Summary
Background
Routine drain placement after breast cancer surgery is standard practice. Anchoring the axillary and mastectomy flaps to the underlying chest wall with sutures has been advocated as a means of avoiding drainage following breast surgery. This study compares outcomes following flap fixation or routine drain placement and uniquely considers the economic implications of each technique.
Patients and Methods
Data on seroma formation and wound infection following mastectomy and axillary clearance were recorded prospectively. Patients underwent either routine drain placement or flap anchoring using subcutaneous tacking sutures without drainage. Equipment and surgical bed costs were provided by our finance department.
Results
Data was available for 135 patients. 76 underwent flap anchoring without drainage and 59 had routine drainage. There was no difference in seroma rates between the two groups: 49% vs. 59% (p = 0.22). However, the length of hospital stay was reduced in the flap fixation group: 1.88 vs. 2.67 days (p < 0.0001). Per patient, flap suturing equated to an estimated financial saving of £ 240.
Conclusions
Flap anchoring resulted in a significantly shorter hospital stay than routine drainage, with a comparable rate of seroma formation. This technique presents a viable alternative to drain placement and could lead to a considerable economic savings.
Key Words: Seroma, Flap fixation, Flap anchoring, Breast cancer
Zusammenfassung
Hintergrund
Die routinemäßige Platzierung einer Drainage gehört zum Standardvorgehen bei Brustkrebsoperationen. Die Verankerung der Achselhöhlen- und Mastektomie-Lap-pen an der darunterliegenden Brustwand ist als Methode zur Vermeidung einer Drainage nach der Brustoperation befürwortet worden. Diese Studie vergleicht die Ergebnisse nach Lappenfixierung und routinemäßiger Drainagesetzung und berücksichtigt im Besonderen die ökonomischen Konsequenzen der einzelnen Techniken.
Patienten und Methoden
Die Daten zur Serombildung und Wundinfektion nach Mastektomie und nach Ausräumung der Achselhöhle wurden prospektiv erfasst. Die Patientinnen wurden entweder einer routinemäßigen Drainagesetzung oder einer Lappenverankerung, bei der subkutane Heftnähte ohne Drainage verwendet wurden, unterzogen. Daten über die Kosten für die Ausrüstung und die chirurgische Bettenbelegung wurden von unserer Finanzabteilung bereitgestellt.
Ergebnisse
Für 135 Patienten waren Daten verfügbar. 76 erhielten eine Lappenverankerung ohne Drainage und 59 bekamen eine routinemäßige Drainage. Zwischen den Seromraten der beiden Gruppen gab es keinen Unterschied: 49% vs. 59% (p = 0,22). Die Länge des Krankenhausaufenthaltes war bei der Gruppe mit Lappenfixierung jedoch verkürzt: 1,88 vs. 2,67 Tage (p < 0,0001). Pro Patient beliefen sich die geschätzten finanziellen Einsparungen durch Lappenvernä-hung auf 240 £.
Schlussfolgerungen
Bei vergleichbarer Serombildungsrate führt die Lappenverankerung verglichen mit der routinemäßigen Drainage zu einem signifikant kürzeren Krankenhausaufenthalt. Diese Technik stellt eine realisierbare Alternative zur Drainagesetzung dar und könnte zu beträchtlichen wirtschaftlichen Einsparungen führen.
Introduction
In March 2008, the National Mastectomy and Breast Reconstruction Audit published preliminary results showing a steady rise in the number of women diagnosed with breast cancer in England and Wales. It identified a 37% increase in the total number of operations performed for breast cancer in the National Health Service (NHS) between 1997 (24,684) and 2006 (33,814) [1]. This increase has had considerable financial implications for health service planning at both local and national levels. Specialist breast units are now under growing pressure to increase patient throughput and limit expenditure whilst continuing to increase the quality of care.
The most expeditious way to reduce surgical costs is to reduce the length of hospital stay (LOS) [2] and this also facilitates high patient throughput. However, a major limiting factor when considering early discharge of breast patients is the formation of wound seromas. A seroma is a collection of acute inflammatory exudates secondary to surgical trauma and the acute phase of wound healing [3, 4]. Many surgeons regard it as an inevitable nuisance rather than a true complication, but it may lead to significant morbidity including delayed wound healing, wound infection or dehiscence, flap necrosis and delayed initiation of adjuvant therapy, as well as prolongation of hospital stay [5, 6]. Several factors have been implicated in the formation of seroma, but the true patho-physiology is still poorly understood [7].
