Abstract
Objective
To investigate the cognitive and neural correlates of discourse impairment in corticobasal syndrome (CBS).
Background
Difficulty communicating is a frequent clinical manifestation in patients with CBS. However, the mechanisms underlying this disabling problem are not well understood.
Methods
Twenty patients with CBS and 8 healthy seniors narrated a picture story. Narratives were analyzed for maintenance of the narrative theme, identification of the overall point of the story (global connectedness), and connectedness between consecutive events (local connectedness). Discourse measures were correlated with performance on cognitive tasks and with cortical atrophy as determined by magnetic resonance imaging voxel-based morphometry.
Results
Patients with CBS referred to the narrative theme significantly less frequently than controls. Global connectedness was intact in only 6 of 20 CBS patients (30%), but preserved in all controls. Local connectedness was significantly diminished in patients relative to controls. Discourse performance in CBS was related to tasks requiring higher-order integration of visual material, but not to basic visuospatial/visuoperceptual, language, or memory function. Discourse impairment was directly related to atrophy in the right parietal lobe and bilateral dorsolateral prefrontal cortex.
Conclusions
Our findings suggest that impaired information integration in CBS, related to parieto-frontal disease, interferes with patients’ ability to narrate a coherent story.
Keywords: corticobasal syndrome, volumetric MRI, discourse, inferior parietal lobe, dorsolateral prefrontal cortex
Discourse refers to the way in which utterances are organized into a coherent and meaningful communication. An example of discourse is narrative, or story-telling, an essential means of communication and a ubiquitous part of daily interaction.1 Successful narrative discourse depends on linguistic as well as nonlinguistic skills, such as working memory, planning and organizing, and theory of mind.1,2 As such, discourse impairment has been demonstrated in patients with neurodegenerative diseases including Alzheimer disease3 and frontotemporal dementia.4 We examine narrative discourse impairment in patients with corticobasal syndrome (CBS), in whom difficulty communicating with others is a common and disabling clinical manifestation.
CBS is a neurodegenerative condition characterized by an asymmetric extrapyramidal disorder with cortical features such as apraxia, the alien-limb phenomenon, and cortical sensory loss.5,6 CBS patients exhibit cognitive impairment in frontal and parietal domains, including executive dysfunction, social disorder, and higher-order visual processing deficits (eg, simultagnosia and difficulty navigating).7,8 Neuropathologic and imaging studies demonstrate parietal, frontal, and basal ganglia involvement.8–10
Prior work has begun to elucidate the neural basis of discourse processing. Frontal and parietal regions have been implicated in discourse using functional magnetic resonance imaging (MRI) studies of healthy subjects during production11 and comprehension2,12,13 tasks. Nonaphasic frontotemporal dementia patients with social and executive dysfunction (SOC/EXEC) produce narratives with decreased organization and coherence, associated with right prefrontal atrophy.4,14 In tests of narrative comprehension, SOC/EXEC patients show relative insensitivity to the internal organization of story events, also associated with prefrontal atrophy.15 Regions of the parietal lobe have been implicated in integrative processes in various domains, from multimodal sensory processing16 to integration of social information into a coherent sense of another’s mental state (theory of mind).17 Of particular importance to discourse, parietal regions are thought to participate in the generation of situation models, or the integrated mental representation of described events.2,13,18 The use of situation models is thought to facilitate comprehension and recall of information being communicated,12,13 and in this way, has substantial functional implication for patients in their interactions with others.
In the present study, we used a semi-structured discourse task to quantify aspects of discourse impairment in patients with CBS and to investigate the cognitive and neural correlates of these difficulties. Participants narrated a children’s wordless picture book to obtain an extended speech sample for which the intended content could be verified. We focused primarily on narrative organization and coherence, measuring patients’ ability to carry the story theme through their narratives, grasp the main point of the story, and connect consecutive story events. We correlated discourse measures with cognitive task performance and cortical atrophy. We expected that patients with CBS would have difficulty creating coherent narratives. We predicted that discourse impairment would correlate with performance on tasks requiring integration of visual information and with parietal and frontal atrophy as measured by MRI voxel-based morphometry.
METHODS
Subjects
Twenty patients diagnosed clinically with CBS by an experienced neurologist (M.G.) and 8 age-matched and education-matched healthy seniors completed the narrative task. Exclusion criteria included primary psychiatric disease, encephalopathy due to a medical condition, and use of sedating medications. Demographic features are summarized in Table 1. This protocol was approved by the University of Pennsylvania Institutional Review Board. Written informed consent was obtained from all participants in this study.
