Abstract
The current epidemic of hospital- and community-acquired methicillin-resistant Staphylococcus aureus (MRSA) infections has caused significant human morbidity, but a protective vaccine is not yet available. Prior infection with S. aureus is not associated with protective immunity. This phenomenon involves staphylococcal protein A (SpA), an S. aureus surface molecule that binds to Fcγ of immunoglobulin (Ig) and to the Fab portion of VH3-type B cell receptors, thereby interfering with opsonophagocytic clearance of the pathogen and ablating adaptive immune responses. We show that mutation of each of the five Ig-binding domains of SpA with amino acid substitutions abolished the ability of the resulting variant SpAKKAA to bind Fcγ or Fab VH3 and promote B cell apoptosis. Immunization of mice with SpAKKAA raised antibodies that blocked the virulence of staphylococci, promoted opsonophagocytic clearance, and protected mice against challenge with highly virulent MRSA strains. Furthermore, SpAKKAA immunization enabled MRSA-challenged mice to mount antibody responses to many different staphylococcal antigens.
Staphylococcus aureus is the leading cause of bloodstream, lower respiratory tract, skin, and soft tissue infections in the United States (Klevens et al., 2007). Methicillin-resistant S. aureus (MRSA) strains are isolated in more than half of all community and hospital infections (Klevens et al., 2008). MRSA strains harbor methicillin resistance genes, rendering the entire class of β-lactam antimicrobials obsolete as therapeutic agents (Berger-Bächi, 1994). Some MRSA isolates also acquired resistance to vancomycin, the antibiotic of last resort. These strains threaten a return to the preantibiotic era (Chang et al., 2003). Thus, there is an urgent need for vaccines that protect against staphylococcal infection.
S. aureus infection in humans is not associated with the generation of protective immunity, as patients often suffer recurrent bouts of skin and soft tissue infections (Lowy, 1998). Recent advances described several mechanisms for staphylococcal escape from innate host defenses (de Haas et al., 2004; Rooijakkers et al., 2005; Thammavongsa et al., 2009); however, the molecular events underlying the escape from adaptive immune responses during staphylococcal infection are not known. Human diseases caused by S. aureus can be recapitulated in animals. In particular, experimental infections of the lung, skin, or soft tissues and internal organs have been established in mice (Bubeck Wardenburg et al., 2008; Cheng et al., 2009). Using these models and molecular genetics approaches, staphylococcal protein A (SpA), a cell wall–anchored surface protein (Sjöquist et al., 1972), was identified as a crucial virulence factor for lung infections, septicemia, and abscess development (Palmqvist et al., 2002; Gómez et al., 2004; Cheng et al., 2009).
The vast majority of clinical S. aureus isolates express SpA (Forsgren, 1970; Shopsin et al., 1999), which binds to the Fcγ portion of most Ig subclasses (Jensen, 1958; Lindmark et al., 1983), VH3 type B cell receptors (Sasso et al., 1989), von Willebrand factor (vWF; Hartleib et al., 2000), and TNFR1 (Gómez et al., 2004). Interaction of SpA with B cell receptors (IgM) leads to clonal expansion and subsequent cell death of B cell populations with effects on adaptive and innate immune responses (Forsgren and Quie, 1974; Forsgren et al., 1976; Goodyear and Silverman, 2004; Silverman and Goodyear, 2006). In contrast, SpA binding to the Fcγ of Ig interferes with opsonophagocytic clearance of staphylococci by polymorphonuclear leukocytes (Peterson et al., 1977). SpA is synthesized as a precursor with an N-terminal signal peptide and a C-terminal sorting signal for covalent anchoring to the cell wall (Schneewind et al., 1992). The N-terminal part of mature SpA is comprised of four or five 56–61-residue Ig binding domains (Sjödahl, 1977), which fold into triple helical bundles connected by short linkers (Deisenhofer, 1981). The C-terminal region X is comprised of Xr, a highly repetitive yet variable octapeptide, and Xc, a domain of unique sequence which abuts the cell wall anchor structure of SpA (Guss et al., 1984; Schneewind et al., 1995).
As a result of the attribute of simultaneously binding Fcγ and Fab, SpA vaccines with neutralizing antibodies and protective immunity have hitherto not been reported (Greenberg et al., 1989). We wondered whether antibodies that neutralize the immunosuppressive properties of SpA could affect the outcome of S. aureus infections.
