Abstract
The past ten years have seen new approaches to elucidating genetic pathways regulating sleep. The emerging theme is that sleep-like states are conserved in evolution, with similar signaling pathways playing a role in animals as distantly related as flies and humans. We review the evidence for the presence of sleep states in non-mammalian species including zebrafish (Danio rerio), fruit flies (Drosophila melanogaster), and round worms (Caenorhabditis elegans). We describe conserved sleep-regulatory molecular pathways with a focus on cAMP and Epidermal Growth Factor (EGF) signaling; neurotransmitters with conserved effects on sleep and wake regulation, including dopamine and GABA; and a conserved molecular response to sleep deprivation involving the chaperone protein BiP/GRP78.
Introduction
An increasing appreciation of the importance of sleep and the impact of sleep disorders on health has underlined the need for a basic understanding of sleep and sleep regulation. Until recently studies of sleep have largely focused on humans and other mammalian systems (e.g. rat, mouse, hamster, dog, cat and monkey). These studies have largely depended upon the electroencephalogram (EEG) to distinguish between wakefulness and sleep. . However, as EEG waves arise from the underlying neural architecture, non-mammalian species with significantly different neuroanatomies can not be expected to produce the same EEG wave forms associated with sleep/wake cycles in mammals. Even if this were not the case, it is extremely challenging to record electrophysiological signals from small model organisms without perturbing their behavior. A major advance in our ability to study sleep in non-mammalian species, came with the appreciation that behavioral criteria alone are sufficient for the identification of sleep in many model systems [1, 2]. This review argues that the study of sleep in multiple non-mammalian model systems has led to identification of conserved molecular pathways that regulate sleep and wakefulness.
The chief behavioral properties of sleep, which distinguish it from quiet wakefulness, or other states of quiescence such as torpor and hibernation are: 1) a period of quiescence associated with a species-specific posture; 2) an increased arousal threshold (reduced responsiveness to external stimuli); 3) quick reversibility to wakefulness; 4) homeostasis; and 5) interaction with the circadian clock and/or expression of clock genes. Sleep is reversible since sleeping animals will awaken and move given a sufficiently strong stimulus. Sleeping animals demonstrate an elevated arousal threshold, for instance showing no response to sensory stimuli when asleep that would evoke a clear response when awake. Sleep homeostasis is demonstrated by there being elevated sleep propensity and deeper and longer recovery sleep period following sleep deprivation. In normal animals the timing of sleep is coupled to a clock mechanism. However, a functioning circadian clock is not essential for sleep to occur. Sleep still occurs in organisms despite changes that abolish their circadian rhythm, for instance from lesions of the suprachiasmatic nucleus in rats [3] and from mutations of clock genes in both mice [4, 5] and Drosophila [6, 7]. Moreover, circadian clocks also occur in species such as cyanobacteria and neurospora (for review see [8]) in which there is no evidence of a sleep-like state. Sleep can therefore occur without a clock mechanism and conversely, circadian clocks can function without sleep as an output. The use of behavioral criteria initially showed that insects, the cockroach [1] and the honey bee (see [9] and references therein), have a sleep-like state. However, despite 30-years of research into the circadian rhythm in the fruit fly Drosophila melanogaster (reviewed in [10, 11]), the demonstration that quiescence in Drosophila is a sleep-like state was relatively recent [6, 12] (see Table 1). Moreover, a sleep state has recently been identified using behavioral criteria in two additional non-mammalian model systems; the zebrafish, Danio rerio [13-15] (see Table 1) and the round worm, Caenorhabditis elegans [16, 17] (see Table 1 and Text Box 1). Both of these model systems are genetically tractable and possess easily visualized nervous systems allowing sleep researchers unparalleled opportunities for manipulation of neural circuitry (see [13-17] and references therein). The identification of a sleep state in such distantly-related species indicates that sleep is a basic biological process. While the fundamental aspects of sleep are preserved in disparate species, there are species-specific elaborations of the process.
Table 1. Evidence for a sleep state in three non-mammalian model organisms.
| Model Organism |
Behavioral Criterion For Sleep | ||||
|---|---|---|---|---|---|
| Species- Specific Posture |
Increased Arousal Threshold |
Quick Reversibility |
Homeostasis | Interaction with Circadian Clock |
|
|
Caenorhabditis elegans (worm) |
×a[16]b | ×[16] | ×[16] | ×[16] | |
|
Drosophila melanogaster (fruit fly) |
×[6] | ×[6, 12] | ×[6, 12] | ×[6, 12] | ×[6, 12] |
|
Danio rerio (zebrafish) |
×[13, 15] | ×[14, 15] | ×[15] | ×[13, 15] |
×[13-15, 67] |
An X indicates that evidence for this behavioral criterion has been demonstrated.