TABLE 1.
Demographic Characteristics of CBS Patients and Controls
| Controls | CBS | |
|---|---|---|
| Number, male/female | 2/6 | 9/11 |
| Mean age (SD), y | 69.4 (3.9) | 67.4 (9.8) |
| Mean education (SD), y | 15.9 (2.2) | 14.5 (2.3) |
| Mean disease duration (SD), y | — | 3.9 (2.0) |
| Mean MMSE (SD), max = 30 | 30.0 (0.0) | 22.7 (6.2) |
CBS indicates corticobasal syndrome; MMSE, Mini-Mental State Examination.
Materials
The narrative procedure was described in detail previously.4 Briefly, participants narrated the children’s wordless picture story, Frog, Where Are You? by Mercer Mayer.19 Black and white drawings relate the story of a boy and his dog whose pet frog escapes. The story then details their search for the frog, which they ultimately find. The story has an internal structure, involving 30 events grouped into 7 episodes, each consisting of an orientation, 1 or more complicating actions, and a resolution. We chose story-book narration over unstructured conversation or autobiographical tales so we could assess narrative content relative to clearly defined target information. We avoided fairy tales, where the theme and sequence of events are over-learned, as this would impede our ability to judge how participants organize a story.
Narrative Procedure
All text was covered with heavy paper stock. Participants reviewed the pictures before speaking to familiarize themselves with the story. Subjects were asked to narrate the story as if telling it to a child, which helped place the narrative in the context of a verbal interaction between 2 people, creating a more naturalistic discourse task. Subjects viewed the book while speaking to minimize reliance on memory for story material. Narratives were digitally recorded and transcribed by trained individuals using the signal processing software, Praat,20 following transcription conventions described previously.4
Narrative Analysis
Two investigators (R.G.G. and S.A.) coded the transcripts with 85% agreement. Coding conflicts were resolved by consensus. As our focus is narrative organization, the analysis centered on the following 3 discourse measures adapted from previous work.4,21
Narrative Theme
This measure represents participants’ ability to maintain the story theme (ie, search for the frog) throughout their narratives. Participants received 1 point for initially noting that the frog was lost and an additional point for each instance of mentioning the search there-after.
Global Connectedness
This measure captures whether subjects identified the point of the story. Subjects showed “global connectedness” if they realized that the frog found at the end of the story was the frog who initially escaped. If they mentioned a frog, but did not indicate that it was the lost frog, the event was scored as “globally unconnected.” A score of “missing” was used if the event was not mentioned.
Local Connectedness
This measure refers to conceptual connectedness between consecutive story events based on rhetorical devices such as sequencing adverbials, pronouns referring to previously mentioned characters, definite versus indefinite determiners, and statements of cause and effect. An event was scored as “locally unconnected” if there were no such devices, and the event did not follow logically from that preceding it. Events that were not mentioned were coded as “missing.”
In addition to the discourse measures described above, we evaluated 2 general aspects of the narratives. We assessed the accuracy with which participants described each event. An event description was “accurate” if it included at least part of the expected content. A description was scored as “error” if any aspect was factually inaccurate and as “missing” if the event was not mentioned. Finally, speech fluency was measured in words per minute. The word count incorporated all complete words, including repeated content in self-edits and restarts.
Neuropsychologic Testing
CBS patients and controls performed the Folstein Mini-Mental State Examination,22 as well as tests of visuospatial and visuoperceptual ability, executive function, language, and declarative memory.
Visuospatial and Visuoperceptual Ability
Participants were asked to identify items shown from an unusual view, estimate the location of dots in space, and match faces from the Benton facial recognition task.23 Participants also copied geometric designs (circle, rectangle, diamond, and cube) to demonstrate the ability to appreciate elements of a visually presented item and recreate a coherent representation.24
Executive Function
Digit span was measured in the forward and reverse directions. Verbal fluency was assessed by asking participants to name as many unique words as possible in 1 minute beginning with each of the letters, F, A, and S. Subjects were also asked to list as many animals as possible in 1 minute.
Language
Visual confrontation naming was assessed using the Boston Naming Test (BNT).25 The Pyramids and Palm Trees (PPT) task assessed semantic memory by asking subjects to make associative judgments about the meaning of pairs of pictures (PPT-pictures) and words (PPT-words).26
Memory
Episodic memory was assessed using a 10-word list-learning task. We recorded the number of words recited after each of the 3 successive trials, recalled after a 2-minute distracted delay, and recognized from a set of randomly intermixed list and nonlist words.
