Summary
Although a current decline in breast cancer incidence and mortality is being observed, the disease continues to be the most common malignancy among women. Breast cancer is a worldwide public health problem that causes substantial personal and social burdens. While we do not yet know exactly what causes the disease, we know a large number of risk factors that are linked to breast cancer. In particular, hormonal factors seem to play a key role in the causation of the disease. The aim of this paper is to review the current knowledge of established and suspected risk factors of breast cancer from an epidemiologic point of view.
Key Words: Breast cancer, Risk factor, Epidemiology
Zusammenfassung
Trotz eines Rückgangs sowohl der Inzidenz als auch der Mortalität des Brustkrebses bleibt die Erkrankung die häufigste maligne Neubildung bei Frauen, verbunden mit erheblichen persönlichen Belastungen und gesellschaftlichen Kosten. Obwohl wir bisher nicht genau wissen, wie Brustkrebs entsteht, kennen wir doch eine Reihe von Risikofaktoren, die mit der Erkrankung in Verbindung stehen. Insbesondere hormonellen Faktoren kommt eine Schlüsselrolle in der Entstehung der Erkrankung zu. Ziel des vorliegenden Artikels ist die Zusammenfassung des aktuellen Kenntnisstandes zu etablierten und vermuteten Risikofaktoren des Brustkrebses aus epidemiologischer Sicht.
Introduction
With over 1 million new breast cancer cases each year, breast cancer is the most common malignancy among women worldwide [1]. Estimates from Europe assume a number of cancer cases of 370,100 and a number of deaths of 129,900 in 2004 [2]. During her lifetime, a woman who survives to the age of 85 will have a 1-in-9 chance to develop breast cancer. However, depending on the risk factor profile, the probability of developing breast cancer is not homogeneously distributed across all women. For primary and secondary prevention of breast cancer, it is important to identify high-risk women. This review will summarise the results from recent studies on factors that have been determined to influence the breast cancer risk. Table 1 provides an overview of the main established risk factors of breast cancer.
Table 1.
Established risk factors in breast cancer
| Risk factor | RR | High-risk group or unit of risk increase | Comparing group | References |
|---|---|---|---|---|
| Age | 7.8a | age ≥ 50 | age < 50 | [3] |
| Age at menarche | 1.1–1.2 | 1 year earlier menarche | – | [16] |
| Age at menopause | 1.03 | 1 year delay in menopause | – | [17] |
| Age at first birth | 0.97 | each year younger at first birth | – | [43] |
| Breastfeeding | 0.96 | per every 12 months of breastfeeding | – | [43] |
| Nulliparous | 1.3 | nulliparous women | at least 1 full-term pregnancy | [42] |
| Body height | 1.07 | per 5-cm increment | – | [21] |
| Body mass index | 0.94b, 1.03c | per BMI increase of 2 kg/m2 | – | [33] |
| Breast density | 4.7 | density in ≥75 % of the mammogram | density > 10% of the mammogram | [19] |
| Alcohol consumption | 1.07 | per each additional 10 g alcohol/day | – | [32] |
| Physical activity | 0.97 | per 7 MET hours recreational activity per week | – | [33] |
| Hormone replacement | 1.02 | per year of use of HRT among | – | [17] |
| therapy | current or recent users | |||
| Oestradiol | 2 | high levels of plasma concentration | low levels of plasma concentration | [24] |
| BRCA1 / BRCA2 | > 30 (BRCAl)d, 11 (BRCA2)d | BRCA1/2 mutation carriers | general population rates | [12] |
| Family history of breast cancer | 1.8 | one affected first-degree relative | no affected first-degree relative | [8] |
| Previous benign breast biopsy | 4.4 | atypical hyperplasia | no biopsy and normal mammogram | [27] |
RR, Relative risk; BRCA, breast cancer gene; BMI, body mass index; MET, metabolic equivalent.
Calculated as quotient of age-standardised SEER incidence rates.
Premenopausal women.
Tostmenopausal women.
For women > 50 years of age.
