Part of the sympathetic flight-or-fight response is an increase in intracellular cyclic AMP (cAMP) that raises the rate of action potential generation in the heart pacemaker, the sinoatrial (SA) node (Robinson and Siegelbaum, 2003). The increased firing rate in the SA node increases the heart rate and cardiac output necessary to deliver more oxygen and nutrients to the muscles for the flight-or-fight response. How cAMP increases the rate of the SA node has been debated for a long time. Recently, there has been a hot debate between groups promoting a model in which hyperpolarization-activated cyclic nucleotide–gated (HCN) channels are the main regulator of the heart rate, and groups promoting a model in which intracellular Ca2+ oscillations affecting currents through Na+/Ca2+ exchangers are the main regulator of the heart rate (Lakatta and DiFrancesco, 2009). There are several candidate targets for the increased cAMP in the SA node during sympathetic stimulation (Fig. 1). For example, cAMP-activated protein kinases (PKA) have been shown to phosphorylate ryanodine receptors and other Ca2+-cycling proteins in the SA node, leading to altered calcium cycles and increased diastolic currents through Na+/Ca2+ exchangers (Lakatta et al., 2010). In contrast, HCN channels have earlier been shown to be directly modulated by cAMP in the SA node by a mechanism that is independent of PKA activation (DiFrancesco and Tortora, 1991). In this issue, Liao et al. show that HCN channels in the SA node are also modulated by PKA, and suggest that this PKA modulation of HCN channels might contribute to the increased firing rate in the SA node in response to sympathetic stimulation. In addition, the result that PKA directly phosphorylates HCN channels might further reduce some of the confusion in the field regarding how sympathetic stimulation affects heart rate, as PKA and HCN channels were previously thought to act through different pathways in the SA node to increase the heart rate.
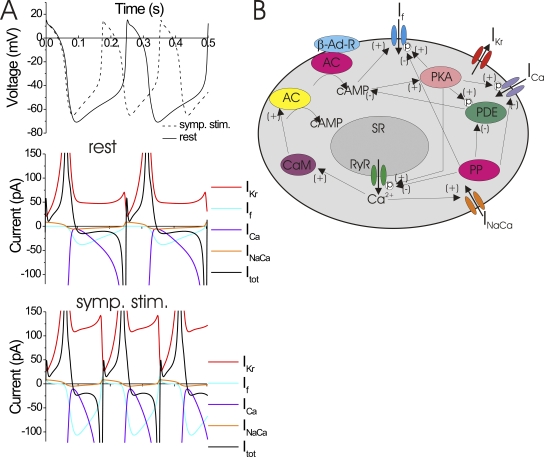
Figure 1.
Sympathetic stimulation of heart rate in the SA node. (A) Simulated voltage (top) and currents (bottom) in an SA node cell during rest (solid lines) and sympathetic stimulation (dashed lines). Sympathetic stimulation was simulated by shifting the voltage dependence of HCN channels to more depolarized potentials while all other parameters were the same (model from Elinder et al., 2006, based on a rabbit SA node model from Zhang et al., 2000). Only the currents through HCN (If), T-type and L-type Ca (combined to one ICa current for display), Herg channels (IKr), and Na+/Ca2+ exchangers (INaCa) are shown. In this model, sympathetic stimulation increases the inward HCN currents, thereby increasing the rate of depolarization and the action potential firing (dashed lines). Notice that the net (total) current (Itot) is very small during diastole (a few pA), and that it is the sum of many different currents of larger size with opposite polarity. The small net current during diastole is one of the reasons for the difficulty in clearly assigning one channel as generating the currents that drive the pacemaking in the SA node. (B) SA node cell with some of the possible pathways for the effect of sympathetic stimulation on pacemaking (Lakatta et al., 2010). β-adrenergic–stimulated G protein–coupled receptors (β-Ad-R) activate adenylyl cyclases (red AC) that produce cAMP. Rising concentrations of cAMP activate PKA that directly modulates HCN channels and other protein targets. PKA phosphorylates ryanodine receptors (RyR) and SERCA Ca2+ pumps that then alters the Ca2+ cycling from the sarcoplasmic reticulum (SR) and alters cytosolic Ca2+ oscillations. Increased Ca2+ levels also activate basal adenylyl cyclase (yellow AC) through calmodulin (CaM). Increased cytosolic Ca2+ also increases the inward currents through Na+/Ca2+ exchangers (INaCa). Protein phosphatases (PP) dephosphorylate HCN channels, as well as inhibit phosphodiesterases (PDE) that otherwise would break down cAMP.
