Abstract
Cocaine is a highly addictive drug that exerts its effects by increasing the levels of released dopamine in the striatum, followed by stable changes in gene transcription, mRNA translation, and metabolism within medium spiny neurons in the striatum. The multiple changes in gene and protein expression associated with cocaine addiction suggest the existence of a mechanism that facilitates a coordinated cellular response to cocaine. Here, we provide evidence for a key role of miRNAs in cocaine addiction. We show that Argonaute 2 (Ago2), which plays an important role in miRNA generation and execution of miRNA-mediated gene silencing, is involved in regulation of cocaine addiction. Deficiency of Ago2 in dopamine 2 receptor (Drd2)–expressing neurons greatly reduces the motivation to self-administer cocaine in mice. We identified a distinct group of miRNAs that is specifically regulated by Ago2 in the striatum. Comparison of miRNAs affected by Ago2 deficiency with miRNAs that are enriched and/or up-regulated in Drd2-neurons in response to cocaine identified a set of miRNAs that are likely to play a role in cocaine addiction.
The ability of neurons to adapt to environmental signals and to memorize novel experiences determines the individual patterns of human and animal behavior. In turn, the individual differences in environmental adaptation and behavior depend on changes in gene expression. The fine-tuning of gene expression could be achieved at different levels, including regulation by microRNAs (miRNAs). miRNAs are small, noncoding RNAs that have the ability to negatively control messenger RNA (mRNA) levels or mRNA translation of their target genes (He and Hannon, 2004). These miRNA functions are executed by the multiprotein RNA-induced silencing complex (RISC). At the core of RISC are the miRNA-generating enzyme Dicer (Hutvágner et al., 2001) and the Argonaute (Ago) proteins that bind miRNAs (Lingel et al., 2003; Song et al., 2003; Yan et al., 2003) and mediate miRNA-dependent mRNA decay or translational suppression (Hammond et al., 2001). The human and mouse genome carry four independent Ago genes that encode Ago1, 2, 3, and 4. The Ago proteins are structurally and functionally similar. However, only Ago2 is able to control mRNA expression by “slicing” of mRNAs that are fully complementary to specific miRNAs (Liu et al., 2004; Song et al., 2004).
In addition to its mRNA-regulating function, Ago2 contributes to the generation of miRNAs from their precursors (Diederichs and Haber, 2007; O’Carroll et al., 2007). Importantly, this poorly understood function of Ago2 appears to be selective and affects only a fraction of miRNAs within each cell (O’Carroll et al., 2007).
The ability of Ago2 to contribute to the generation of specific miRNAs provides an opportunity to relate physiological changes caused by Ago2 deficiency to expression changes of specific miRNAs in various cell types, including neurons.
There are >300 miRNAs expressed in the adult mouse brain (Lagos-Quintana et al., 2002; Krichevsky et al., 2003; Miska et al., 2004). It is very likely that many of these miRNAs have highly specialized physiological functions. However, identification of these functions in a systematic fashion, e.g., by ablating individual miRNAs, is challenging. We argue that, by narrowing the number of miRNAs that may contribute to specific physiological processes in the brain, one can gain access to individual miRNAs that may play a key role in specialized adult brain function. Similar approaches have led to the discovery of the essential and unique role of the Ago2-dependent miRNA miR-451 in erythropoiesis (O’Carroll et al., 2007; Cheloufi et al., 2010; Rasmussen et al., 2010).
We used Ago2 deficiency to address the potential involvement of miRNAs in cocaine addiction. The addictive potential of cocaine is related largely to its ability to increase the neurotransmitter dopamine in the striatum. Increased levels of released dopamine alter the activity of the dopamine 1 and dopamine 2 receptor (Drd1 and Drd2) expressing medium spiny neurons (MSNs) in the striatum (Girault and Greengard, 2004). Administration of cocaine leads to rapid activation and/or up-regulation of genes such as Creb1, Fos, FosB, Jun, and Cdk5r1, all of which play a role in the formation of cocaine addiction (Bibb et al., 2001; McClung and Nestler, 2003; Chao and Nestler, 2004; Heiman et al., 2008). Recent data suggest that regulation of expression levels of these genes by cocaine may involve miRNAs (Chandrasekar and Dreyer, 2009).
Here, we show that ablation of Ago2, specifically in mouse Drd2-expressing neurons, alleviates cocaine addiction as judged by a reduction in the animal’s motivation to self-administer the drug. Reduced animal dependence on cocaine is associated with selective down-regulation of a specific set of miRNAs in the Ago2-deficient striatum. Some of these Ago2-dependent miRNAs are present within the pool of the Drd2-enriched and cocaine-induced miRNAs. We speculate that this particular group of Ago2-dependent, induced by cocaine and Drd2-enriched (ADICD) miRNAs plays an important role in cocaine addiction. In silico analysis of the putative targets of ADICD miRNAs combined with 3′ UTR luciferase reporter assays highlights their potential involvement in regulation of genes important for the development of cocaine addiction, such as Cdk5r1 and the transcription factors FosB and Mef2d. Our studies provide evidence for a key role of Ago2 in cocaine addiction and suggest ADICD miRNAs as potential regulators of addictive behavior.
RESULTS AND DISCUSSION
Ago2 is dispensable for neuronal survival
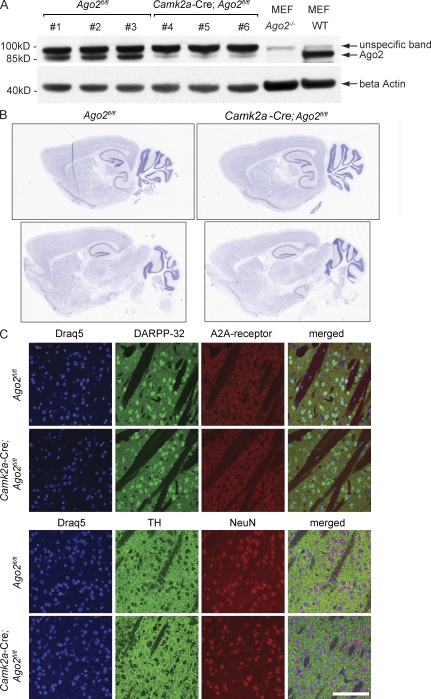
Deficiency in Ago2 in specialized adult brain areas has been achieved by Cre-mediated recombination of the loxP-modified Ago2 alleles (O’Carroll et al., 2007). The expression of Cre recombinase in postnatal forebrain neurons or in Drd2-expressing neurons has been driven by the neuron-specific Camk2a (Casanova et al., 2001) or Drd2 promotors (Gong et al., 2007), respectively. Cre-mediated modification of the Ago2 gene resulted in the loss of the functional Ago2 alleles and the loss of Ago2 protein expression in the striatum (Fig. 1 A).