Reference source(s) for evidence.
Text Box 1: Sleep-like states in the nematode Caenorhabditis elegans.
C. elegans behavioral quiescence, which consists of cessation of both locomotion and feeding, has been observed during three different stages: During normal larval development in the lethargus periods, during the adult stage under conditions of satiety, and during the dauer stage, a long-lived alternative third larval stage that is induced by harsh environmental conditions. There is evidence that the first two of these quiescence periods have sleep-like properties. The evidence is strongest for lethargus, which occurs between each of the four larval stages and between the fourth larval stage and the adult stage [68]. Expression of lethargus is time-locked to expression of the C. elegans orthologue of the circadian gene period [20]. The quiescence observed during lethargus has the following behavioral sleep-like properties:
It is associated with reduced animal responsiveness to mechanical and olfactory stimuli.
The behavioral quiescence and the reduced responsiveness are reversible to strong stimulation of the animal.
Following partial deprivation of quiescence, subsequent quiescence occurs earlier and is more consolidated.
Following partial deprivation of quiescence, decreased responsiveness to olfactory stimuli occurs earlier and is more profound than in non-deprived animal. The C. elegans cGMP-dependent protein kinase (PKG) functions in sensory neurons to promote quiescence and reduced responsiveness during lethargus [16]. PKG is required for the quiescence promoting effects of Epidermal Growth Factor (EGF) [16] and is also required for the quiescence associated with the adult satiety response [69], indicating that this adult quiescence is regulated in a similar fashion to lethargus. Whereas EGF is one of the signals for induction of the sleep-like behavior associated with lethargus, TGF-β is a likely signal for induction of the quiescence associated with the adult satiety response [69, 70].
Sleep characteristics specific to different model systems
Zebrafish show rest-activity rhythms that are synchronous with the day-night cycle, being active during the day and quiescent at night [15, 18, 19]. In the zebrafish, light potently and directly promotes wakefulness by a mechanism that likely does not involve a resetting of the circadian clock [15]. In adult zebrafish, light powerfully suppresses sleep with no homeostatic response for up to seven days [15]. Therefore, research in this model system has uncovered a, as yet to be understood, direct interaction of light with the arousal system.
In C. elegans, the short life-cycle of the worm is not conducive to long periods of quiescence regulated by circadian factors (Text Box 1). Behavioral quiescence in C. elegans is concentrated during four discrete times during larval development called lethargus periods. Timing of lethargus is time-locked to expression of the C. elegans gene lin-42 [20], an orthologue of the period, a gene which oscillates in phase with the sleep period and regulates sleep timing in Drosophila and mammals. This provides a molecular connection between a “clock mechanism” and lethargus. Whereas the timing of mammalian and Drosophila period action is regulated by light, an external time giver, the timing of C. elegans lethargus is regulated by developmental time, an internal time giver. Quiescence has also been observed in adult worms (Text Box 1).
Conserved signaling pathways regulating sleep
As in the circadian field (reviewed in [10, 11]), discoveries about fundamental molecular mechanisms in one model system have led to insights in others.
Analysis of the sleep phenotype of Drosophila mutants with decreased and increased cAMP levels led to the conclusion that cAMP promotes wakefulness in Drosophila [21] (see Figure 1A). Reduced levels of cAMP in the adenylate cyclase mutant rutabaga are associated with increased sleep, while increased levels of cAMP in the phosphodiesterase mutant dunce are associated with decreased sleep [21]. Further, by analyzing the effects of reduced and elevated activity of the transcription factor CREB--one of the cAMP dependent kinase targets--it was found that CREB promotes wakefulness in flies [21]. This led to the hypothesis that in mammals, CREB promotes wakefulness. In the mouse, as in flies, mutants with reduced CREB activity have reduced wakefulness [22]. Thus, there is a molecular mechanism involving CREB that promotes wakefulness in both flies and mice. While the role of CREB has not yet been assessed in C. elegans lethargus regulation, reduction of function mutations of the cyclic nucleotide phosphodiesterase gene pde-4 and gain of function mutations of the adenylate cylcase gene acy-1, both of which increase cAMP levels, increase sensory responsiveness during lethargus [16], suggesting that cAMP signaling affects sleep-like behavior in a similar fashion in C. elegans. Therefore, cAMP signaling has a phylogenetically conserved role in promoting wake behavior (see Figure 1A). Future studies need to address the effects of altered cAMP signaling on behavioral quiescence in C. elegans as well as on zebra fish sleep. In addition, such studies should aim to provide mechanistic insight into how cAMP signaling promotes wakefulness.