HCN channels are also called pacemaker channels due to their presumed effect on the cardiac heart rate (Robinson and Siegelbaum, 2003). There are four members in the mammalian HCN channel family: HCN1–4 (Robinson and Siegelbaum, 2003). HCN4 is the most prevalent in the SA node, but HCN1 and HCN2 have also been found in the SA node in some species (Moroni et al., 2001). HCN channels belong to the superfamily of voltage-gated ion channels. HCN channels are tetrameric channels with six transmembrane domains per subunit (Robinson and Siegelbaum, 2003; Zagotta et al., 2003). Like other voltage-gated channels in this superfamily, HCN channels have many positive charges in the fourth transmembrane domain that functions as the voltage sensor (Männikkö et al., 2002). Most members in the superfamily are activated by depolarization. In contrast, HCN channels are activated by hyperpolarization due to some unknown mechanism (Männikkö et al., 2002). The currents through HCN channels in the SA node are called If. The subscript “f” stands for “funny” because of its funny characteristics (for example, being activated by hyperpolarization instead of depolarization). Under physiological conditions, HCN channels are activated by hyperpolarizations in the normal range of diastolic membrane potentials. So during the hyperpolarized diastolic membrane potential between action potentials, HCN channels will increase their open probability. Open HCN channels conduct both Na+ and K+, but at these negative membrane potentials they mainly let Na+ into the cells. In the model proposed by proponents for the HCN pacemaker role in SA node, it is this inward Na+ current through HCN channels (together with Ca2+ inflow through voltage-activated Ca2+ channels, inward currents through Na+/Ca2+ exchangers, and decaying outward K+ currents) at the diastolic membrane potentials that is thought to depolarize the pacemaker cells to threshold to trigger the next action potential and to generate a rhythmic firing pacemaker (Fig. 1 A).
In addition to the increased HCN channel activation by hyperpolarization, HCN channels also show an increased activation in response to the binding of cAMP to a domain in the C terminus called the cyclic nucleotide–binding domain (CNBD) (DiFrancesco and Tortora, 1991; Wainger et al., 2001; Zagotta et al., 2003) (Fig. 2). Increases in the cytosolic cAMP concentration have been shown to shift the voltage dependence of HCN channel activation to more depolarized potentials, thereby increasing the number of open HCN channels during the diastolic hyperpolarization (DiFrancesco and Tortora, 1991; Gauss et al., 1998; Ludwig et al., 1998; Santoro et al., 1998) (Fig. 2). This will lead to an increased current through HCN channels, which causes a faster depolarization during diastole. The increased rate of depolarization means that the threshold for firing will be reached earlier and the firing rate will increase (Fig. 1 A). This direct effect of cAMP was shown earlier to not require the activity of PKA, and the reversible modulation of HCN channels by sympathetic/parasympathetic stimulations was persistent in the presence of phosphatase inhibitors (Accili et al., 1997). It was therefore thought that cAMP affects HCN channels directly and that PKA was not involved in HCN channel regulation. Here, Liao et al. show evidence that PKA phosphorylation also shifts the voltage dependence of activation of HCN channels to more depolarized potentials, which also would increase the firing rate in the SA node.
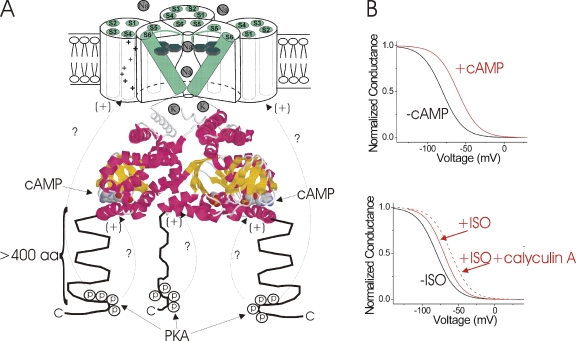
Figure 2.
Separate, but interacting PKA phosphorylation and cAMP-binding domains? (A) Model of HCN4 channel (only three out of the four subunits are shown for clarity). The six transmembrane domains (S1–S6) are shown in green, with the positively charged S4 functioning as the voltage sensor. The cAMP-binding domain is based on the crystal structure of the C terminus of HCN2 (Zagotta et al., 2003), showing the cAMP-binding pocket. The >400–amino acid long C-terminal region after the CNBD is shown as unstructured, and the region with PKA sites that modulates HCN channel function is indicated with arrows. How phosphorylation of these distal C-terminal sites affect the voltage dependence is not clear. This region could interact with other cytosolic domains, such as the CNBD or the transmembrane domains, and the voltage sensor directly (arrows with question marks). (B) Schematic drawings showing the effects of cAMP and phosphatase inhibition on the voltage dependence of activation of HCN channels. Direct application of cAMP in the absence of ATP reversibly shifts the voltage dependence of activation of If in excised patches (DiFrancesco and Tortora, 1991). The phosphatase inhibitor calyculin A increased the ISO-induced shift in the voltage dependence of activation of If in SA node, suggesting some synergy between the effect of phosphatase activity and cAMP on HCN channels (Accili et al., 1997).
