Summary
Breast cancer is a rare disease in men representing nearly 1% of the total breast cancer cases worldwide. Due to the low incidence, there are no randomized clinical studies giving information on the optimal diagnostics and therapy for male breast cancer patients. Therefore, treatment recommendations are derived from established guidelines for breast cancer in women. However, the lack of awareness of this disease leads to its detection at a later stage in men associated with a worse prognostic outcome. The gender-specific differences in breast cancer are among others related to the differing genetic and hormonal environment and the anatomic constitution in men. For example, males have a much higher percentage of hormone receptor-positive tumors but a significantly lower fraction of carcinomas overexpressing HER2. This review focuses on epidemiology, pathogenesis, and clinical findings of male breast cancer, and discusses current findings available to treat this disease. To optimize disease outcome and tolerability of treatment, these data should be considered to improve the therapeutic index of male breast cancer patients.
Key Words: Breast cancer, Male, Etiology, Pathology, Therapy
Zusammenfassung
Das Mammakarzinom des Mannes ist eine seltene Erkrankung. Es macht ungefähr 1% aller Mammakarzinome aus. Daten aus randomisierten klinischen Studien, die Diagnostik und Therapie untersuchen, sind aufgrund der niedrigen Inzidenz derzeit nicht verfügbar. Deshalb werden Therapieempfehlungen zumeist von den etablierten Leitlinien für Frauen mit Brustkrebs abgeleitet. Fehlende klinische Erfahrung im Umgang mit dem Mammakarzinom des Mannes führt leider häufig zu einer verspäteten Diagnose, was eine schlechtere Prognose bedingen kann. Die geschlechtsspezifischen Unterschiede des Mammakarzinoms sind unter anderem durch unterschiedliche genetische und hormonelle Bedingungen und anatomische Verhältnisse gegeben. So haben Männer zum Beispiel viel häufiger Hormonrezeptor-positive und HER2-negative Karzinome. Diese Übersicht fasst neue Erkenntnisse zu Epidemiologie, Pathogenese und Klinik des Mammakarzinoms beim Mann zusammen. Darüber hinaus werden aktuelle Strategien zur Therapie dargestellt. Um eine bestmögliche Prognose und Verträglichkeit der Behandlung von männlichen Patienten zu gewährleisten, sollten diese Informationen für entsprechende Therapieplanungen zur Verfügung stehen.
Introduction
Male breast cancer (MBC) is a relatively rare disease accounting for less than 1% of all cases of cancer in men. Because of its rarity, most information about this disease has been obtained from small, single-institutional, or retrospective studies, or by extrapolation from breast cancer trials in women. Occurrence of breast cancer has been reported in males aged 5–93 years, with a peak at approximately 71 years. The bimodal age distribution seen in women is absent in men, and the incidence increases exponentially with age. Hyperestrogenization resulting from genetic disorders, gonadal dysfunction, obesity, or excess alcohol increase the risk whereas gynecomastia does probably not. Presentation is usually a lump or nipple inversion, but is often late, with more than 40% of individuals having stage III or IV disease. Most tumors are ductal, and 10% are ductal carcinoma in situ. Surgery is usually mastectomy with axillary lymphonodectomy or sentinel node biopsy. Indications for radiotherapy are similar to female breast cancer. Because 90% of tumors are estrogen receptor (ER)-positive, tamoxifen is standard adjuvant therapy, but some individuals could also benefit from chemotherapy. Preoperative chemotherapy is a treatment option in primary inoperable cases. Hormonal therapy is the main treatment for metastatic disease, but chemotherapy can also provide tumor response. National initiatives are increasingly needed to improve information and support for MBC patients.
Epidemiology and Risk Factors
Relative to the disease in women, MBC is rare, accounting for less than 1% of all cases of breast carcinoma with an incidence of 1 in 100,000 men in Europe [1]. The National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program noted that the incidence of MBC increased by 26% from 1973 to 1998 [2]. MBC rates vary widely between countries. In central African countries, a substantially higher proportion of MBC cases (6–15%) has been reported. The reasons of this geographic variability remain unclear. The relatively high rates have been attributed to endemic infectious diseases causing liver damage, leading to hyperestrogenism [3]. By contrast, the annual incidence of MBC in Japan is less than 5 per million, in parallel with the lower than average incidence of female breast cancer in that country. Mortality rates in Europe remained stable between 1982 and 1992 [4]. The etiology of MBC remains as poorly understood as that of female breast cancer, but genetic disorders and an imbalance by various mechanisms in the estrogen-testosterone ratio are probably implicated.