Statistical Analysis
Independent sample t tests were used to compare narrative theme maintenance, accuracy, and speech rate between CBS patients and controls. Global connectedness was scored in a binary fashion (“yes” or “no”), thus groups were compared on this measure using the χ2 test. The local connectedness measure yielded control data with a skewed distribution, thus the Mann-Whitney U test was used to compare CBS patients and controls on this measure. Individual patients’ scores on each neuropsychologic task were converted to z scores based on the performance of 24 age-matched and education-matched controls. Using only cognitive tests on which the CBS group was significantly impaired (mean z scorer≤ −1.96, P < 0.05), correlation analyses were performed between test performance and maintenance of the narrative theme. Narrative theme maintenance was selected for these correlation analyses because of the parametric properties of this measure. Correlation analyses were performed in 17 CBS patients who completed cognitive testing within 1 year of the narrative task. All statistical analyses were performed using SPSS 15.0 (SPSS Inc, Chicago, IL).
Imaging Procedure
Six patients with CBS had a brain MRI within 1 year of the narrative task. Images were collected using a SIEMENS Trio 3.0 T scanner with a high resolution T1-weighted 3-dimensional spoiled gradient-echo sequence, at TR = 1620 m seconds, TE = 3 m seconds, slice thickness = 1 mm, flip angle = 15 degrees, matrix = 195 × 256, and in-plane resolution = 0.9 × 0.9 mm. Voxel-based morphometry was used to identify areas of cortical atrophy and to correlate atrophy with narrative theme maintenance. All image processing was performed using the unified segmentation algorithm implemented in SPM5 (www.fil.ion.ucl.ac.uk/spm5). Cortical volumes were coregistered to the Montreal Neurological Institute template. Gray matter tissue was determined using a single generative model, which combines segmentation, bias correction, and spatial normalization using affine transformation and deformation of tissue probability maps.27 The segmentation algorithm calculates a Bayesian probability for each voxel of each tissue group in the volume, based on a priori MRI information. Lastly, the modulated gray matter volumes were smoothed in SPM5 with an 8-mm full width at half maximum Gaussian filter to minimize individual gyral variations.
Statistical analyses were conducted in SPM5. T tests were used to contrast gray matter volume between CBS patients and a group of 12 age-matched controls to identify areas of significant cortical atrophy. An explicit mask defined by the SPM5 a priori gray matter tissue probability map was used. We identified clusters consisting of at least 100 adjacent voxels and an extent significant at P < 0.01 where the cluster contained a peak with z score ≥ 3.09 (P < 0.001). We also performed a regression analysis between cortical volume and narrative theme maintenance. Explicit masking was used for this whole-brain analysis so that we could test the relationship between theme maintenance and brain areas known to be significantly atrophied from the prior analysis. We did not assess for regions where theme maintenance was related to nonatrophied areas because it would be difficult to interpret the role of these nonatrophied regions in patients’ poor discourse performance. For the regression analysis, we used a 100-voxel extent and a statistical height threshold of P < 0.05 with a stringent criterion of P< 0.05 (false discovery rate-corrected) for identifying significant clusters. Coordinates for each accepted cluster were converted to Talairach space.28
RESULTS
Narrative Measures
Narrative discourse was significantly impaired in patients with CBS, as summarized in Table 2. CBS patients mentioned the narrative theme less often than controls [t(26) = 3.55, P = 0.001]. Global connectedness was intact in all controls, but only 6 of 20 patients (30%) realized that the frog found at the end of the story was the frog who initially escaped [χ2(1) = 11.20, P = 0.001]. Local connectedness, or the number of connected events as a proportion of mentioned events, was lower in CBS patients relative to controls (U = 28.00, P < 0.005). The mean speech rate in patients with CBS was approximately one-half that of controls [t(26) = 4.69, P < 0.001].
TABLE 2.
Performance on Narrative Measures in CBS Patients and Controls
| Controls | CBS | P | |
|---|---|---|---|
| Narrative theme maintenance | |||
| Mean number of instances (SD) | 7.0 (1.9) | 2.7 (3.2) | 0.001 |
| Global connectedness | |||
| Percent successful individuals | 100 | 30 | 0.001 |
| Local connectedness | |||
| Mean percent connected events (SD) |
100 (0) | 75 (30) | 0.005 |
| Accuracy | |||
| Mean percent accurate events (SD) | 93 (4) | 62 (21) | 0.001 |
| Speech rate | |||
| Mean words/min (SD) | 142.4 (21.2) | 77.4 (36.5) | 0.001 |
CBS indicates corticobasal syndrome.