Figure 1.
Ago2 is dispensable for brain organization and neuronal maintenance in the adult brain. (A) Conditional inactivation of Ago2 in adult neurons. Expression of Ago2 was analyzed by Western blotting of the striatal protein lysates derived from three Ago2fl/fl mice (lanes 1–3), and three Camk2a-Cre; Ago2fl/fl mice (lanes 4–6). The lysates derived from Ago2−/− and WT mouse embryonic fibroblasts (MEF) were used as controls for the specificity of the anti-Ago2 antibodies. Equal protein concentration in the samples was controlled by β actin loading. (B and C) Ago2 deficiency in the forebrain does not affect brain morphology. (B) The overall brain morphology of 12-wk-old Camk2a-Cre; Ago2fl/fl and Ago2fl/fl control mice was analyzed using standard Nissl-stain (n = 3/genotype). Two representative images from sagittal brain sections of mice of both genotypes are shown. (C) Saggital brain sections of Ago2fl/fl and Camk2a-Cre; Ago2fl/fl mice are shown (n = 3/genotype). Striatal morphology was analyzed by visualizing Drd1 and Drd2 MSNs (top) using antibodies against the MSN-enriched protein DARPP-32 (green) and the Drd2-MSN–specific adenosine 2A (A2A) receptor (red); the dopaminergic terminals (bottom) were visualized by expression of the dopamine-producing enzyme TH (green), and neuronal nuclei in the striatum were visualized by using the neuron-specific marker NeuN (red). The nucleus of each cell was visualized using Draq5 DNA staining (blue). Bar, 100 µm.
Deficiency in Ago2 in neurons in the striatum or Drd2-expressing neurons specifically has no impact on the overall morphology of these well-defined brain regions (Fig. 1 B and not depicted). Furthermore, as judged by the expression of MSN markers such as dopamine- and cAMP-regulated phosphoprotein of Mr 32 kD (DARPP-32), the adenosine 2A (A2A) receptor, and the dopaminergic neuron marker tyrosine hydroxylase (TH), deficiency in Ago2 has no apparent impact on the number, morphology, or distribution of MSNs or their dopaminergic innervation in the striatum in vivo (Fig. 1 C). The apparent absence of a role for Ago2 in neuronal survival is in sharp contrast to the deleterious impact of Dicer deficiency in postnatal neurons (Kim et al., 2007; Schaefer et al., 2007). Similar to Dicer-deficient cerebellar Purkinje cells (Schaefer et al., 2007), postnatal forebrain-specific loss of Dicer (Camk2a-Cre; Dicerfl/fl; Fig. S1, A–C; Davis et al., 2008), as well as deficiency of Dicer in Drd2-expressing neurons (Drd2-Cre; Dicerfl/fl; Fig. S1, D and E), triggers neuronal loss and ensuing behavioral abnormalities, followed by the premature death of the animals (Fig. S1 and not depicted).
Drd2-specific ablation of Ago2 decreases the motivation to self-administer cocaine
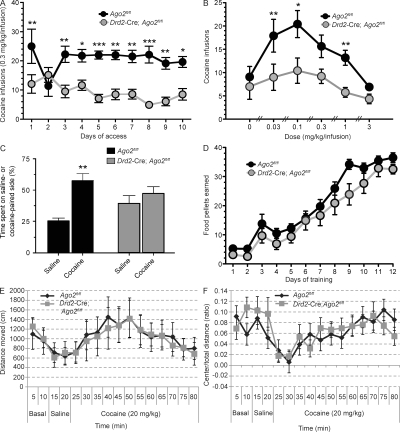
The majority of drugs that are abused by humans, including cocaine, are also self-administered by animals. Self-administration of drugs is generally considered to be the most direct measure of the reinforcing properties of a drug, and recent studies have shown that mice robustly self-administer cocaine (Caine et al., 2002). To investigate the role of Ago2 and Ago2-dependent miRNAs in the development of cocaine addiction, we permitted Drd2-Cre; Ago2fl/fl and Ago2fl/fl control mice to self-administer cocaine (0.3 mg/kg/infusion) in their home cage environment. The number of cocaine infusions on each day over 10 consecutive days was recorded (Fig. 2 A). We found that ablation of Ago2 in Drd2-expressing neurons leads to a significant decrease of daily cocaine self-administration in these mice as compared with controls (Fig. 2 A; F = 24.1, P < 0.001). Furthermore, characterization of the cocaine dose–response (D–R) curve in each group of mice revealed a profound downward shift of the D–R curve in the mutant mice (Fig. 2 B; F = 9.1, P = 0.01). Downward shifts in the cocaine D–R curve are considered as a decrease in the motivation to consume cocaine (Piazza et al., 2000), supporting the notion that the loss of Ago2 in Drd2-expressing neurons attenuates the reinforcing effects and thereby leads to a decreased motivation to consume the drug. The reduction of the reinforcing effects of cocaine as the underlying mechanism for the decreased cocaine intake in those mice is further supported by an almost complete lack of preference for an environment that the animal was trained to associate with the administration of cocaine. Whereas control mice preferentially seek the environment in which they receive cocaine over an environment that has been associated with administration of saline, the conditional place preference to cocaine is abolished in Drd2-Cre; Ago2fl/fl mice (Fig. 2 C; F = 7.76, P = 0.009).
Figure 2.