Hormonal alterations due to testicular disease may be an important factor. There is an association with Klinefelter's syndrome which may account for approximately 3% of MBC. People with Klinefelter's syndrome – characterized by the addition of at least one X chromosome to the normal XY karyotype (47XXY) – have testicular dysgenesis, gynecomastia, low testosterone concentrations, and increased gonadotrophins. The risk of breast cancer in these individuals is 20–50 times higher than in 46XY men [5]. In addition, males who have had mumps orchitis, undescended testes or testicular injury are at increased risk of breast cancer due to androgen deficiency or excess estrogens. Cirrhosis of the liver, characterized by increased estrogen levels, may predispose to MBC. Exogenous estrogens have occasionally been associated with causing MBC. Cases of transsexuals developing breast cancer on exogenous estrogens have been reported, and some reports exist of breast cancer in men receiving estrogens for prostate cancer [3]. One study found that serum levels of estradiol and estrone were higher in MBC patients, but did not find any differences between hormone levels of MBC patients and matched controls [6]. Another risk factor, obesity, may act by affecting estrogen levels by peripheral aromatization of androgens. Thus, feminization genetically or by environmental exposure appears to increase risk. Obesity doubles the risk of developing MBC [7]. Environmental factors include increased risk for certain occupations, such as men employed in workplaces with chronic heat and radiation exposure and electromagnetic fields, which is supposed to suppress testicular function [7]. Other elements that have been considered as etiological factors include drugs, head trauma (by increasing prolactin production), local chest trauma, smoking, history of rapid weight gain, or amphetamine use [8].
A meta-analysis revealed that the risk was significantly increased in males with the following characteristics: never married, benign breast disease, gynecomastia, Jewish ancestry, or history of breast cancer in first-degree relatives [1]. However, most individuals of either gender who develop breast cancer have no apparent risk factor for the disease, and the majority of male patients have no detectable hormone imbalances. Several studies looking at gynecomastia in MBC have not shown a higher incidence of gynecomastia than population incidences. Histological data indicated that the incidence of gynecomastia in mastectomy specimens from MBC cases was 21% which is less than the incidence of 40–55% reported at autopsy of unselected cases. There is no convincing evidence to link gynecomastia with MBC [9].
Genetics
A family history of breast cancer, in man or woman, is certainly a risk factor. The BRCA2 gene shows evidence of linkage to breast cancer-prone families in whom MBC cases have occurred, and BRCA2 may prove a useful marker for identifying males who are at increased risk. BRCA1, unlike BRCA2, is not strongly associated with MBC. Sisters and daughters of MBC patients have a 2- to 3-fold increased risk of developing breast cancer [10]. A family history of breast cancer confers a relative risk of 3.98 [11], and 20% of men with breast cancer have a first-degree relative with the disease. 5–10% of female breast cancers are thought to result from autosomal dominant inheritance, particularly BRCA1 and, less frequently, BRCA2 mutations. For men, it is estimated to be between 4% and 40%. The risk increases with increasing numbers of first-degree relatives affected and with young age at diagnosis in affected relatives. Families in which breast cancer has occurred and at least 1 male has been affected, have been reported to have a 60–76% chance of carrying BRCA2 mutations [12]. MBC in patients with BRCA2 mutations tends to present at a younger age and may be associated with a poorer survival. Because of the prevalence of these mutations in MBC patients, genetic counseling and testing should be considered. Other genes have been investigated for a potential role in the etiology of MBC, but none have clearly been associated with an increased risk. Mutations in the androgen receptor gene, PTEN (Cowden's syndrome), and mismatch repair genes (hMLHl) have been reported in male patients with breast cancer [13, 14]. However, none of these genes have been demonstrated to have a causal association with MBC. Further studies are needed to elucidate their role.
Clinical Features
The most common symptom of MBC is a painless lump which is centrally located in 70–90%. Because of the predominant central location and its anatomical neighborhood, nipple involvement is an early event, with retraction in 9%, discharge in 6%, and ulceration in 6% [3]. Paget's disease is rare, being the presenting feature in only 1%. Up to 20% of patients notice changes in the areola [15]. Less commonly, patients have symptoms of breast tenderness, swelling, nipple itching, and symptoms of distant metastasis. Axillary adenopathy suspicious for metastasis is clinically detected in 40–55% of patients at the time of presentation [16, 17]. The rarity of MBC and therefore the low index of suspicion of both patients and their doctors have been largely responsible for delay in diagnosis. Recent series show a mean delay of 6–10 months. This delay leads to progression of disease before presentation. More than 40% of men with breast cancer present with stage III/IV disease. Therefore, men have a worse prognosis than women because of the extent of the disease at the time of diagnosis [18]. As a result of the paucity of breast tissue in males, the tumornode-metastasis system may not be appropriate in MBC because of early chest wall spread.
