The ErbB Family – a New Target for Breast Cancer Therapy
The members of the family of ErbB receptors (ErbB1–4) play an essential role in cell proliferation and survival. Their overexpression or mutation is a common feature in a large variety of human cancers and linked with poor response to therapy and shorter survival times. Nevertheless, overexpressed or mutated receptors may still be responsive to regulation, and their inhibition eventually leads to reduced proliferation, induction of apoptosis in tumor cells, and a regression of metastases [1,2,3,4,5]. The 4 ErbB receptors are structurally well conserved and consist of an extracellular domain responsible for ligand binding, a helical transmembrane region, and an intracellular protein tyrosine kinase (TK) domain [6]. When activated by ligand binding, homodimerization or heterodimerization of these receptors occurs, resulting in autophosphorylation and finally activation of different signal transduction pathways within the cell [1].
As ErbB1 (epidermal growth factor receptor, EGFR) is overexpressed in about one third of breast cancers and overexpression of ErbB2 (HER2) occurs in 20–25% of these tumors, ErbB-targeting therapies emerged as an attractive treatment modality for breast cancer [7]. The first agent developed to target ErbB2 was the monoclonal antibody trastuzumab, targeting the extracelluar ErbB2 domain. An alternative approach is the development of small molecules which penetrate the cell membrane and block the intracellular TK activity by competitive inhibition.
Lapatinib: Molecular Profile and Mechanism of Action
Lapatinib as a small molecule is the first oral, dual inhibitor of both ErbB1 and ErbB2. By reversibly binding to the cytoplasmic ATP-binding site of the receptor TK, lapatinib blocks receptor phosphorylation and prevents subsequent downstream signaling. This is in contrast to trastuzumab which acts primarily by stimulating antibody-dependent cellular cytotoxicity (ADCC) [8, 9]. The rationale for the development of a dual inhibitor is threefold: Firstly, the combined blockage of two receptors involved in tumor proliferation may result in a synergistic inhibition of tumor cell growth [10]. Secondly, the simultaneous inhibition of ErbB1 and ErbB2 is thought to be more effective at preventing the formation of heterodimers containing ErbB1 and ErbB2 [11]. Finally, this may result in a more complete inhibition of different signal transduction pathways of the partly redundant intracellular signaling network [12].
Lapatinib may overcome trastuzumab resistance linked to the overexpression of a truncated form of ErbB2 (p95) which can be detected in an estimated 9% of breast cancer cells [13]. This truncated version lacks the extracellular receptor domain so that trastuzumab is unable to bind to the cancer cells. The membrane-associated p95 fragment shows high kinase activity in vitro and is characterized by an increased transforming potency and a worse outcome [5, 13, 14].
Another mechanism conferring resistance to trastuzumab therapy is loss of the tumor suppressor gene PTEN (phosphatase and tensin homologue deleted on chromosome 10), which occurs in about 50% of all breast cancers and correlates with a high potential of invasion and poorer prognosis. Significant loss of PTEN expression is seen in some 20–25% of HER2-positive breast cancers [15,16,17,18]. Activating mutations in PIK3CA have also been found in approximately 25% of primary breast cancers, and these mutations occur almost exclusively in PTEN-positive samples [17, 18]. Since both the loss of PTEN and oncogenic mutations in PIK3CA lead to activation of PI3K/AKT signaling [19], one would expect that both genes can contribute to the prediction of response to trastuzumab. Trastuzumab partially works by activating PTEN, which in turn leads to an inhibition of downstream signaling. PTEN deficiency was identified as a predictive marker for resistance to the monoclonal antibody in vitro, in vivo, and in the clinical setting [16]. Evidence that lapatinib may overcome this resistance comes from a neoadjuvant trial in patients with inflammatory breast cancer (IBC): After 8 weeks of lapatinib monotherapy, a high response rate was reported even in patients with PTEN deficiency or low PTEN expression [20].
