Abstract
Post-menopausal women have an increased risk of developing a number of degenerative pathological conditions, linked by the common theme of excessive inflammation. Systemic estrogen replacement (in the form of hormone replacement therapy) is able to accelerate healing of acute cutaneous wounds in elderly females, linked to its potent antiinflammatory activity. However, in contrast to many other age-associated pathologies, the detailed mechanisms through which estrogen modulates skin repair, particularly the cell type–specific role of the two estrogen receptors, ERα and ERβ, has yet to be determined. Here, we use pharmacological activation and genetic deletion to investigate the role of both ERα and ERβ in cutaneous tissue repair. Unexpectedly, we report that exogenous estrogen replacement to ovariectomised mice in the absence of ERβ actually delayed wound healing. Moreover, healing in epidermal-specific ERβ null mice (K14-cre/ERβL2/L2) largely resembled that in global ERβ null mice. Thus, the beneficial effects of estrogen on skin wound healing are mediated by epidermal ERβ, in marked contrast to most other tissues in the body where ERα is predominant. Surprisingly, agonists to both ERα and ERβ are potently antiinflammatory during skin repair, indicating clear uncoupling of inflammation and overall efficiency of repair. Thus, estrogen-mediated antiinflammatory activity is not the principal factor in accelerated wound healing.
Wounds to the skin heal via a complex series of overlapping tightly regulated phases. With increasing age these become disrupted, and repair efficiency is reduced. Chronically impaired healing in the elderly is a major area of unmet clinical need that constitutes a substantial drain on global health services and leads to significant patient morbidity. In women the transition to delayed healing coincides with menopause, where hormone levels, particularly estrogen, rapidly fall. We have previously reported that estrogen replacement substantially accelerates healing in both aged humans and estrogen-depleted animal models (Ashcroft et al., 1997). Moreover, estrogen protects against the development of chronic nonhealing wounds (Margolis et al., 2002). We currently believe estrogen to be a key pleiotropic factor (Emmerson et al., 2009), beneficially influencing a range of cutaneous cell types involved in multiple aspects of wound repair including inflammation, re-epithelialization, angiogenesis, matrix deposition, and remodelling. In fact, at the level of gene expression, estrogen appears more important than intrinsic ageing in the ontogenesis of pathological repair (Hardman and Ashcroft, 2008). Unfortunately, recent research has highlighted the potential detrimental impact of long-term hormone replacement therapy (HRT) use, namely an increased risk of breast cancer, stroke, and coronary heart disease (Anderson et al., 2004). Thus, there is a fundamental need to further elucidate cutaneous estrogen signaling with a view to selectively exploit the beneficial aspects to promote healing.
In both mice and humans, estrogen signals through two related but distinct nuclear hormone receptors, estrogen receptor (ER) α and β, which function as either homo- or hetero-dimers. The receptors, which share >95% homology in their DNA-binding domain, are differentially expressed throughout mammalian tissues (for review see Dahlman-Wright et al., 2006). In human skin ER isoform distribution reportedly varies depending on cell type, source, and time: ERβ is suggested to be the predominant isoform in human scalp keratinocytes (Thornton et al., 2003b); although other studies describe both receptors in neonatal foreskin-derived keratinocytes (Verdier-Sevrain et al., 2004), dermal fibroblasts (Haczynski et al., 2004), and hair follicles and sebaceous glands (Thornton et al., 2003a). Despite detailed knowledge of human skin ER distribution, but curiously not mouse, understanding of the role that each receptor plays in cutaneous healing is severely lacking.
Agonists selective for each estrogen receptor are currently being used in vivo to elucidate the specific roles of ER isoforms in a range of tissues. In addition, several iterations of ER null mice have been generated, each improving upon the previous, after initial issues with lack of splice variant deletion (for review see Antal et al., 2008). These mice have been extensively characterized with phenotypes reported in a range of tissues. Both ERα−/− and ERβ−/− mice display pronounced reproductive phenotypes (for review see Couse and Korach, 1999). In peripheral tissues, however, ERα appears to predominate, with ERα−/− mice displaying abnormalities in mammary, bone, neurological, vascular, metabolic, and other tissues. Thus, although ERα is clearly of fundamental physiological importance, the respective role of ERβ is more contentious. Despite numerous reports describing pathological phenotypes in global ERβ−/− mice (for review see Couse and Korach, 1999), a recently generated constitutive-Cre ERβ(L/L) null mouse appears virtually devoid of peripheral tissue pathology (Antal et al., 2008).
The limited data that does exist on the role of ERs in the skin is mostly based around studies of hair biology. In male mice, hair cycling appears mediated by ERα, whereas catagen transition is ERβ regulated (Movérare et al., 2002; Ohnemus et al., 2005). Recently the protective effect of estrogen on skin flap necrosis has been reported to be mediated by ERα (Toutain et al., 2009). To date, however, no study has investigated the effect of ERβ-specific deletion, or the role of ER isoforms in skin wound healing in female mice. In this study, using a combination of in vivo and in vitro methodologies, we demonstrate very different roles for the two ERs during skin wound healing. Specifically, the beneficial effects of estrogen in skin are mediated through ERβ, with signaling through ERα alone having a detrimental influence on repair. To our knowledge, skin healing is unique in that it is the first tissue where; (a) ERs have been demonstrated to have such clearly opposed physiological effects and (b) ERβ-mediated influence is so predominant.