Histopathology
The predominant histological type of disease is invasive ductal which forms more than 90% of all male breast tumors. Much rarer tumor types include invasive lobular tumors, papillomas, and medullary lesions. Lobular carcinoma, previously not thought to occur in men due to lack of lobular differentiation, has now been reported in a few cases. Ductal carcinoma in situ of the male breast is slightly more common, representing 10% of all cases. However, due to the delayed diagnosis and the lack of sufficient screening programs, the proportion of preinvasive tumor stages is low [19, 20]. In large series reporting tumor grade, 12–20% were grade I, 54–58% grade II, and 17–33% of breast cancers were grade III which is comparable with findings from female breast cancer [21].
Male breast cancers have high rates of hormone receptor expression. Approximately 90% of male breast cancers express the ER, and 81% express the progesterone receptor. As in female breast cancer, the rates of hormone receptor positivity increase with increasing patient age [22]. In women, the age-specific rates of ER-negative breast cancer stop increasing at age 50, but rates of ER-positive cancer continue to increase after age 50. In men, the pattern of ER-negative tumors was less clear because of the small number of cases, but the incidence rate appeared to increase at all ages, although at a slower rate than ER-positive tumors. The propensity for receptor positivity in men may be a result of the low level of circulating estrogen in the male system. This is similar to that observed in postmenopausal women, which has led to the speculation that receptor-positive breast cancers may result from aberrant steroid receptor upregulation and a consequent constitutive activation of downstream targets.
In contrast, the HER2 proto-oncogene is less likely to be overexpressed in cancers of the male breast. Recent series found that approximately 11% of male breast cancers are both HER2 gene amplified and protein overexpressed [23]. Due to the small number of cases, the prognostic significance of HER2 is not sufficiently evaluated. The role of the androgen receptor in MBC is unclear. The reported rates of androgen receptor expression have ranged from 34 to 95%, but this receptor has not been associated with breast cancer prognosis [24].
Diagnosis
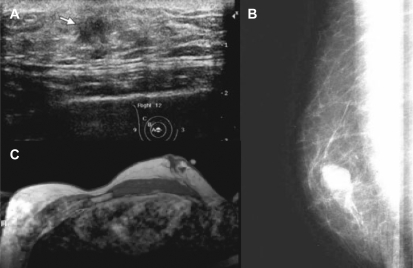
In most cases diagnosis is made by triple assessment: Clinical assessment, mammography or ultrasonography, and core biopsy (fig. 1). Ultrasound-guided core biopsy is preferred because it enables a definitive diagnosis of invasive breast cancer to be made. Mammography in men with breast lesions is an effective diagnostic technique with a sensitivity of 92% and a specificity of 90%. Microcalcifications are less common in male than in female breast cancer and have irregular spiculated edges [25,26,27]. However, depending on size and volume of the male breast, the diagnostic value of mammography could be limited. On ultrasonography, invasive cancers are typically solid, and all solid lesions require biopsy. Ultrasonography can also be a useful adjunct and provide information regarding nodal involvement. The diagnostic impact of magnetic resonance imaging (MRI) for the male breast is not sufficiently examined. In small studies, the MRI features of benign breast diseases and breast cancers in male patients seemed to be comparable to those seen in female patients, and therefore its diagnostic use should be limited to corresponding applications [28].
Fig. 1.
Diagnostics for male breast cancer: A ultrasound, B mammography, C magnetic resonance mammography (images were kindly provided by the department of radiology, University Hospital Bonn, Germany, Chair: Professor H. Schild).
No structured psychosocial studies have been reported in MBC but it is likely that there is substantial undiagnosed and untreated morbidity in such cases. Interviews of male patients indicated several major areas of concern: delay in diagnosis, shock, stigma, causal factors related to body image, and lack of informational and emotional support. Focus group discussions have suggested a particular need for sex-specific information on MBC together with provision of support [29].
Staging and Prognosis
Staging of MBC is the same as that in women using the TNM system [30]. The most important prognostic indicators are stage at diagnosis and lymph node status. MBC most commonly develops in the central retro-areolar/nipple area which has the greatest lymphatic drainage in the breast. Therefore, it is postulated that MBC has a propensity for nodal metastasis. Estimates for overall 5-year survival are around 40–65% but when grouped by stage at presentation, 5-year survival is 75–100% for stage I disease, 50–80% for stage II disease, and falling to 30–60% for stage III disease [31].