Several techniques have been reported to prevent or reduce seroma formation, but no single method has consistently been shown to be effective [8, 9]. The standard practice of most surgeons is to site one or two suction drains deep to the skin flaps following breast or axillary surgery. These are typically left in situ until fluid drainage is less than 30–50 ml/day, although this practice varies. Despite this strategy, seromas requiring aspiration still occur in 10–80% of patients following drain removal, and drains may cause pain and predispose to infection [10]. The routine use of drains, though acceptable to many patients, may delay hospital discharge whilst surgeons wait for drainage volumes to decrease.
Financial pressures and service provision targets have led breast units to explore ways to limit seroma formation whilst enabling early hospital discharge. Several studies have addressed the practice of early discharge of patients with drains in situ. This has been shown to be safe provided there has been adequate patient education and coordination of inpatient and outpatient facilities [11,12,13,14,15,16,17]. However, patient acceptance rates with this practice vary between 24 and 41% [12, 13] and suitable patients are typically younger and have support at home [12]. Patients have also expressed concerns with personal care, posture in bed, pain from the drain site, and wound care following discharge with drains in situ [12, 18]. There is therefore a considerable cohort of patients for whom early discharge with drains in situ is not appropriate or who suffer increased anxiety as a result. Breast units continue to search for alternative solutions to the challenge of seroma prevention and prolonged hospital stay.
There is now considerable evidence that skin flap fixation to reduce dead space may be as effective as routine drainage at lowering seroma rates. Halsted [19] first described flap fixation in 1913 and others have since described techniques of anchoring flaps to close dead space. In 1993, a randomised controlled trial (RCT) by Coveney et al. [20] demonstrated that fixation of the skin flaps using multiple tacking sutures to close the dead space reduced seroma formation in patients undergoing mastectomy. Subsequently, an RCT by Purushotham et al. [21] demonstrated that mastectomy without drainage does not increase seroma formation when this technique is applied. Other studies have found similar results, but the use of routine drainage has remained standard practice in most specialist units [6, 22]. These published reports have not previously addressed the additional operating time, cosmetic or cost implications of this surgical technique.
In an attempt to reduce costs and expedite early hospital discharge whilst maintaining a high-quality service, we compared rates of seroma formation and LOS following either routine drain placement or flap fixation and examined the financial implications of each technique.
Patients and Methods
Data on seroma formation and wound infection rates following mastectomy and/or axillary clearance were recorded prospectively by specialist breast care nurses from October 2006 to February 2008. Patients were allocated to either suction drain placement or flap fixation, depending on the admitting consultant.
Data was also collected on patient demographics, use of post-operative antibiotics, tumour grade, lymph node status, body mass index (BMI), smoking status and LOS. Male patients and patients who underwent immediate reconstruction were excluded.
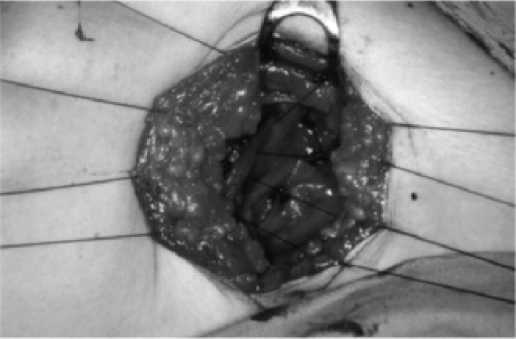
Patients undergoing flap fixation had flaps anchored to the underlying muscle using multiple rows of interrupted 2/0 polyglactin (vicryl) sutures. Sutures were placed approximately 2.5 cm apart and all sutures were buried (fig. 1). Care was taken not to include the long thoracic nerve in the suture when anchoring the axillary flaps. Patients having drainage had one or two Redivac drains sited beneath the mastectomy flaps and in the axilla (if axillary dissection was performed). Drains were removed when draining less than 50 ml of serous fluid per day. Where appropriate, patients were discharged with their drains in situ, which were then removed in the community. Where district nurses were not available, some patients were discharged and subsequently returned to the ward for drain removal.
Fig. 1.
Photograph illustrating flap suturing in the axilla.
Results
Data was available for 135 patients. There was no difference in seroma rates between the drain group (59%) and the flap fixation group (49%) (p = 0.22) (table 1).
Table 1.
Seroma rates and length of stay
| Flap anchoring | Routine drainage | p | |
|---|---|---|---|
| Number | 76 | 59 | |
| Hospital stay, mean ± SD | 1.88 ± 0.96 | 2.67 ± 1.01 | < 0.0001a |
| Seroma | 37 (49%) | 35 (59%) | 0.22b |
| Wound infectionc | 6 | 1 |
Mann-Whitney test.