RESULTS AND DISCUSSION
ER isoform-selective agonists confer contrasting effects on skin repair
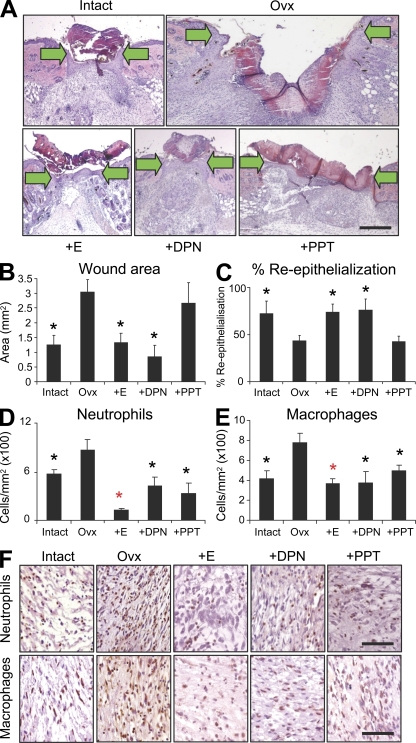
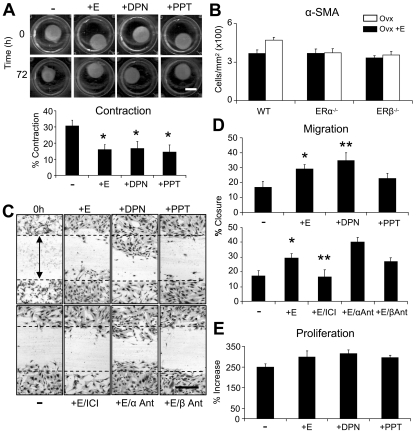
In light of severely limited mouse ER expression data in the literature we first profiled receptor localization in mouse skin. We report that both receptors are widely expressed, with immunohistochemical localization to keratinocytes, hair follicles, fibroblasts, and endothelial and inflammatory cells (Fig. S1). To address the independent contribution of each receptor to skin repair we systemically treated estrogen-deprived mice with agonists specific for ERα or ERβ and generated incisional wounds. After 3 d the degree of in vivo repair was quantified with respect to ovariectomised (Ovx) mice (Fig. 1, A–C). DPN (a 70-fold selective ERβ agonist) significantly reduced wound area, to a level comparable with that of 17β-estradiol, simultaneously promoting restoration of the epidermis. In stark contrast, signaling through ERα (PPT treatment; 410-fold selective ERα agonist) had no effect on these global measures of healing.
Figure 1.
The ERβ agonist, DPN, accelerates wound healing. (A) Representative H&E-stained sections of full-thickness incisional wounds (d 3) from mice with intact ovaries, ovariectomised (Ovx), and Ovx mice systemically treated with 17β-estradiol (E), DPN (ERβ agonist), or PPT (ERα agonist) (arrows indicate wound margins). (B and C) DPN accelerates healing equally to 17β-estradiol quantified by reduced wound area (B) and increased re-epithelialization. (D and E) Both PPT and DPN are potently antiinflammatory when compared with untreated Ovx mice. (F) Representative immunohistochemistry for wound neutrophils and macrophages, respectively. Data shown as mean ± SEM are representative of two independent experiments (n = 6). Bar, 200 µm (A); 50 µm (F). Black asterisk, P < 0.05; red asterisk, P < 0.01 with respect to Ovx.
Intriguingly, both agonists were equally antiinflammatory with respect to wound macrophage and neutrophil recruitment (Fig. 1, D–F). ER-mediated effects on inflammation are important for numerous other body systems: the ERα agonist PPT is both neuroprotective and antiinflammatory in neurodegenerative disorders such as Alzheimer's disease (Tiwari-Woodruff et al., 2007); ERα mediates the protective effect of estrogen on skin necrosis in a skin flap perfusion model (Toutain et al., 2009). Notably, in the brain ERβ agonist treatment has no antiinflammatory effects (Tiwari-Woodruff et al., 2007), although Toutain et al. (2009) unfortunately failed to investigate the role of ERβ in their skin flap model. Our agonist treatment data show that although signaling through ERα alone is sufficient to reduce skin inflammation in vivo, as widely reported in other tissues, it does not accelerate wound repair. Thus, we identify a clear uncoupling of inflammation and repair in the context of ER signaling. Current thinking would suggest a tightly regulated inflammatory response is essential to appropriate repair, with either excessive inflammation (chronic wounds) or macrophage ablation leading to delayed healing (Mirza et al., 2009).
ERβ null mice display a profound wound-healing phenotype
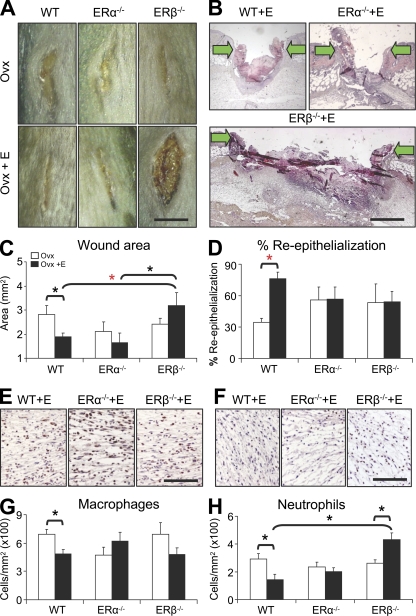
The next logical step was to investigate the effect of ER-specific deletion. Thus, incisional wounds were generated in wild-type (WT), ERα null (ERα−/−), and ERβ null (ERβ−/−) mice, in the absence of estrogen (Ovx) or after exogenous estrogen replacement (Ovx plus s.c. 17β-estradiol pellet). Healing was delayed in the absence of estrogen in all three genotypes (Fig. 2, A, C, and D), implying a limited role for ER signaling in the absence of a ligand. However, upon exogenous estrogen replacement a clear genotype-specific response was revealed, with ERβ−/− wounds substantially increased in size (Fig. 2, A–C). Delayed healing in ERβ−/− corroborates our ER isoform-specific agonist treatment data (Fig. 1; where DPN-mediated acceleration indicates the importance of ERβ). Mechanistically, estrogen can modulate keratinocyte, fibroblast, and inflammatory cell functions. Thus, delayed healing in estrogen-treated ERβ−/− mice may reflect defective wound contraction, delayed re-epithelialization, excessive inflammation, altered angiogenesis, or a combination of these processes.