Ago2 controls the motivation to self-administer cocaine in mice. (A) Ago2 controls cocaine intake in mice. Cocaine self-administration was measured for Drd2-Cre; Ago2fl/fl (n = 12) and Ago2fl/fl control littermates (n = 10). Graph represents the number of cocaine infusions (0.3 mg/kg/infusion) earned under a FR5TO20 s schedule of reinforcement over 10 consecutive days. (B) Ago2 deficiency in Drd2-expressing MSNs results in a downward shift in the cocaine D–R curve. The unit dose of cocaine available for self-administration was varied according to a within-subjects Latin square design (0.3 mg/kg/infusion), and the effects of responding to each dose of cocaine were assessed in Drd2-Cre; Ago2fl/fl mice (n = 12) and Ago2fl/fl controls (n = 10). Data are presented as number of cocaine infusions earned on the last day of access to each dose of cocaine. (C) Loss of Ago2 in Drd2 neurons abolishes conditional place preference to cocaine. Drd2-Cre; Ago2fl/fl (n = 11) and Ago2fl/fl control (n = 5) mice were tested for their preference for an environment associated with cocaine versus saline injections and the percentage of time (%) spent in the saline or cocaine-paired side is shown. (D) Ago2 does not control food reward. The response for food reward was assessed in Drd2-Cre; Ago2fl/fl (n = 12) and Ago2fl/fl (n = 10) littermate controls. Mice responded for food under a FR5TO20 s schedule of reinforcement. No differences in operant performance were detected between genotypes. (E and F) Ago2 does not control acute cocaine-induced locomotor activity (E) or cocaine-induced anxiety (F). Open field analysis was used to measure locomotor activity and thigmotaxis in Drd2-Cre; Ago2fl/fl and Ago2fl/fl control mice (n = 8/genotype) at basal level (10 min), followed by 100 µl i.p. saline injection (for 10 min), followed by acute cocaine injection (20 mg/kg i.p., 60 min). The bar graphs represent the total distance moved (in centimeters; E) and the ratio of total distance moved in the center versus total arena (F). Statistical analysis was performed using a two-way ANOVA (A–D) and a repeated measure two-way ANOVA (E and F). Data are shown as means; error bars represent ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Importantly, the absence of Ago2 in Drd2 MSNs in the striatum has no apparent effect on various basal behaviors that could confound the motivation to self-administer cocaine (Fig. 2, D–F, and Fig. S2). The substantial reduction of cocaine self-administration reflects neither impaired learning of the self-administration task nor a broader alteration of the reward response, as Drd2-Cre; Ago2fl/fl mice show no difference in responding to food rewards (Fig. 2 D) or in their preference for a mild natural reward such as sucrose (Fig. S2 F). Moreover, the Drd2-Cre; Ago2fl/fl and Ago2fl/fl control mice display a similar increase in locomotor activity and anxiety after an acute injection of cocaine (Fig. 2, E and F), demonstrating that loss of Ago2 in Drd2-expressing neurons does not affect the sensitivity to the anxiogenic or locomotion-inducing effects of acute cocaine, but differentially regulates the motivation to consume the drug. Finally, Drd2-Cre; Ago2fl/fl mice show normal basal locomotor and exploratory activity and display no signs of anxiety or depression-like behavior (Fig. S2, A–E). Collectively, these findings indicate that Ago2 and the Ago2-regulated miRNAs in Drd2-expressing neurons play a crucial role in the development of cocaine addiction by regulating the motivation to self-administer the drug.
Ago2 regulates miRNA expression in the striatum
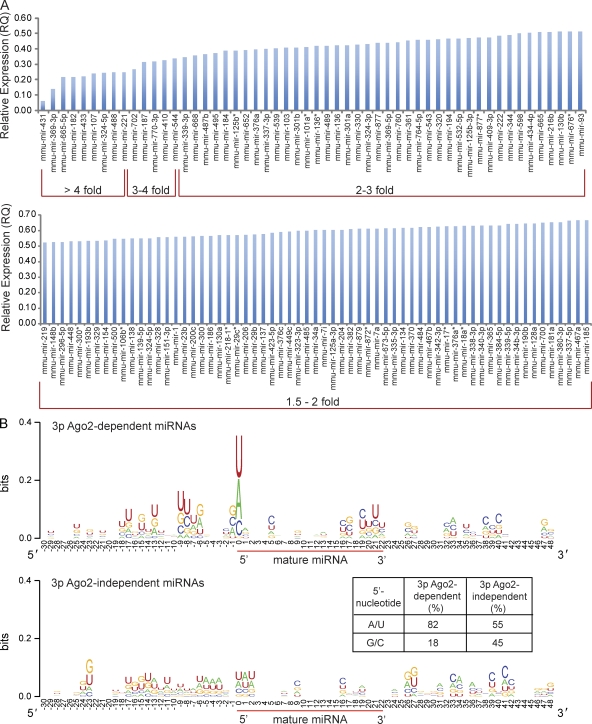
In agreement with previously published data (O’Carroll et al., 2007), we found that Ago2, but none of the other Ago proteins, all of which are ubiquitously expressed in the brain (Fig. S3 A), contributes to the generation of a large fraction of mature miRNAs. Although the expression levels of over 94% of miRNAs in the striatum of Ago1−/−, Ago3−/−, and Ago4−/− mice were identical to their expression in the wild-type striatum, ∼25% of the analyzed miRNAs (94/376 or 119/517) in the Ago2 deficient striatum showed a reduction in their expression levels (Fig. S3, B and C). More than 85% of the down-regulated miRNAs changed their expression between 1.5- and 3-fold (relative quantification [RQ] value = 0.67–0.34; Fig. 3 A). The magnitude of reduction was similar to that found previously in fibroblasts and erythroid cells, which revealed a relatively broad, albeit modest, impact of Ago2 deficiency on expression levels of affected miRNAs (Diederichs and Haber, 2007; O’Carroll et al., 2007). The list of down-regulated miRNAs is topped by 15 miRNAs that are profoundly affected (3–17-fold reduced expression) in the absence of Ago2 in postnatal neurons (Fig. 3 A).
Figure 3.