Although event descriptions were less accurate in patients compared with controls [t(26) = 4.15, P < 0.001; Table 2], we found that poor performance on discourse measures could not be easily explained by reduced event-description accuracy. After excluding 7 patients with fewer than 50% accurate events, significant differences remained between the CBS (n = 13) and control (n = 8) groups in all discourse measures: narrative theme maintenance [t(19) = 2.34, P < 0.05], global connectedness [χ2(1) = 6.46, P < 0.05], and local connectedness (U = 28.00, P < 0.05).
Neuropsychologic Test Results
Table 3 shows that patients have disproportionate difficulty on visually mediated tasks. Narrative theme maintenance was related to the PPT-pictures (r = 0.70, P < 0.05) and the geometric design copy (r = 0.49, P = 0.061) tasks, which are visually mediated measures involving semantic interpretation and higher-level organization.
TABLE 3.
Z Scores for Performance on Selected Cognitive Tasks in CBS Patients
| Mean Z-score (SD)* | |
|---|---|
| Dot location estimation | −7.25 (10.26) |
| Geometric design copy (constructional) | −4.51 (3.84) |
| Unusual views | −3.55 (5.75) |
| Pyramids and Palm Trees—pictures | −3.41 (3.40) |
| Boston Naming Test | −2.72 (2.50) |
| Verbal fluency—animals | −2.13 (0.73) |
| Pyramids and Palm Trees—words | −2.08 (2.92) |
| Memory list recall | −1.76 (0.71) |
| Verbal fluency—F, A, S | −1.60 (1.00) |
| Reverse digit span | −1.53 (1.30) |
| Face recognition | −1.37 (1.19) |
Z-scores based on data from 24 age-matched and education-matched control subjects.
Performance on elementary visuospatial/visuoperceptual tasks (ie, estimating the location of dots in space and identifying objects shown from an unusual view) did not correlate with narrative theme maintenance. To show further that discourse performance is not explained by elementary visual processing deficits, we excluded 9 patients with significant impairment on basic visuospatial/visuoperceptual tasks (defined as average z scorer≤ −1.96, P < 0.05, on the dot location estimation, unusual views, and Benton facial recognition tasks). With these patients excluded, significant differences remained between the CBS (n = 8) and control (n = 8) groups in narrative theme maintenance [t(14) = 2.75, P < 0.05], global connectedness [χ2(1) = 5.33, P < 0.05], and local connectedness (U = 8.00, P < 0.005).