Figure 2.
Exogenous estrogen treatment delays wound healing in ERβ null (ERβ−/−) mice. (A and B) Representative morphology and histology at d 3 after wounding. (arrows indicate wound margins). (C) Wound area quantification reveals delayed healing in Ovx ERβ−/− mice after 17β-estradiol treatment (E). (D) Estrogen fails to accelerate re-epithelialization in ERα null (ERα−/−) or ERβ−/− mice. (E–H) Quantification of wound inflammatory cells, with representative immunohistochemistry (E and F), reveals increased neutrophil numbers in estrogen-treated ERβ−/− mice. Data shown as mean ± SEM are representative of two independent experiments (n = 5–7). (B) “ERβ−/−+E” image has been auto stitched from two individual images. Bars: (A) 6 mm; (B) 600 µm; (E and F) 150 µm. Black asterisk, P < 0.05; red asterisk, P < 0.01.
Estrogen is potently antiinflammatory in WT mice, inhibiting local wound recruitment of innate immune cells in vivo (Fig. 2, E–H) and dampening lipopolysaccharide-induced cytokine production in vitro (Emmerson et al., 2009). Immunohistochemical characterization of wound cell infiltrate in ER null mice revealed key quantitative differences in local inflammation. Estrogen replacement in ERα−/− mice failed to dampen inflammation, whereas ERβ−/− mice were characterized by a significant and specific increase in wound neutrophils (Fig. 2, E–H). It is generally accepted that excessive neutrophil infiltration plays a causative role in pathologically delayed healing; however, the role of macrophages appears more subtle (Diegelmann, 2003). Recent studies have revealed macrophages to be beneficial only at specific stages of tissue repair (Mirza et al., 2009). It is tempting to speculate that increased neutrophil numbers could play a role in the delayed healing of the ERβ−/− mouse phenotype.
Collectively, these in vivo data strongly suggest that signaling though ERα in the absence of ERβ is in fact detrimental to healing. Moreover, we observed marked differences between agonist-treated mice (allowing ER heterodimerization) and ER null mice (exclusively ER homodimers). For example, the ERβ agonist DPN strongly promoted re-epithelialization in vivo (Fig. 1), whereas estrogen treatment of ovariectomised ERα−/− mice (Fig. 2) did not alter re-epithelialization. Thus, signaling through ERβ leads to entirely different phenotypes depending on the presence or absence of ERα. This implies that a proportion of skin ERβ-mediated effects are through ERα inhibition or alternatively that ER heterodimers are physiologically important. Such “yin-yang” ER interactions have been classically described in cancer, which is fascinating as clear analogies have been drawn between cancer progression and tissue repair (Dvorak, 1986). ERα classically promotes tumor proliferation through regulation of the cell cycle, whereas ERβ is anti-proliferative (Paruthiyil et al., 2004). Indeed, it has been proposed, but not shown, that many of the physiological effects of ERβ in other tissues are in fact due to direct antagonistic effects on ERα-mediated transcription (Lindberg et al., 2003). Skin could provide an additional new model to investigate such yin-yang ER interactions.
Delayed healing in global ERβ−/− is recapitulated in epidermal-specific ERβ null mice in the absence of excessive inflammation
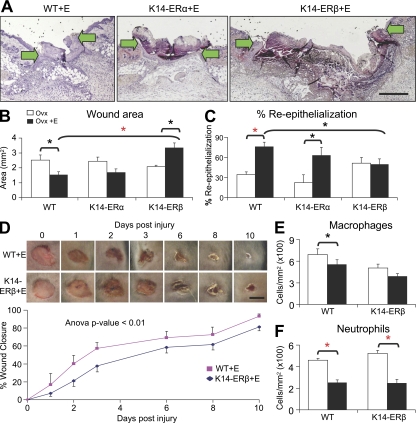
Estrogen is a known keratinocyte mitogen (Emmerson et al., 2009), yet paradoxically, neither ERα−/− nor ERβ−/− mice displayed increased re-epithelialization after estrogen treatment (Fig. 2 D). Mouse keratinocytes express both ERs (Fig. S1) with a recent report indicating that agonists specific for either ER can promote the in vitro migration of the human keratinocyte cell line NCTC 2544 (Merlo et al., 2009). To further investigate mouse epidermal ER function we wounded epidermal-specific (K14-cre) knockout mice, K14-cre/ERαL2/L2 (K14-ERα), K14-cre/ERβL2/L2 (K14-ERβ), and WT litter mates. Estrogen treatment of Ovx K14-ERα mice led to pronounced and significant acceleration of re-epithelialization (Fig. 3 C). In contrast, estrogen treatment of Ovx K14-ERβ mice failed to promote re-epithelialization (Fig. 3 C) and led to a significant delay in overall healing (Fig. 3, A and B). Comparison of the temporal healing profile between estrogen-treated K14-ERβ and WT mice shows that the observed difference in repair at d 3 after wounding is maintained over subsequent stages of healing (Fig. 3 D). That K14-ERβ mice essentially phenocopy global ERβ−/− mice indicates estrogen's beneficial influence on healing is predominantly mediated by epidermal ERβ, and presumably involves paracrine signaling to additional cell types (e.g., vascular, fibroblast) that also functionally influence the healing response.
Figure 3.