Ago2 regulates the expression of miRNAs in striatal neurons. (A) Ago2-dependent miRNAs. The bar graph displays miRNAs that are down-regulated in the striatum of Camk2a-Cre; Ago2fl/fl mice as compared with Ago2fl/fl control mice (n = 6/genotype). The miRNAs are ordered according to the degree of their down-regulation. (B) Ago2-dependent 3p miRNAs are enriched for A/U at their 5′ position. Logo diagrams display all 3p Ago2-dependent and 3p Ago2-independent miRNAs in the striatum of Camk2a-Cre; Ago2fl/fl mice as compared with Ago2fl/fl control mice (n = 6/genotype). The height of each row corresponds to the sequence conservation at specific positions, and the height of individual letters within a row corresponds to the prevalence of that specific nucleotide (nt). Sequences ranging from 30 nt upstream to 48 nt after the first nucleotide of the mature miRNA sequence are depicted. The 5′-nt of the mature miRNA sequence is located at position 0 (indicated by the beginning of the red bar). 3p miRNAs down-regulated in the striatum of Camk2a-Cre; Ago2fl/fl (top) show higher occurrence of A or U at the 5′-nt of the mature miRNA than 3p miRNAs that are unaffected by the loss of Ago2 (bottom). The table displays the percentage of Ago2-dependent (left) and Ago2–independent (right) 3p miRNAs that have an A/U versus a C/G at their 5′-position.
Dependence of miRNA generation on Ago2 appears to be biased toward miRNAs originating from the 3p versus the 5p hairpin arm of the premiRNAs. Over 70% of Ago2-dependent miRNAs that are reduced greater than twofold in the Ago2-deficient striatum are generated from the 3p arm of their corresponding premiRNA (35 out of 49). Furthermore, Ago2-dependent, but not Ago2-independent, 3p miRNAs display a clear enrichment of miRNAs that have either an A or U as the 5′ nucleotide in the mature miRNA sequence (Fig. 3 B). Recent data showed that miRNAs with either an A or U, rather than a G or C, at the 5′ position of the guide strand of the mature sequence bind with up to 30-fold higher affinity to Ago2 (Frank et al., 2010). We argue that the high affinity of 5′-A/U–rich miRNAs to Ago2 might contribute to their dependence on Ago2, whereas miRNAs that display a low affinity to Ago2 might be “rescued” by other Ago proteins.
The majority of miRNAs affected by Ago2 deficiency in the striatum are expressed in Drd2 neurons, where they are associated with Ago2. Using mice that express transgenic FLAG-tagged Ago2 selectively in Drd2 cells (Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2; unpublished data), we found that out of 376 well annotated miRNAs, 230 miRNAs are associated with the Ago2–RISC complex in Drd2 neurons. (Fig. S4, A and B). The spectrum of Ago2-bound miRNAs in Drd2-expressing neurons includes almost the entire spectrum of Ago2-dependent miRNAs in the striatum of Camk2a-Cre; Ago2fl/fl mice (Fig. S4 B). These results further support the suggested correlation between miRNA down-regulation in response to Ago2 deficiency and miRNA association with the Ago2–RISC complex. As an example, the profound reduction of miR-431 (which has an U at the 5′ position of the mature miRNA sequence) in Ago2-deficient neurons (RQ = 0.06; Fig. 3 A) correlates with the great enrichment of miR-431 in the Drd2-cell expressed Ago2–RISC complex (Fig. S4 B).
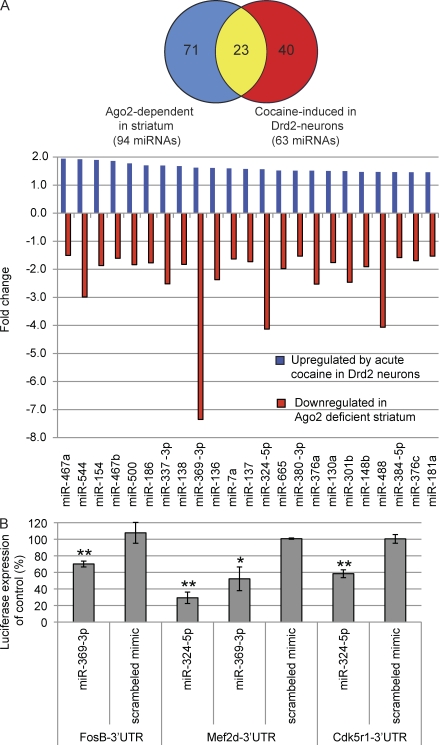
Acute administration of cocaine leads to an increase of 63 miRNAs in Drd2-neurons of wild-type mice (fold change > 1.45, P < 0.05; Fig. 4 A). Of those 63 cocaine-induced miRNAs, 23 miRNAs are Ago2 dependent (Fig. 4 A).
Figure 4.
Ago2-dependent miRNAs in Drd2 neurons are induced by cocaine and regulate genes involved in cocaine addiction. (A) Ago2-regulated miRNAs in Drd2 neurons are induced by cocaine. The Venn diagram shows the number of miRNAs that are down-regulated (>1.5 fold) in the striatum of Camk2a-Cre; Ago2fl/fl mice (blue; n = 6/genotype) and miRNAs that are up-regulated (>1.45-fold) in Drd2 neurons in response to acute cocaine as compared with saline injections (red; n = 3/genotype). The overlapping miRNAs are shown in yellow. The bar graph displays all 23 miRNAs that are up-regulated in response to acute cocaine in Drd2 neurons (blue bars) and down-regulated in the absence of Ago2 in the striatum (red bars). RQ of each miRNA was calculated using the ΔΔCt method; a maximum standard deviation for the ΔCt values of 0.8 was used as a cut-off for each miRNA. (B) Ago2-dependent, induced by cocaine and Drd2-enriched (ADICD) miRNAs regulate genes important in cocaine addiction. The bar graph displays the average percentage of luciferase expression of FosB-, Mef2d-, and Cdk5r1- 3′ UTR-luciferase vectors in the presence of miR-369-3p, miR-324-5p, or a scrambled control as compared with their respective vector controls. Each sample was analyzed in triplicates, and three independent experiments were performed. Statistical analysis was performed using Student’s t test. Error bars indicate + SEM. *, P < 0.05; **, P < 0.01.