Lapatinib peak plasma levels occur 3–6 h after oral administration. The effective plasma half-life is approximately 24 h. The elimination of lapatinib occurs through hepatic metabolism, primarily through cytochrome P450 (CYP)3A4 and excretion in the feces. Therefore, inducers and inhibitors of CYP3A4 may alter the metabolism of lapatinib. Given the significance of CYP3A4 in drug metabolism, lapatinib should be used with caution when dosed concurrently with medications that are substrates, inhibitors, or inducers of CYP3A4. Additionally, antacids that modify gastric pH may affect absorption of lapatinib and should generally be avoided for 1 h before and after lapatinib dosing. Lapatinib should also be used with great caution in patients with severe hepatic dysfunction [21]. Moreover, lapatinib potentially interacts with warfarin and quinazoline derivatives to increase the international normalized ratio (INR) and bleeding. Lastly, lapatinib should not be taken with grapefruit or grapefruit juice. Table 1 lists the potential drug-drug interactions with lapatinib.
Table 1.
Lapatinib: Potential drug-drug interactions
| CYP3A4 inhibition | CYP3A4 induction |
|---|---|
| Lapatinib concentration ↑ | Lapatinib concentration ↓ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Premedication before paclitaxel, oral glucocorticoids of ≤1.5 mg dexamethasone (or equivalent) and short-term use of steroids (up to 2 weeks) permitted.
Early Clinical Data with Lapatinib
Phase I studies designed to determine the feasibility, safety, and efficacy of lapatinib gave a strong positive signal for the activity of this dual TK inhibitor in heavily pretreated patients with solid tumors overexpressing ErbB1 and/or ErbB2. Four partial remissions with a response duration of 3–8 months were seen in patients with ErbB2-positive breast cancer after failure of trastuzumab, with lapatinib in doses ranging from 650 to 1,200 mg/day [22]. The most frequently reported drug-related adverse events (grade 1–4) were diarrhea in 42%, rash in 31%, nausea in 13%, and fatigue in 10% of patients. Only 4 of the total 67 patients experienced a grade 3 adverse event, and there were no grade 4 events.
In the phase I trial EGF 10023 in 54 pretreated and trastuzumab-resistant patients, the combination of lapatinib in doses ranging from 750 to 1,500 mg daily with trastuzumab resulted in 1 complete and 7 partial remissions, as well as long lasting stabilizations in a further 11 patients [23]. The trial defined an optimally tolerated regimen with lapatinib at a dose of 1,000 mg per day and trastuzumab at the standard dose. The most common adverse events were diarrhea, rash, fatigue, nausea, vomiting, and anorexia without an increase in incidence compared to the monotherapies. The simultaneous application of both anti-ErbB2 therapies did not change the pharmacokinetic properties of either drug. Based on these data and positive results concerning efficacy and safety in several other trials in which lapatinib was examined in combination with cytotoxics such as paclitaxel, docetaxel, or capecitabine, the clinical development of the dual TK inhibitor has been continued.
Clinical Phase II Studies with Lapatinib
In the phase II EGF 20009 trial, lapatinib (1,500 mg sid or 500 mg bid) as first-line monotherapy induced partial responses in 24% and disease stabilization in 51% of 138 patients with locally advanced or metastatic ErbB2-positive (as determined by fluorescent in situ hybridization (FISH)) breast cancer [24]. These findings of overall anti-tumor efficacy are comparable with response rates reported for trastuzumab as first-line monotherapy in ErbB2-positive metastatic breast cancer [25]. Moreover, EGF 20009 was the first study reporting activity of lapatinib against brain metastases.
In most women with metastatic ErbB2-overexpressing breast cancer treated with trastuzumab, the tumor will eventually develop resistance to this monoclonal antibody. Therefore, two phase II trials (EGF 20002, EGF 20008) evaluated lapatinib monotherapy as an alternative anti-ErbB2 therapy in ErbB2-positive breast cancer after trastuzumab failure [26]. These studies confirmed the activity of this dual TK inhibitor in patients with disease progression after trastuzumab-containing regimens already reported in the early phase I trials [23].