A large study of 335 male patients found that if nodal status is used to compare male and female breast cancer, then the prognosis is the same in both [32]. The less favorable outcome in men is due to a more advanced stage at presentation and, importantly, the later average age at presentation in men resulting in higher levels of comorbidity. Moreover, there is evidence that adjuvant hormonal and chemotherapy are less often used in male than in female breast cancer which for chemotherapy may relate to the later age at presentation. It is known that, with the consideration of prognostic factors such as tumor size, grade, and axillary nodal involvement, the overall survival rate for men and women is nearly identical [33].
Therapy
Local therapy for breast cancer is generally similar in men and women. Most men are treated with modified radical mastectomy with axillary lymph node dissection or sentinel node biopsy [34]. Retrospective studies indicate that the outcome for men is equally good when treated with breast conserving surgery at least in early stages [35]. Axillary lymph node dissection is clearly an important component of therapy, because men in whom lymph node dissection was omitted have a poorer outcome [36]. Several small studies have been published that have established the feasibility of sentinel node biopsy in the male patient with breast cancer. No difference was found concerning local relapse and overall survival compared to findings in female breast cancer [37,38,39,40].
Radiotherapy in men treated with mastectomy is performed more often than in women perhaps because of the more advanced tumor stages and/or nipple or skin involvement. As in female breast cancer, the standard radiation dose is 50 Gy in 25 fractions. Radiation therapy does appear to be effective in preventing local recurrences in male patients [34]. Most of the common sites of relapse in MBC are located at the chest wall and supraclavicular areas which should be considered for planning adjuvant radiotherapy [41]. Retrospective studies that investigated the effects of radiotherapy in MBC, reported 5-year locoregional recurrence rates ranging from 3 to 20% [31, 42,43,44,45]. Unless there is massive axillary node involvement, regional node irradiation should avoid treating the whole axilla and be limited to the upper axillary nodes and internal mammary chain.
Most available information on adjuvant therapies for MBC comes from small retrospective series [46,47,48,49]. Because of the lack of prospective data on men treated with adjuvant chemotherapy and hormonal therapy, the optimal treatment for men with breast carcinoma remains unknown. Data from small series suggest that male patients with breast carcinoma will benefit from adjuvant systemic therapy, and that the magnitude of the benefit is similar to that seen in women. Chemotherapy regimens for adjuvant and metastatic therapy should be selected in accordance to the established guidelines for breast cancer in women. Given the established benefit of chemotherapy in women and the suggestive evidence in men, most clinicians use similar guidelines for adjuvant chemotherapy in male and female patients. Donegan et al. [50] noted that adjuvant chemotherapy improves prognosis in MBC with axillary metastasis. Yildirim et al. [36] showed that the use of any adjuvant chemotherapy was associated with improved 5-year survival in 121 men with breast cancer.
Recent data for breast cancer in women suggest that particularly patients with HER2-positive status benefit from anthracycline-containing regimens [51]. Due to a HER2-positive rate of approximately 11% in MBC, indication for adjuvant treatment with anthracyclines should be restricted. Other reports indicated a trend towards improved survival or survival benefit in node-positive and ER-negative patient subsets. The role of preoperative chemotherapy in MBC is not well-defined and should be restricted to locally advanced or inflammatory diseases.
The role of taxanes or dose-dense chemotherapy in MBC has not been adequately established [36, 50]. No information exists about the role of adjuvant trastuzumab in MBC. Only 1 case was published demonstrating response to treatment with trastuzumab in the metastatic setting [52]. However, since very substantial therapeutic benefit is observed after adjuvant trastuzumab in women with HER2-positive breast cancer, adjuvant trastuzumab should be considered for men with high risk, HER2-positive breast cancer similar to indications in women.
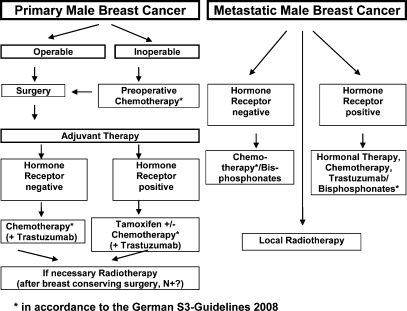
In the metastatic setting, chemotherapy can offer substantial palliation to men with hormone-refractory breast cancer. There are no data about the activity or tolerance of bevacizumab or other angiogenesis inhibitors. Adjuvant hormonal therapy is widely accepted in MBC patients with hormone receptor-positive tumors. Due to the fact that more than 90% of breast cancers in men are hormone receptor-positive, an endocrine responsiveness is currently accepted. Retrospective series that have evaluated tamoxifen in the adjuvant setting have shown a reduced risk of breast cancer recurrence and death [48, 49, 53]. The therapy algorithm for adjuvant treatment of MBC is summarized in figure 2.
Fig. 2.
Therapy algorithm for treatment of male breast cancer.