Ago2-dependent and cocaine-induced miRNAs in Drd2 cells can control expression of genes essential for cocaine addiction
We argue that the contribution of Ago2 to cocaine addiction depends on the function of the Ago2-dependent miRNAs. In the Drd2 cells, these miRNAs are split between two groups. The first group includes Ago2-dependent miRNAs that are greatly enriched in Drd2-neurons (Fig. S4 B). The second group includes all Ago2-dependent miRNAs that are enriched in Drd2 neurons and are induced by acute cocaine administration (Fig. 4 A). We speculate that those ADICD miRNAs play a role in cocaine addiction. The 23 identified ADICD miRNAs (Fig. 4 A) include miRNAs that have a potential to control genes that play a crucial role in the development of addiction. As an example, in silico predicted targets of ADICD miRNAs (using TargetScan 5.0) include cocaine-induced neuronal plasticity genes such as Creb1 (targeted by 18/23 Ago2-dependent miRNAs), Fos (3/23), FosB (6/23), Fosl2 (12/23), Mef2 (3/23), Cdk5r1 (6/23), and Bdnf (11/23), all of which play an important role in regulation of cocaine addiction (Bibb et al., 2001; McClung and Nestler, 2003; Chao and Nestler, 2004; Heiman et al., 2008; Pulipparacharuvil et al., 2008). Using a standard 3′ UTR luciferase assay, in which binding of miRNAs to specific target gene 3′ UTRs is followed by a reduction of luciferase expression, we were able to confirm several of the in silico predicted ADICD miRNAs gene targets. As an example, ADICD miRNAs miR-369-3p and miR-324-5p can both regulate the expression of the neuronal plasticity genes Mef2d and FosB (miR-369-3p) or Cdk5r1 (miR-324-5p; Fig. 4 B).
In summary, our studies suggest that the important role of Ago2 in cocaine addiction involves the presence or generation of specific miRNAs that contribute to the stable changes in gene expression patterns that define neuronal cell plasticity and ultimately regulate the motivation to consume cocaine. We speculate that some or potentially all of the 23 identified ADICD miRNAs contribute to cocaine addiction. The current findings provide a foundation for future studies that will identify the contribution of individual ADICD miRNAs to various facets of addictive behavior in mice and possibly humans.
MATERIALS AND METHODS
Generation of neuron-specific Dicer and Ago2 mutant and FLAG-Ago2 mice
Dicerfl/fl, Ago2fl/fl, Ago1−/−, Ago3−/−, and Ago4−/− mice were provided by A. Tarakhovsky (The Rockefeller University, New York, NY). Dicerfl/fl (Yi et al., 2006) and Ago2fl/fl (O’Carroll et al., 2007) mice were bred to Camk2a-Cre (provided by G. Schuetz, German Cancer Research Center, Heidelberg, Germany; Casanova et al., 2001) and Drd2-Cre (Gong et al., 2007) and Drd2-eGFP (ER44 line from GENSAT; Heintz, 2004) mice (provided by N. Heintz, The Rockefeller University, New York, NY) to generate Camk2a-Cre; Dicerfl/fl, Drd2-Cre; Drd2-eGFP; Dicerfl/fl, Camk2a-Cre; Ago2fl/fl, Drd2-Cre; Ago2fl/fl, and Dicerfl/fl and Ago2fl/fl control mice. Genotyping was performed as previously described (Casanova et al., 2001; Heintz, 2004; Yi et al., 2006; Gong et al., 2007; O’Carroll et al., 2007). Transgenic mice expressing a Cre-inducible FLAG-HA2-tagged Ago2 under the control of the Rosa26 promoter were generated as previously described (Sasaki et al., 2006; the STOP-eGFP-ROSA26TV vector was a gift from K. Rajewsky, Harvard Medical School, Boston, MA). Rosa-Stopfl/fl-Flag-Ago2 mice were bred to Drd2-Cre mice to generate mice that express FLAG-HA2-Ago2 only in Drd2 cells (Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2). All mice were backcrossed to the C57BL/6 background for >5 generations. Mice were housed under standard laboratory conditions at the Rockefeller University Comparative Bioscience Center. Protocols were approved by the Rockefeller University Institutional Animal Care and Use Committee.
Brain morphology analysis
Brains of 16-d-old Camk2a-Cre; Dicerfl/fl, 8- and 16-wk-old Drd2-Cre; Drd2-eGFP; Dicerfl/fl, and 12-wk-old Camk2a-Cre; Ago2fl/fl mice and their sex- and age-matched littermate controls were perfused and processed as previously described (Schaefer et al., 2007). For IHC, sections were incubated with anti-GFP (1:5,000; Abcam) and anti-TH (1:500; Millipore) antibodies and visualized by the avidin–biotin–peroxidase complex method as described (Schaefer et al., 2007). DARPP-32 (1:1,000; Novus Biologicals), NeuN (1:1,000; Millipore), TH (1:500; Millipore), and A2A (1:100; Millipore) were visualized using immunofluorescence analysis (Alexa Fluor 546/488–labeled goat anti–mouse/anti–rabbit IgGs [H+L] dilution 1:500; Invitrogen). Draq5 (Biostatus Limited) was used to counterstain the nucleus and sections were mounted using Prolong Gold antifade (Invitrogen). Sagittal brain sections of 16-d-old Camk2a-Cre; Dicerfl/fl and 12-wk-old Camk2a-Cre; Ago2fl/fl and their respective littermate controls were visualized using standard Nissl-stain (FD NeuroTechnologies). All brain sections were visualized on a LSM510 confocal microscope (Carl Zeiss, Inc.) using 10× and 40× (oil) magnifications. Eight-bit (512X512) images were acquired using LSM software and scale bars were added using ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/ij).
Taqman real-time RT-PCR analysis
Total RNA was extracted from the striatum of 12-wk-old Camk2a-Cre; Ago2fl/fl, Ago1−/−, Ago3−/−, and Ago4−/− (O’Carroll et al., 2007) mice and their age- and sex-matched littermate controls (n = 2–6/genotype), and mRNA quantity and quality were assessed as described previously (Schaefer et al., 2009).
The purified RNA samples were assayed by qRT-PCR for relative gene expression using predesigned Taqman gene expression assays from Applied Biosystems, as recommended by the manufacture. The following TaqMan gene expression assays were used: AGO1 (Mm00462977_m1), AGO2 (Mm00838341_m1), AGO3 (Mm01188531_mH), AGO4 (Mm01188520_m1), and GAPDH (Mm99999915_g1). Relative expression (ΔCt) and quantification (RQ = 2−ΔΔC) for each mRNA were calculated using RQ Manager Software, and the ΔΔCt method as suggested. Calculation of standard deviation (SDΔΨt = [SDtarget2+SDref2]1/2) and error bars (RQ1 = 2^[−{ΔΔCt + SDΔΔCt}], and RQ2 = 2^[−{ΔΔCt − SDΔΔCt}]) was performed according to Applied Biosystems technical literature part number 4371095 Rev B.