RESULTS AND DISCUSSION
SpA is a virulence factor for lethal S. aureus infections
The contribution of the spa gene toward lethal S. aureus challenge has thus far not been appreciated. To address this, we generated the isogenic spa deletion variant S. aureus Newman Δspa. After intraperitoneal challenge with 2 × 108 CFU of wild-type S. aureus Newman, 60% of animals succumbed to challenge. In contrast, animals infected with the isogenic mutant resulted in only 25% mortality (Fig. S1 A). In addition, the spa mutant displayed a consistent survival defect when examined in naive mouse blood (see Fig. 3 D). These results suggest that SpA is a crucial virulence factor for lethal infections of S. aureus in mice.
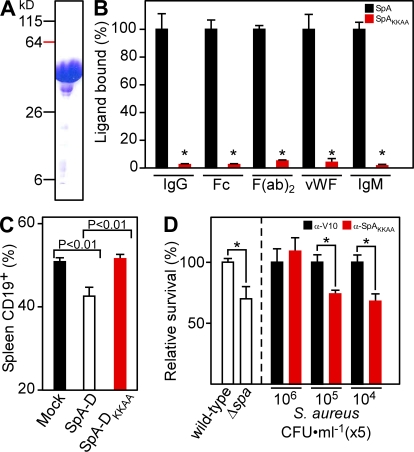
Figure 3.
Full-length nontoxigenic SpA elicits antibodies that stimulate opsonophagocytic clearance of staphylococci. (A) Full-length SpAKKAA was purified on Ni-NTA sepharose and analyzed by Coomassie blue–stained SDS-PAGE. (B) ELISA examining the association of immobilized SpA or SpAKKAA with human IgM, IgG, and its Fc or F(ab)2 fragments or vWF (n = 3). *, P < 0.01. (C) CD19+ B lymphocytes in splenic tissue of 6-wk-old BALB/c mice (n = 5) that had been mock immunized or treated with SpA or SpAKKAA were quantified by FACS. (D, Left) Anticoagulated mouse whole blood (lepirudin) was incubated with 5 × 105 CFU S. aureus Newman or its isogenic spa variant and survival measured (n = 3). (D, Right) Opsonophagocytic clearance of staphylococci (5 × 104, 5 × 105, or 5 × 106 CFU) was measured in the presence of affinity-purified V10 (αV10) or SpAKKAA-specific antibodies in naive mouse whole blood (n = 3). *, P < 0.05. Data are the means and error bars represent ±SEM. Results in A–D are representative of three independent analyses.
SpA-DKKAA cannot bind to immunoglobulin and trigger B cell apoptosis
Guided by amino acid homology, the triple α-helical bundle structure of Ig binding domains (Deisenhofer, 1981), and their atomic interactions with Fab VH3 (Graille et al., 2000) or Fcγ (Gouda et al., 1998), we selected glutamine 9 and 10, as well as aspartate 36 and 37, as critical for the association of SpA with immunoglobulin (Fig. 1, A and B; and Fig. S2, A and B). To test this, substitutions Gln9Lys, Gln10Lys, Asp36Ala, and Asp37Ala were introduced into the D domain to generate SpA-DKKAA (Fig. 1 B). The ability of isolated SpA-D or SpA-DKKAA to bind human IgG or IgM was analyzed by affinity chromatography and ELISA (Fig. 1, C and D). Polyhistidine-tagged SpA-D, as well as full-length SpA, retained human IgG on Ni-NTA, whereas SpA-DKKAA or a negative control (sortase A; Mazmanian et al., 1999) did not (Fig. 1, C and D). A similar result was observed with vWF (Hartleib et al., 2000), which, along with TNFR1 (Gómez et al., 2004), can also bind SpA via glutamines 9 and 10 (Gómez et al., 2006; Fig. 1 D). Human Ig encompasses ∼50% VH3-type IgG (Cook and Tomlinson, 1995). Human Fcγ and F(ab)2 fragments, as well as IgM, all bound to full-length SpA or SpA-D but not to SpA-DKKAA (Fig. 1 D). Injection of SpA-D into the peritoneal cavity of mice resulted in B cell expansion followed by apoptotic collapse of CD19+ (or CD45R+) lymphocytes in the spleen tissue of BALB/c or C57BL/6 mice (Goodyear and Silverman, 2003; Fig. 1 E). B cell superantigen activity was not observed after injection with SpA-DKKAA, and TUNEL-staining of splenic tissue failed to detect the increase in apoptotic cells that follows injection of SpA or SpA-D (Fig. 1 E; and Fig. S2, E–G).