Non-Modifiable Risk Factors
Age
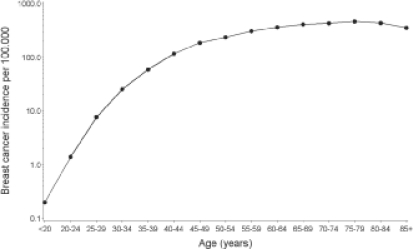
As for most diseases, age is a well-documented risk factor for breast cancer. Breast cancer incidence is relatively low before the age of 30 and then increases dramatically until the age of 80. For example, in 2005, the Surveillance, Epidemiology, and End Results (SEER) incidence was 42.8 per 100,000 for women aged < 50 and 335.5 for women aged 50 and older (SEER areas: San Francisco, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, and Atlanta) [3]. Mortality also increases with age, ranging from 5.2 per 100,000 for women aged < 50 to 73.3 per 100,000 for women aged 50 and older [3]. The rate of increase in breast cancer incidence with age is declining at around the age of 50, confirming the important role of hormonal factors in breast cancer aetiology (fig. 1).
Fig. 1.
Age-specific breast cancer incidence (per 100,000) (data derived from SEER Cancer Statistic Review, 2000–2004 [3]).
Race, Ethnicity, Geographical Variation, and Migration
The observed breast cancer incidence rates vary approximately 5-fold between countries. The highest age-standardised rates (world standard) were observed in the USA and Northern Europe (50–100 per 100,000), the lowest in Asia (10–30 per 100,000) [4]. There are also important racial and ethnic differences in breast cancer incidence. In 2005, the age-adjusted incidence in women aged 50 and older was 351.9 per 100,000 in Caucasians and 292.2 per 100,000 in African Americans. However, in women younger than 50, the incidence rates were the same, with 43.4 per 100,000 in both ethnic groups [3]. Despite lower overall incidence rates of breast cancer in African Americans, the mortality rates from breast cancer were higher than in Caucasians [3]. Differences in access to care as well as biological factors such as higher incidence of aggressive breast cancers and higher tumour grades have been named as the causes [5].
Migration studies of Asians migrated to the USA have shown that the breast cancer risk increases throughout several generations and is nearing that among U.S. whites [6, 7]. These findings suggest that lifestyle and environmental factors of the host country have a substantial impact on breast cancer risk.
Genetic Factors / Family History of Breast Cancer
Women with a family history of breast cancer have an increased risk of developing the disease. A major pooled analysis of 52 epidemiological studies including 58,209 cases and 101,986 controls found that women with 1, 2, and 3 or more affected first-degree relatives have a relative risk (RR) of 1.8 (99% confidence interval (CI) 1.7–1.9), 2.9 (2.4–3.6), and 3.9 (2.0–7.5), respectively, compared to women without affected relatives [8]. The estimated lifetime probability of developing breast cancer for women with 0, 1, and 2 affected first-degree relatives is 7.8, 13.3, and 21.1%, respectively. However, 8 of 9 women who develop the disease do not have an affected mother, sister, or daughter [8].
Hereditary breast cancers are often characterised by an early age of diagnosis and an excess of bilateral breast cancer [9]. On average, 5–10% of all breast cancers are attributable to genetic predisposition. However, it is not known yet how many relevant breast cancer genes exist. Two major susceptibility genes, BRCA1 and BRCA2, were identified in the 1990s [10, 11]. The estimated cumulative risks in BRCA1 or BRCA2 mutation carriers by the age of 70 years were 65% (95% CI 44–78%), and 45% (31–56%), respectively [12]. Further genes such as p53 and PTEN are also associated with a high risk of breast cancer [9]. However, regardless of the comparatively high breast cancer risk in mutation carriers, the frequency of these mutations in the population is rare [9].
An increasing number of identified genes is associated with a more moderate risk of breast cancer [13,114,15]. Because mutations in some of these genes are expected to be more common in the general population, the proportion of breast cancers attributable to these genes may be substantial.
Age at Menarche / Age at Menopause
Age at menarche is a chronological indicator of the onset of ovarian activity as well as a predictor of ovulatory frequency during adolescence and hormone levels in young adults [16].
There is evidence of decreased breast cancer risk by later age at menarche. Generally, the risk decreased by 10–24% with each year of delay in menarche [16].
In contrast, age at menopause is a marker of the cessation of ovarian activity. The risk of breast cancer increases with the later onset of menopause, with risk increasing by about 3% for each year of delay in menopause [17]. The effect is similar in women whose menopause was natural and for those whose menopause was the result of bilateral oophorectomy [17].