Fisher's exact test.
Culture-confirmed infection.
Seroma rates in obese patients (BMI > 30) were not statistically different to patients with BMI < 30 in either treatment group (table 2). There was no difference in post-operative antibiotic use or culture-positive infection rates. Tumour grade, lymph node status and smoking had little impact on the seroma rates (tables 1 and 3).
Table 2.
Characteristics of patients who developed seromas
| Flap sutured group | Routine drainage group | All patients with seroma | |
|---|---|---|---|
| BMI | |||
| < 30 | 8/15 (53%) | 21/34 (62%) | 29/49 (59%) |
| > 30 | 12/26 (46%) | 5/8 (63%) | 17/34 (50%) |
| Lymph node statusa | |||
| Positive | 3/12 (25%) | 7/13 (54%) | 10/25 (40%) |
| Negative | 12/19 (63%) | 5/8 (63%) | 17/27 (63%) |
Data is for axillary node clearance alone.
Table 3.
Patient characteristics (all patients)
| Flap-sutured group, n = 76 | Routine drainage group, n = 59 | p | |
|---|---|---|---|
| Age, yearsa | 66 | 65 | NS |
| BMPa | 27.5 | 25.5 | NS |
| Axillary node clearance | 62 | 43 | NS |
| Mastectomy | 49 | 37 | NS |
| Smokers | 5 | 10 | NS |
| Antibiotic use | 19/76 | 8/59 | NS |
| Seromas < 100 ml | 7/21 (33%) | 17/28 (61%) |
NS = Not significant.
Median.
Discussion
The number of operations performed for breast cancer in the NHS increased by 37% between 1997 and 2006 and continues to escalate [1]. This has had inevitable financial and service implications at both local and national levels, with specialist breast units being placed under increasing pressure to minimise costs whilst maintaining a high-quality service.
Over the past three decades, surgical services have altered considerably in an attempt to reduce the LOS and surgical costs and to increase patient satisfaction. Pre-admission clinics are now in place to minimise preoperative stay, and day surgical units are used for minor procedures, avoiding the use of inpatient beds [23]. The process of patient discharge has also been made considerably more efficient, and written summaries and medications are often now organised the night before discharge is anticipated. Specialist units also provide effective outpatient services to facilitate early discharge, and peer review processes and department audits monitor inappropriate admissions, LOS and other performance-related outcomes.
By the mid 1990s many of these measures were in place but the reported post-operative hospital stay following breast cancer surgery in the UK was still 5–7 days [7, 24, 25]. Efforts to reduce LOS and thereby reduce surgical costs have continued since then, and in 2009 breast units frequently discharge patients after a single overnight stay provided suitable prior arrangements have been made. Patients who require longer admission are usually elderly with poor social support or those who are monitored until drainage volumes decline. Several studies have examined the feasibility of early discharge of patients with drains in situ, and this has been shown to be safe and effective for selected patients provided district nurse facilities are in place, although patient satisfaction with this practice varies [12, 13, 21, 26].
In this study, we compared the median LOS after flap fixation or wound drainage. Where appropriate, patients with drains were discharged early with their drains in situ, but median LOS was still longer (2.67 vs. 1.88 days (p < 0.0001)) in the drain group than in the flap fixation group, whilst rates of post-operative seroma and wound infection were comparable (table 3). This difference in LOS had considerable financial implications.
In our breast unit, the estimated cost of a single surgical bed is £ 296 per day. 77% of this is due to medical, nursing and pathology costs, 17% due to building and construction and 7% due to drugs, dressings and other medical equipment.
For every 100 patients undergoing breast cancer surgery in our unit, our data suggest that approximately 79 bed days could be saved by employing the flap fixation technique, which would equate to a financial saving of over £ 23,000 (table 4). Allowing for equipment costs, a technique of flap fixation without drainage could lead to a total saving of over £ 24,000 per 100 patients treated (table 4), as well as decreased district nurse requirements and reductions in surgical cancellations through increased bed availability. This would represent a considerable financial saving whilst causing no adverse impact on patient outcome.
Table 4.