Figure 1.
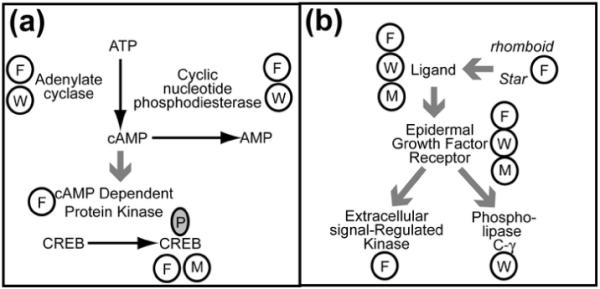
Schematic showing pathways that have been identified by more than one model systems as having an impact on sleep. (a) The cAMP–CREB pathway has been shown to impact sleep /wake behavior in the fly, worm, and mammalian systems. Mutations of genes which code for the enzymes adenylate cyclase and cyclic AMP phosphodiesterase in Drosophila melanogaster and in Caenorhabditis elegans have led to the following conclusions: elevation of cAMP promotes wake behavior and reduction of cAMP, at least in the fly, promotes sleep. Over expression of a murine cAMP dependent kinase and expression of an activated form of CREB in the fly leads to decreased sleep. Mice lacking the α and δ isoforms of CREB have low CREB activity and decreased wakefulness. Therefore CREB activity is involved in the maintenance of wakefulness in both the mouse and the fly. (b) The Epidermal Growth Factor Receptor (EGFR). EGFR ligands increased sleep and suppressed activity in both mammals and fruit flies. Mice with reduced EGFR function have increased quiescence. Phosphorylation of an extracellular signal-regulated kinase (ERK) is a downstream event correlated with the sleep induction by EGF in the fly. In the worm, mutants with reduced EGFR function show increased activity during the sleep-like state of lethargus and over expression of the single EGF ligand, LIN-3, causes quiescence during periods of normal activity. This LIN-3 effect requires phospholipase C-γ.
Steps in each of the pathways are labeled by the enzyme or receptor; substrates of enzymatic reactions are connected by black arrows next to the enzyme responsible for the step; large grey arrows indicate binding and/or activation; a circled F indicates evidence from Drosophila, a circled W indicates evidence from C. elegans, a circled M indicates evidence from a mammalian system, and a circled P indicates phosphorylation.
The discoveries of the sleep-regulatory roles of the epidermal growth factor (EGF) pathway illustrate the utility of using simple model organisms to better understand the impacts of a pathway upon sleep. The original observations as to the role of EGF were made in mammals, but mechanistic questions were then asked in invertebrates. In rabbits, intracerebroventricular injection of EGF increases non-REM sleep [23]; in hamster an EGF receptor (EGFR) ligand, transforming growth factor alpha (TGF-α), suppresses wheel running activity when chronically infused into the third ventricle [24], and mice with the waved-2 mutation, which have reduced EGFR activity, have increased locomotor activity [24]. In mammals, since EGFR is one of four receptor tyrosine kinases (reviewed in [25]) and since there is only one allele of waved-2, there have been inconsistent results with respect to wheel-running activity [26]. By contrast, in flies there is only one receptor family member and four ligands (reviewed in [27]), and more available genetic tools. In Drosophila, genetic constructs that increase the release of EGFR ligands increase sleep, while inhibition of ligand release decreases total sleep by shortening sleep bout durations [28]. This effect is dependent on a functional EGFR and correlates with increased phosphorylation of ERK, a downstream target of EGF-signaling [28] (see Figure 1B). The site of action for the effect of EGF on sleep was localized to the pars intercerebralis, a Drosophila brain area that is developmentally and functionally analogous to the hypothalamus in vertebrates [28].
In C. elegans, there is also a single EGF receptor (EGFR), LET-23 [29, 30], and mutants with loss of function of this receptor show increased activity during the sleep-like state of lethargus [17]. Over expression of the EGFR ligand LIN-3 leads to quiescent behavior during periods of normal activity. By exploiting the power of the transparent C. elegans neuroanatomy, Van Buskirk and Sternberg [17] were able to identify that this action of EGF signaling on sleep was mediated by a single neuron called ALA. The down stream EGFR target regulating the worm sleep state is phospholipase C-γ [17]. Therefore, EGF receptor signaling has a conserved sleep-promoting function, and studies in worms and flies have added molecular insight into this signaling pathway.