Breast Density
The radiological appearance of the breast varies interindividually because of variations in breast tissue composition. Fat is radiolucent and appears dark on a mammogram, whereas epithelium and stroma are radiodense and therefore appear light.
Mammographic density of the breast, i.e. a high proportion of radiodense tissue, is a strong and independent risk factor for breast cancer [18, 19]. Risk of breast cancer is approximately 5 times increased in women with density in more than 75% of the breast compared with women with density in less than 10% of the breast [19]. About one third of breast cancer cases was attributable to density of 50% or more of the breast [18]. It has been hypothesised that the combined effects of cell proliferation and genetic damage to proliferating cells by mutagens may explain the positive association of high breast density with increased risk for breast cancer [20]. Because breast density is mainly genetically determined (63%), the identification of responsible genes could provide insights into the biology of the breast [18].
Body Height
Numerous studies have investigated the relation of body height and breast cancer risk. Most of them showed an increased risk with increasing body height. A pooled analysis of prospective cohort studies showed an RR of 1.02 (95% CI 0.96–1.10) per height increment of 5 cm in premenopausal women and 1.07 (1.03–1.12) in postmenopausal women [21]. A large cohort study of Danish women also showed that high stature at 14 years of age and peak growth at an early age were independent risk factors for breast cancer [22]. The reason for the association of increased risk with increasing body height is not fully understood; however, the attained height is determined by genetic and environmental factors, whereas especially childhood energy intake seems to play a role in breast cancer genesis. Furthermore, body height is a marker for the number of susceptible breast cells, or more precisely, the mammary gland mass, which reflects the total number of mammary cells and can be correlated with mammary cells at risk for transformation [23].
Endogenous Hormones
Several cohort studies have examined the association of serum concentration of endogenous hormones with breast cancer. Pooled data from the Endogenous Hormones and Breast Cancer Collaborative Group provide strong evidence that plasma hormone levels predict breast cancer risk in postmenopausal women [24]. The risk of breast cancer increased with increasing concentrations of different sex hormones; among others, total oestradiol and testosterone. The RR for postmenopausal women in the highest quintile of oestradiol was 2.0 (95% CI 1.5–2.7) compared to women in the lowest quintile. A positive association has also been postulated for further endogenous hormones such as insulin-like growth factor (IGF). Although some studies showed an increased risk of breast cancer with increasing concentration of IGF-I especially in premenopausal women, the overall effect is marginal [25].
Prior History of Breast Biopsy with Benign Diagnosis
The results of clinical follow-up studies have indicated that women with biopsy-proven benign breast disease are at increased risk of subsequent breast cancer [26]. A recent study showed that women with atypical hyperplasia have a hazard ratio (HR) of 4.4 (95% CI 2.7–7.1) for the development of breast cancer compared with women with no known breast biopsy experience and a normal mammogram, whereas women with benign breast diseases without atypia (including adenosis, apocrine metaplasia, calcifications and ductal hyperplasia) have an HR of 1.9 (1.8–2.0) [27]. The increased risk seems to be modified by breast density and hormonal status [26, 27].
Modifiable or Potentially Modifiable Risk Factors
Diet
Because of the large international variations of breast cancer rates, it has been suggested that diet could play a role in cancer aetiology. Although there are many studies that investigated the association of diet and breast cancer risk, up to date there is no consistent and strong association [28]. Especially the association of fat intake [29], soy intake [30] and consumption of fruits and vegetables [31] with breast cancer risk were investigated in pooled analyses. Overall, there is limited evidence that a high total fat consumption could increase the risk of breast cancer, whereas high soy intake as well as fruit and vegetable consumption may prevent breast cancer. This effect may be ascribed to the antioxidant properties of selected nutrients, influence on DNA repair, DNA mutations, DNA adducts, metabolic detoxification, stimulation of growth factors, and potential anti-oestrogenic influences [28].