FLAG-Ago2–associated RNA immunoprecipitation from Drd2-neurons
The striata of transgenic mice expressing a FLAG-HA2-tagged Ago2 only in Drd2 cells (Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2) were dissected, and Ago2 cross-linking immunoprecipitation (CLIP) experiments were performed as previously described (Chi et al., 2009), with some minor modifications: 30 µl magnetic beads precoupled to the M2 FLAG antibody (M8823; Sigma-Aldrich) have been used for each immunoprecipitate (IP; 2 striata/IP) after preblocking the beads with 2% BSA, 1% t-RNAs, and 1% salmon sperm for 1.5 h at 4°C. Immunoprecipitated RNA has been de–cross-linked and purified for miRNA qRT-PCR analysis as previously described (Chi et al., 2009) described. Enrichment of individual miRNAs in Drd2 neurons were analyzed by comparison of Ago2-associated miRNA expression values between Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2 and Rosa-Stopfl/fl-Flag-Ago2 control IPs (n = 4) using TaqMan qRT-PCR analysis. miRNAs that showed a greater than twofold enrichment (P < 0.05) over control IP in the Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2 IP were considered enriched in Drd2 neurons. For identification of Ago2-associated miRNAs that are induced by cocaine in Drd2 neurons, Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2 mice were injected with 20 mg/kg cocaine or saline (n = 3/genotype) and sacrificed 2 h later. MiRNAs that changed expression >1.45-fold (SD < 0.8) in acute cocaine versus saline-treated samples were considered as induced by acute cocaine in Drd2 cells. TargetScan Mouse 5.1 (www.targetscan.org) was used for mouse gene target predictions for ADICD miRNAs.
Western blot analysis of immunoprecipitated FLAG-Ago2, IP input, and unbound fraction of Drd2-Cre; Rosa-Stopfl/fl-Flag-Ago2 and Rosa-Stopfl/fl-Flag-Ago2 control mice was performed using NuPage 4–12% Bis-Tris Gels, MES running buffer, and SeeBlue Plus2 prestained standard (all Invitrogen) following the advanced ECL protocol (GE Healthcare) using Ago2 (ab32381; rabbit; 1:1,000; Abcam) and Flag (ab1162; rabbit; 1:1,000; Abcam) antibodies. Membranes were stained before antibody incubation for 5 min in Ponceau solution to control for equal protein loading.
miRNA analysis
Total RNA or Ago2-immunoprecipitated RNA was analyzed using TaqMan Rodent qRT-PCR analysis (Megaplex MicroRNA Card A and B; Applied Biosciences). 10–90 ng of RNA for each sample was reverse transcribed and amplified following the ABI Megaplex protocol as recommended (part number 4399721 Rev. B). Relative expression and RQ for each miRNA were calculated using RQ Manager Software and the ΔΔCt method, as recommended. Mouse miRNAs that changed >1.5-fold in the mutant samples relative to control were assigned as up- or down-regulated (RQ ≥ 1.5 or RQ ≤ 0.667, respectively). Calculation of SD was performed according to Applied Biosciences technical literature as follows: SDΔCt = (SDtarget2 + SDref2)1/2. A maximum SD of 0.8 was allowed for ΔCt values for all TaqMan Rodent MicroRNA Card applications.
79 nt-long sequences of all 3p miRNAs affected by the loss of Ago2 in the striatum of Camk2a-Cre; Ago2fl/fl mice were used to generate Logos using WebLogo 2.8.2 (http://weblogo.berkeley.edu/) and compared with all Ago2-independent 3p miRNAs.
Target validation
Standard 3′ UTR luciferase assays were performed to validate in silico target predictions. 200 ng FosB, Mef2d, or Cdk5r1 3′ UTR-luciferase vectors (GeneCopoeia) were cotransfected with 20 pmol miRIDIAN mimics (Thermo Fisher Scientific) into N2a cells using lipofectamine 2000 (Invitrogen). 24 h after transfection, cells were lysed and assayed for firefly and renilla luciferase expression using the Dual-Luciferase Reporter Assay kit (Promega). Following the Promega kit protocol, Victor3V (Wallac 1420) Plate Reader (Perkin Elmer) was used to read the absorbance. Each analysis has been done in triplicates, and three independent experiments have been performed.
Behavioral analysis
All behavioral tests were performed on 8–24-wk-old mutant mice and their respective age and sex matched littermate controls between 9:00 a.m. and 5:00 p.m. Experiments were conducted by an experimenter blind to the genotypes of the mice. GraphPad Prism version 4.03 for Windows (GraphPad Software) was used for statistical analysis of the data. All procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Cocaine self-administration experiments were conducted at The Scripps Research Institute and approved by the Institutional Animal Care and Use Committee.
Locomotor activity in the open field.
Locomotion, exploratory, and thigmotaxis/anxiety behavior was measured using the open field analysis as previously described (Schaefer et al., 2009). For measurements of cocaine-induced locomotor and anxiety responses, mice were recorded for 10 min in the novel environment, followed by 10 min after sterile saline injection (100 μl i.p.) and 60 min after injection of 20 mg/kg cocaine i.p. (Sigma-Aldrich; dissolved in saline). Open-field data were analyzed using a two-way repeated measure analysis of variance (ANOVA) with Bonferoni posttest and Student’s t test.
Light and dark chamber.
The light and dark chamber test was used to determine basal anxiety–related behavior. Anxiety-related behavior was measured by comparing the total time spent in the light using a computer-operated Photobeam activity system (AccuScan Instruments). Statistical analysis was performed using Student’s t test.
Tail suspension test.
Mice were tested for their degree of immobility behavior in the tail suspension test. The Square Test Cubicle (Med Associates, Inc.) equipped with a strain gauge was used. The total duration of immobility during a single trial of 6 min was calculated as the time the force of the mouse’s movements was below a preset threshold. Statistical analysis was performed using Student’s t test.
Sucrose preference test.
Sucrose preference of Drd2-Cre; Ago2fl/fl and their sex- and age-matched littermate controls (n = 5 mice/genotype) was measured as previously described (Schaefer et al., 2009).
Cocaine place preference.