One of the key uses of model systems such as Drosophila and C. elegans is in gene discovery. Studies in C. elegans of the gene egl-4, which encodes a cGMP-dependent protein kinase (PKG) [31] illustrates this approach. egl-4 gain of function mutants show increased quiescence and reduced responsiveness during the adult stage, normally a wake period, while egl-4 loss-of-function mutants show reduced quiescence and increased responsiveness during lethargus, a sleep period [16]. This would argue that PKG promotes sleep.
In Drosophila, increased PKG activity is also associated with more sleep suggesting that in flies too, this gene promotes sleep-like behavior [16]. This finding, if confirmed with additional fly genetic analysis, would point to a phylogenetically conserved role for PKG in promoting sleep. That this conservation may extend to mammals is suggested by a preliminary report that PKG inhibition in the basal forebrain, reduces subsequent sleep [32].
Finally, core clock genes have been shown to have non-circadian roles in regulating sleep and sleep homeostasis in mammals [33-36] and Drosophila [6, 7], and is another example of a likely conserved pathway. In mammalian systems, these effects are likely mediated by actions of clock genes outside the suprachiasmatic nucleus (SCN) [33].
Conserved functions of neurotransmitters
While of great value in detecting genes involved in behavior, one ostensible disadvantage of using invertebrate animal models to study sleep is that the neuroanatomy is distinctly different. This may be a particular issue for sleep research as compared to circadian research. Molecular clocks can function in isolated cells [37], while sleep is determined by circuits (reviewed in [38]). Nevertheless, although the structure of the nervous system differs, there is evidence of conservation in the action of certain neurotransmitters.
Dopamine is implicated in the arousal system of mammals, as evidenced by the following: there is reduced sleep in mice lacking the dopamine reuptake transporter (DAT) [39]; amphetamines, which increase dopamine concentrations in the brain, decrease sleep (reviewed in [40]); destruction of dopaminergic neurons in the ventral periaqueductal gray matter of rats increases sleep [41]; and daytime sleepiness is observed in patients with Parkinson’s disease, in which there is a loss of dopaminergic neurons (reviewed in [42]). Drosophila also sleep less when fed amphetamines, and sleep more when dopamine synthesis is inhibited [43]. In addition, a mutation of the Drosophila dopamine transporter fumin leads to less sleep [44]. Thus, dopamine plays a similar role in sleep/wake control in both mammals and Drosophila. Its role in sleep control has not yet been studied in zebrafish or C. elegans.
Hypocretin (orexin) was identified simultaneously in dogs and mice as being critical in the pathogenesis of the human disorder narcolepsy. The narcoleptic dog model was found to be a null for the hypocretin 2 receptor [45] while a genetic knockout mouse that lacks the peptide has all of the features of narcolepsy [46]. Hypocretin is also found in zebrafish [14, 15]. To date, no homologous molecule has been reported in Drosophila or C. elegans. In zebrafish, as in mammals, hypocretin is found in cells in the lateral hypothalamus but unlike mammals, which have two hypocretin receptors, fish have only one receptor [14, 15]. The projections of these cells is controversial, with one study reporting that, as in mammals, there are projections to other wake-active catecholaminergic groups [14], while another study did not confirm this projection [15]. The role of hypocretin in zebrafish sleep regulation is also an unsettled question. Overexpression of the peptide leads to less sleep and a reduced arousal threshold in zebrafish larva [14], an observation compatible with the known role of hypocretin in wake promotion in mammals [47, 48]. Surprisingly, adult zebrafish lacking a functional hypocretin receptor also have less sleep [15], a phenotype that is opposite to what is expected based on mammalian research. Further research on the role of hypocretin in zebrafish and other model systems is needed.
GABA is a major inhibitory neurotransmitter in the mammalian nervous system and pharmacological agents directed against the GABAA receptor are used to treat insomnia in humans (reviewed in [49]). Neurons of the major sleep active area of the mammalian brain, the ventrolateral preoptic area (VLPO), express GABA and the inhibition of wake active brain regions by projections from the VLPO is hypothesized to be the mechanism of sleep onset [50]. Sleep onset regulation by a GABAA receptor has recently also been shown in Drosophila [51]. Flies with mutations of a Drosophila GABAA receptor gene Resistant to dieldrin have significantly decreased sleep latencies [51]. Hyperpolarizing all GABA producing neurons deceased total sleep and sleep bout duration, as well as increased wake bout duration and sleep latency [51]. These data indicate that GABA is a sleep promoting neurotransmitter conserved between mammals and insects.