Epidermal-specific ERβ null (K14-ERβ) mice phenocopy ERβ−/− mice. (A) Representative H&E-stained sections from estrogen (E)-treated wild-type (WT), K14-ERα, and K14-ERβ mice. (B and C) Estrogen treatment of K14-ERβ mice specifically delays healing, shown by increased wound area (B) with no increase in re-epithelialization (C). (D) Temporal monitoring of excisional wound closure reveals that delayed healing (K14-ERβ) is maintained through later time points. (E and F) Quantification of wound inflammatory cell numbers reveals antiinflammatory effects for estrogen on neutrophils in WT and K14-ERβ (E). Data shown as mean ± SEM are representative of two independent experiments (n = 6). (A) “K14-ERβ+E” image has been auto stitched from two individual images. Bars: (A) 400 µm; (D) 4 mm. Black asterisk, P < 0.05; red asterisk, P < 0.01.
In this context it is interesting to draw comparisons to other tissues where complex stromal–epithelial interactions occur, such as mammary gland or prostate. The mammary gland undergoes highly orchestrated morphological change during development and pregnancy, for which epithelial–mesenchymal cross talk is absolutely essential. ER null mouse experiments indicate that ERα triggers ductal growth, whereas ERβ is involved much later in terminal differentiation. Interestingly, although ERα expression in mammary epithelium is heterogeneous, estrogen induces proliferation in all epithelial cells, implying paracrine effects on ERα-negative epithelial cells (Mallepell et al., 2006). Amphiregulin, an EGF receptor ligand, is a prime candidate for mediating these effects in vivo. Similar keratinocyte–keratinocyte and keratinocyte–fibroblast interactions almost certainly occur during skin wound healing, where EGF/FGF receptors play a well-documented role. However, our data suggest that ERβ is the key player in these epithelial–mesenchymal events.
Fascinatingly, the inflammatory response in delayed-healing wounds from epidermal-specific K14-ERβ mice appeared equivalent to that in WT mice (Fig. 3, E and F). That K14-ERβ mice display delayed healing concomitant with reduced inflammation provides yet more evidence for uncoupling of inflammation and healing. Moreover, this phenotype strongly suggests that inflammatory cell changes (i.e., increased neutrophil infiltration) are not a principal causative factor in delayed healing in global ERβ−/− mice (Fig. 2 H). To investigate this further we wounded mice with LysM-cre–mediated inflammatory cell-specific deletion of ERβ (Fig. S2). These mice were essentially phenotypically normal with reduced wound area and dampened inflammation in response to estrogen treatment. This strongly implies that inflammatory cell-specific ERβ does not contribute to the global ERβ−/− delayed wound healing phenotype, reinforcing the importance of keratinocyte-expressed ERβ in estrogen's beneficial effects on wound healing.
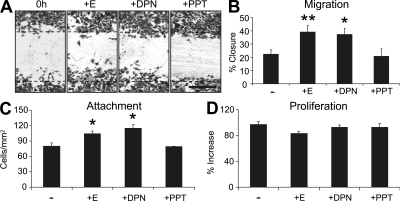
To further clarify the role of each ER in keratinocyte function we performed in vitro scratch wound migration assays. Treatment of WT keratinocytes with the ERβ agonist DPN promoted migration, whereas the ERα agonist PPT had no influence on scratch wound closure (Fig. 4, A and B). These data fit perfectly with in vivo agonist treatment (Fig. 1 D) and epidermal-specific ER deletion data (Fig. 3 C). It is unclear why Merlo et al. (2009) report enhanced in vitro migration in response to both PTT and DPN. They used a cell line, whereas we used more physiologically relevant primary keratinocytes. In our primary keratinocytes both 17β-estradiol and DPN, but not PPT, significantly increased adhesion to noncoated (Fig. 4 C) and collagen-coated (unpublished data) tissue culture plates. Interestingly, the migration/adhesion effects are proliferation independent, as proliferation rate is not influenced by either 17β-estradiol or ER agonists (Fig. 4 D).
Figure 4.
Estrogen modulates mouse keratinocyte function through ERβ in vitro. (A and B) ERβ agonist (DPN; 10-6M) promotes keratinocyte migration to the same extent as 17β-estradiol (E; 10-7 M), whereas ERα agonist (PPT; 10-6M) has no effect. (C) Keratinocyte attachment is increased with E and DPN treatment. (D) E, DPN, or PPT treatment has no effect on proliferation after 48 h. Data shown as mean ± SEM, in triplicate wells, and are representative of two independent experiments. Bar: (A) 300 µm. *, P < 0.05; **, P < 0.01.
ER isoforms differentially regulate fibroblast migration in vitro
Fibroblasts represent an excellent candidate cell type for nonkeratinocyte-mediated effects of ERs on wound healing, with estrogen known to influence multiple skin fibroblast functions (Emmerson et al., 2009). As the observed in vivo delayed healing phenotype in ERβ−/− mice (Fig. 2) involved substantial wound gaping, we asked whether ER-mediated effects on fibroblast contraction could be involved. In a cell-based contraction assay 17β-estradiol, DPN, or PPT treatment all inhibited collagen disc contraction (Fig. 5 A), suggesting that signaling through ERα versus ERβ was unlikely to differentially alter contraction in vivo. Moreover, equal numbers of α-smooth muscle actin (SMA)–positive cells were observed in WT and global ERα−/− and ERβ−/− wounds in vivo (Fig. 5 B). Although these data suggest minimal involvement of fibroblast-mediated contraction, the observed phenotype (wound gaping) does suggest inherent differences in tensional forces upon wounding. Wound contraction is a complex process that is also affected by numerous factors, for example balance between collagen synthesis and deposition, and thus early changes may be involved in the observed phenotype.
Figure 5.