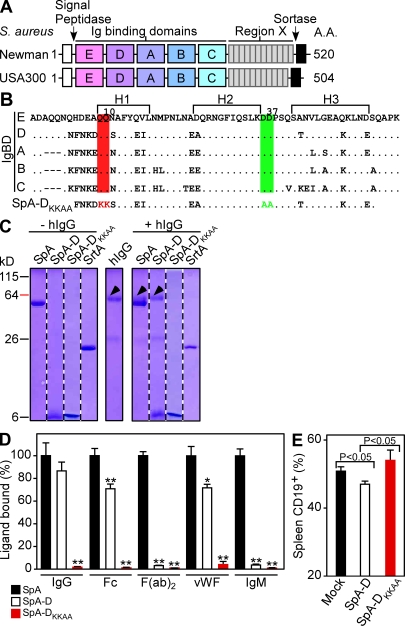
Figure 1.
Generation of a nontoxigenic SpA vaccine. (A) SpA of S. aureus Newman and USA300 LAC harbors an N-terminal signal peptide (white boxes), five Ig binding domains (E, D, A, B, and C), variable region X, and C-terminal sorting signal (black boxes). (B) Amino acid sequence of the five Ig binding domains, as well as nontoxigenic SpA-DKKAA, with the positions of triple α-helical bundles (H1, H2, and H3), as well as glutamine (Q, red) 9 and 10 and aspartate (D, green) 36 and 37 as indicated. (C) Coomassie blue–stained SDS-PAGE of SpA, SpA-D, SpA-DKKAA, or sortase A purified on Ni-NTA sepharose in the presence or absence of human immunoglobulin (hIgG). (D) ELISA examining the association of immobilized SpA, SpA-D, or SpA-DKKAA with human IgG, as well as its Fc or F(ab)2 fragments, vWF and IgM. Statistical significance of SpA-DKKAA binding to each ligand was compared against SpA-D, and SpA-D binding was compared against SpA (n = 3). *, P < 0.05; **, P < 0.01. (E) CD19+ B lymphocytes in splenic tissue of 6-wk-old BALB/c mice (n = 6) that had been mock immunized or treated with SpA-D or SpA-DKKAA were quantified by FACS. Data are the means and error bars represent ±SEM. Results in C–E are representative of three independent analyses.
Antibodies against SpA-DKKAA protect against MSSA and MRSA infections
Naive BALB/c mice were injected with 50 µg each of purified SpA, SpA-D, or SpA-DKKAA emulsified in CFA and boosted with the same antigen emulsified in IFA. IgG responses to immunization were examined by ELISA with SpA-DGGSS and SpA-DKKAA. SpA-DGGSS harbors amino acid substitutions at the same positions as SpA-DKKAA; however, glutamines 9 and 10 were each replaced with glycine and aspartic acids 36 and 37 with serine. Similar to SpA-DKKAA, SpA-DGGSS does not interact with human IgG (unpublished data). It is of note that similar antibody titers were measured with SpA-DGGSS and SpA-DKKAA antigen, indicating that the four amino acid substitutions do not diminish the reactivity of antibodies raised with heterologous antigens (IgG titers against SpA-DKKAA vs. SpA-DGGSS, P = 0.8315). After immunization of mice with either SpA-D or SpA-DKKAA, we observed a 10-fold higher titer of SpA-specific antibodies for the nontoxigenic variant as compared with the B cell superantigen (P < 0.0001; Table I). Antibody titers raised by immunization with full-length SpA were higher than those elicited by SpA-D (P = 0.0022), which is likely a result of the larger size and reiterative domain structure of this antigen (Table I). Nevertheless, even SpA elicited lower antibody titers than SpA-DKKAA (P = 0.0003), which encompasses only 50 aa of the mature 520-residue protein. Immunized mice were challenged by intravenous inoculation with S. aureus Newman, and the ability of staphylococci to seed abscesses in renal tissues was examined by necropsy 4 d after challenge (Cheng et al., 2009). In homogenized renal tissue of mock (PBS/adjuvant) immunized mice, a mean staphylococcal load of 6.46 log10 CFU g−1 was enumerated (Table I). Immunization of mice with SpA or SpA-D led to a reduction in staphylococcal load; however, SpA-DKKAA–vaccinated animals displayed an even greater 3.07 log10 CFU g−1 reduction of S. aureus Newman in renal tissues (P < 0.0001; Table I). Abscess formation in kidneys was analyzed by histopathology (Fig. S3 A-H). Mock immunized animals harbored a mean of 3.7 (±1.2) abscesses per kidney (Table I). Vaccination with SpA-DKKAA reduced the mean number of abscesses to 0.5 (±0.4; P = 0.0204), whereas immunization with SpA or SpA-D did not cause a significant reduction in the number of abscess lesions (Table I). Lesions from SpA-DKKAA–vaccinated animals were smaller in size, with fewer infiltrating PMNs, and characteristically lacked staphylococcal abscess communities (Cheng et al., 2009; Fig. S3, A–H). Abscesses in animals that had been immunized with SpA or SpA-D displayed the same overall structure of lesions in mock immunized animals (Fig. S3, A–H).