Alcohol Consumption
Many observational studies have investigated the association of alcohol consumption and breast cancer. Overall, alcohol consumption seems to be the only lifestyle factor with convincing evidence for increased risk of breast cancer [32, 33]. A pooled analysis of 53 epidemiological studies showed a 7% increase for each additional 10 g per day intake of alcohol in pre- and postmenopausal women [32]. No threshold of risk increasing was identified and no evidence of a different contribution of types of beverages was found [33]. The RR of breast cancer was 1.3 (1.2–1.4) for an intake of 35–44 g per day, and 1.46 for an intake > 45 g per day compared with women who reported no drinking of alcohol. To explain these observations, a number of mechanisms have been assumed, amongst others that alcohol intake increases oestrogen concentration, that alcohol intake decreases the DNA repair efficiency or that alcohol intake stimulates the metabolism of carcinogens such as acetaldehyde [34].
Smoking
Results of epidemiological studies assessing the association between smoking and breast cancer risk have been inconclusive. A pooled analysis of 53 epidemiological studies concluded that smoking has little or no independent effect on breast cancer risk [32]. However, recently published investigations showed that smoking may increase the risk of breast cancer in special subgroups of women, such as in women with N-acetyltransferase 2 slow acetylation genotypes and in premenopausal women who started smoking before the breast tissue was completely differentiated [35, 36]. Band et al. found a substantially increased risk of breast cancer in premenopausal women who had been pregnant and who started smoking within 5 years of menarche, and in nulliparous women who smoked 20 cigarettes daily or more or had smoked 20 cumulative packyears or more [35]. In contrast, postmenopausal women whose body mass index (BMI) increased from age 18 to current and who started to smoke after a first full-term pregnancy had a significantly reduced risk of breast cancer. The authors concluded that the carcinogenic effect of tobacco smoke is dominant in breast tissue before a complete differentiation, whereas in postmenopausal women the anti-oestrogenic effect is dominating.
It has been suggested that passive smoking could increase the risk of breast cancer in women who have never smoked. However, a recent meta-analysis found no association, independent of the time of onset of exposure (childhood vs. adult) [37].
Physical Activity
Several studies have found that there is an association between physical activity and reduced breast cancer risk. However, the results are not entirely consistent across the studies. A systematic review concluded that the evidence for an inverse association of physical activity and breast cancer risk is stronger for post- than for premenopausal women [38]. A meta-analysis of cohort studies showed a 3% decreased risk per 7 metabolic equivalent (MET) hours of recreational activity per week in postmenopausal women [33]. To a large extent, the mechanisms by which physical activity prevents breast cancer are unclear. It is known that physical activity has a beneficial influence on certain menstrual characteristics, body size and serum hormone levels. It is therefore assumed that physical activity reduces breast cancer risk primarily through hormone-related pathways.
Obesity
The relation between obesity and breast cancer risk strongly depends on menopausal status. In premenopausal women obesity decreases the risk of breast cancer, whereas in postmenopausal women obesity is associated with an increased risk of breast cancer. In a pooled analysis of prospective cohort studies, premenopausal women with a BMI exceeding 31 kg/m2 had an RR of 0.5 (95% CI 0.3–0.8) compared to women with a BMI < 21 [21]. In contrast, postmenopausal women with a BMI > 28 had an RR of 1.3 (95% CI 1.1–1.5) compared to women with a BMI < 21. It is assumed that in premenopausal women, obesity may protect from breast cancer by causing more frequent anovulatory menstrual cycles, which in turn results in decreased oestradiol and progesterone levels. In postmenopausal women, ovarian oestrogen production is diminished and oestrogen is derived mainly from the aromatisation of androstenedione which occurs primarily in adipose tissue.
The association of waist-to-hip ratio (WHR), a marker of central obesity, with breast cancer risk is not as clear as with the BMI. In postmenopausal women, the positive association is generally confirmed. On the other hand, a systematic review found that in premenopausal women there was a positive association only with adjustment for BMI, suggesting that central obesity may be specifically associated with an increased risk of breast cancer [39].
Some studies reported that weight gain during adult life increases the risk of breast cancer among postmenopausal women, whereas weight loss after menopause is associated with a decreased risk of breast cancer [40, 41]. A recent study reported that a weight gain of more than 25 kg since the age of 18 years was associated with an RR of 1.5 (95% CI 1.3–1.7) of invasive breast cancer compared to women who had maintained their weight [41].