Moreover, there was no correlation between narrative theme maintenance and language or executive tasks such as BNT, PPT-words, and verbal fluency. To examine the influence of naming deficits on discourse performance, we excluded 8 patients with significant impairment on the BNT (z scorer≤ −1.96, P < 0.05). Again, differences remained between CBS patients (n = 9) and controls (n = 8) in narrative theme maintenance [t(15) = 2.93, P = 0.01], global connectedness [χ2(1) = 4.65, P < 0.05], and local connectedness (U = 12.00, P < 0.01).
Imaging Results
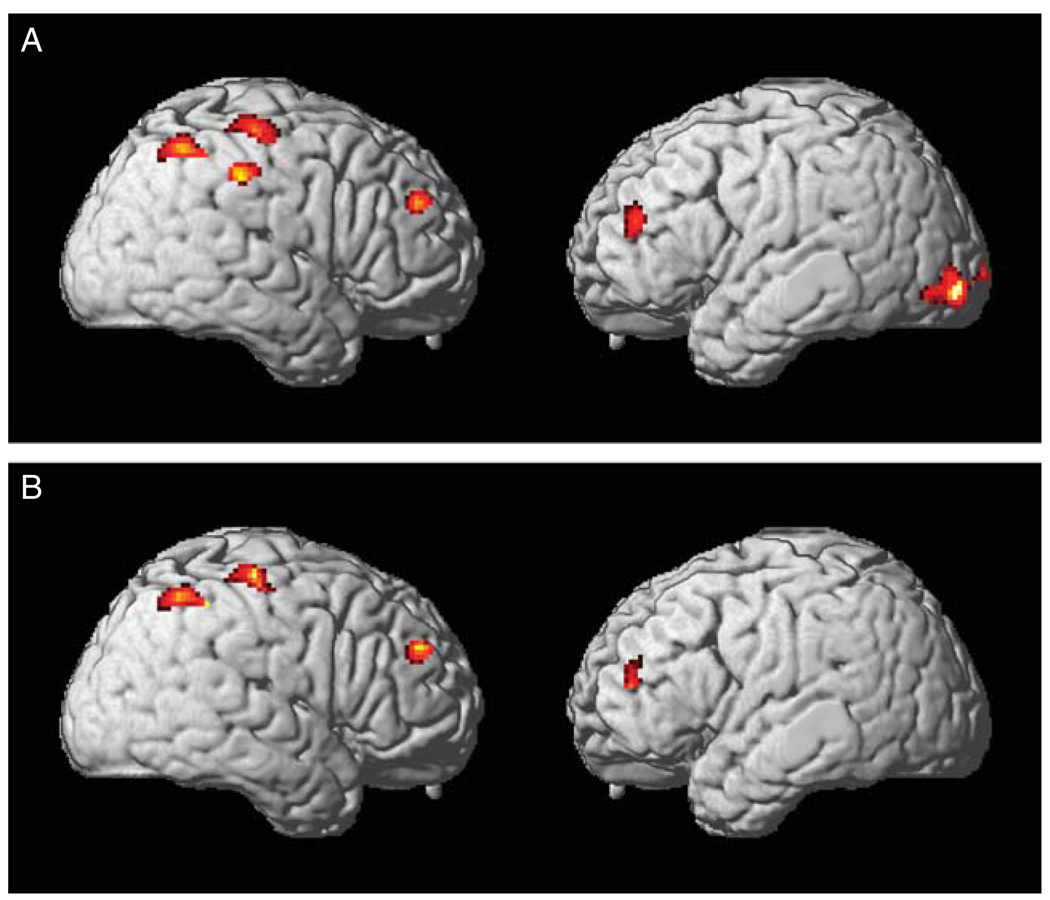
Table 4 and Figure 1A summarize regions of significant cortical atrophy in CBS patients relative to controls. We found significant atrophy in the right inferior parietal lobe [Brodmann area (BA) 40], right postcentral gyrus (BA 1/2/3), bilateral middle frontal gyri (BA 9/10/46), and left inferior occipital lobe (BA 18). Regression analyses showed that narrative theme maintenance was significantly related to right inferior parietal (BA 40), right postcentral (BA 1/3), and bilateral dorsolateral prefrontal (BA 9/10/46) atrophy (Table 4 and Fig. 1B).
TABLE 4.
Volumetric MRI Analysis in Patients With CBS
| Coordinates | ||||
|---|---|---|---|---|
| Peak Anatomic Locus (Brodmann area) | X | Y | Z | Z-score |
| Areas of Cortical Atrophy in CBS Patients Relative to Controls | ||||
| Right inferior parietal (40) | 48 | −48 | 50 | 3.63 |
| Right parietal (2/40) | 51 | −25 | 40 | 3.49 |
| Right middle frontal (9/46) | 42 | 46 | 25 | 3.49 |
| Right postcentral gyrus (1/3) | 50 | −18 | 58 | 3.91 |
| Left middle frontal (10/46) | −50 | 46 | 20 | 3.60 |
| Left inferior occipital (18) | −28 | −88 | −4 | 3.25 |
| Areas of Cortical Atrophy Correlated With Theme Maintenance | ||||
| Right inferior parietal (40) | 57 | −38 | 48 | 3.50 |
| Right middle frontal (9) | 44 | 50 | 29 | 4.19 |
| Right postcentral gyrus (1/3) | 46 | −18 | 58 | 3.54 |
| Left middle frontal (10/46) | −50 | 45 | 12 | 3.26 |
CBS indicates corticobasal syndrome; MRI, magnetic resonance imaging.
FIGURE 1.
Results of volumetric MRI in patients with CBS showing (A) regions of cortical atrophy; and (B) regions of correlation between narrative theme maintenance and cortical atrophy. CBS indicates corticobasal syndrome; MRI, magnetic resonance imaging.
DISCUSSION
We analyzed narrative speech to characterize discourse impairment in patients with CBS. Relative to controls, patients showed diminished narrative organization and coherence. They had difficulty maintaining the story theme and reduced connectedness between events. A majority of patients failed to identify the overall point of the story. The imaging analysis related discourse impairment to the disruption of a parietal-frontal network in CBS.