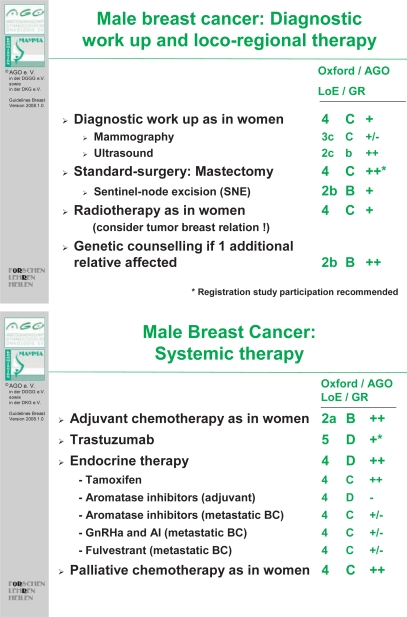
The role of adjuvant aromatase inhibitors in MBC has not been determined, but in view of the success of adjuvant gonadal ablation and adjuvant aromatase inhibitors in women with breast cancer, additional studies using these interventions for MBC are warranted. Preclinical data suggest that aromatase inhibitors may be less effective in men. In animal models, chronic administration of aromatase inhibitors was associated with significant increases in follicle-stimulating hormone (FSH) and testosterone, and no change in levels of estradiol. A possible explanation for this finding is that the increase in FSH and testosterone could lead to an increase in substrate for aromatization via a feedback loop. Currently, the Southwest Oncology Group has activated S0511 which evaluates the combination of anastrozole and goserelin in men with hormone receptor-positive metastatic breast cancer. In addition to assessing response rates and survival, this protocol will measure hormone levels, and will bank tissue and serum samples [54, 55]. A combination of aromatase inhibitor or tamoxifen and an ER-degrading agent (fulvestrant) could also be appropriate. Fulvestrant has no effect on estrogen synthesis, but it destroys ERs in the tumor cell, thus blocking further proliferation and inhibiting tumor growth. Treatment guidelines from the ‘Arbeitsgemeneinschaft für Gynäkologische Onkologie (AGO)’ for MBC with the available level of evidence are summarized in figure 3.
Fig. 3.
Treatment guidelines from the Arbeitsgemeinschaft für Gynäkologische Onkologie (AGO), 2008 (ago-online.de).
Perspectives
There is a substantial need to obtain more detailed information about the etiology, pathogenesis, and clinical aspects of MBC as well as therapeutic options. Support systems for men with breast cancer are rudimentary, and need more resources and research at a national rather than a local level. Future studies with a focus on disease biology are crucial to advance the understanding of MBC and to optimize the care of all male patients. However, due to the rare incidence, prospective studies are lacking and will be difficult to initiate. National protocols for both information and support for men diagnosed with breast cancer are needed. Building a specially focused tumor registry and including MBC patients in a prospective design will allow investigators to obtain reliable information related to the therapeutic response and biology of MBC.
The German Breast Group (GBG) in cooperation with the ‘Arbeitsgemeinschaft für Gynäkologische Onkologie’ (AGO) are planning a register study to document the clinical features and pathobiology of MBC. The study will be performed in a retrospective and prospective design to consider data about treatment modalities, toxicity, and patient survival. Furthermore, tumor tissue and blood samples will be collected for a concomitant translational program. The study will be initiated in the second half of 2008. Further details on the study protocol will be presented on the GBG/AGO homepages [56].
