Summary
Breast cancer is increasing in developing countries, and Colombia has a double burden from cervical and breast cancer. Suitable guidelines for breast cancer early detection are needed, and the Breast Health Global Initiative provides a favorable framework for breast cancer control in low resource nations. The Colombian National Cancer Institute developed evidence-based guidelines for breast cancer early detection in which coordinated early detection in symptomatic women and hospital-based screening in women aged 50–69 are recommended. A pilot project to evaluate programmatic approaches (opportunistic screening) was designed, and it is expected that organized hospital-based screening for breast cancer will represent a move towards population-based screening in the near future in accordance with country specific conditions.
Key Words: Breast neoplasms: early detection, mass screening; Developing countries; Colombia
Zusammenfassung
Die Brustkrebsrate steigt in den Entwicklungsländern, und Kolumbien kämpft sowohl mit Brust- als auch Ge-bährmutterhalskrebs. Geeignete Richtlinien zur Brustkrebsfrüherkennung werden dringend gebraucht. Die Breast Health Global Initiative bietet ein für die Brustkrebskontrolle in ärmeren Ländern geeignetes System. Das Kolumbianische National Cancer Institute hat evidenzbasierte Richtlinien für die Brustkrebsfrüherkennung entwickelt, in denen die koordinierte Früherkennung bei symptomatischen Frauen sowie ein klinisches Screening von Frauen der Altersgruppe 50–69 empfohlen werden. Ein Pilotprojekt zur Evaluierung programmatischer Ansätze (opportunistisches Screening) wurde ins Leben gerufen, und es wird erwartet, dass das von Kliniken organisierte Brustkrebsscreening ein Schritt hin zum umfassenden Screening in der nahen Zukunft – gemäß den landesspezifischen Gegebenheiten – ist.
Introduction
Colombia is a low-middle income country with a middle risk for incidence or mortality from breast cancer [1]; however, mortality from breast cancer is increasing dramatically [2], currently being the second cause of cancer incidence (age-standardized incidence rate (ASR) 30.3 per 100,000) and the third cause of cancer mortality (ASR 12.5 per 100,000) in the female population [1]. Due to the current weak regulations regarding breast cancer early detection in Colombia, the abovementioned situation leads us to foresee a high burden of breast cancer in the near future if no action is taken. The Colombian health system is comprised of 4 different health plans. These include: general health insurance (mainly through private insurance companies equivalent to American Health Maintenance Organizations (HMOs)) for employees and their families (36.3% of the population); subsidized health insurance for the lowest income population (42.9%); and public hospital assistance for people without any health insurance (20.7%). An additional plan corresponds to prepaid medical care which is affordable only for the high income population (5%) and is allowed only for people already affiliated to the general health insurance [3].
Screening, diagnosis, and treatment of breast cancer for women with prepaid medical care is done on demand, and mammography screening should be done every 2 years starting at 50 years of age for women with general health insurance, but HMOs are only obligated to screen 20% of their female affiliates. There are no mandatory early detection programs for the remaining health plans [4], and timely access to diagnosis and treatment for positively screened women under HMOs is uncertain [5]. The purpose of this paper is to describe the experience of the National Cancer Institute of Colombia (NCIC) in introducing breast cancer early detection programs.
Burden of Breast Cancer in Colombia
In 2002, breast cancer accounted for 5,526 new cases (14.3% of cancer cases) and 2,253 deaths (10.4% of cancer deaths) among Colombian women [2, 6]. Colombia is a typical western society where postmenopausal women have the highest risk of breast cancer, different from incidence rates observed in eastern countries such as Japan or India [1]. However, between 40 and 45% of new cases occur in women younger than 50 years as in other nations [1].
Several data indicate a special moment in the epidemiological transition. The main cause of incidence and mortality from cancer for women is cervical cancer with rates among the highest in the world [1]. Despite that, we are not a high-risk nation for breast cancer. The incidences of cervical and breast cancer do not differ greatly (36.4 and 30.3 per 100,000), indicating a double burden of disease with components from developed and developing nations [1].