Little attention has been devoted to discourse in CBS. A case study demonstrated discourse impairment in a 60-year-old woman with CBS using the Pragmatic Protocol, a detailed assessment of videotaped conversation.29,30 Only 38% of protocol items were judged “appropriate.” The authors described impaired topic management, turn-taking, comment cohesion, and inclusion of relevant information.30 This description of naturalistic discourse (ie, conversation) in a patient with CBS complements our findings using a semi-structured discourse task with clearly defined target content, which enabled quantitative assessment of narratives in a large group of CBS patients.
Correlation analyses were performed to identify neuropsychologic factors that contribute to discourse impairment in CBS patients. Patients with CBS have visual processing deficits, as shown in this study (Table 3) and by others.8,31 Thus it is essential to demonstrate that difficulty perceiving elements of a pictured scene does not account for patients’ poor narrative performance. In fact, performance on basic visuospatial/visuoperceptual tasks did not correlate with narrative theme maintenance. In addition, after excluding patients with significant deficits on basic visual tasks, the CBS group still showed impaired discourse-level performance relative to controls. Likewise, the CBS group was impaired on the BNT, raising the possibility that difficulty naming pictured items interfered with production of a coherent story. However, BNT performance did not correlate with narrative theme maintenance, and discourse deficits remained after patients with significant naming difficulty were excluded from analysis. In addition, discourse-level deficits remained after patients with poor event-description accuracy for any reason were excluded from analysis. Cognitive testing also did not reveal significant memory impairment in the CBS group, and participants could consult the book while narrating. In summary, we feel patients’ difficulty in creating coherent narratives cannot be entirely accounted for by trouble perceiving and naming elements of scenes or by inability to remember story events.
Instead we found that narrative theme maintenance was related to performance on the PPT-pictures and the geometric design copy tasks. These tasks require patients to integrate and derive meaning from visual material. Thus we propose that difficulty integrating information into a coherent, meaningful whole contributes to narrative impairment in CBS. Consider the following attempt to describe a scene from a patient with CBS as an illustration:
“… there’s a frog … in a jar—and there’s slippers there and there’s uh a shirt and a sock and boots and a stool and a window and a bed and a lamp …”
This patient correctly identifies objects in the scene, demonstrating her ability to perceive and name individual items. However, she focuses locally on each object in the scene and does not capture how these items together convey what is happening in the scene. This difficulty extracting the gist of a given scene could impede situation model building over the course of narration.2,13,18 In addition, we speculate that this deficit is not restricted to integrating elements of single visual scenes, rather that CBS patients have a higher-level deficit integrating described events into a coherent narrative. Future studies using nonpictorial materials are needed to demonstrate the contribution of this more broadly defined integrative deficit to discourse impairment in CBS.
Volumetric MRI revealed parietal and frontal atrophy in CBS patients relative to controls, consistent with studies of patients with autopsy-proven corticobasal degeneration.8,10 Atrophy in inferior occipital visual association areas and the postcentral gyrus is consistent with visual processing deficits and cortical sensory loss seen in CBS.31,32 Narrative theme maintenance was related to atrophy in a parieto-frontal network, including the right inferior parietal lobe and bilateral dorsolateral prefrontal cortex. Right hemisphere damage has been shown to interfere with discourse processing,33 and functional MRI (fMRI) studies in healthy adults have demonstrated right hemisphere contribution to discourse.2,34,35 In an fMRI study of healthy subjects during story comprehension, Xu and colleagues2 showed increased activation in right hemisphere regions associated with narrative-level processing. Moreover, right hemisphere activity increased at story conclusion, presumably when events come together to form a coherent representation of the story as a whole.
Furthermore, the relation between theme maintenance and parieto-frontal atrophy suggests that parietal and frontal areas involved in higher-order information processing may function during discourse to integrate events into a coherent narrative. Previous studies have demonstrated parietal and frontal involvement in narrative production4,11,14 and comprehension,2,15 as well as in tasks that manipulate the coherence among sets of sentences.12,13 Yarkoni and coworkers13 performed an fMRI study during which young healthy subjects read blocks of scrambled sentences or blocks of sentences comprising a coherent story. The authors demonstrated bilateral posterior parietal cortex activation at story onset in both conditions, which remained elevated in the scrambled-sentence condition. They attributed this pattern to involvement of posterior parietal cortex in the initial construction of a situation model and in updating the model when conflict between an event and the global representation arises (as would occur often in the scrambled-sentence condition). They also argued that a network of frontal and temporal structures participate in situation model maintenance over time and in strategic coherence-building processes.13
Using the Frog Story paradigm, our group examined narratives from frontotemporal dementia patients with semantic dementia, progressive nonfluent aphasia, and nonaphasic patients with SOC/EXEC.4 Narrative impairment in patients with semantic dementia and progressive nonfluent aphasia was related to the linguistic difficulties characteristic of each disorder—that is, to lexical retrieval deficits and simplified grammatical output, respectively. Like CBS patients, SOC/EXEC patients showed impaired discourse-level performance. In SOC/EXEC patients, reduced story organization and coherence was associated with poor performance on executive tasks and with prefrontal atrophy.4 We hypothesize that in CBS, the additional parietal disease contributes to impaired information integration and difficulty telling a coherent story.