Cost analysis
| Item | Cost per item, £ | Number of items required per patient (dependant on technique used) | Estimated total cost per 100 patients, £ |
|---|---|---|---|
| Suction drain and bottle | 4.73 | 2 (D) | 946 |
| 2.0 vicryl | 1.92 | 2 (FA) | 384 |
| Silk drain stitch | 0.97 | 1 (D) | 97 |
| Single surgical bed/24 h | 296 | 2.67 (D) | 79,032 (D) |
| (1 bed day) | 1.88 (FA) | 55,648 (FA) | |
| Total cost per 100 patients having routine drainage | 80,075 | ||
| Total cost per 100 patients having flaps anchored | 56,032 | ||
| Total saving achieved using flap anchoring technique per 100 patients | 24,043 | ||
D = Routine drainage group, FA = flap anchoring group.
In this study there was no significant difference in seroma rates between the drain group (59%) and the flap fixation group (49%) (p = 0.22) (table 1). However, seroma rates in both treatment groups were relatively high compared to published rates. One explanation for this is that we excluded patients having breast-conserving surgery and minor axillary dissections (sentinel node/axillary sampling), which are known to be considerably less frequently affected by seroma [27]. It is also possible that our nurse specialists had a relatively low threshold for aspiration. Most commentators regard a small collection of serous fluid as an inevitable post-operative event, and generally only larger seromas causing pain or threatening wound integrity should be aspirated. In our study, where data were available, 1/3 of seromas aspirated from the flap fixation group, and 61% from the drainage group, were less than 100 ml in volume. In these cases, aspiration may have been unnecessary.
We acknowledge that there may be a degree of selection bias in this study. Patients’ care was determined by their assigned consultant surgeon. This process was random but not truly randomised. Due to differences in practice, one consultant used drains in every case and the other employed the technique of flap fixation.
In this study, flap anchoring without drainage resulted in a significantly shorter hospital stay without any increase in the incidence of seroma formation or wound infection. We suggest that the policy of routine drainage should be reviewed. Patient care should be individualised, to account for age, geographic location, social support and the general medical fitness of each patient. Preoperative counselling, nursing (both hospital-based nurse specialists and community nurses), accessibility of seroma aspiration clinics and patient understanding must also all be considered when deciding upon treatment policy [18]. Flap fixation may present a viable alternative to routine drain placement and could lead to considerable financial savings. At a national level, these could potentially sum-mate to savings of several million pounds.
Conflict of Interest
The authors can confirm that there are no conflicts of interest and that no funding or sponsorship was granted in order to undertake this research.
References
- 1.The National Mastectomy and Breast Reconstruction Audit. www.healthcarecommission.org.uk, November 2008.
- 2.Tartter PI, Beck G, Fuchs K. Determinants of hospital stay after modified radical mastectomy. Am J Surg. 1994;168:320–324. doi: 10.1016/s0002-9610(05)80157-6. [DOI] [PubMed] [Google Scholar]
- 3.Watt-Boolsen S, Nielsen VB, Jensen J, Bak S. Post mastectomy seroma. A study of the nature and origin of seroma after mastectomy. Dan Med Bull. 1989;36:487–489. [PubMed] [Google Scholar]
- 4.Watt-Boolsen S, Jacobsen K, Blichert-Toft M. Total mastectomy with special reference to surgical technique, extent of axillary dissection and complications. Acta Oncol. 1988;27:663–665. doi: 10.3109/02841868809091765. [DOI] [PubMed] [Google Scholar]
- 5.Aitken D, Minton J. Complications associated with mastectomy. Surg Clin North Am. 1983;63:1331–1352. doi: 10.1016/s0039-6109(16)43192-0. [DOI] [PubMed] [Google Scholar]
- 6.Chilson TR, Chan FD, Lonser RR, et al. Seroma prevention after modified radical mastectomy. Am Surg. 1992;58:750–754. [PubMed] [Google Scholar]
- 7.Agrawal A, Ayantunde AA, Cheung KL. Concepts of seroma formation and prevention in breast cancer surgery. ANZ J Surg. 2006;76:1088–1095. doi: 10.1111/j.1445-2197.2006.03949.x. [DOI] [PubMed] [Google Scholar]