Age at First Birth / Parity / Breastfeeding
Age at first birth, childbearing, and breastfeeding are strong correlated factors which account for a final cell differentiation of breast tissue. The earlier this final differentiation is completed the lower the susceptibility to carcinogens. A meta-analysis of large Scandinavian epidemiological studies found that nulliparous women have a 30% increased risk compared with women who have had at least 1 full-term pregnancy [42]. Additionally, an increasing protective effect was found with increasing number of full-term pregnancies. For each birth, the risk was reduced by about 7% [43]. Besides parity, an early age at first birth also reduces the breast cancer risk. Women giving first birth after the age of 35 years have a 40% increased risk compared to those with a first birth before the age of 20 years [42]. Independent of parity, the effect of breastfeeding was evaluated in a review of 47 epidemiological studies [43]. The result showed that the longer women breastfed, the more they were protected against breast cancer. The risk of breast cancer decreased by 4.3% for every 12 months of breastfeeding [43].
Hormone Replacement Therapy / Hormonal Contraceptives
A large number of data exists regarding the relation of use of hormone replacement therapy (HRT) and risk of breast cancer. Results from a review of 51 epidemiological studies and from the Women's Health Initiative (WHI) randomised trial provide convincing evidence that current HRT use increases breast cancer risk [17, 44]. The amount of risk depends strongly on hormonal constituents, duration of HRT use and time of cessation. Overall, the risk of getting breast cancer is higher in oestrogen plus progestogen users, increases with advancing duration of use, and nearly disappears after about 5 years after ending HRT use. The average RR reported in a recent review of epidemiological studies is 1.2 (95% CI 1.0–1.4) with current use of oestrogen alone and 1.7 (1.4–2.2) with current use of oestrogen plus progestogen [45]. Since HRT has been communicated as a risk factor for breast cancer, the use of HRT has decreased substantially in the general population over the last years. It has been suspected that the recent decline in breast cancer incidence is partly attributable to the decrease in utilisation of HRT [46].
Many epidemiological studies have investigated whether hormonal contraceptives might affect breast cancer risk. Overall, there is only a small increase in the risk of having breast cancer diagnosed in current users of oral contraceptives [47]. Women currently taking combined oral contraceptives have an RR of 1.2 (95% CI 1.1–1.3) compared to never users, but there is no evidence of an increase in the risk more than 10 years after stopping the use [47].
Electromagnetic Fields
Besides the risk factors described above, several further factors were investigated regarding their association with breast cancer risk. Ionising radiation is an established breast cancer risk factor especially in young girls in whom differentiation of breast tissue is not completed. The risk increases linearly with the dose of ionising radiation [48].
It has also been suggested that exposures to electromagnetic fields increase the risk of breast cancer. However, most of the recent studies reported no increased risks [49].
Conclusions
This review offers a summary of known and expected risk factors for breast cancer. Risk factors that are associated with increased oestrogen levels, such as age at menarche, age at menopause, and obesity as well as hormonal factors per se play a key role in the aetiology of breast cancer.
Breast density is a strong risk factor for breast cancer, although it is unknown whether a reduction in breast density would reduce the risk of breast cancer. Nevertheless, the implementation of breast density in risk prediction models improves the identification of high-risk women for preventive interventions [50].
Modifiable risk factors, such as alcohol consumption, low physical activity, obesity, and HRT use, increase the risk of breast cancer marginally. However, because the prevalence of these risk factors is high, estimates of the population-attributable risk showed that about 40% of breast cancer cases could be prevented by these risk factors [51]. Moreover, prevention of breast cancer by modifiable risk factors will also reduce the incidence of other chronic diseases, as diabetes type 2 or cardiovascular diseases [52, 53].