EGF100151 Pivotal Trial and Other Phase III Trials
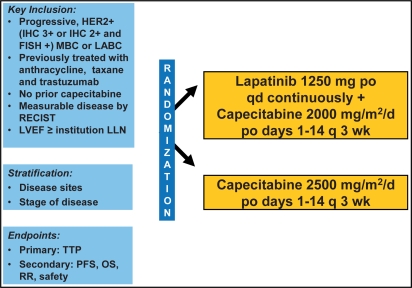
The evaluation of lapatinib in combination with capecitabine in the pivotal EGF 100151 trial was based on the encouraging results of Schwartz et al. [28] who reported the clinical activity of this regimen and an adverse event profile similar to that of the single agents. The phase III trial compared capecitabine at the standard dose (2,500 mg/m2 daily over 14 days every 3 weeks) with the combination of capecitabine (2,000 mg/m2 daily over 14 days every 3 weeks) and lapatinib (1,250 mg daily; fig. 1) [29]. Eligible for the study were patients with ErbB2-positive, locally advanced or metastatic breast cancer who had progressed after treatment with anthracyclines, taxanes, and trastuzumab [29]. Treatment was given until progression of disease or manifestation of intolerable adverse events. Both arms were well balanced in terms of patient and disease characteristics. The primary endpoint of the trial was time to progression (TTP), secondary endpoints were progression-free survival, overall survival, overall response rate, and safety.
Fig. 1.
Design of the phase III study EGF 100151 [29]. Randomized as of April 3, 2006 n = 399. Patients on treatment until progression or unacceptable toxicity, then followed for survival.
At the time of the first interim analysis, the primary endpoint TTP met the pre-specified criteria for early reporting on the basis of superiority of the combination arm. The independent Data and Safety Monitoring Committee therefore recommended termination of enrollment [29]. In total, 399 patients had been recruited, and 198 women had been randomly assigned to the combination of capecitabine and lapatinib, 201 to capecitabine only.
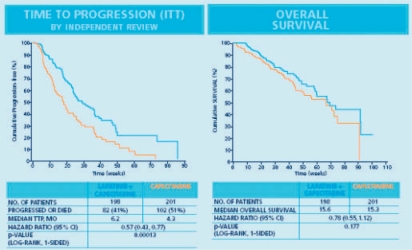
In the updated analysis reported by Cameron et al. [30], 47 patients in the combination arm (23.7%) and 28 patients in the capecitabine alone arm (13.9%) showed a clinical response (p = 0.017). With 82 events in the combination arm compared to 102 events in the capecitabine alone arm, there was a clinically meaningful and significant advantage favoring the combination treatment with a hazard ratio of 0.57 (p = 0.00013). Median TTP was 6.2 months for patients receiving capecitabine plus lapatinib, compared to 4.3 months for patients treated by capecitabine monotherapy (fig. 2). Overall survival as a secondary endpoint was comparable in both arms with 15.6 months for lapatinib plus capecitabine and 15.3 months for capecitabine as monotherapy (fig. 2). Despite EGF 100151 being powered to show a significant survival benefit for the combination, after the study had been stopped early, a significant difference could not be observed at the time of analysis due to a lack of required events. The survival improvement increased over time from the pre-planned interim analysis to the updated analysis 5 months later with the respective hazard rates of 0.92 and 0.78 [29, 30].
Fig. 2.
Phase III study EGF 100151: time to progression (ITT population) and event-free survival (ITT population) [29]. Clinical outcome data for 399 randomized patients.