The evidence for the role of serotonin in sleep/wake control in mammals is not straightforward. Although brain serotonin release is reduced during sleep, lesions of serotonergic projections in the brain, or pharmacological depletion of serotonin in the brain, lead to insomnia (reviewed in [52]). 5-HT1a receptor knockout mice have increased REM sleep and do not show REM sleep rebound following REM deprivation [53]. Additionally, intra peritoneal injection of 5-HT can increase either wakefulness or sleep, depending on the timing of injection and the dosage [54]. In Drosophila, genetic and pharmacologic manipulations that increase serotonin levels increase sleep, and mutants lacking the 5HT1A receptor sleep less, suggesting that in Drosophila, serotonin promotes sleep [55]. The similarities between the role of serotonin in sleep/wake control in mammals and Drosophila is thus not as clear as that for dopamine and GABA.
Potassium channels
The first reported Drosophila forward genetic screen focused on sleep phenotypes led to the discovery of potassium channels as fundamental regulators of sleep behavior. Specifically, the potassium channel shaker was identified as a modulator of sleep by screening 9,000 mutant lines for a short sleep phenotype [56]. To extend these findings in Drosophila to mammals, Cirelli and colleagues examined mice lacking the α subunit of a shaker channel (Kcna2). These mice spent 21% more time awake than heterozygous siblings [57]. There may also be a role for shaker channels in sleep in humans; auto immunity to Shaker-type potassium channels (Kv1.1 and Kv1.2) is associated with Morvan’s syndrome, which can manifest as insomnia among other neurological symptoms [58].
A key molecular response to sleep deprivation is also conserved
It is not only sleep/wake signaling mechanisms that are conserved but so too are molecular responses to sleep deprivation. In Drosophila [12], in rat cortex [59, 60], mouse cortex and hypothalamus [61], and avian brain [62] sleep deprivation leads to up regulation of GRP78/BiP, an endoplasmic resident (ER) molecular chaperone and heat shock family protein. Up-regulation of BiP indicates ER stress and activation of the unfolded protein response (UPR) (for review see [63]). In addition to BiP induction, other aspects of the UPR have been found following sleep deprivation in mouse cortex [64].
Conclusions
Research in model systems offers substantial advantages for identifying and studying genes involved in regulating complex behavior. They can be considered hypothesis-generating studies. Using these systems, the cAMP, EGF and cGMP pathways have been identified as being involved in sleep-wake control. In contrast to circadian clocks, which are regulated by a relatively small set of genes that are primarily dedicated to the function of timing, the regulation of sleep and wakefulness involves a number of signaling pathways that have been previously implicated in other biological processes. Therefore, the specificity for sleep/wake control is likely to be explained by signaling events that are regulated both temporally and spatially, i.e., the specificity for sleep/wake control comes from the neuronal circuitry. While there are differences in the relevant neuronal circuitry, some aspects that include dopamine promoting wakefulness and GABA promoting sleep seem to be conserved at least between Drosophila and mice. While differences in neuronal circuitry might be seen as a disadvantage, they might also confer advantages. There may be a conserved neuronal circuitry involving core neurotransmitters which regulate sleep/wake states in multiple model systems. As species specific sleep/wake behaviors became more complex, specialized additional neuronal groups have likely evolved to regulate these functions.
Moving forward, there is a need to identify additional signaling mechanisms regulating sleep/wake control, and to understand how they interact so that a more complete picture at a molecular level is obtained. It is essential to understand mechanisms within the context of the relevant circuitry so that we can determine, for example, what leads to the switch of sleep-active neurons being inactive to active, with the converse for wake-active systems. Identifying additional neurons involved in regulation of sleep in Drosophila and lethargus in C. elegans are important future directions. As illustrated by the recent work on the role of hypocretin in the regulation of zebrafish sleep, an important future direction for research will be to more fully understand the neuroanatomical similarities and differences between fish and mammals.