ERs differentially modulate fibroblast migration in vitro. (A) Fibroblast-mediated contraction of collagenous discs was inhibited by 17β-estradiol (E), ERβ agonist (DPN; 10-6M), or ERα agonist (PPT; 10-6M). (B) Immunohistochemical quantification of wound α-SMA. (C and D) E and DPN promoted fibroblast migration compared with untreated cells (−). Co-treatment with E and the ER antagonist (ICI; 10-7 M) blocked migration, whereas cotreatment with ERα antagonist (MMP; 10-5M) increased migration. PHTPP, ERβ antagonist (10-5M). (E) Fibroblast proliferation assay. Data shown as mean ± SEM using triplicate wells (A, C–E) or 5–7 animals per group (B) are representative of two independent experiments. Bars: (A) 400 µm; (D) 600 mm. *, P < 0.05; **, P < 0.01.
We have previously shown that 17β-estradiol treatment accelerates WT mouse fibroblast migration in vitro (Emmerson et al., 2009). The ERβ agonist DPN also substantially accelerated in vitro scratch assay migration, whereas the ERα agonist PPT had no significant effect (Fig. 5, C and D). Receptor-specific function was confirmed by cotreatment with 17β-estradiol and ER-specific antagonists (Fig. 5 D); blocking both ERs (ICI) abolished estrogen-promoted migration; specifically blocking ERα (MPP) led to dramatically accelerated migration (> twofold increase), whereas specifically blocking ERβ (PHTPP) had no significant effect on estrogen's migration promoting ability. As in keratinocytes, these treatments had no effect on fibroblast proliferation (Fig. 5 E), indicating that altered proliferation does not contribute to the migration-promoting effects of 17β-estradiol or DPN. Thus, promotion of fibroblast migration may contribute to the observed accelerated healing in DPN-treated mice (Fig. 1).
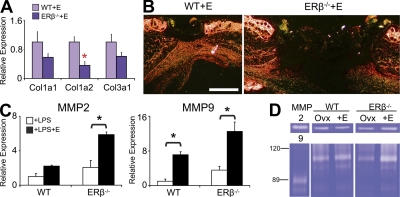
Excessive protease activity and reduced matrix deposition contribute to delayed healing in estrogen-treated ERβ−/− mouse wounds
Altered systemic hormone levels have been linked to changes in skin collagen deposition and turnover during wound healing (Gilliver et al., 2007). In light of clear ER-specific effects on fibroblast function, we next assessed aspects of wound matrix synthesis, deposition, and remodeling (Fig. 6). We demonstrate reduced gene expression of the major skin collagen species in delayed-healing estrogen-treated ERβ−/− wounds (Fig. 6 A) accompanied by greatly reduced wound collagen protein deposition/remodelling, assessed via Picro-Sirius red staining (Fig. 5 B). Of mechanistic importance, we show that the specific increase in wound neutrophil influx in estrogen-treated ERβ−/− wounds is accompanied by an increase in inflammatory cell gelatinase expression and crucially wound gelatinase activity (Fig. 5, C and D). Gelatinases (MMP-2 and MMP-9) are induced after wounding, are pathologically overexpressed in chronic wounds, and play an important role in granulation tissue remodelling (Wysocki et al., 1993). Though we have previously reported increased MMP-9 protein levels in delayed-healing Ovx mouse wounds (Emmerson et al., 2009), we have not explored the effect of in vivo 17β-estradiol treatment on wound protease activity. Collectively, these data suggest that inappropriately excessive local gelatinase activity is a principal contributing factor to reduced collagen deposition and delayed wound healing in ERβ−/− mice. However, the phenotypes of estrogen-treated LysM-ERβ (Fig. S2) and K14-ERβ (Fig. 3) mice suggests that increased inflammation and protease activity in estrogen-treated ERβ−/− mice is in fact secondary to delayed healing (i.e., noncausative), once again indicating complex paracrine interactions. Of note, by d 7 after wounding this ERβ-specific difference in collagen deposition, assessed by Picro-Sirius red staining, was no longer apparent (unpublished data), indicating the observed d 3 phenotype is due to an extended lag-phase of healing in the ERβ−/− mice (see also Fig. 3 D), possibly due to differences in wound contraction (see Fig. 5).
Figure 6.
Altered matrix deposition and protease activity in ERβ null (ERβ−/−) mice. (A and B) Wound collagen content determined by expression of major collagen species (qPCR) and Picro-Sirius staining was reduced in estrogen (E)-treated ERβ−/− mice. (C) E-treated peritoneal macrophages from ERβ−/− mice display increased MMP-2 and MMP-9 expression. (D) Via zymography, MMP-9 activity was strongly increased in E-treated ERβ−/− wounds. Data from two independent experiments are shown as mean ± SEM using cells in triplicate wells (C) or four animals per group (A). (B and D) Representative of at least four animals per group from three independent experiments. (B) “ERβ−/−+E” image has been auto stitched from two individual images. Bar, 800 µm. Black asterisk, P < 0.05; red asterisk, P < 0.01.
In summary, our novel data reveal the beneficial effects of estrogen on skin healing to be predominantly mediated though keratinocyte ERβ (Fig. S3). This fascinating finding appears at odds with the recent suggestion that ERβ−/− mice display relatively few global phenotypic abnormalities (Antal et al., 2008). Indeed, we suggest that the role of ERβ may be revealed only in response to perturbation of homeostasis or in pathophysiological conditions. Limited previous studies have suggested that ERα plays a predominant role in the skin (Toutain et al., 2009). That ERβ has been identified as the major player during skin repair further corroborates previous reports linking specific human ERβ gene polymorphisms with predisposition to chronic nonhealing wounds (Ashworth et al., 2008). In light of the detrimental side-effects of estrogen treatment in post-menopausal women, specifically increased cancer incidence, there is currently much interest in the development of estrogenic alternatives. Selective estrogen receptor modulators (SERMs) have been, and are currently being, developed that differentially bind ERs with tissue-specific agonist/antagonist profiles. In fact, we have recently demonstrated the potential of clinically relevant SERMs (tamoxifen and raloxifene) to accelerate wound repair in vivo. The novel findings from this study are of fundamental importance and should now permit the development of strategies to selectively exploit beneficial ERβ-mediated signaling events to accelerate wound healing in the elderly.