The significant improvement in efficacy by combining capecitabine with lapatinib was achieved without a significant increase in serious toxicities or cardiotoxic events. In 5 patients (4 in the combination arm, 1 in the capecitabine arm), an asymptomatic decrease of the left ventricular ejection fraction (LVEF) was reported but did not lead to discontinuation of treatment. On the basis of these results, the authors concluded that lapatinib in combination with capecitabine is an effective new regimen in ErbB2-positive breast cancer and can be regarded as a new treatment standard after failure of trastuzumab [29]. For this reason, the treatment with lapatinib in combination with capecitabine for trastuzumab failure after pretreatment with anthracyclines and taxanes was incorporated in the national treatment guidelines of the AGO (Arbeits-gemeinschaft Gynäkologische Onkologie) [31].
A second phase III study (EGF30001) evaluated lapatinib (1,500 mg daily) in combination with paclitaxel (175 mg/m2 every 3 weeks) versus paclitaxel alone in 579 patients with advanced or metastatic breast cancer in the first-line setting [32]. Patients were untested or negative for ErbB2. The primary endpoint was TTP. This study revealed a differential treatment effect in ErbB2-negative and ErbB2-positive patients. In the total study population as well as in the ErbB2-negative cohort (n = 401), both treatments were equally active in terms of the primary endpoint TTP. In contrast, in patients with tumors tested ErbB2-positive in the predefined central analysis, the combination of lapatinib and paclitaxel was superior to paclitaxel alone: women in the combination arm had a median TTP of 7.9 months compared to only 5.2 months for the paclitaxel-treated women (p = 0.0007; hazard ratio: 0.56). There was a trend in overall survival favoring the combination treatment with 24 months versus 19 months for the paclitaxel alone arm (p = 0.160). This result is comparable to data from Slamon et al. [33] who compared trastuzumab plus paclitaxel versus paclitaxel only and reported a median overall survival of 25 versus 18 months. Other efficacy parameters, such as TTP, response rate, and median duration of response, are also within the same range.
Activity of Lapatinib against Brain Metastases
The treatment of patients with advanced breast cancer developing metastases in the central nervous system (CNS) continues to be a challenge. In women with ErbB2-positive breast cancer, the estimated incidence of brain metastases is about 30% [34,35,36]. Possible reasons for this observed increased incidence of brain metastases include: i) more effective systemic treatments resulting in a longer overall survival; ii) more sensitive diagnostic tools, e.g. dynamic contrast enhanced magnetic resonance imaging (MRI); and iii) an increased awareness of this problem in patients and physicians.
Young age (premenopausal status), negative hormone receptor status, and infiltrating ductal carcinoma histology were identified as risk factors associated with the development of CNS metastases in a multivariate analysis [37]. The 1-year survival rate for breast cancer patients with brain metastases is about 25%, and median survival is shorter in patients with ErbB2-positive disease compared to ErbB2-negative disease [37]. As the blood-brain barrier shows permeability for lipidsoluble substances below a molecular weight of 400 Da via lipid-mediated free diffusion, the monoclonal antibody trastuzumab (molecular weight: 148 kD) is unable to penetrate into the CNS under physiologic circumstances [27]. Clearly therefore, drugs which have the potential to penetrate the blood-brain barrier and target ErbB2-overexpressing metastases in the CNS are urgently needed. In an exploratory analysis of the pivotal trial EGF 100151, the frequency of brain metastases as site of first progression was significantly lower in the combination group of lapatinib and capecitabine than in the capecitabine alone arm (4 (8%) vs. 13 (6%); p value = 0.045) [30].
The activity of lapatinib against brain metastases was further evaluated in a study presented by Lin et al. [38]. In this study, 241 patients with radiologically documented progressive brain metastases after prior cranial irradiation and trastuzumab therapy received lapatinib (750 mg bid) single agent therapy. In the case of progressive disease, patients continued to an extension arm and received a combination of lapatinib with capecitabine. The majority of patients (87%) presented with CNS metastases and systemic disease. Fifteen patients (6%) responded to lapatinib with a partial regression of brain metastases, 102 patients (42%) with disease stabilization. In an exploratory analysis, 19 patients (7%) experienced durable volumetric reductions of the CNS disease of at least 50% with a median absolute volumetric reduction of 3.1 cm3. A total of 46 patients (19%) experienced a volumetric reduction of at least 20% (absolute tumor reduction: 1.9 cm3). The median progression-free survival for the whole study population was 15.1 weeks. In lapatinib responders this interval was prolonged to a median of 25.3 weeks. Forty patients in the extension arm received the combined treatment of lapatinib and capecitabine. Here, a volumetric reduction of at least 50% was observed in 8 patients (20%) and of at least 20% in 16 patients (40%; table 2). Based on these interesting results, the combination of lapatinib plus capecitabine can be regarded as a promising new approach to treating CNS disease in ErbB2-positive breast cancer. Clearly, further evaluation of lapatinib in combination with other cytotoxic drugs and radiotherapy is warranted.
