) or explore new strategies such as regenerative medicine. New drugs with ongoing or planned phase III studies in this field include oral compounds such as NO-independent stimulators and activators of cyclic guanosine monophosphate, tyrosine kinase inhibitors (platelet-derived growth factor inhibitors), tissular dual endothelin receptor antagonists, prostanoids and non-prostanoid prostacyclin receptor agonists, and inhaled vasoactive intestinal peptide. The efficacy of these new compounds needs to be demonstrated ‘on top of’ the available approved PAH drug therapies in order to avoid any delay in the initiation of effective medications. Therefore, a combination approach is required also in this case.
Future study designs
The future decisive challenge is the identification of the most appropriate study designs to demonstrate the efficacy-to-safety ratio of combination strategies either with already approved drugs or with novel therapies. The replication of the traditional phase III strategy (placebo-controlled design in treatment-naïve patients, 6MWT as primary endpoint assessed after 3–4 months of treatment) appears not to be suitable for practical and ethical reasons. In fact, the inclusion of patients on background effective therapies will reduce our ability to demonstrate a difference between the ‘placebo-treated group’ and the ‘actively treated group’, in particular, if exercise capacity is the primary endpoint. This phenomenon was observed in the more recently completed RCTs in which the treatment effect on the 6MWT ranged from 15 to 25 m16,18,19,26 when compared with the traditional 35 to 55 m observed in historical monotherapy studies. A possible solution is the adoption of different primary endpoints, such as TtCW, accepted by the regulatory agencies. This approach also presents challenges, including the objective and uniform definition of this composite endpoint and the sample size and/or duration of the study, which can be either pre-specified or based on the number of observed events. Additional problems of multicentre and international studies are linked to the country-related heterogeneity of the PAH-approved medications, the different attitudes for hospitalization in different geographic areas, and the availability of centres with experience in combination therapy.
Initial appropriate answers to solve these difficulties have been given by the regulatory agencies and by the investigators. In fact, in the recently published ‘Guideline on the Clinical Investigations of Medicinal Products for the Treatment of PAH’,28 the Committee for Medicinal Products for Human Use (CHMP) of the EMA clarified the requirements for the approval of PAH medications, indicating the characteristics of acceptable primary endpoints, including 6MWT and TtCW. Randomized controlled trials adopting novel designs and TtCW as primary endpoint have already been initiated. For example, a morbidity and mortality primary endpoint has been adopted in the SERAPHIN study (Study with Endothelin Receptor Antagonist in Pulmonary arterial Hypertension to Improve cliNical outcome), testing the efficacy of a new tissular dual endothelin receptor antagonist, and in the AMBITION study (A randomized, double-blind, placebo-controlled, multicentre study of first-line combination therapy with AMBrIsentan and Tadalafil vs. monotherapy in subjects with pulmonary arterial hypertensION), testing the efficacy of the initial combination therapy of a selective endothelin receptor antagonist and a phosphodiesterase type-5 inhibitor when compared with monotherapy using single compounds.
Conclusions
In conclusion, the clear recent progress in the treatment of PAH supported by the concordant results of recent meta-analyses need to be further extended because the current treatment strategy is still not satisfactory. This requires a joint effort between regulatory agencies, patient associations, investigators, and industry for the development and completion of additional new RCTs. There is no time for sterile discussions about the extent of current achievements based on others' published papers. Let us fight the battle against PAH ‘on the field’ together. Our patients deserve this commitment.
Funding
Funding to pay the Open Access publication charges for this article was provided by Dipartimento Cardiovascolare, Università di Bologna.
Conflict of interest: N.G. has participated in advisory board activities for Actelion, Pfizer, United Therapeutics, Eli-Lilly, Bayer-Schering, Encysive, and GlaxoSmithKline, Mondobiotec, given paid lectures for Actelion, Pfizer, Bayer-Schering, and Encysive. The Institute of Cardiology of the University of Bologna has received research grants from Actelion, Pfizer, United Therapeutics, Eli-Lilly, Bayer-Schering, Encysive and GlaxoSmithKline. M.P. and A.M., had nothing to be declared.