First, Liao et al. showed that the PKA inhibitor PKI blocks the effect of the β-adrenergic agonist isoprenaline (ISO) to modulate the If currents in the SA node, suggesting that PKA modulates HCN channels. To directly test this, the authors then tried the effect of PKA on heterologously expressed HCN4 channels, the most highly expressed HCN channel in the SA node. PKA shifted the voltage dependence of activation of HCN4 channels to more depolarized potentials in Chinese hamster ovary cells. In contrast, one earlier study reported that PKA activation increased the HCN current amplitude without shifting the voltage activation (Accili et al., 1997). In this issue, Liao et al. show that activation of PKA shifts the voltage dependence of activation of HCN channels in the SA node by 11 mV. One word of caution when comparing different studies of HCN channels is that HCN channels sometimes exhibit rundown, which affects both the voltage dependence and current amplitude (Robinson and Siegelbaum, 2003). The mechanism of this rundown is not completely clear, but it could be the result of PIP2 breakdown or washout of other cytosolic components during whole cell or excised patch recordings (Pian et al., 2006). So, the small PKA-induced shifts in the voltage dependence of HCN channels might be masked by rundown (which shifts the voltage dependence in the opposite direction than PKA) in some preparations. In the present study, Liao et al. furthermore showed direct phosphorylation of HCN4 channels by making GST fusion protein with HCN4 channels and measuring phosphorylation of HCN4 residues directly in vitro. By mass spectroscopy, they identified up to 13 different PKA phosphorylated sites. By a deletion and mutagenesis strategy, they could pinpoint a region in the distal C terminus containing four PKA sites that was necessary for the PKA modulation of HCN4 channels (Fig. 2 A). This PKA modulation region is distinct from the earlier found CNBD that binds cAMP (Fig. 2 A). From these studies, it seems clear that PKA can phosphorylate HCN4 and that PKA phosphorylation alters the voltage dependence of HCN4 activation. In addition, these studies show that PKA is necessary for the sympathetic stimulation of HCN channels in the SA node.
But, what about the earlier direct cAMP effects on HCN channels that was supposedly independent of PKA activation? These direct effects of cAMP were previously shown to be due to direct binding of cAMP to the CNBD in another region of the C terminus of HCN channels (Fig. 2 A). In addition, these direct effects of cAMP persisted in the presence of phosphatase inhibitors and did not require PKA. Therefore, PKA phosphorylation and cAMP binding are separate phenomena. However, one intriguing finding in the present study is that there is no effect of the β-adrenergic agonist ISO on If in the SA node in the presence of the PKA inhibitor PKI. ISO should raise the cAMP level even in the presence of PKI; therefore, one would expect that ISO in the presence of PKI would still modulate HCN channels through the direct binding of cAMP to HCN channels (Fig. 1 B).
One possibility is that there is some direct synergy between direct cAMP modulation of HCN channels and PKA phosphorylation of HCN channels. In an earlier study, there was indeed evidence for a synergy between inhibition of phosphatase activity and direct cAMP binding in modulating HCN channels in the SA node (Accili et al., 1997) (Fig. 2 B). In that study, phosphatase inhibitors increased the ISO-induced voltage shift of HCN channels (Accili et al., 1997). Another possibility is that the cAMP levels are already saturating in the presence of PKI, or that not enough adenylyl cyclase is activated by ISO in the presence of the PKA inhibitor PKI. PKA has been proposed to regulate a calcium-dependent adenylyl cyclase through a mechanism involving PKA phosphorylation of ryanodine receptors and increases in internal Ca2+ levels (Lakatta et al., 2010) (Fig. 1 B). So, in the presence of PKI, ISO might not generate enough cAMP in SA node cells to significantly raise the level of cAMP. However, the finding in this issue by Liao et al. that PKA and cAMP both directly affect HCN channels leaves open the possibility that there is a direct synergy between cAMP and PKA modulation of HCN channels. That would suggest that the direct cAMP-binding effect requires a phosphorylated HCN channel and that cAMP has no effect on dephosphorylated HCN channels. This would explain why ISO retains its (reversible) effects in the presence of phosphatase inhibitors (which could render all HCN channels phosphorylated) (Accili et al., 1997), but has no effect in the presence of PKA inhibitors (which could render all HCN channels dephosphorylated). It could also explain the synergy found between phosphatase inhibitors and cAMP levels in earlier studies (Accili et al., 1997) if not all HCN channels are phosphorylated before the application of the phosphatase inhibitor. Earlier, this synergy was hypothesized to be at the level of cAMP concentrations, using a model where endogenously active phosphatases would reduce cAMP levels by activating, for example, phosphodiesterases (Accili et al., 1997). Finally, this synergy might resolve some of the controversy in the field regarding the mechanism of action of the sympathetic stimulation in the SA node. Inhibitors of PKA have previously been used in some studies to discriminate between sympathetic effects mediated by HCN channels and effects mediated by other Ca2+-handling proteins (such as ryanodine receptors and SERCA pumps) in the SA node (Lakatta and DiFrancesco, 2009) (Fig. 1 B). The possibility that even the direct effect of cAMP on HCN channels requires PKA phosphorylation will make studies based on the use of PKA inhibitors less able to discriminate between different mechanisms of action for sympathetic stimulation in the SA node. It is clear that more research is necessary to determine the mechanism of this synergy and to detangle the different mechanism of pacemaking in the SA node. The finding that HCN channels are modulated by PKA is clearly helping to explain a part of the molecular mechanism for modulating pacemaking in the SA node. But more pieces to the puzzle are needed to fully understand how pacemaking is regulated in the SA node.