References
- 1.Sasco AJ, Lowenfels AB, Pasker-de Jong P. Review article: epidemiology of male breast cancer. A meta-analysis of published case-control studies and discussion of selected aetiological factors. Int J Cancer. 1993;53:538–549. doi: 10.1002/ijc.2910530403. [DOI] [PubMed] [Google Scholar]
- 2.Nahleh Z, Girnius S. Male breast cancer: a gender issue. Nat Clin Pract Oncol. 2006;3:428–437. doi: 10.1038/ncponc0564. [DOI] [PubMed] [Google Scholar]
- 3.Agrawal A, Ayantunde AA, Rampaul R, Robertson JF. Male breast cancer: a review of clinical management. Breast Cancer Res Treat. 2007;103:11–21. doi: 10.1007/s10549-006-9356-z. [DOI] [PubMed] [Google Scholar]
- 4.La Vecchia C, Levi F, Lucchini F. Descriptive epidemiology of male breast cancer in Europe. Int J Cancer. 1992;51:62–66. doi: 10.1002/ijc.2910510113. [DOI] [PubMed] [Google Scholar]
- 5.Hsing AW, McLaughlin JK, Cocco P, Co Chien HT, Fraumeni JF., Jr Risk factors for male breast cancer (United States) Cancer Causes Control. 1998;9:269–275. doi: 10.1023/a:1008869003012. [DOI] [PubMed] [Google Scholar]
- 6.Scheike O, Svenstrup B, Frandsen VA. Male breast cancer. II. Metabolism of oestradiol-17 beta in men with breast cancer. J Steroid Biochem. 1973;4:489–501. doi: 10.1016/0022-4731(73)90064-2. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JR, Moysich KB, Swede H. Epidemiology of male breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:20–26. [PubMed] [Google Scholar]
- 8.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367:595–604. doi: 10.1016/S0140-6736(06)68226-3. [DOI] [PubMed] [Google Scholar]
- 9.Wells S. Gynecomastia and male breast cancer. Radiol Technol. 1994;65:331–333. [PubMed] [Google Scholar]
- 10.Basham VM, Lipscombe JM, Ward JM, Gayther SA, Ponder BA, Easton DF, Pharoah PD. BRCA1 and BRCA2 mutations in a population-based study of male breast cancer. Breast Cancer Res. 2002;4:R2. doi: 10.1186/bcr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblatt KA, Thomas DB, McTiernan A, Austin MA, Stalsberg H, Stemhagen A, Thompson WD, Curnen MG, Satariano W, Austin DF. Breast cancer in men: aspects of familial aggregation. J Natl Cancer Inst. 1991;83:849–854. doi: 10.1093/jnci/83.12.849. [DOI] [PubMed] [Google Scholar]
- 12.Ford D, Easton DF, Stratton M, Narod S, Goldgar D, Devilee P, Bishop DT, Weber B, Lenoir G, Chang-Claude J, Sobol H, Teare MD, Struewing J, Arason A, Scherneck S, Peto J, Rebbeck TR, Tonin P, Neuhausen S, Barkardottir R, Eyfjord J, Lynch H, Ponder BA, Gayther SA, Zelada-Hedman M. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudmundsdottir K, Thorlacius S, Jonasson JG, Sigfusson BF, Tryggvadottir L, Eyfjord JE. CYP17 promoter polymorphism and breast cancer risk in males and females in relation to BRCA2 status. Br J Cancer. 2003;88:933–936. doi: 10.1038/sj.bjc.6600839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohayon T, Gal I, Baruch RG, Szabo C, Friedman E. CHEK2∗1100delC and male breast cancer risk in Israel. Int J Cancer. 2004;108:479–480. doi: 10.1002/ijc.11603. [DOI] [PubMed] [Google Scholar]
- 15.Scheike O. Male breast cancer. 5. Clinical manifestations in 257 cases in Denmark. Br J Cancer. 1973;28:552–561. doi: 10.1038/bjc.1973.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller KS, Rosen PP, Schottenfeld D, Ashikari R, Kinne DW. Male breast cancer: a clinicopathologic study of 97 cases. Ann Surg. 1978;188:60–65. doi: 10.1097/00000658-197807000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgen PI, Wong GY, Vlamis V, Potter C, Hoffmann B, Kinne DW, Osborne MP, McKinnon WM. Current management of male breast cancer. A review of 104 cases. Ann Surg. 1992;215:451–457. doi: 10.1097/00000658-199205000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano SH. A review of the diagnosis and management of male breast cancer. Oncologist. 2005;10:471–479. doi: 10.1634/theoncologist.10-7-471. [DOI] [PubMed] [Google Scholar]
- 19.Clark JL, Nguyen PL, Jaszcz WB, Jatoi A, Niehans GA. Prognostic variables in male breast cancer. Am Surg. 2000;66:502–511. [PubMed] [Google Scholar]