Table I.
Active immunization of mice with SpA vaccines
| Antigen | Staphylococcal load and abscess formation in renal tissue | |||||
| Log10 CFU g−1a | P valueb | Reduction (log10 CFU g−1)c | IgG titerd | Number of abscessese | P-valueb | |
| S. aureus Newman challenge | ||||||
| Mock | 6.46 ± 0.25 | N/A | N/A | <100 | 3.7 ± 1.2 | N/A |
| SpA | 3.95 ± 0.56 | 0.0003 | 2.51 | 1,706 ± 370 | 2.1 ± 1.2 | 0.3581 |
| SpA-D | 4.43 ± 0.41 | 0.0001 | 2.03 | 381 ± 27 | 1.5 ± 0.8 | 0.1480 |
| SpA DKKAA | 3.39 ± 0.50 | <0.0001 | 3.07 | 5,600 ± 801 | 0.5 ± 0.4 | 0.0204 |
| S. aureus USA300 (LAC) challenge | ||||||
| Mock | 7.20 ± 0.24 | N/A | N/A | <100 | 4.0 ± 0.8 | N/A |
| SpA | 6.81 ± 0.26 | 0.2819 | 0.39 | 476 ± 60 | 3.3 ± 1.0 | 0.5969 |
| SpA-D | 6.34 ± 0.52 | 0.1249 | 0.86 | 358 ± 19 | 2.2 ± 0.6 | 0.0912 |
| SpA-DKKAA | 6.00 ± 0.42 | 0.0189 | 1.20 | 3,710 ± 1147 | 1.6 ± 0.6 | 0.0277 |
| SpAKKAA | 3.66 ± 0.76 | 0.0001 | 3.54 | 10,200 ± 2476 | 1.2 ± 0.5 | 0.0109 |
Means ± SEM of staphylococcal load calculated as log10 CFU g−1 in homogenized renal tissues 4 d after infection in cohorts of 15–20 BALB/c mice per immunization. A representative of three independent and reproducible animal experiments is shown.
Statistical significance was calculated with the unpaired two-tailed Students t test and p-values were recorded.
Reduction in bacterial load calculated as log10 CFU g−1.
Means ± SEM of five randomly chosen serum IgG titers were measured before staphylococcal infection by ELISA using SpA-DKKAA or SpA-DGGSS as antigens.
Histopathology of hematoxylin-eosin–stained thin-sectioned kidneys from 10 animals. The mean number of abscesses per kidney was recorded and averaged again for the final mean ± SEM.
We wondered whether SpA-DKKAA immunization could protect mice against MRSA strains and selected the USA300 LAC isolate for animal challenge (Diep et al., 2006). This highly virulent CA-MRSA strain spread rapidly throughout the United States, causing significant human morbidity and mortality. Compared with adjuvant control mice, SpA-DKKAA–immunized animals harbored a 1.20 log10 CFU g−1 reduction in bacterial load of infected kidney tissues. Histopathology examination of renal tissue after S. aureus USA300 challenge revealed that the mean number of abscesses was reduced from 4.0 (±0.8) to 1.6 (±0.6; P = 0.0277). In contrast, SpA or SpA-D immunization did not cause a significant reduction in bacterial load or abscess formation (Table I; and Fig. S3, I–L and N–Q).