Conflict of Interest
The authors have declared no conflict of interest.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002: cancer incidence, mortality and prevalence worldwide. IARC CancerBase No 5, version 2.0. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller AB, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK: SEER Cancer Statistics Review, 1975–2005, National Cancer Institute, Bethesda, MD. http://seer cancer gov/csr/1975_2005/
- 4.Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: Cancer incidence, mortality and prevalence worldwide. Lyon: IARC Press; 2001. [Google Scholar]
- 5.Morris GJ, Mitchell EP. Higher incidence of aggressive breast cancers in African-American women: a review. J Natl Med Assoc. 2008;100:698–702. doi: 10.1016/s0027-9684(15)31344-4. [DOI] [PubMed] [Google Scholar]
- 6.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85:1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 7.Stanford JL, Herrinton LJ, Schwartz SM, Weiss NS. Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology. 1995;6:181–183. doi: 10.1097/00001648-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Collaborative Group on Hormonal Factors in Breast Cancer Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 9.Turnbull C, Rahman N. Genetic predisposition to breast cancer: past, present, and future. Annu Rev Genomics Hum Genet. 2008;9:321–345. doi: 10.1146/annurev.genom.9.081307.164339. [DOI] [PubMed] [Google Scholar]
- 10.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 11.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The CHEK2 Breast Cancer Case-Control Consortium: CHEK2∗1100delC and susceptibility to breast cancer: a collaborative analysis involving 10,860 breast cancer cases and 9,065 controls from 10 studies. Am J Hum Genet. 2004;74:1175–1182. doi: 10.1086/421251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson D, Duedal S, Kirner J, McGuffog L, Last J, Reiman A, Byrd P, Taylor M, Easton DF. Cancer risks and mortality in heterozygous ATM mutation carriers. J Natl Cancer Inst. 2005;97:813–822. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- 15.Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, Struewing JP, Morrison J, Field H, Luben R, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447:1087–1093. doi: 10.1038/nature05887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein L. Epidemiology of endocrine-related risk factors for breast cancer. J Mammary Gland Biol Neoplasia. 2002;7:3–15. doi: 10.1023/a:1015714305420. [DOI] [PubMed] [Google Scholar]
- 17.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 18.Boyd NF, Rommens JM, Vogt K, Lee V, Hopper JL, Yaffe MJ, Paterson AD. Mammographic breast density as an intermediate phenotype for breast cancer. Lancet Oncol. 2005;6:798–808. doi: 10.1016/S1470-2045(05)70390-9. [DOI] [PubMed] [Google Scholar]
- 19.Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, Jong RA, Hislop G, Chiarelli A, Minkin S, Yaffe MJ. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 20.Martin LJ, Boyd NF. Mammographic density. Potential mechanisms of breast cancer risk associated with mammographic density: hypotheses based on epidemiological evidence. Breast Cancer Res. 2008;10:201. doi: 10.1186/bcr1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 22.Ahlgren M, Melbye M, Wohlfahrt J, Sorensen TI. Growth patterns and the risk of breast cancer in women. N Engl J Med. 2004;351:1619–1626. doi: 10.1056/NEJMoa040576. [DOI] [PubMed] [Google Scholar]
- 23.Trichopoulos D, Lagiou P, Adami HO. Towards an integrated model for breast cancer etiology: the crucial role of the number of mammary tissue-specific stem cells. Breast Cancer Res. 2005;7:13–17. doi: 10.1186/bcr966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 25.Sugumar A, Liu YC, Xia Q, Koh YS, Matsuo K. Insulin-like growth factor (IGF)-I and IGF-binding protein 3 and the risk of premenopausal breast cancer: a metaanalysis of literature. Int J Cancer. 2004;111:293–297. doi: 10.1002/ijc.20253. [DOI] [PubMed] [Google Scholar]
- 26.Schnitt SJ. Benign breast disease and breast cancer risk: potential role for antiestrogens. Clin Cancer Res. 2001;7:4419s–4422s. [PubMed] [Google Scholar]
- 27.Ashbeck EL, Rosenberg RD, Stauber PM, Key CR. Benign breast biopsy diagnosis and subsequent risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:467–472. doi: 10.1158/1055-9965.EPI-06-0394. [DOI] [PubMed] [Google Scholar]