MATERIALS AND METHODS
Animals and wounding.
All animal studies were approved by the UK Government Home Office (Project licence 40/3203) following local ethics committee approval. ERα−/− (Esr1) and ERβ−/− (Esr2) have been described previously (Dupont et al., 2000). Novel conditional ER null mice were generated as follows. Epidermal-specific deletion of ERα and ERβ was obtained by crossing the previously described ERαL2/L2 (Dupont et al., 2000) and ERβL2/L2 (Antal et al., 2008) mice with the extensively characterized K14-cre line (Li et al., 2001). Inflammatory cell-specific deletion was obtained by crossing ERβL2/L2 mice with the previously characterized LysM-cre line (Clausen et al., 1999). In each case, Cre-negative homozygous floxed mice were used as littermate controls. 10-wk-old female C57/BL6 mice with intact ovaries and 10-wk-old C57/BL6 mice that had undergone ovariectomy 1 mo previously were wounded (two equidistant 1-cm full-thickness skin incisional wounds left to heal by secondary intention or two 6-mm excisional wounds to temporally monitor healing) following our established protocol (Emmerson et al., 2009). PPT (4,4’,4”-(4-Propyl-[1H]-pyrazole-1,3,5-triyl; Tocris), DPN (2,3-bis(4-Hydroxyphenyl)-propionitrile; Tocris), or vehicle (5% DMSO in maize oil) was injected at 330 µg/kg/day on days −1, 0, and +1 with respect to time of wounding. Female ERα−/−, ERβ−/−, K14-cre/ERαL2/L2, K14-cre/ERβL2/L2, LysM-cre/ERβL2/L2, and WT littermates were also ovariectomised and wounded as above. Exogenous estrogen was administered at the time of wounding by s.c. implantation of a 0.05-mg, 21-d, slow-release 17β-estradiol pellet (Innovative Research of America) with successful estrogen replacement confirmed by enzyme immunoassay on serum samples.
Histology and immunohistochemistry.
Histological sections were prepared from wound tissue fixed in 10% buffered formalin saline and embedded in paraffin. 6-µm sections were stained with hematoxylin and eosin, Sirius red (in picric acid), or subjected to immunohistochemical analysis with the following antibodies: anti-ERα rabbit polyclonal (Santa Cruz Biotechnology, Inc.); mouse monoclonal anti-ERβ (AbD Serotec); anti-neutrophil rat polyclonal (Thermo Fisher Scientific); anti–Mac-3 rat polyclonal (BD); and anti–α-SMA rabbit polyclonal (Sigma-Aldrich). Bound primary antibody was detected using the VECTASTAIN ABC kit (Vector Laboratories), NovaRed substrate, and counterstained with hematoxylin. Images were captured using a microscope (Eclipse E600; Nikon) and a SPOT camera (Image Solutions Inc.) and total cell numbers, granulation tissue wound area, and re-epithelialization were quantified using Image Pro Plus software (Media Cybernetics). Specifically, re-epithelialization was determined by dividing the sum length of both neo-epithelial tongues by the distance the epidermis would have to migrate to fully close the wound. Picro-Sirius red staining was visualized using plane-polarized light where larger collagen fibers appear red, orange, or yellow and thinner fibers green. Excisional wound closure was determined from planimetry of macroscopic wound images.
Quantitative real-time PCR (qPCR).
Total RNA was isolated from frozen wound tissue by homogenizing in Trizol reagent (Invitrogen) or from cultured macrophages using the RNeasy mini kit (QIAGEN) with cDNA synthesis, and qPCR was performed as described previously (Gilliver et al., 2007; SYBR Green core kit; Eurogentec). Each sample was serially diluted over three orders of magnitude, and expression ratios normalized to the mean of three separate reference primers (18s rRNA, Gapdh, and Ywahz) with all samples analyzed concurrently. Full primer sequences are listed in Table S1.
Gelatin zymography.
Total protein was extracted from mouse wound tissue using denaturing, nonreducing buffer. 50 µg protein was assessed for gelatinase activity, as described previously (Gilliver et al., 2007). In brief, samples were separated by SDS-PAGE alongside human MMP-2 and MMP-9 reference standards. Gels were washed in 2.5% Triton X-100 (Sigma-Aldrich), incubated for 16 h at 37°C in assay buffer (100 mM Tris, 30 mM CaCl2, 0.02% [wt/vol], sodium azide, 0.05% [vol/vol]; Brij 35, pH 7.9) and stained with Coomassie brilliant blue G for 20 min and destained in 1% acetic acid and 30% methanol.
Primary cell assays.
Keratinocytes and dermal fibroblasts were isolated from neonatal mice mouse skin as described previously (Emmerson et al., 2009). Mouse macrophages were isolated by peritoneal lavage with PBS and cultured as described previously (Emmerson et al., 2009). Functional assays were performed in phenol red–free media containing charcoal-stripped serum and mitomycin C where appropriate. Fibroblasts were cultured in PSA-supplemented DMEM (Lonza) and keratinocytes in CnT-02 media (CELLnTEC) at 35°C and 5% CO2. Treatments (10−7 M 17β-estradiol, 10−6 M PPT, 10−6 M DPN, 10−7 M ICI 182780, 10−5 M MPP dihydrochloride, 10−5 M PHTPP) were consistent across all assays.
Scratch migration assay.