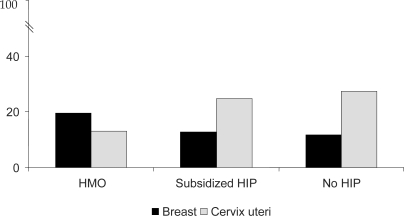
Geographical patterns of mortality from breast cancer in Colombia show an increased risk around big urban centers as opposed to geographical patterns of mortality from cervical cancer in which rural areas account for the highest risk [7]. While cervical cancer has been related to low socioeconomic and education levels as well as to limited access to health care, risk factors for breast cancer such as early menarche, postmenopausal obesity, low parity, and use of exogenous hormones are more prevalent among women with high levels of education and better access to health care in Colombia [8]. These data correspond to observations from developed countries where women within the highest socioeconomic level and urban communities have a greater risk of developing breast cancer [9, 10]. However, in Colombia, women under HMOs have the greatest number of years of life lost due to breast cancer while women without any health insurance the least (fig. 1). The latter situation contradicts observations in developed countries where mortality from breast cancer relates to low socioeconomic level and limited access to health care [11, 12]. The differences might be explained by lack of extended public health programs that guarantee proper access to early detection and treatment of breast cancer in Colombia, even for women with health insurance.
Fig. 1.
Years of life lost (YLL) from breast and cervical cancer in Colombia 2000-2004 (%). HMO = health maintenance organizations, HIP = health insurance plan. Source: YLL from cancer, NCI Colombia 2007.
The scarce data on clinical stage at diagnosis show a great difference between private (prepaid medical care) and public centers (59.3 and 19.7% of early stages, respectively: cancer in situ, stage I, and stage IIA) [13, 14]. In addition, mortality/incidence rates in Colombia are higher than those observed in developed countries and in other Latin American nations with similar or lower national gross domestic product [1]. These rates indicate undersupplied breast care services in Colombia whether for screening, diagnosis, or treatment. Accordingly, in a 2004 national health survey, only 10% of women over 50 years of age reported a mammography in the previous 2 years; the screening rate under HMOs was 17%, under the subsidized health insurance plan 3%, and among women without health insurance 2% [8].
Development of Guidelines for Breast Cancer Early Detection in Colombia
In order to tackle the problem, the NCIC began the development of evidence-based guidelines for breast cancer early detection in 2005. 3 groups with complementary actions and responsibilities were created [15]. A steering committee designed the entire process, promoted institutional commitment, and developed strategies to engage stakeholders such as oncologists and other breast cancer specialists, scientific societies, non-governmental organizations, and patient associations. A methodological committee defined clinical questions, selected the technical team, trained the team on evidence-based medicine and literature review, designed strategies for literature search, and performed a national consensus conference (to discuss specific country conditions). Finally, the technical team conducted the literature search and review, classified the evidence, and set up the recommendations according to the evidence and the consensus conference [15].
The guidelines developed by the Breast Health Global Initiative offered the framework for the process [16, 17], and the purpose was to move from a limited to an enhanced scenario for breast cancer early detection in Colombia. The main recommendations in the NCIC guidelines aim to strengthen early detection in symptomatic women based on breast self examination (BSE) and clinical breast examination (CBE); to establish opportunistic screening with mammography every 2 years and CBE yearly in women aged 50–69; and to implement breast cancer early detection programs which will improve health professional training, screening rates, access to diagnosis and treatment, and quality control. Additional recommendations on health education, breast cancer registries (surveillance), and research were also made [15].
The promotion of early detection in symptomatic women takes into account the high percentage of cases in women under 50, and is proposed on complementary basis with opportunistic screening for women aged 50 and over. Opportunistic screening (understood as hospital-based rather than population-based screening) is viewed as an opportunity to move forward in the introduction of breast cancer early detection programs, and is based on the economic restrictions for implementing massive breast cancer screening. Additionally, it corresponds to the current situation where women in urban communities and/or those who have better access to health care account for higher mortality rates. Recommendations on program development consider the lack of impact if no program is organized, and place special emphasis on follow-up of positive screening results and quality control for mammography and CBE as health technologies deserving high performance standards.