References
- 1.Galiè N, Hoeper M, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Klepetko W, Joendeau G, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G. Guidelines on diagnosis and treatment of pulmonary hypertension: the Task Force on Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology and of the European Respiratory Society. Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 2.Peacock AJ, Murphy NF, McMurray JJV, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104–109. doi: 10.1183/09031936.00092306. doi:10.1183/09031936.00092306. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. doi:10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 4.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43(Suppl. 12):S25–S32. doi: 10.1016/j.jacc.2004.02.033. doi:10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth S. Pulmonary circulation: development and pathology. J Am Coll Cardiol. 2009;54:S3–S9. doi: 10.1016/j.jacc.2009.04.009. doi:10.1016/j.jacc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Morrell N, Adnot S, Archer S, Dupuis J, Jones P, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. doi:10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Lloyd-Jones P, Maitland ML, Michelakis E, Morrell N, Newman B, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel N, Yuan JX, Humber DM. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. doi:10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Machado R, Eickelberg O, Elliott CG, Geraci M, Hanoaka M, Loyd J, Newman J, Phillips JA, Soubrier F, Trembath R, Chung WK. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S32–S42. doi: 10.1016/j.jacc.2009.04.015. doi:10.1016/j.jacc.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N, Manes A, Palazzini M, Negro L, Romanazzi S, Branzi A. Pharmacological impact on right ventricular remodelling in pulmonary arterial hypertension. Eur Heart J Suppl. 2007;9(Suppl. _H):H68–H74. [Google Scholar]
- 11.Robin ED. The kingdom of the near-dead: the shortened unnatural life history of primary pulmonary hypertension. Chest. 1987;92:330–334. doi: 10.1378/chest.92.2.330. doi:10.1378/chest.92.2.330. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Manes A, Negro L, Palazzini M, Bacchi Reggiani ML, Branzi A. A meta-analysis of randomized controlled trials in pulmonary arterial hypertension. Eur Heart J. 2009;30:394–403. doi: 10.1093/eurheartj/ehp022. doi:10.1093/eurheartj/ehp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peacock AJ, Naeije R, Galiè N, Rubin L. End-points and clinical trial design in pulmonary arterial hypertension: have we made progress? Eur Respir J. 2009;34:231–242. doi: 10.1183/09031936.00107108. doi:10.1183/09031936.00107108. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Badesch DB, Delcroix M, Fleming TR, Gaine SP, Galiè N, Gibbs JS, Kim NH, Oudiz RJ, Peacock A, Provencher S, Sitbon O, Tapson VF, Seeger W. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(Suppl. 1):S97–S107. doi: 10.1016/j.jacc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group [see comments] N Engl J Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. doi:10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 16.Simonneau G, Rubin L, Galiè N, Barst RJ, Fleming T, Frost A, Engel PJ, Kramer MR, Burgess G, Collings L, Cossons N, Sitbon O, Badesch BD For the Pulmonary Arterial Hypertension combination Study of Epoprostenol and Sildenafil (PACES) Study Group. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension. Ann Intern Med. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 17.Macchia A, Marchioli R, Marfisi R, Scarano M, Levantesi G, Tavazzi L, Tognoni G. A meta-analysis of trials of pulmonary hypertension: a clinical condition looking for drugs and research methodology. Am Heart J. 2007;153:1037–1047. doi: 10.1016/j.ahj.2007.02.037. doi:10.1016/j.ahj.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 18.Galiè N, Brundage BH, Ghofrani HA, Oudiz RJ, Simonneau G, Safdar Z, Shapiro S, White RJ, Chan M, Beardsworth A, Frumkin L, Barst RJ on behalf of the Pulmonary Arterial Hypertension and Response to Tadalafil (PHIRST) Study Group. Tadalafil therapy for pulmonary arterial hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. doi:10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin VV, Benza RL, Rubin L, Channick R, Voswinckel R, Tapson V, Robbins I, Olschewski H, Rubenfire M, Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. doi:10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Macchia A, Marchioli R, Tognoni G, Scarano M, Marfisi R, Tavazzi L, Rich S. Systematic review of trials using vasodilators in pulmonary arterial hypertension: why a new approach is needed. Am Heart J. 2010;159:245–257. doi: 10.1016/j.ahj.2009.11.028. doi:10.1016/j.ahj.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 21.Channick RN, Simonneau G, Sitbon O, Robbins IM, Frost A, Tapson VF, Badesch DB, Roux S, Rainisio M, Bodin F, Rubin LJ. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. doi:10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 22.Olschewski H, Simonneau G, Galiè N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Sitbon O, Speich R, Hoeper M, Behr J, Winkler J, Seeger W for the AIR Study Group. Inhaled iloprost in severe pulmonary hypertension. N Engl J Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. doi:10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 23.Galiè N, Ghofrani HA, Torbicki A, Barst RJ, Rubin LJ, Badesch D, Fleming T, Parpia T, Burgess G, Branzi A, Grimminger F, Kurzyna M, Simonneau G the Sildenafil Use in Pulmonary Arterial Hypertension (SUPER) Study Group. Sildenafil citrate therapy for pulmonary arterial hypertension. New Engl J Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. doi:10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Olschewski H, Oudiz RJ, Torres F, Frost A, Ghofrani HA, Badesch DB, McGoon MD, McLaughlin VV, Roecker EB, Gerber MJ, Dufton C, Wiens BL, Rubin LJ. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. doi:10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 25.Galiè N, Rubin LJ, Hoeper M, Jansa P, Al-Hiti H, Meyer GMB, Chiossi E, Kusic-Pajic A, Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): a double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. doi:10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin VV, Oudiz RJ, Frost A, Tapson VF, Murali S, Channick RN, Badesch DB, Barst RJ, Hsu HH, Rubin LJ. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174:1257–1263. doi: 10.1164/rccm.200603-358OC. doi:10.1164/rccm.200603-358OC. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Barst RJ, Robbins IM, Channick RN, Galiè N, Boonstra A, Rubin LJ, Horn EM, Manes A, Simonneau G. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J. 2004;24:353–359. doi: 10.1183/09031936.04.00028404. doi:10.1183/09031936.04.00028404. [DOI] [PubMed] [Google Scholar]
- 28.Committee for Medicinal Products for Human Use. Guideline on the Clinical Investigation of Medicinal Products for the Treatment of Pulmonary Arterial Hypertension. EMEA website. http://www.ema.europa.eu/pdfs/human/ewp/35695408enfin.pdf. (28 February 2010) [Google Scholar]