Acknowledgments
I thank Dr. F. Elinder for the simulations for Fig. 1 A and Drs. F. Elinder and G. Dahl for comments on the manuscript.
The work in my laboratory is funded by National Institutes of Health (grant HL095920) and the American Heart Association (grant 10GRNT4150069).
References
- Accili E.A., Redaelli G., DiFrancesco D. 1997. Differential control of the hyperpolarization-activated current (i(f)) by cAMP gating and phosphatase inhibition in rabbit sino-atrial node myocytes. J. Physiol. 500:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. 1991. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 351:145–147 10.1038/351145a0 [DOI] [PubMed] [Google Scholar]
- Elinder F., Mannikko R., Pandey S., Larsson H.P. 2006. Mode shifts in the voltage gating of the mouse and human HCN2 and HCN4 channels. J. Physiol. 575:417–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss R., Seifert R., Kaupp U.B. 1998. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 393:583–587 10.1038/31248 [DOI] [PubMed] [Google Scholar]
- Lakatta E.G., DiFrancesco D. 2009. What keeps us ticking: a funny current, a calcium clock, or both? J. Mol. Cell. Cardiol. 47:157–170 10.1016/j.yjmcc.2009.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E.G., Maltsev V.A., Vinogradova T.M. 2010. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 106:659–673 10.1161/CIRCRESAHA.109.206078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Zong X., Jeglitsch M., Hofmann F., Biel M. 1998. A family of hyperpolarization-activated mammalian cation channels. Nature. 393:587–591 10.1038/31255 [DOI] [PubMed] [Google Scholar]
- Männikkö R., Elinder F., Larsson H.P. 2002. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature. 419:837–841 10.1038/nature01038 [DOI] [PubMed] [Google Scholar]
- Moroni A., Gorza L., Beltrame M., Gravante B., Vaccari T., Bianchi M.E., Altomare C., Longhi R., Heurteaux C., Vitadello M., et al. 2001. Hyperpolarization-activated cyclic nucleotide-gated channel 1 is a molecular determinant of the cardiac pacemaker current I(f). J. Biol. Chem. 276:29233–29241 10.1074/jbc.M100830200 [DOI] [PubMed] [Google Scholar]
- Pian P., Bucchi A., Robinson R.B., Siegelbaum S.A. 2006. Regulation of gating and rundown of HCN hyperpolarization-activated channels by exogenous and endogenous PIP2. J. Gen. Physiol. 128:593–604 10.1085/jgp.200609648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R.B., Siegelbaum S.A. 2003. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu. Rev. Physiol. 65:453–480 10.1146/annurev.physiol.65.092101.142734 [DOI] [PubMed] [Google Scholar]
- Santoro B., Liu D.T., Yao H., Bartsch D., Kandel E.R., Siegelbaum S.A., Tibbs G.R. 1998. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 93:717–729 10.1016/S0092-8674(00)81434-8 [DOI] [PubMed] [Google Scholar]
- Wainger B.J., DeGennaro M., Santoro B., Siegelbaum S.A., Tibbs G.R. 2001. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 411:805–810 10.1038/35081088 [DOI] [PubMed] [Google Scholar]
- Zagotta W.N., Olivier N.B., Black K.D., Young E.C., Olson R., Gouaux E. 2003. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 425:200–205 10.1038/nature01922 [DOI] [PubMed] [Google Scholar]
- Zhang H., Holden A.V., Kodama I., Honjo H., Lei M., Varghese T., Boyett M.R. 2000. Mathematical models of action potentials in the periphery and center of the rabbit sinoatrial node. Am. J. Physiol. Heart Circ. Physiol. 279:H397–H421 [DOI] [PubMed] [Google Scholar]