For dissemination and implementation of guidelines, the level of knowledge and application is judged as the result of determinant factors and specific strategies [18, 19]. Accordingly, regulatory issues and formal education of health professionals are identified as the main determinants for application of the NCIC guidelines and the strategies proposed include: development/adaptation of complementary guidelines for diagnosis and treatment; diffusion of guideline contents among stakeholders for breast cancer screening; introduction of guideline contents in graduate and postgraduate programs; evaluation of guidelines through pilot projects; cost-effectiveness analysis; and advocacy for policy development.
Pilot Project for the Introduction of Breast Cancer Early Detection Programs
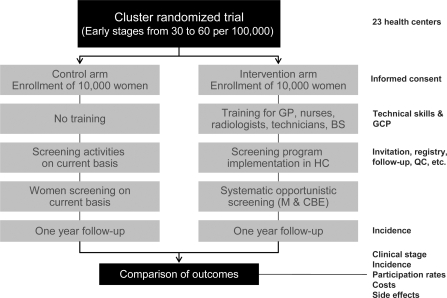
Following strategies defined for dissemination of guidelines, a pilot project was designed to evaluate programmatic approaches to opportunistic screening. Primary objectives for the pilot are to estimate the impact of the NCIC guidelines on breast cancer downstaging, to estimate the impact of opportunistic screening on exposure to mammography and CBE, and to estimate the costs for implementing opportunistic programs in the Colombian health system. A cluster randomized trial with 2 groups of women from Bogota was proposed, where randomization units correspond to primary health centers. One group will undergo opportunistic breast cancer screening with mammography plus CBE (offered systematically to women aged 50–69 who attend primary health centers on their own, regardless of motivation), and the second group will have no intervention (fig. 2). Each branch is considered as a cohort with a basal incidence of breast cancer stage I or II. For sample size, we sought out 23 centers and 20,000 women to establish a greater early breast cancer incidence rate with screening. In the non-intervention group, early breast cancer basal incidence is a known event. All centers randomly allocated to the intervention group will receive training for implementing hospital-based breast cancer screening. The target groups include radiographers (technicians), radiologists, and general physicians. The staff for screening, diagnosis, and treatment will correspond to the HMO staff. The reading of mammograms will be centralized as much as possible, keeping in line with standard HMO practice. The general practitioners will undergo training in CBE (Barton's method) and mammography reports (BIRADS). Silicone phantoms will be used as initial practice for CBE, and 3 practical sessions in a breast clinic will be performed 15, 30, and 45 days after the practice with silicone phantoms. At 1-year follow-up, downstaging at diagnosis will be the main outcome; additional outcomes will include incidence, participation rates, costs, and side effects. Clinical stage at diagnosis has been defined according to the TNM nomenclature. In order to verify quality standards for mammography equipment, radiology centers will be visited prior to patient recruitment. The quality control program will cover basal requirements (machine features and calibration), a standard report on image quality in every mammography report, a second reading of mammograms by an expert radiologist (about 20%), and permanent evaluation of CBE skills among general practicioners.
Fig. 2.
Design of the pilot project for early detection of breast cancer in Colombia (women aged 50-69). GP = general practitioners; BS = breast surgeons; GCP = good clinical practice; HC = health centers; QC = quality control; M = mammography; CBE = clinical breast examination.
Conclusion
Developing countries such as Colombia have an increasing breast cancer burden. The development of suitable recommendations for limited resource nations is necessary, and the Breast Health Global Initiative guidelines are an important asset for this purpose. The implementation of organized hospital-based screening with the characteristics previously described in Colombia is a remarkable step forward for the future implementation of massive breast cancer screening programs.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancer Base No. 5, version 2.0. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Murillo R, Quintero A, Piñeros M, Bravo MM, Cendales R, Wiesner C, Lizcano LA: Framework for cancer control in Colombia. Serie Doc Tecnicos INC 2004, no 1.