There is also the need to translate these findings into humans. Ultimately, as in the circadian field, this approach holds the promise of identifying gene variants that alter sleep and confer risk for common sleep disorders. Sleep duration [65], response to sleep deprivation [66], and the common sleep disorder insomnia [66] are to some extent heritable. Thus, it is to be expected that model systems will continue to contribute to the identification of key genes regulating sleep.
Outstanding Questions Box.
What are the downstream events associated with cAMP, EGF and cGMP pathways?
What are the neuronal circuits responsible for sleep/wake control in the fly, worm and zebrafish and where in these circuits do signaling pathways act?
What conserved neuronal groups are found in these circuits?
What can we learn about the core function of neurotransmitters based on their study in model systems?
Are there conserved sleep functions?
Are variants of conserved genes in signaling pathways responsible, at least in part, for individual variation in sleep duration and response to sleep deprivation in humans?
Below are the responses to the five general comments included in the editorial decision for the TINS-D-08-00006R1 document.
-
Title. Given the focus of the paper is on the use of non-mammalian model systems to study sleep. I would suggest making this more explicit in the title, perhaps by changing it to “Conservation of sleep: Insights from non-mammalian model systems”.
This has been done
-
Abstract. … For instance, what are the names of the sleep regulatory pathways that are conserved across phyla? When revising please do not let the abstract exceed 120 words.
We have added details about the pathways and removed the sentence concerning the future human studies. The abstract is now 110 words.
-
I have suggested some minor textual changes in the Introduction and Discussion.
We have rewritten or removed unclear sentences and expanded the text for clarity and background information where appropriate. We have accepted your suggestions with occasional minor modifications.
-
Textbox 1 is currently written in a very “list-like” manner. I would suggest either re-working the text here, or perhaps converting this textbox into a table. If so, I would suggest including information about non-mammalian systems (e.g., C. Elegans and Drosophilia).
We thank the editor for this suggestion. We have removed the text box on the zebrafish Danio rerio and have added a Table (Table 1) that indicates the evidence for the behavioral characteristics of sleep for the three non-mammalian model organisms we discuss in the review and cites the reference(s) where this evidence is presented. We now have Table 1, Text Box 1 (detailed information on C. elegans sleep states), Figure 1, and the Outstanding Questions Box.
-
The length of the text for Figure 1 is quite long (>400 words). Please try to condense the text, and where possible incorporate some of it directly into the main body of the paper.
We have shortened the text for Figure 1 considerably, down to 275 words, as there was a lot of explanation already present in the main text.
Acknowledgements
We thank Daniel Barrett and Jennifer Montoya for help in manuscript preparation. This work was supported by NIH grants P01 AG17628 (to J.E.Z, N.N., and A.I.P) and K08 NS48914 (to D.M.R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tobler I. Effect of forced locomotion on the rest-activity cycle of the cockroach. Behav Brain Res. 1983;8:351–360. doi: 10.1016/0166-4328(83)90180-8. [DOI] [PubMed] [Google Scholar]
- 2.Hendricks JC, et al. The need for a simple animal model to understand sleep. Prog Neurobiol. 2000;61:339–351. doi: 10.1016/s0301-0082(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 3.Baker FC, et al. Persistence of sleep-temperature coupling after suprachiasmatic nuclei lesions in rats. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2005;289:R827–838. doi: 10.1152/ajpregu.00093.2005. [DOI] [PubMed] [Google Scholar]
- 4.Shiromani PJ, et al. Sleep rhythmicity and homeostasis in mice with targeted disruption of mPeriod genes. Am J Physiol Regul Integr Comp Physiol. 2004;287:R47–57. doi: 10.1152/ajpregu.00138.2004. [DOI] [PubMed] [Google Scholar]