Mice were tested in place conditioning devices (Med Associates, Inc.). The experiment consisted of three phases: habituation (two sessions), conditioning (six sessions), and preference testing (one session). During the habituation sessions mice were permitted to freely explore the apparatus for 30 min to determine whether any mice had an intrinsic preference for a particular choice compartment. Mice that spent >70% of their time in a particular choice compartment on day 2 were excluded from further analyses. During the conditioning phase, mice were injected with cocaine (15 mg/kg) every second day for a total of three injections, and confined to one of the two choice compartments (counterbalanced between groups). On alternating days mice were injected with saline and confined to the other (non–cocaine-paired) choice compartment for 30 min. After six conditioning trials (three cocaine, three saline; days 3–8), a 30 min preference test was conducted (day 9). Immediately before the preference test session, mice received a saline injection and were allowed to explore the entire apparatus for 30 min. The time spent in either the cocaine- or saline-paired choice compartments, but not in the connecting compartment between the two choice compartments, was recorded. Entrance into and movements within the two choice compartments were automatically recorded via photobeams in the walls of the apparatus, with the data collected on a PC computer. Statistical analysis was performed using a two-way ANOVA.
Cocaine self-administration.
Mice (>8-wk-old males) were anesthetized using isoflurane vapor (1–3% in oxygen) and prepared with intravenous catheters. In brief, the catheters consisted of a 6-cm length of silastic tubing fitted to a guide cannula (Plastics One) that was bent at a curved right angle and encased in dental acrylic. The catheter tubing was passed subcutaneously from the animals’ back to the right jugular vein, and a 1-cm length of the catheter tip was inserted and secured into the vein. All subjects were allowed to recover for at least 96 h before access to cocaine self-administration. Catheters were flushed daily with physiological sterile saline solution (0.9% wt/vol) containing heparin (10–50 USP U/ml). Catheter integrity was tested with the ultra short-acting barbiturate anesthetic Brevital (Eli Lilly).
Mice were mildly food restricted to maintain a body weight of 85–90%. Mice were then trained to press an “active” lever for 20 mg food pellets on a fixed ratio 5 time-out 20 s (FR5TO20 s) schedule of reinforcement during 30-min sessions. Mice were permitted to self-administer food until stable intake of >25 pellets per 30 min was achieved. Mice were then permitted to self-administer cocaine on an FR5TO20 s schedule during 60-min daily sessions (FR5TO20 s = 5 active lever responses resulted in the delivery of one 0.3 mg/kg cocaine infusion [35.25 µl/infusion, delivered over 3 s] that initiated a 20-s time-out period signaled by a light cue located above the lever). Responding on the active lever during the post-infusion time-out period was recorded, but was without consequence. For the D–R curve analysis, mice were permitted access to each cocaine dose for at least five consecutive sessions. Between the different cocaine doses, mice were permitted to respond to the training dose of the drug (0.3 mg/kg/infusion) for at least three sessions to ensure that their intake returned to baseline levels. Statistical analysis was performed using a two-way ANOVA. Cocaine was obtained from the National Institute on Drug Abuse.
Online supplemental material
Fig. S1 shows that the miRNA-generating enzyme Dicer is essential for neuronal cell survival in Camk2a-Cre; Dicerfl/fl and Drd2-Cre; Dicerfl/fl mice. Fig. S2 shows that the selective ablation of Ago2 in Drd2-expressing MSNs has no effect on basal mouse behavior such as locomotion, exploration, anxiety, mood, and response to a natural reward such as sucrose. Fig. S3 shows that Ago 1, 2, 3, and 4 are ubiquitously expressed in the mouse brain, but that only Ago2 regulates the expression of a large group of miRNAs in the striatum. Fig. S4 shows that Ago2-dependent miRNAs are associated with the Ago2–RISC complex in Drd2 neurons and displays the 20 greatest Drd2-enriched and Ago2-dependent miRNAs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100451/DC1.
Acknowledgments
We thank Alexander Tarakhovsky for providing the Dicerfl/fl, Ago1−/−, Ago2fl/fl, Ago3−/−, and Ago4−/− mice and helpful discussions; Nathaniel Heintz and GENSAT for providing the Drd2-eGFP and Drd2-Cre mice; Guenther Schuetz for providing the Camk2a-Cre mice; Klaus Rajewsky for the STOP-eGFP-ROSA26TV vector; Silas Mann for miRNA data analysis; Selina Riddick for animal work; and Elisabeth Griggs for her help in the preparation of the manuscript.
This work was supported in part by National Institutes of Health grants DA10044 and MH074866 (P. Greengard), DA025962 (A. Schaefer), and DA025983 (P. Kenny). A. Schaefer was the recipient of a German Research Foundation fellowship (SCHA 1482/1-1). H.-I. Im was the recipient of a Ruth L. Kirschstein NARSAD award (DA027281).
The authors have no competing financial interest.