- 3.Proyecto así vamos en salud: Aseguramiento. www.asivamosensalud.org/areas/aseguramiento.htm.
- 4.República de Colombia Ministerio de Salud Resolución 412 de 2000. Bogotá, Ministerio de Salud de Colombia, 2000.
- 5.Wiesner C. Psychological, social, and clinical determinants of breast cancer early detection in Bogota, Colombia. Rev Col Cancerol. 2007;11:7–15. [Google Scholar]
- 6.Piñeros M, Hernández G, Bray F. Increasing mortality rates of common malignancies in Colombia: an emerging problem. Cancer. 2004;101:2285–92. doi: 10.1002/cncr.20607. [DOI] [PubMed] [Google Scholar]
- 7.Murillo R, Piñeros M, Hernández G. Atlas de mortalidad por cáncer en Colombia. Bogotá: INCIGAC; 2004. [Google Scholar]
- 8.Ojeda G, Ordoñez M, Ochoa LH. Encuesta nacional de demografía y salud. Bogotá: Profamilia; 2005. Salud sexual y reproductiva en Colombia. [Google Scholar]
- 9.Farley TA, Flannery JT. Late-stage diagnosis of breast cancer in women of lower socioeconomic status: public health implications. AJPH. 1989;79:1508–12. doi: 10.2105/ajph.79.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert SA, Strombom I, Trentham-Dietz A, Hampton JM, McElroy JA, Newcomb PA, Remington PL. Socioeconomic risk factors for breast cancer: distinguishing individual- and community-level effects. Epidemiology. 2004;15:442–50. doi: 10.1097/01.ede.0000129512.61698.03. [DOI] [PubMed] [Google Scholar]
- 11.Rutqvist LE, Bern A, Stockholm Breast Cancer Study Group Socioeconomic gradients in clinical stage at presentation and survival among breast cancer patients in the Stockholm area 1977-1997. Int J Cancer. 2006;119:1433–9. doi: 10.1002/ijc.21949. [DOI] [PubMed] [Google Scholar]
- 12.Katz SJ, Zemencu JK, Hofer TP. Breast cancer screening in the United States and Canada, 1994: socioeconomic gradients persist. AJPH. 2000;90:799–803. doi: 10.2105/ajph.90.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Instituto Nacional de Cancerologia . Anuário estadístico 2006. Bogotá: INC; 2007. [Google Scholar]
- 14.Robledo JF, Caicedo JJ, Deantonio R. Análisis de sobrevida en una cohorte de 1328 pacientes con carcinoma de seno. Rev Col Cirugía. 2005;20:6–14. [Google Scholar]
- 15.Instituto Nacional de Cancerologia . Recomendaciones para la detección temprana de cáncer de mama en Colombia. Bogotá: INC; 2006. [Google Scholar]
- 16.Anderson BO, Shyyan R, Eniu A, Smith RA, Yip CH, Bese NS, Chow LW, Masood S, Ramsey SD, Carlson RW. Breast cancer in limited-resource countries: an overview of the Breast Health Global Initiative 2005 guidelines. Breast J. 2006;12(suppl 1):S3–15. doi: 10.1111/j.1075-122X.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 17.Smith RA, Caleffi M, Albert US, Chen TH, Duffy SW, Franceschi D, Nyström L, Global Summit Early Detection and Access to Care Panel Breast cancer in limited-resource countries: early detection and access to care. Breast J. 2006;12(suppl 1):S16–26. doi: 10.1111/j.1075-122X.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 18.Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, Whitty P, Eccles MP, Matowe L, Shirran L, Wensing M, Dijkstra R, Donaldson C. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8:iii–iv. doi: 10.3310/hta8060. 1-72. [DOI] [PubMed] [Google Scholar]
- 19.Ouimet M, Landry R, Amara N, Belkhodja O. What factors induce health care decision-makers to use clinical guidelines? Evidence from provincial health ministries, regional health authorities and hospitals in Canada. Soc Sci Med. 2006;62:964–76. doi: 10.1016/j.socscimed.2005.06.040. [DOI] [PubMed] [Google Scholar]