- 5.Debruyne JP, et al. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron. 2006;50:465–477. doi: 10.1016/j.neuron.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Hendricks JC, et al. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 7.Hendricks JC, et al. Gender dimorphism in the role of cycle (BMAL1) in rest, rest regulation, and longevity in Drosophila melanogaster. J Biol Rhythms. 2003;18:12–25. doi: 10.1177/0748730402239673. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap JC. Molecular bases for circadian clocks. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 9.Sauer S, et al. Sleep deprivation in honey bees. J Sleep Res. 2004;13:145–152. doi: 10.1111/j.1365-2869.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosato E, et al. Molecular genetics of the fruit-fly circadian clock. Eur J Hum Genet. 2006;14:729–738. doi: 10.1038/sj.ejhg.5201547. [DOI] [PubMed] [Google Scholar]
- 11.Vallone D, et al. Start the clock! Circadian rhythms and development. Dev Dyn. 2007;236:142–155. doi: 10.1002/dvdy.20998. [DOI] [PubMed] [Google Scholar]
- 12.Shaw PJ, et al. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 13.Zhdanova IV, et al. Melatonin promotes sleep-like state in zebrafish. Brain Research. 2001;903:263–268. doi: 10.1016/s0006-8993(01)02444-1. [DOI] [PubMed] [Google Scholar]
- 14.Prober DA, et al. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–13410. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yokogawa T, et al. Characterization of sleep in zebrafish and insomnia in hypocretin receptor mutants. PLoS Biol. 2007;5:2379–2397. doi: 10.1371/journal.pbio.0050277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raizen DM, et al. Lethargus is a Caenorhabditis elegans sleep-like state. Nature. 2008;451:569–572. doi: 10.1038/nature06535. [DOI] [PubMed] [Google Scholar]
- 17.Van Buskirk C, Sternberg PW. Epidermal growth factor signaling induces behavioral quiescence in Caenorhabditis elegans. Nat Neurosci. 2007;10:1300–1307. doi: 10.1038/nn1981. [DOI] [PubMed] [Google Scholar]
- 18.Hurd MW, et al. Circadian rhythms of locomotor activity in zebrafish. Physiol Behav. 1998;65:465–472. doi: 10.1016/s0031-9384(98)00183-8. [DOI] [PubMed] [Google Scholar]
- 19.Cahill GM, et al. Circadian rhythmicity in the locomotor activity of larval zebrafish. Neuroreport. 1998;9:3445–3449. doi: 10.1097/00001756-199810260-00020. [DOI] [PubMed] [Google Scholar]
- 20.Jeon M, et al. Similarity of the C. elegans developmental timing protein LIN-42 to circadian rhythm proteins. Science. 1999;286:1141–1146. doi: 10.1126/science.286.5442.1141. [DOI] [PubMed] [Google Scholar]
- 21.Hendricks JC, et al. A non-circadian role for cAMP signaling and CREB activity in Drosophila rest homeostasis. Nat Neurosci. 2001;4:1108–1115. doi: 10.1038/nn743. [DOI] [PubMed] [Google Scholar]
- 22.Graves LA, et al. Genetic evidence for a role of CREB in sustained cortical arousal. J Neurophysiol. 2003;90:1152–1159. doi: 10.1152/jn.00882.2002. [DOI] [PubMed] [Google Scholar]
- 23.Kushikata T, et al. Epidermal growth factor enhances spontaneous sleep in rabbits. Am J Physiol. 1998;275:R509–514. doi: 10.1152/ajpregu.1998.275.2.R509. [DOI] [PubMed] [Google Scholar]
- 24.Kramer A, et al. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- 25.Pinkas-Kramarski R, et al. ErbB receptors and EGF-like ligands: cell lineage determination and oncogenesis through combinatorial signaling. J Mammary Gland Biol Neoplasia. 1997;2:97–107. doi: 10.1023/a:1026343528967. [DOI] [PubMed] [Google Scholar]
- 26.Mrosovsky N, et al. Masking in waved-2 mice: EGF receptor control of locomotion questioned. Chronobiol Int. 2005;22:963–974. doi: 10.1080/07420520500395086. [DOI] [PubMed] [Google Scholar]
- 27.Shilo BZ. Regulating the dynamics of EGF receptor signaling in space and time. Development. 2005;132:4017–4027. doi: 10.1242/dev.02006. [DOI] [PubMed] [Google Scholar]
- 28.Foltenyi K, et al. Activation of EGFR and ERK by rhomboid signaling regulates the consolidation and maintenance of sleep in Drosophila. Nat Neurosci. 2007;10:1160–1167. doi: 10.1038/nn1957. [DOI] [PubMed] [Google Scholar]
- 29.Aroian RV, et al. The let-23 gene necessary for Caenorhabditis elegans vulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. doi: 10.1038/348693a0. [DOI] [PubMed] [Google Scholar]
- 30.Hill RJ, Sternberg PW. The gene lin-3 encodes an inductive signal for vulval development in C. elegans. Nature. 1992;358:470–476. doi: 10.1038/358470a0. [DOI] [PubMed] [Google Scholar]
- 31.Stansberry J, et al. A cGMP-dependent protein kinase is implicated in wild-type motility in C. elegans. J Neurochem. 2001;76:1177–1187. doi: 10.1046/j.1471-4159.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalinchuk A, et al. On the role of cGMP in nitric oxide-mediated regulation of recovery sleep in the basal forebrain. Sleep. 2006;29:A17. [Google Scholar]