Fibroblast or keratinocyte sheets were scratch wounded (sterile pipette tip) and immediately treated. Cells were incubated in serum and PSA-supplemented DMEM (Lonza) or CnT-02 media (CELLnTEC) at either 37°C or 35°C and 5% CO2 for 24 h (fibroblasts) or 48 h (keratinocytes). Images were captured on a microscope (Eclipse E600; Nikon) with a SPOT camera and software (Image Solutions Inc.), and degree of cell migration was determined using Image Pro-Plus software (Media Cybernetics).
Proliferation assay.
Fibroblasts or keratinocytes were seeded in 96-well plates at a density of 5 × 103 cells/well. Cells were incubated for 48 h (keratinocytes) or 96 h (fibroblasts) and proliferation measured using the CellTiter96 AQueous MTS-based kit (Promega) relative to a calibration curve determined from wells known to contain between 5 × 103 and 105 fibroblasts.
Attachment assay.
Keratinocytes were incubated at 37°C and 5% CO2 for 3 h in uncoated or collagen IV–coated (BD) 12-well plates. Unattached cells were removed by PBS washes. Attached keratinocytes were stained with 2% crystal violet and quantified using Image Pro-Plus software (Media Cybernetics).
Contraction assay.
The contractile activity of dermal fibroblasts was assessed using an established contraction assay (Emmerson et al., 2009). In brief, 1.5 × 106 cells/ml were resuspended in chilled collagen I matrix/DMEM solution (Vitrogen 100). Aliquots of this cell/collagen/DMEM solution were incubated for 1 h under mineral oil in a Teflon-lined, die-cut Teflon washer containing a 12-well plate. Gelated cell disks were incubated in DMEM at 37°C and 5% CO2 for 24 h. Teflon washers and sheets were then removed and wells treated with DMEM/10−7 M phorbol 12,13-dibutyrate (Sigma-Aldrich) plus 17β-estradiol/agonists. Discs were visualized via a flat-bed scanner every 24 h and reduction in volume quantified over a 4-d period (Image Pro-Plus; Media Cybernetics).
Macrophage treatment assay.
ERβ−/− and WT littermate control peritoneal macrophages were resuspended in RPMI 1640 medium and plated at a concentration of 106 cells. Individual wells were then treated with bacterial lipopolysaccharide (1 µg/ml) and incubated for 2 h at 37°C and 5% CO2 and then treated for a further 3 h with 17β-estradiol (10−7 M). Samples were then processed for RNA extraction and qPCR.
Statistical analysis.
Statistical differences were determined using Student's t tests, ANOVA (with post-hoc testing), or Mann–Whitney U tests for nonparametric data (SimFit; William Bardsley, University of Manchester, Manchester, UK). A P-value of <0.05 was considered significant.
Online supplemental material.
Fig. S1 shows immunohistochemical expression of ERα and ERβ in mouse skin. Fig. S2 shows the wound-healing phenotype of inflammatory cell-specific LysM-cre/ERβL2/L2 mice. Fig. S3 shows a schematic illustration of the proposed contribution of estrogen receptors during wound healing. The sequences of the PCR primers used in RT-PCR analysis are shown in Table S1. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100500/DC1.
Acknowledgments
This work was supported by funding from Research Into Ageing and European Union EWA (LSHM-CT-2005-518245).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- DPN
- 2,3-bis(4-Hydroxyphenyl)-propionitrile
- ER
- estrogen receptor
- MPP dihydrochloride
- 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- Ovx
- ovariectomised
- PHTPP
- 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- PPT
- 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl
- qPCR
- quantitative real-time PCR
- WT
- wild type
References
- Anderson G.L., Limacher M., Assaf A.R., Bassford T., Beresford S.A., Black H., Bonds D., Brunner R., Brzyski R., Caan B., et al. ; Women's Health Initiative Steering Committee 2004. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 291:1701–1712 10.1001/jama.291.14.1701 [DOI] [PubMed] [Google Scholar]
- Antal M.C., Krust A., Chambon P., Mark M. 2008. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERbeta-null mutant. Proc. Natl. Acad. Sci. USA. 105:2433–2438 10.1073/pnas.0712029105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft G.S., Dodsworth J., van Boxtel E., Tarnuzzer R.W., Horan M.A., Schultz G.S., Ferguson M.W. 1997. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 3:1209–1215 10.1038/nm1197-1209 [DOI] [PubMed] [Google Scholar]
- Ashworth J.J., Smyth J.V., Pendleton N., Horan M., Payton A., Worthington J., Ollier W.E., Ashcroft G.S. 2008. Polymorphisms spanning the 0N exon and promoter of the estrogen receptor-beta (ERbeta) gene ESR2 are associated with venous ulceration. Clin. Genet. 73:55–61 10.1111/j.1399-0004.2007.00927.x [DOI] [PubMed] [Google Scholar]
- Clausen B.E., Burkhardt C., Reith W., Renkawitz R., Förster I. 1999. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8:265–277 10.1023/A:1008942828960 [DOI] [PubMed] [Google Scholar]
- Couse J.F., Korach K.S. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr. Rev. 20:358–417 10.1210/er.20.3.358 [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K., Cavailles V., Fuqua S.A., Jordan V.C., Katzenellenbogen J.A., Korach K.S., Maggi A., Muramatsu M., Parker M.G., Gustafsson J.A. 2006. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol. Rev. 58:773–781 10.1124/pr.58.4.8 [DOI] [PubMed] [Google Scholar]