Footnotes
Abbreviations used:
- ADCID
- Argo2-dependent, induced by cocaine and DRD2-enriched
- ANOVA
- analysis of variance
- miRNA
- microRNA
- MSN
- medium spiny neuron
- RISC
- RNA-induced silencing complex
- RQ
- relative quantification
- TH
- tyrosine hydroxylase
References
- Bibb J.A., Chen J., Taylor J.R., Svenningsson P., Nishi A., Snyder G.L., Yan Z., Sagawa Z.K., Ouimet C.C., Nairn A.C., et al. 2001. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 410:376–380 10.1038/35066591 [DOI] [PubMed] [Google Scholar]
- Caine S.B., Negus S.S., Mello N.K., Patel S., Bristow L., Kulagowski J., Vallone D., Saiardi A., Borrelli E. 2002. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J. Neurosci. 22:2977–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova E., Fehsenfeld S., Mantamadiotis T., Lemberger T., Greiner E., Stewart A.F., Schütz G. 2001. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 31:37–42 10.1002/gene.1078 [DOI] [PubMed] [Google Scholar]
- Chandrasekar V., Dreyer J.L. 2009. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol. Cell. Neurosci. 42:350–362 10.1016/j.mcn.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Chao J., Nestler E.J. 2004. Molecular neurobiology of drug addiction. Annu. Rev. Med. 55:113–132 10.1146/annurev.med.55.091902.103730 [DOI] [PubMed] [Google Scholar]
- Cheloufi S., Dos Santos C.O., Chong M.M., Hannon G.J. 2010. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature. 465:584–589 10.1038/nature09092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S.W., Zang J.B., Mele A., Darnell R.B. 2009. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 460:479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T.H., Cuellar T.L., Koch S.M., Barker A.J., Harfe B.D., McManus M.T., Ullian E.M. 2008. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 28:4322–4330 10.1523/JNEUROSCI.4815-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederichs S., Haber D.A. 2007. Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell. 131:1097–1108 10.1016/j.cell.2007.10.032 [DOI] [PubMed] [Google Scholar]
- Frank F., Sonenberg N., Nagar B. 2010. Structural basis for 5′-nucleotide base-specific recognition of guide RNA by human AGO2. Nature. 465:818–822 10.1038/nature09039 [DOI] [PubMed] [Google Scholar]
- Girault J.A., Greengard P. 2004. The neurobiology of dopamine signaling. Arch. Neurol. 61:641–644 10.1001/archneur.61.5.641 [DOI] [PubMed] [Google Scholar]
- Gong S., Doughty M., Harbaugh C.R., Cummins A., Hatten M.E., Heintz N., Gerfen C.R. 2007. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J. Neurosci. 27:9817–9823 10.1523/JNEUROSCI.2707-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S.M., Boettcher S., Caudy A.A., Kobayashi R., Hannon G.J. 2001. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 293:1146–1150 10.1126/science.1064023 [DOI] [PubMed] [Google Scholar]
- He L., Hannon G.J. 2004. MicroRNAs: small RNAs with a big role in gene regulation. Nat. Rev. Genet. 5:522–531 10.1038/nrg1379 [DOI] [PubMed] [Google Scholar]
- Heiman M., Schaefer A., Gong S., Peterson J.D., Day M., Ramsey K.E., Suárez-Fariñas M., Schwarz C., Stephan D.A., Surmeier D.J., et al. 2008. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 135:738–748 10.1016/j.cell.2008.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N. 2004. Gene expression nervous system atlas (GENSAT). Nat. Neurosci. 7:483 10.1038/nn0504-483 [DOI] [PubMed] [Google Scholar]
- Hutvágner G., McLachlan J., Pasquinelli A.E., Bálint E., Tuschl T., Zamore P.D. 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 293:834–838 10.1126/science.1062961 [DOI] [PubMed] [Google Scholar]
- Kim J., Inoue K., Ishii J., Vanti W.B., Voronov S.V., Murchison E., Hannon G., Abeliovich A. 2007. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 317:1220–1224 10.1126/science.1140481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A.M., King K.S., Donahue C.P., Khrapko K., Kosik K.S. 2003. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA. 9:1274–1281 10.1261/rna.5980303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. 2002. Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12:735–739 10.1016/S0960-9822(02)00809-6 [DOI] [PubMed] [Google Scholar]
- Lingel A., Simon B., Izaurralde E., Sattler M. 2003. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature. 426:465–469 10.1038/nature02123 [DOI] [PubMed] [Google Scholar]
- Liu J., Carmell M.A., Rivas F.V., Marsden C.G., Thomson J.M., Song J.J., Hammond S.M., Joshua-Tor L., Hannon G.J. 2004. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 305:1437–1441 10.1126/science.1102513 [DOI] [PubMed] [Google Scholar]
- McClung C.A., Nestler E.J. 2003. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat. Neurosci. 6:1208–1215 10.1038/nn1143 [DOI] [PubMed] [Google Scholar]
- Miska E.A., Alvarez-Saavedra E., Townsend M., Yoshii A., Sestan N., Rakic P., Constantine-Paton M., Horvitz H.R. 2004. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol. 5:R68 10.1186/gb-2004-5-9-r68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Carroll D., Mecklenbrauker I., Das P.P., Santana A., Koenig U., Enright A.J., Miska E.A., Tarakhovsky A. 2007. A Slicer-independent role for Argonaute 2 in hematopoiesis and the microRNA pathway. Genes Dev. 21:1999–2004 10.1101/gad.1565607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza P.V., Deroche-Gamonent V., Rouge-Pont F., Le Moal M. 2000. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci. 20:4226–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulipparacharuvil S., Renthal W., Hale C.F., Taniguchi M., Xiao G., Kumar A., Russo S.J., Sikder D., Dewey C.M., Davis M.M., et al. 2008. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 59:621–633 10.1016/j.neuron.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K.D., Simmini S., Abreu-Goodger C., Bartonicek N., Di Giacomo M., Bilbao-Cortes D., Horos R., Von Lindern M., Enright A.J., O’Carroll D. 2010. The miR-144/451 locus is required for erythroid homeostasis. J Exp Med. 207:1351–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Derudder E., Hobeika E., Pelanda R., Reth M., Rajewsky K., Schmidt-Supprian M. 2006. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 24:729–739 [DOI] [PubMed] [Google Scholar]
- Schaefer A., O’Carroll D., Tan C.L., Hillman D., Sugimori M., Llinas R., Greengard P. 2007. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 204:1553–1558 10.1084/jem.20070823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A., Sampath S.C., Intrator A., Min A., Gertler T.S., Surmeier D.J., Tarakhovsky A., Greengard P. 2009. Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron. 64:678–691 10.1016/j.neuron.2009.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.J., Liu J., Tolia N.H., Schneiderman J., Smith S.K., Martienssen R.A., Hannon G.J., Joshua-Tor L. 2003. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat. Struct. Biol. 10:1026–1032 10.1038/nsb1016 [DOI] [PubMed] [Google Scholar]
- Song J.J., Smith S.K., Hannon G.J., Joshua-Tor L. 2004. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 305:1434–1437 10.1126/science.1102514 [DOI] [PubMed] [Google Scholar]
- Yan K.S., Yan S., Farooq A., Han A., Zeng L., Zhou M.M. 2003. Structure and conserved RNA binding of the PAZ domain. Nature. 426:468–474 10.1038/nature02129 [DOI] [PubMed] [Google Scholar]
- Yi R., O’Carroll D., Pasolli H.A., Zhang Z., Dietrich F.S., Tarakhovsky A., Fuchs E. 2006. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat. Genet. 38:356–362 10.1038/ng1744 [DOI] [PubMed] [Google Scholar]