SpA-DKKAA antibodies neutralize immunoglobulin binding activities of SpA
Rabbits were immunized with SpA-DKKAA, and specific antibodies were purified on SpA-DKKAA affinity column and analyzed by SDS-PAGE (Fig. 2 A, lane 1). SpA-DKKAA–specific IgG was cleaved with pepsin to generate Fcγ and F(ab)2 fragments (Fig. 2 A, lane 2), the latter of which were repurified by affinity chromatography on SpA-DKKAA column (Fig. 2 A, lane 3). Binding of human IgG or vWF to SpA or SpA-D was perturbed by SpA-DKKAA–specific F(ab)2, indicating that SpA-DKKAA–derived antibodies can block the Fcγ- and vWF-binding properties of SpA (Fig. 2, B and C).
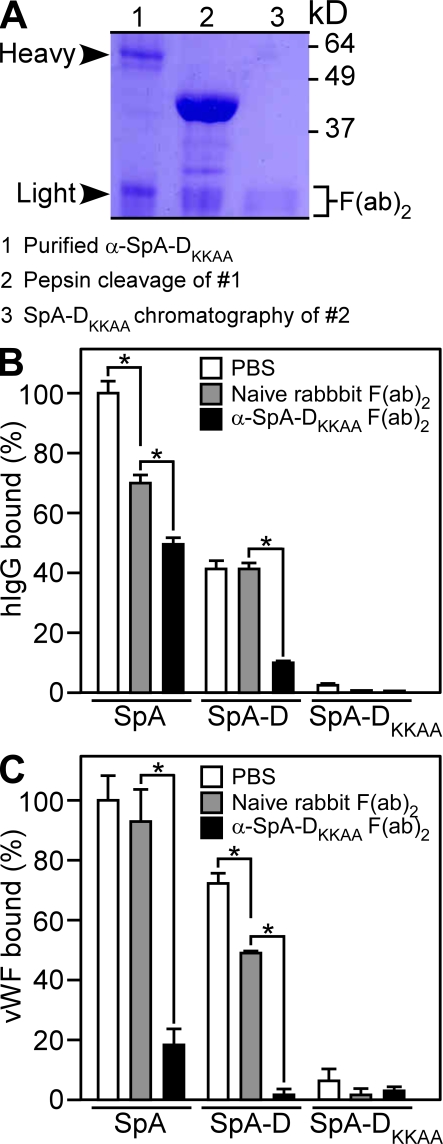
Figure 2.
Antibodies raised by the nontoxigenic SpA vaccine block the association of immunoglobulin and vWF with SpA. (A) Rabbit antibodies raised against SpA-DKKAA were affinity purified on a matrix with immobilized antigen and analyzed by Coomassie blue–stained SDS-PAGE under reducing conditions (lane 1, α-SpA-DKKAA). SpA-DKKAA antibodies were treated with pepsin to separate Fc and F(ab)2 (lane 2). The resulting F(ab)2 fragments were repurified by affinity chromatography on SpA-DKKAA (lane 3). (B and C) SpA-DKKAA specific F(ab)2 was added to wild-type SpA, SpA-D, or SpA-DKKAA, and the association with human IgG (B; n = 3) or vWF (C; n = 3) was measured. Data are the means and error bars represent ±SEM. Results in A–C are representative of three independent analyses. *, P < 0.01.