Parieto-frontal dysfunction in CBS may compromise other aspects of communication central to discourse such as inference and theory of mind. CBS patients show poor story theme maintenance, despite the fact that the book contains many instances in which pictured events prompt the speaker to mention searching for a lost frog. Many of these prompts require inference about an action (eg, if the boy looks in a hole, we infer that he is looking for something, and if the frog was lost, we infer that he is looking for the frog). The ability to draw inferences has been associated with parts of the frontoparietal network36 affected in CBS. In everyday story-telling, speakers repeatedly refer to the theme to help the listener follow the tale. This facet of communication involves theory of mind, or the ability to infer the mental states of others.37 Current models propose that theory of mind is subserved by a frontoparietal network including prefrontal cortex and the temporo-parietal junction17,38 and may therefore be compromised in CBS as well.
When interpreting our results, an important caveat to consider is that the narrative task depends on picture perception. The absence of a correlation between narrative performance and basic visuospatial and visuoperceptual tasks suggests that discourse impairment in CBS patients is not accounted for by elementary visual processing deficits. Moreover, discourse-level deficits remained in the CBS group after excluding patients with significant impairment on such tasks. The picture-book paradigm has the advantage of eliciting a rich extended speech sample for which intended content can be easily verified (unlike unstructured conversation or autobiographical tales) without relying on an overly familiar story line (as in fairy tales). Future work will examine discourse using semi-structured conversation and nonpictorial stimuli to obtain converging evidence for difficulty integrating discourse information in CBS patients.
With this caveat in mind, our findings are consistent with the hypothesis that CBS patients have impaired information integration, related to parieto-frontal disease, which interferes with their ability to tell a coherent story. Difficulty communicating through story-telling devastates a central aspect of human interaction with substantial consequences for patients in their daily lives.
ACKNOWLEDGMENTS
The authors thank the individuals who participated in this study.
Supported by an American Academy of Neurology Foundation Clinical Research Training Fellowship grant, as well as by NIH grants NS44266, AG17586, AG15116, and NS53488.
REFERENCES
- 1.Mar RA. The neuropsychology of narrative: story comprehension, story production and their interrelation. Neuropsychologia. 2004;42:1414–1434. doi: 10.1016/j.neuropsychologia.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Xu J, Kemeny S, Park G, et al. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Chapman SB, Zientz J, Weiner M, et al. Discourse changes in early Alzheimer disease, mild cognitive impairment, and normal aging. Alzheimer Dis Assoc Disord. 2002;16:177–186. doi: 10.1097/00002093-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ash S, Moore P, Antani S, et al. Trying to tell a tale: discourse impairments in progressive aphasia and frontotemporal dementia. Neurology. 2006;66:1405–1413. doi: 10.1212/01.wnl.0000210435.72614.38. [DOI] [PubMed] [Google Scholar]
- 5.Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- 6.Mahapatra RK, Edwards MJ, Schott JM, et al. Corticobasal degeneration. Lancet Neurol. 2004;3:736–743. doi: 10.1016/S1474-4422(04)00936-6. [DOI] [PubMed] [Google Scholar]
- 7.Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology. 1999;53:1969–1974. doi: 10.1212/wnl.53.9.1969. [DOI] [PubMed] [Google Scholar]
- 8.Murray R, Neumann M, Forman MS, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–1283. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- 9.Soliveri P, Monza D, Paridi D, et al. Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology. 1999;53:502–507. doi: 10.1212/wnl.53.3.502. [DOI] [PubMed] [Google Scholar]