In the ongoing phase II EGF 107671 trial, the combination of lapatinib and capecitabine will be compared to lapatinib plus topotecan in patients with ErbB2-overxpressing metastatic breast cancer after prior irradiation and trastuzumab-based therapy. A German phase I/II trial will explore a combined modality treatment of radiotherapy plus lapatinib and capecitabine in patients with ErbB2-overexpressing breast cancer and at least 2 newly diagnosed brain metastases (R. Fietkau, personal communication). Ongoing neoadjuvant and adjuvant trials (Neo-ALTTO and ALTTO) with lapatinib will show whether prevention of CNS disease is achievable by early treatment with this small molecule.
Lapatinib in the Treatment of Inflammatory Breast Cancer
With an incidence of only 1–6%, inflammatory breast cancer (IBC) is a relatively rare disease. IBC is diagnosed clinically, affected women report with redness, induration, pain, and a sudden onset of symptoms. Before the introduction of systemic therapy, patients with IBC had a poor prognosis with an overall survival at 5 years of less than 5%. Since 1990, more aggressive treatment approaches with systemic therapies and higher rates of mastectomy have improved outcome with a significant prolongation of recurrence-free survival (p = 0.01), but without increase in overall survival [39]. Of crucial importance for this moderate improvement was the introduction of modern cytotoxic regimens with anthracyclines and taxanes as well as dose-dense chemo therapy. Thus, primary systemic treatment before surgery is nowadays considered the standard for IBC.
Nevertheless, the outcome of patients with this disease is still poor, and additional treatment approaches are urgently needed. As IBC is characterized by overexpression of ErbB1 in about 30% and of ErbB2 in 40% of cases, therapy with lapatinib would be a logical strategy. First results of phase I studies showed that lapatinib can induce responses in heavily pretreated women with inflammatory ErbB1/ErbB2-positive breast cancer after failure of trastuzumab [40]. Based on these encouraging results, single-drug lapatinib was evaluated in the phase II EGF 103009 study in 49 patients with anthracyclin-refractory IBC with or without ErbB2 overexpression [41]. In the ErbB2-negative cohort, 8.3% of patients responded to lapatinib with a partial response and 17% with a stabilization of the disease. In patients with ErbB2-overexpressing tumors, lapatinib showed a much higher activity and induced partial responses in 62% and disease stabilization in 21% of the patients. 69% of these responders had tumors with PTEN deficiency which is thought to be associated with resistance to trastuzumab.
In the phase II EGF 102580 study, lapatinib in combination with paclitaxel was evaluated as neoadjuvant therapy in women with or without ErbB2-overexpressing primary IBC [42]. Patients were treated for 2 weeks by lapatinib as monotherapy. After a tumor biopsy on day 14, lapatinib was combined with paclitaxel, and the treatment continued for another 12 weeks until surgery. In the cohort of patients with ErbB2-overexpressing tumors (n = 30), the combined neoadjuvant treatment induced complete responses in 3 (10%) and partial responses in 20 (67%) patients. Moreover, 10 patients (30%) in this cohort had already responded to lapatinib as monotherapy. The rate of pathological complete remissions was 17%. Four of the 5 patients in cohort B with ErbB2-negative tumors had a partial response, but there were no complete and pathological complete remissions. In the biomarker analysis, phosphorylated ErbB2 was identified as a predictor of response to lapatinib therapy.