- 33.Franken P, et al. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 2007;8:87. doi: 10.1186/1471-2202-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wisor JP, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viola AU, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 36.Naylor E, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herzog ED, et al. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 38.Fuller PM, et al. Neurobiology of the sleep-wake cycle: sleep architecture, circadian regulation, and regulatory feedback. J Biol Rhythms. 2006;21:482–493. doi: 10.1177/0748730406294627. [DOI] [PubMed] [Google Scholar]
- 39.Wisor JP, et al. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleckenstein AE, et al. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 41.Lu J, et al. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 43.Andretic R, et al. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Kume K, et al. Dopamine is a regulator of arousal in the fruit fly. J Neurosci. 2005;25:7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 46.Chemelli RM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 47.Moore RY, et al. The hypocretin neuron system: an arousal system in the human brain. Arch Ital Biol. 2001;139:195–205. [PubMed] [Google Scholar]
- 48.Baldo BA, et al. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 2003;464:220–237. doi: 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- 49.Harrison NL. Mechanisms of sleep induction by GABA(A) receptor agonists. J Clin Psychiatry. 2007;68(Suppl 5):6–12. [PubMed] [Google Scholar]
- 50.Saper CB, et al. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 51.Agosto J, et al. Modulation of GABA(A) receptor desensitization uncouples sleep onset and maintenance in Drosophila. Nat Neurosci. 2008;11:354–359. doi: 10.1038/nn2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jouvet M. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 53.Boutrel B, et al. Involvement of 5-HT1A receptors in homeostatic and stress-induced adaptive regulations of paradoxical sleep: studies in 5-HT1A knock-out mice. J Neurosci. 2002;22:4686–4692. doi: 10.1523/JNEUROSCI.22-11-04686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrow JD, et al. Effects of serotonergic activation by 5-hydroxytryptophan on sleep and body temperature of C57BL/6J and interleukin-6-deficient mice are dose and time related. Sleep. 2008;31:21–33. doi: 10.1093/sleep/31.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan Q, et al. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 56.Cirelli C, et al. Reduced sleep in Drosophila Shaker mutants. Nature. 2005;434:1087–1092. doi: 10.1038/nature03486. [DOI] [PubMed] [Google Scholar]
- 57.Douglas CL, et al. Sleep in Kcna2 knockout mice. BMC Biol. 2007;5:42. doi: 10.1186/1741-7007-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kleopa KA, et al. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129:1570–1584. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 59.Terao A, et al. Gene expression in the rat brain during sleep deprivation and recovery sleep: an Affymetrix GeneChip study. Neuroscience. 2006;137:593–605. doi: 10.1016/j.neuroscience.2005.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 61.Mackiewicz M, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31:441–457. doi: 10.1152/physiolgenomics.00275.2006. [DOI] [PubMed] [Google Scholar]
- 62.Jones S, et al. Molecular correlates of sleep and wakefulness in the brain of the white-crowned sparrow. J Neurochem. 2008;105:46–62. doi: 10.1111/j.1471-4159.2007.05089.x. [DOI] [PubMed] [Google Scholar]
- 63.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 64.Naidoo N, et al. Sleep deprivation induces the unfolded protein response in mouse cerebral cortex. J Neurochem. 2005;92:1150–1157. doi: 10.1111/j.1471-4159.2004.02952.x. [DOI] [PubMed] [Google Scholar]
- 65.Klei L, et al. Heritability of morningness-eveningness and self-report sleep measures in a family-based sample of 521 Hutterites. Chronobiol Int. 2005;22:1041–1054. doi: 10.1080/07420520500397959. [DOI] [PubMed] [Google Scholar]
- 66.Watson NF, et al. Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep. 2006;29:645–649. doi: 10.1093/sleep/29.5.645. [DOI] [PubMed] [Google Scholar]
- 67.Hurd MW, Cahill GM. Entraining signals initiate behavioral circadian rhythmicity in larval zebrafish. J Biol Rhythms. 2002;17:307–314. doi: 10.1177/074873002129002618. [DOI] [PubMed] [Google Scholar]
- 68.Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- 69.You YJ, et al. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Kim SK. Global analysis of dauer gene expression in Caenorhabditis elegans. Development. 2003;130:1621–1634. doi: 10.1242/dev.00363. [DOI] [PubMed] [Google Scholar]