- 28.Michels KB, Mohllajee AP, Roset-Bahmanyar E, Beehler GP, Moysich KB. Diet and breast cancer: a review of the prospective observational studies. Cancer. 2007;109:2712–2749. doi: 10.1002/cncr.22654. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Warner SA, Spiegelman D, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, Graham S, et al. Types of dietary fat and breast cancer: a pooled analysis of cohort studies. Int J Cancer. 2001;92:767–774. doi: 10.1002/1097-0215(20010601)92:5<767::aid-ijc1247>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Warner SA, Spiegelman D, Yaun SS, Adami HO, Beeson WL, van den Brandt PA, Folsom AR, Fraser GE, Freudenheim JL, Goldbohm RA, et al. Intake of fruits and vegetables and risk of breast cancer: a pooled analysis of cohort studies. JAMA. 2001;285:769–776. doi: 10.1001/jama.285.6.769. [DOI] [PubMed] [Google Scholar]
- 32.Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Jr, Coates RJ, Liff JM, Talamini R, Chantarakul N, et al. Alcohol, tobacco and breast cancer – collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Cancer Research Fund / American Institute for Cancer Research . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington D.C.: AICR; 2007. [Google Scholar]
- 34.Seitz HK, Becker P. Alcohol metabolism and cancer risk. Alcohol Res Health. 2007;30:38–41, 44-47. [PMC free article] [PubMed] [Google Scholar]
- 35.Band PR, Le ND, Fang R, Deschamps M. Carcinogenic and endocrine disrupting effects of cigarette smoke and risk of breast cancer. Lancet. 2002;360:1044–1049. doi: 10.1016/S0140-6736(02)11140-8. [DOI] [PubMed] [Google Scholar]
- 36.Ambrosone CB, Kropp S, Yang J, Yao S, Shields PG, Chang-Claude J. Cigarette smoking, N-acetyltrans-ferase 2 genotypes, and breast cancer risk: pooled analysis and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:15–26. doi: 10.1158/1055-9965.EPI-07-0598. [DOI] [PubMed] [Google Scholar]
- 37.Pirie K, Beral V, Peto R, Roddam A, Reeves G, Green J. Passive smoking and breast cancer in never smokers: prospective study and meta-analysis. Int J Epidemiol. 2008;37:1069–1079. doi: 10.1093/ije/dyn110. [DOI] [PubMed] [Google Scholar]
- 38.Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18:137–157. doi: 10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 39.Harvie M, Hooper L, Howell AD. Central obesity and breast cancer risk: a systematic review. Obes Rev. 2003;4:157–173. doi: 10.1046/j.1467-789x.2003.00108.x. [DOI] [PubMed] [Google Scholar]
- 40.Feigelson HS, Patel AV, Teras LR, Gansler T, Thun MJ, Calle EE. Adult weight gain and histopathologic characteristics of breast cancer among postmenopausal women. Cancer. 2006;107:12–21. doi: 10.1002/cncr.21965. [DOI] [PubMed] [Google Scholar]
- 41.Eliassen AH, Colditz GA, Rosner B, Willett WC, Hankinson SE. Adult weight change and risk of postmenopausal breast cancer. JAMA. 2006;296:193–201. doi: 10.1001/jama.296.2.193. [DOI] [PubMed] [Google Scholar]
- 42.Ewertz M, Duffy SW, Adami HO, Kvale G, Lund E, Meirik O, Mellemgaard A, Soini I, Tulinius H. Age at first birth, parity and risk of breast cancer: a meta-analysis of 8 studies from the Nordic countries. Int J Cancer. 1990;46:597–603. doi: 10.1002/ijc.2910460408. [DOI] [PubMed] [Google Scholar]
- 43.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet. 2002;360:187–195. doi: 10.1016/S0140-6736(02)09454-0. [DOI] [PubMed] [Google Scholar]
- 44.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative randomized trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 45.Collins JA, Blake JM, Crosignani PG. Breast cancer risk with postmenopausal hormonal treatment. Hum Reprod Update. 2005;11:545–560. doi: 10.1093/humupd/dmi028. [DOI] [PubMed] [Google Scholar]
- 46.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 47.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data on 53 297 women with breast cancer and 100 239 women without breast cancer from 54 epidemiological studies. Lancet. 1996;347:1713–1727. doi: 10.1016/s0140-6736(96)90806-5. [DOI] [PubMed] [Google Scholar]
- 48.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feychting M, Forssen U. Electromagnetic fields and female breast cancer. Cancer Causes Control. 2006;17:553–558. doi: 10.1007/s10552-005-9008-3. [DOI] [PubMed] [Google Scholar]
- 50.Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 51.Sprague BL, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Hampton JM, Newcomb PA. Proportion of invasive breast cancer attributable to risk factors modifiable after menopause. Am J Epidemiol. 2008;168:404–411. doi: 10.1093/aje/kwn143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S, Laakso M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 53.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15:239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]