- Diegelmann R.F. 2003. Excessive neutrophils characterize chronic pressure ulcers. Wound Repair Regen. 11:490–495 10.1046/j.1524-475X.2003.11617.x [DOI] [PubMed] [Google Scholar]
- Dupont S., Krust A., Gansmuller A., Dierich A., Chambon P., Mark M. 2000. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Dvorak H.F. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315:1650–1659 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- Emmerson E., Campbell L., Ashcroft G.S., Hardman M.J. 2009. Unique and synergistic roles for 17beta-estradiol and macrophage migration inhibitory factor during cutaneous wound closure are cell type specific. Endocrinology. 150:2749–2757 10.1210/en.2008-1569 [DOI] [PubMed] [Google Scholar]
- Gilliver S.C., Ruckshanthi J.P., Atkinson S.J., Ashcroft G.S. 2007. Androgens influence expression of matrix proteins and proteolytic factors during cutaneous wound healing. Lab. Invest. 87:871–881 10.1038/labinvest.3700627 [DOI] [PubMed] [Google Scholar]
- Haczynski J., Tarkowski R., Jarzabek K., Wolczynski S., Magoffin D.A., Czarnocki K.J., Ziegert M., Jakowicki J., Jakimiuk A.J. 2004. Differential effects of estradiol, raloxifene and tamoxifen on estrogen receptor expression in cultured human skin fibroblasts. Int. J. Mol. Med. 13:903–908 [PubMed] [Google Scholar]
- Hardman M.J., Ashcroft G.S. 2008. Estrogen, not intrinsic aging, is the major regulator of delayed human wound healing in the elderly. Genome Biol. 9:R80 10.1186/gb-2008-9-5-r80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chiba H., Warot X., Messaddeq N., Gérard C., Chambon P., Metzger D. 2001. RXR-alpha ablation in skin keratinocytes results in alopecia and epidermal alterations. Development. 128:675–688 [DOI] [PubMed] [Google Scholar]
- Lindberg M.K., Movérare S., Skrtic S., Gao H., Dahlman-Wright K., Gustafsson J.A., Ohlsson C. 2003. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol. Endocrinol. 17:203–208 10.1210/me.2002-0206 [DOI] [PubMed] [Google Scholar]
- Mallepell S., Krust A., Chambon P., Brisken C. 2006. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc. Natl. Acad. Sci. USA. 103:2196–2201 10.1073/pnas.0510974103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis D.J., Knauss J., Bilker W. 2002. Hormone replacement therapy and prevention of pressure ulcers and venous leg ulcers. Lancet. 359:675–677 10.1016/S0140-6736(02)07806-6 [DOI] [PubMed] [Google Scholar]
- Merlo S., Frasca G., Canonico P.L., Sortino M.A. 2009. Differential involvement of estrogen receptor alpha and estrogen receptor beta in the healing promoting effect of estrogen in human keratinocytes. J. Endocrinol. 200:189–197 10.1677/JOE-08-0442 [DOI] [PubMed] [Google Scholar]
- Mirza R., DiPietro L.A., Koh T.J. 2009. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am. J. Pathol. 175:2454–2462 10.2353/ajpath.2009.090248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movérare S., Lindberg M.K., Faergemann J., Gustafsson J.A., Ohlsson C. 2002. Estrogen receptor alpha, but not estrogen receptor beta, is involved in the regulation of the hair follicle cycling as well as the thickness of epidermis in male mice. J. Invest. Dermatol. 119:1053–1058 10.1046/j.1523-1747.2002.00637.x [DOI] [PubMed] [Google Scholar]
- Ohnemus U., Uenalan M., Conrad F., Handjiski B., Mecklenburg L., Nakamura M., Inzunza J., Gustafsson J.A., Paus R. 2005. Hair cycle control by estrogens: catagen induction via estrogen receptor (ER)-alpha is checked by ER beta signaling. Endocrinology. 146:1214–1225 10.1210/en.2004-1219 [DOI] [PubMed] [Google Scholar]
- Paruthiyil S., Parmar H., Kerekatte V., Cunha G.R., Firestone G.L., Leitman D.C. 2004. Estrogen receptor beta inhibits human breast cancer cell proliferation and tumor formation by causing a G2 cell cycle arrest. Cancer Res. 64:423–428 10.1158/0008-5472.CAN-03-2446 [DOI] [PubMed] [Google Scholar]
- Thornton M.J., Taylor A.H., Mulligan K., Al-Azzawi F., Lyon C.C., O'Driscoll J., Messenger A.G. 2003a. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J. Investig. Dermatol. Symp. Proc. 8:100–103 10.1046/j.1523-1747.2003.12181.x [DOI] [PubMed] [Google Scholar]
- Thornton M.J., Taylor A.H., Mulligan K., Al-Azzawi F., Lyon C.C., O'Driscoll J., Messenger A.G. 2003b. Oestrogen receptor beta is the predominant oestrogen receptor in human scalp skin. Exp. Dermatol. 12:181–190 10.1034/j.1600-0625.2003.120209.x [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S., Morales L.B., Lee R., Voskuhl R.R. 2007. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc. Natl. Acad. Sci. USA. 104:14813–14818 10.1073/pnas.0703783104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain C.E., Brouchet L., Raymond-Letron I., Vicendo P., Bergès H., Favre J., Fouque M.J., Krust A., Schmitt A.M., Chambon P., et al. 2009. Prevention of skin flap necrosis by estradiol involves reperfusion of a protected vascular network. Circ. Res. 104:245–254: 12p: 254 10.1161/CIRCRESAHA.108.182410 [DOI] [PubMed] [Google Scholar]
- Verdier-Sevrain S., Yaar M., Cantatore J., Traish A., Gilchrest B.A. 2004. Estradiol induces proliferation of keratinocytes via a receptor mediated mechanism. FASEB J. 18:1252–1254 [DOI] [PubMed] [Google Scholar]
- Wysocki A.B., Staiano-Coico L., Grinnell F. 1993. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J. Invest. Dermatol. 101:64–68 10.1111/1523-1747.ep12359590 [DOI] [PubMed] [Google Scholar]