A phase III study (EGF108838) with lapatinib in patients with recurrent IBC with ErbB2 overexpression is ongoing. In this trial, lapatinib as single drug is compared to the combination of lapatinib with pazopanib, a multi-TK inhibitor of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) receptors. The primary endpoint is progression-free survival.
In conclusion, based on the encouraging data of several phase II and phase III trials, lapatinib appears to be a promising new treatment for ErbB2-positive breast cancer patients who have progressed on trastuzumab. Based on the data of the phase III trial with lapatinib in combination with capecitabine, lapatinib was licensed by the FDA in the US in March 2007 and received a positive opinion by the EMEA in December 2007.
References
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson RI, Gee JMW, Harper ME, et al. EGFR and cancer prognosis. Eur J Cancer. 2001;3(suppl 4):S9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 4.Nahta R, Hortobagyi G, Esteva FJ. Growth factor receptors in breast cancer: potential for therapeutic intervention. Oncologist. 2003;8:5–17. doi: 10.1634/theoncologist.8-1-5. [DOI] [PubMed] [Google Scholar]
- 5.Xia W, Liu H, Ho P, et al. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB2 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW 572016. Oncogene. 2004;23:646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 6.Hynes NE, Lane HA. ErbB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 7.Moy G, Goss P. Lapatinib: current status and future directions in breast cancer. Oncologist. 2006;11:1047–1057. doi: 10.1634/theoncologist.11-10-1047. [DOI] [PubMed] [Google Scholar]
- 8.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastu-zumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 9.Arrnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer. 2006;94:259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burris HA., 3rd Dual kinase inhibition in the treatment of breast cancer. Initial experience with the EGFR/ErbB2-inhibitor lapatinib. Oncologist. 2004;9(suppl 3):10–15. doi: 10.1634/theoncologist.9-suppl_3-10. [DOI] [PubMed] [Google Scholar]
- 11.Rusnak DW, Affleck K, Cockerill SG, et al. The characterization of novel dual ErbB-2/EGFR tyro-sine kinase inhibitors: potential therapy for cancer. Cancer Res. 2001;61:7196–7203. [PubMed] [Google Scholar]
- 12.Nelson MH, Dolder CR. Lapatinib: a novel dual tyrosine kinase inhibitor with activity in solid tumors. Ann Pharmacother. 2006;40:261–269. doi: 10.1345/aph.1G387. [DOI] [PubMed] [Google Scholar]
- 13.Saez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER2-positive breast cancer. Clin Cancer Res. 2006;12:424–431. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- 14.Christianson TA, Doherty JK, Lin YJ, et al. NH2-terminally truncated HER2/neu-protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998;58:5123–5129. [PubMed] [Google Scholar]
- 15.Fujita T, Doihara H, Kawasaki K, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94:247–252. doi: 10.1038/sj.bjc.6602926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Saal LH, Holm K, Maurer L, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and erbB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2254–2259. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 18.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of tratsuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Isakoff SJ, Engelman JA, Irie HY, et al. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 20.Xia W, Husain I, Liu L, et al. Lapatinib antitumor activity is not dependant upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 2007;67:1170–1175. doi: 10.1158/0008-5472.CAN-06-2101. [DOI] [PubMed] [Google Scholar]
- 21.Moy B, Goss P. Lapatinib-associated toxicity and practical management recommendations. Oncologist. 2007;12:756–765. doi: 10.1634/theoncologist.12-7-756. [DOI] [PubMed] [Google Scholar]
- 22.Burris HA, 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016): a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinoma. J Clin Oncol. 2005;13:1–9. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 23.Storniolo A, Burris A, Pegram M, et al. A phase I, open-label study of lapatinib (GW572016) plus trastuzumab: active regimens. Proc Am Soc Clin Oncol. 2005;23 abstr 559. [Google Scholar]
- 24.Gomez HL, Chavez MA, Doval DC, et al.: Updated biomarker results from a phase II randomized study of lapatinib as first-line treatment for patients with ErbB2-amplified advanced or metastatic breast cancer. 29th Annual SABCS Dec 14-17, 2006, San Antonio, Texas; abstr 1090.