- 10.Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Troiani V, Fernandez-Seara MA, Wang Z, et al. Narrative speech production: an fMRI study using continuous arterial spin labeling. Neuroimage. 2008;40:932–939. doi: 10.1016/j.neuroimage.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin-Loeches M, Casado P, Hernandez-Tamames JA, et al. Brain activation in discourse comprehension: a 3t fMRI study. Neuroimage. 2008;41:614–622. doi: 10.1016/j.neuroimage.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 13.Yarkoni T, Speer NK, Zacks JM. Neural substrates of narrative comprehension and memory. Neuroimage. 2008;41:1408–1425. doi: 10.1016/j.neuroimage.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman SB, Bonte FJ, Wong SB, et al. Convergence of connected language and SPECT in variants of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord. 2005;19:202–213. doi: 10.1097/01.wad.0000189050.41064.03. [DOI] [PubMed] [Google Scholar]
- 15.Cosentino S, Chute D, Libon D, et al. How does the brain support script comprehension? A study of executive processes and semantic knowledge in dementia. Neuropsychology. 2006;20:307–318. doi: 10.1037/0894-4105.20.3.307. [DOI] [PubMed] [Google Scholar]
- 16.Mesulam M-M. Behavioral neuroanatomy: large-scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations: functions and syndromes of posterior parietal heteromodal cortex. In: Mesulam M-M, editor. Principles of Behavioral and Cognitive Neurology. 2nd ed. New York: Oxford University Press, Inc; 2000. pp. 39–41. [Google Scholar]
- 17.Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- 18.Van Dijk T, Kintsch W. Strategies of Discourse Comprehension. New York: Academic Press; 1983. [Google Scholar]
- 19.Mayer M. Frog, Where Are You? New York: Penguin Books; 1969. [Google Scholar]
- 20.Praat. Doing phonetics by computer (computer program) 2008 Version 5.0.32. [Google Scholar]
- 21.Reilly J, Losh M, Bellugi U, et al. “Frog, where are you?” Narratives in children with specific language impairment, early focal brain injury, and Williams syndrome. Brain Lang. 2004;88:229–247. doi: 10.1016/S0093-934X(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Benton AL, Sivan AB, Hamsher KdS, et al. Contributions to Neuropsychological Assessment. New York: Oxford University Press; 1983. Facial Recognition: Stimulus and Multiple Choice Pictures. [Google Scholar]
- 24.Welsh KA, Butters N, Hughes JP, et al. Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 26.Howard D, Patterson K. Pyramids and Palm Trees: a Test of Semantic Access From Pictures and Words. Bury St. Edmunds: Thames Valley Publishing Company; 1992. [Google Scholar]
- 27.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 29.Prutting CA, Kirchner DM. A clinical appraisal of the pragmatic aspects of language. J Speech Hear Disord. 1987;52:105–119. doi: 10.1044/jshd.5202.105. [DOI] [PubMed] [Google Scholar]
- 30.Donovan NJ, Kendall DL, Moore AB, et al. Why consider impaired social language usage in a case of corticobasal degeneration? Clin Neuropsychol. 2007;21:190–203. doi: 10.1080/13854040600634584. [DOI] [PubMed] [Google Scholar]
- 31.Bak TH, Caine D, Hearn VC, et al. Visuospatial functions in atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry. 2006;77:454–456. doi: 10.1136/jnnp.2005.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kompoliti K, Goetz CG, Boeve BF, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol. 1998;55:957–961. doi: 10.1001/archneur.55.7.957. [DOI] [PubMed] [Google Scholar]
- 33.Joanette Y, Goulet P. Narrative discourse in right-brain-damaged right-handers. In: Joanette Y, Brownell HH, editors. Discourse Ability and Brain Damage: Theoretical and Empirical Perspectives. New York: Springer Verlag; 1990. [Google Scholar]
- 34.Ferstl EC, Rinck M, von Cramon DY. Emotional and temporal aspects of situation model processing during text comprehension: an event-related fMRI study. J Cogn Neurosci. 2005;17:724–739. doi: 10.1162/0898929053747658. [DOI] [PubMed] [Google Scholar]
- 35.St George M, Kutas M, Martinez A, et al. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain. 1999;122(Pt 7):1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- 36.Liepelt R, Von Cramon DY, Brass M. How do we infer others’ goals from non-stereotypic actions? The outcome of context-sensitive inferential processing in right inferior parietal and posterior temporal cortex. Neuroimage. 2008;43:784–792. doi: 10.1016/j.neuroimage.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behavior Brain Sci. 1978;1:515–526. [Google Scholar]
- 38.Rilling JK, Sanfey AG, Aronson JA, et al. The neural correlates of theory of mind within interpersonal interactions. Neuroimage. 2004;22:1694–1703. doi: 10.1016/j.neuroimage.2004.04.015. [DOI] [PubMed] [Google Scholar]