- 20.Cutuli B, Dilhuydy JM, De Lafontan B, Berlie J, Lacroze M, Lesaunier F, Graic Y, Tortochaux J, Resbeut M, Lesimple T, Gamelin E, Campana F, Reme-Saumon M, Moncho-Bernier V, Cuilliere JC, Marchal C, G De Gislain, N'Guyen TD, Teissier E, Velten M. Ductal carcinoma in situ of the male breast. Analysis of 31 cases. Eur J Cancer. 1997;33:35–38. doi: 10.1016/s0959-8049(96)00436-4. [DOI] [PubMed] [Google Scholar]
- 21.Ciatto S, Iossa A, Bonardi R, Pacini P. Male breast carcinoma: review of a multicenter series of 150 cases. Coordinating Center and Writing Committee of FONCAM (National Task Force for Breast Cancer), Italy. Tumori. 1990;76:555–558. doi: 10.1177/030089169007600608. [DOI] [PubMed] [Google Scholar]
- 22.Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi G. Breast carcinoma in men: a population-based study. Cancer. 2004;101:51–57. doi: 10.1002/cncr.20312. [DOI] [PubMed] [Google Scholar]
- 23.Rudlowski C, Friedrichs N, Faridi A, Fuzesi L, Moll R, Bastert G, Rath W, Buttner R. Her-2/neu gene amplification and protein expression in primary male breast cancer. Breast Cancer Res Treat. 2004;84:215–223. doi: 10.1023/B:BREA.0000019953.92921.7e. [DOI] [PubMed] [Google Scholar]
- 24.Kidwai N, Gong Y, Sun X, Deshpande CG, Yeldandi AV, Rao MS, Badve S. Expression of androgen receptor and prostate-specific antigen in male breast carcinoma. Breast Cancer Res. 2004;6:R18–R23. doi: 10.1186/bcr733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Appelbaum AH, Evans GF, Levy KR, Amirkhan RH, Schumpert TD. Mammographic appearances of male breast disease. Radiographics. 1999;19:559–568. doi: 10.1148/radiographics.19.3.g99ma01559. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Chantra PK, Larsen LH, Barton P, Rohitopakarn M, Zhu EQ, Bassett LW. Imaging characteristics of malignant lesions of the male breast. Radiographics. 2006;26:993–1006. doi: 10.1148/rg.264055116. [DOI] [PubMed] [Google Scholar]
- 27.Newman J. Breast cancer in men and mammography of the male breast. Radiol Technol. 1997;69:17–28. [PubMed] [Google Scholar]
- 28.Morakkabati-Spitz N, Schild HH, Leutner CC, von Falkenhausen M, Lutterbey G, Kuhl CK. Dynamic contrast-enhanced breast MR imaging in men: preliminary results. Radiology. 2006;238:438–445. doi: 10.1148/radiol.2382041312. [DOI] [PubMed] [Google Scholar]
- 29.Peate I. Caring for men with breast cancer: causes, symptoms and treatment. Br J Nurs. 2001;10:975–981. doi: 10.12968/bjon.2001.10.15.5262. [DOI] [PubMed] [Google Scholar]
- 30.Hecht JR, Winchester DJ. Male breast cancer. Am J Clin Pathol. 1994;102:S25–S30. [PubMed] [Google Scholar]
- 31.Cutuli B, Lacroze M, Dilhuydy JM, Velten M, De Lafontan B, Marchal C, Resbeut M, Graic Y, Campana F, Moncho-Bernier V. Male breast cancer: results of the treatments and prognostic factors in 397 cases. Eur J Cancer. 1995;31A:1960–1964. doi: 10.1016/0959-8049(95)00366-5. [DOI] [PubMed] [Google Scholar]
- 32.Guinee VF, Olsson H, Moller T, Shallenberger RC, van den Blink JW, Peter Z, Durand M, Dische S, Cleton FJ, Zewuster R. The prognosis of breast cancer in males. A report of 335 cases. Cancer. 1993;71:154–161. doi: 10.1002/1097-0142(19930101)71:1<154::aid-cncr2820710125>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Willsher PC, Leach IH, Ellis IO, Bourke JB, Blamey RW, Robertson JF. A comparison outcome of male breast cancer with female breast cancer. Am J Surg. 1997;173:185–188. doi: 10.1016/s0002-9610(97)89592-x. [DOI] [PubMed] [Google Scholar]
- 34.Scott-Conner CE, Jochimsen PR, Menck HR, Winchester DJ. An analysis of male and female breast cancer treatment and survival among demographically identical pairs of patients. Surgery. 1999;126:775–780. [PubMed] [Google Scholar]
- 35.Gough DB, Donohue JH, Evans MM, Pernicone PJ, Wold LE, Naessens JM, O'Brien PC. A 50-year experience of male breast cancer: is outcome changing? Surg Oncol. 1993;2:325–333. doi: 10.1016/0960-7404(93)90063-5. [DOI] [PubMed] [Google Scholar]
- 36.Yildirim E, Berberoglu U. Male breast cancer: a 22-year experience. Eur J Surg Oncol. 1998;24:548–552. doi: 10.1016/s0748-7983(98)93608-3. [DOI] [PubMed] [Google Scholar]