- 8.Eroglu E, Oral S, Unal E, et al. Reducing seroma formation with fibrin glue in an animal mastectomy model. Eur J Surg Oncol. 1996;22:137–139. doi: 10.1016/s0748-7983(96)90567-3. [DOI] [PubMed] [Google Scholar]
- 9.Jain PK, Sowdi R, Anderson AD, MacFie J. Randomised clinical trial investigating the use of drains and fibrin sealant following surgery for breast cancer. Br J Surg. 2004;91:54–50. doi: 10.1002/bjs.4435. [DOI] [PubMed] [Google Scholar]
- 10.Porter K, O'Connor S, Rimm E, Lopez M. Electrocautery as a factor in seroma formation following mastectomy. Am J Surg. 1999;176:8–11. doi: 10.1016/s0002-9610(98)00093-2. [DOI] [PubMed] [Google Scholar]
- 11.Wagman LD, Terz JJ, Hill LR, et al. Evaluation of a short stay program for patients undergoing mastectomy. J Surg Oncol. 1989;41:98–101. doi: 10.1002/jso.2930410209. [DOI] [PubMed] [Google Scholar]
- 12.Boman L, Bjorvell H, Cedermark B, et al. Effects of early discharge from hospital after surgery for primary breast cancer. Eur J Surg. 1993;159:67–73. [PubMed] [Google Scholar]
- 13.Holcombe C, West N, Mansel RE, et al. The satisfaction and savings of early discharge with drain in situ following axillary lymphadenectomy in the treatment of breast cancer. Eur J Surg Oncol. 1995;21:604–606. doi: 10.1016/s0748-7983(95)95133-4. [DOI] [PubMed] [Google Scholar]
- 14.Burke CC, Zabka CL, McCarver KJ, Singletary SE. Patient satisfaction with 23 hours ‘short-stay’ observation following breast cancer surgery. Oncol Nurs Forum. 1997;24:645–651. [PubMed] [Google Scholar]
- 15.Tan LR, Guenther JM. Outpatient definitive breast cancer surgery. Am Surg. 1997;63:865–867. [PubMed] [Google Scholar]
- 16.Orr RK, Ketcham AS, Robinson DS, et al. Early discharge after mastectomy. A safe way of diminishing hospital costs. Am Surg. 1987;53:161–163. [PubMed] [Google Scholar]
- 17.Bonnema J, Van Wersch AMEA, Van Geel AN, et al. Medical and psychosocial effects of early discharge after surgery for breast cancer: randomised trial. BMJ. 1998;316:1267–1271. doi: 10.1136/bmj.316.7140.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pogson CJ, Adwani A, Ebbs SR. Seroma following breast cancer surgery. Eur J Surg Oncol. 2003;29:711–717. doi: 10.1016/s0748-7983(03)00096-9. [DOI] [PubMed] [Google Scholar]
- 19.Halsted WS. Developments in skin grafting operation for cancer of the breast. JAMA. 1913;60:416. [Google Scholar]
- 20.Coveney EC, O'Dwyer PJ, Geraghty JG, O'Higgins NJ. Effect of closing dead space on seroma formation after mastectomy – a prospective randomised clinical trial. Eur J Surg Oncol. 1993;19:143–146. [PubMed] [Google Scholar]
- 21.Purushotham AD, McLatchie E, Young D, et al. Randomized clinical trial of no wound drains and early discharge in the treatment of women with breast cancer. Br J Surg. 2002;89:286–292. doi: 10.1046/j.0007-1323.2001.02031.x. [DOI] [PubMed] [Google Scholar]
- 22.Schuijtvlot M, Sahu AK, Cawthorn SJ. A prospective audit of the use of a buttress suture to reduce seroma formation following axillary node dissection without drains. Breast. 2002;11:94–96. doi: 10.1054/brst.2001.0366. [DOI] [PubMed] [Google Scholar]
- 23.Pederson SH, Douville LM, Eberlein TJ. Accelerated surgical stay program: a mechanism to reduce health care costs. Ann Surg. 1994;219:374–381. doi: 10.1097/00000658-199404000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bundred N, Maguire P, Reynolds J, et al. Randomised controlled trial of effects of early discharge after surgery for breast cancer. BMJ. 1998;317:1275–1279. doi: 10.1136/bmj.317.7168.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holcombe C, West N, Mansel RE, Horgan K. The satisfaction and savings of early discharge with drain in situ following axillary lymphadenectomy in the treatment of breast cancer. Eur J Surg Oncol. 1995;21:604–606. doi: 10.1016/s0748-7983(95)95133-4. [DOI] [PubMed] [Google Scholar]
- 26.Edwards MJ, Broadwater JR, Bell JL, et al. Economic impact of reducing hospitalisation for mastectomy patients. Ann Surg. 1988;208:330–336. doi: 10.1097/00000658-198809000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budd DC, Cochran RC, Sturtz DL, Fouty WJ., Jr Surgical morbidity after mastectomy operations. Am J Surg. 1978;135:218–220. doi: 10.1016/0002-9610(78)90103-4. [DOI] [PubMed] [Google Scholar]