- 25.Johnston S, Leary A. Lapatinib: a novel EGFR/HER2 tyrosine kinase inhibitor for cancer. Drugs Today (Barc) 2006;42:441–453. doi: 10.1358/dot.2006.42.7.985637. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell KL, Kaplan EH, Franco SX, et al. A phase II, open-label, multi-center study of GW572016 in patients with trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2004;22:3006. [Google Scholar]
- 27.Pardridge WM. Blood-brain barrier delivery. Drug Discov Today. 2007;12:55–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz G, Chu QS, Hammond LA, et al. Phase I clinical, biology, and pharmacokinetic study of the combination of GW572016 and capecitabine in patients with advanced solid tumors. Proc Am Soc Clin Oncol. 2004;23(suppl 14S) abstr 212s. [Google Scholar]
- 29.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 30.Cameron D, Casey M, Press M, et al.: A phase III randomized comparison of lapitinib plus capecitabine versus capecitabine alone in women with advaned breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res Treat 2008 [epub ahead of print]. [DOI] [PubMed]
- 31.AGO-Leitlinie Mamma 2007, www-ago-online.org
- 32.Di Leo A, Gomez H, Aziz Z, et al. Lapatinib plus paclitaxel compared to paclitaxel as first-line therapy in patients with metastatic breast cancer: a phase III randomized double-blind study of 580 patients. Proc ASCO. 2007;25(18S) abstr1011. [Google Scholar]
- 33.Slamon DJ, Leyland-Jones B, Shark S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 34.Bendell JC, Domcheck SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97:2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 35.Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stemmler J, Kahlert S, Siekiera W, et al. Brain metastases (BM) in patients treated with trastuzumab for HER2 overexpressing metastatic breast cancer (MBC): incidence and survival. J Clin Oncol. 2005;23(suppl):131s. abstr1568. [Google Scholar]
- 37.Tham YL, Sexton K, Kramer R. Primary breast cancer phenotypes associated with propensity for central nervous system metastases. Cancer. 2006;107:2521–2522. doi: 10.1002/cncr.22041. [DOI] [PubMed] [Google Scholar]
- 38.Lin NU, Dieras V, Paul D, et al. A phase II study of lapatinib for brain metastases in subjects with ErbB2-positive breast cancer following trastu-zumab-based systemic therapy and cranial radiotherapy. Proc Am Soc Clin Oncol. 2007;27(suppl) abstr 1012. [Google Scholar]
- 39.Panades M, Olivotto IA, Speers CH, et al. Evolving treatment strategies for inflammatory breast cancer: a population-based survival analysis. J Clin Oncol. 2005;23:1941–1950. doi: 10.1200/JCO.2005.06.233. [DOI] [PubMed] [Google Scholar]
- 40.Liauw SL, Benda RK, Morris CG, et al. Inflammatory breast carcinoma: outcomes with trimodality therapy for nonmetastatic disease. Cancer. 2004;100:920–928. doi: 10.1002/cncr.20083. [DOI] [PubMed] [Google Scholar]
- 41.Spector NL, Blackwell K, Hurley J, et al.: EGF 103009, a phase II trial of lapatinib monotherapy in patients with relapsed/refractory inflammatory breast cancer (IBC): Clinical activity and biologic predictors of response. Proc Am Soc of Clin Oncol 2006, abstr 502.
- 42.Cristofanilli M, Boussen H, Baselga J, et al.: A phase II combination study of lapatinib and paclitaxel as a neoadjuvant therapy in patients with newly diagnosed inflammatory breast cancer (IBC). 29th Annual SABCS Dec 14-17, 2006, San Antonio, Texas; abstr 1.