- 37.Albo D, Ames FC, Hunt KK, Ross MI, Singletary SE, Kuerer HM. Evaluation of lymph node status in male breast cancer patients: a role for sentinel lymph node biopsy. Breast Cancer Res Treat. 2003;77:9–14. doi: 10.1023/a:1021173902253. [DOI] [PubMed] [Google Scholar]
- 38.Cimmino VM, Degnim AC, Sabel MS, Diehl KM, Newman LA, Chang AE. Efficacy of sentinel lymph node biopsy in male breast cancer. J Surg Oncol. 2004;86:74–77. doi: 10.1002/jso.20045. [DOI] [PubMed] [Google Scholar]
- 39.Goyal A, Horgan K, Kissin M, Yiangou C, Sibbering M, Lansdown M, Newcombe RG, Mansel RE, Chetty U, Ell P, Fallowfield L, Kissin M. Sentinel lymph node biopsy in male breast cancer patients. Eur J Surg Oncol. 2004;30:480–483. doi: 10.1016/j.ejso.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Port ER, Fey JV, Cody HS, III, Borgen PI. Sentinel lymph node biopsy in patients with male breast carcinoma. Cancer. 2001;91:319–323. [PubMed] [Google Scholar]
- 41.Perkins G, Middleton LP, Garcia SM. Male breast carcinoma: outcomes and predictors of local-regional failure in patients treated without radiation therapy. Breast Cancer Res Treat. 2002;76:121. [Google Scholar]
- 42.Stranzl H, Mayer R, Quehenberger F, Prettenhofer U, Willfurth P, Stoger H, Hackl A. Adjuvant radiotherapy in male breast cancer. Radiother Oncol. 1999;53:29–35. doi: 10.1016/s0167-8140(99)00122-x. [DOI] [PubMed] [Google Scholar]
- 43.Chakravarthy A, Kim CR. Post-mastectomy radiation in male breast cancer. Radiother Oncol. 2002;65:99–103. doi: 10.1016/s0167-8140(02)00210-4. [DOI] [PubMed] [Google Scholar]
- 44.Schuchardt U, Seegenschmiedt MH, Kirschner MJ, Renner H, Sauer R. Role of percutaneous radiotherapy in male breast carcinoma. Strahlenther Onkol. 1996;172:369–375. [PubMed] [Google Scholar]
- 45.Zabel A, Milker-Zabel S, Zuna I, Wannenmacher M, Debus J. External beam radiotherapy in the treatment of male breast carcinoma: patterns of failure in a single institute experience. Tumori. 2005;91:151–155. doi: 10.1177/030089160509100209. [DOI] [PubMed] [Google Scholar]
- 46.Giordano SH, Perkins GH, Broglio K, Garcia SG, Middleton LP, Buzdar AU, Hortobagyi GN. Adjuvant systemic therapy for male breast carcinoma. Cancer. 2005;104:2359–2364. doi: 10.1002/cncr.21526. [DOI] [PubMed] [Google Scholar]
- 47.Bagley CS, Wesley MN, Young RC, Lippman ME. Adjuvant chemotherapy in males with cancer of the breast. Am J Clin Oncol. 1987;10:55–60. doi: 10.1097/00000421-198702000-00013. [DOI] [PubMed] [Google Scholar]
- 48.Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85:629–639. doi: 10.1002/(sici)1097-0142(19990201)85:3<629::aid-cncr13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro G, Swindell R, Harris M, Banerjee S, Cramer A. A review of management of the male breast carcinoma based on an analysis of 420 treated cases. Breast. 1996;5:141–146. [Google Scholar]
- 50.Donegan WL, Redlich PN, Lang PJ, Gall MT. Carcinoma of the breast in males: a multiinstitutional survey. Cancer. 1998;83:498–509. doi: 10.1002/(sici)1097-0142(19980801)83:3<498::aid-cncr19>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 51.Pritchard KI, Messersmith H, Elavathil L, Trudeau M, O'Malley F, Dhesy-Thind B. HER-2 and topoisomerase II as predictors of response to chemotherapy. J Clin Oncol. 2008;26:736–744. doi: 10.1200/JCO.2007.15.4716. [DOI] [PubMed] [Google Scholar]
- 52.Rudlowski C, Rath W, Becker AJ, Wiestler OD, Buttner R. Trastuzumab and breast cancer. N Engl J Med. 2001;345:997–998. [PubMed] [Google Scholar]
- 53.Ribeiro G, Swindell R. Adjuvant tamoxifen for male breast cancer (MBC) Br J Cancer. 1992;65:252–254. doi: 10.1038/bjc.1992.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giordano SH, Hortobagyi GN. Leuprolide acetate plus aromatase inhibition for male breast cancer. J Clin Oncol. 2006;24:e42–e43. doi: 10.1200/JCO.2006.07.2397. [DOI] [PubMed] [Google Scholar]
- 55.Nahleh ZA. Hormonal therapy for male breast cancer: a different approach for a different disease. Cancer Treat Rev. 2006;32:101–105. doi: 10.1016/j.ctrv.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Harbeck N, Gerber B: Breast Cancer in Specific Situations. Arbeitsgemeinschaft für Gynäkologische Onkologie (AGO), 2008. ago-online.de.