SpAKKAA generates improved protective immune responses
To further improve the vaccine properties for nontoxigenic SpA, we generated SpAKKAA, which includes all five Ig binding domains with four amino acid substitutions—Gln9Lys, Gln10Lys, Asp36Ala, and Asp37Ala—in each of its five domains (E, D, A, B, and C; Sjödahl, 1977). Polyhistidine-tagged SpAKKAA was purified by affinity chromatography and analyzed by Coomassie blue–stained SDS-PAGE (Fig. 3 A). Unlike full-length SpA, SpAKKAA did not bind human IgG, Fc and F(ab)2, IgM, or vWF (Fig. 3 B). SpAKKAA failed to display B cell superantigen activity, as injection of the variant into BALB/c or C57BL/6 mice did not cause a depletion of both CD19+ and CD45R+ B cells in splenic tissue (Fig. 3 C; Fig. S2, C and D; and not depicted). SpAKKAA immunization generated higher specific antibody titers than SpA-DKKAA and provided mice with elevated protection against S. aureus USA300 (Table I) or Mu50 challenge (Fig. S3 U; Mu50 is a Japanese MRSA isolate). 4 d after challenge, SpAKKAA-vaccinated animals harbored 3.54 log10 CFU g−1 fewer staphylococci in renal tissues (P = 0.0001) and also caused a greater reduction in the number of abscess lesions (P = 0.0109; Table I; and Fig. S3, I–R). Furthermore, SpAKKAA immunization reduced the mortality of mice that received a lethal S. aureus challenge dose (Fig. S1 B).
Staphylococci persist in mouse organ tissues (Cheng et al., 2009). Over time, abscesses harboring communities of the infectious agent increase in size and eventually rupture, thereby releasing staphylococci into circulation. This initiates the formation of new abscesses and precipitates lethal outcomes 30–60 d after challenge (Cheng et al., 2009). We asked whether SpA-DKKAA or SpAKKAA immunization prevents staphylococcal replication over a longer period of time. 15 d after challenge with S. aureus USA300, immunization with SpA did not generate significant protection of animals compared with the mock control (P = 0.5817; Table S1). In contrast, immunization with SpA-DKKAA caused a 1.56 log10 CFU g−1 reduction (P = 0.0183), whereas SpAKKAA vaccination reduced the load by 2.7 log10 CFU g−1 (P = 0.0059). These data suggest that antibodies against SpA, generated via active immunization using nontoxigenic SpAKKAA, can interfere with bacterial persistence in host tissues.
SpA-specific antibodies generate protective immunity
SpAKKAA was used to immunize rabbits. Rabbit antibodies specific for SpA-DKKAA or SpAKKAA were affinity purified on matrices with immobilized cognate antigen and injected at a concentration of 5 mg × kg−1 body weight into the peritoneal cavity of BALB/c mice (Table II). 24 h later, antibody titers specific for SpA-DKKAA/SpAKKAA were determined in serum and animals challenged by intravenous inoculation with S. aureus Newman. Passive transfer reduced the staphylococcal load in kidney tissues for SpA-DKKAA- (P = 0.0016) or SpAKKAA (P = 0.0005)-specific antibodies. On histopathology examination, both antibodies reduced the abundance of abscess lesions in kidneys of mice challenged with S. aureus Newman (Table II). Compared with control cohorts treated with nonspecific antibody (α-V10), animals that had been injected with SpA-specific antibodies were protected against the B cell superantigen activity of SpA (Fig. S2, C and D). In addition, SpA-specific antibodies (2 µg × ml−1) induced opsonophagocytic clearance of S. aureus Newman inoculated into naive mouse blood (Fig. 3 D) and reduced the mortality associated with lethal staphylococcal challenge (Fig. S1 C). Together these data reveal that disease protection after immunization with SpA-DKKAA or SpAKKAA is conferred by antibodies that bind SpA and neutralize its ability to bind Ig.
Table II.
Passive immunization of mice with antibodies against SpA
| Antibodya | Staphylococcal load and abscess formation in renal tissue | |||||
| Log10 CFU g−1b | P valuec | Reduction (log10 CFU g−1)d | IgG titere | Number of abscessesf | P-valuec | |
| Mock (α-V10) | 7.10 ± 0.14 | N/A | N/A | <100 | 4.5 ± 0.8 | N/A |
| α-SpA-DKKAA | 5.53 ± 0.43 | 0.0016 | 1.57 | 466 ± 114 | 1.9 ± 0.7 | 0.0235 |
| α-SpAKKAA | 5.69 ± 0.34 | 0.0005 | 1.41 | 1,575 ± 152 | 1.6 ± 0.5 | 0.0062 |
Affinity-purified antibodies were injected into the peritoneal cavity of BALB/c mice at a concentration of 5 mg × kg−1 24 h before intravenous challenge with 1 × 107 CFU S. aureus Newman.
Means ± SEM of staphylococcal load calculated as log10 CFU g−1 in homogenized renal tissues 4 d after infection in cohorts of 15 BALB/c mice per immunization. A representative of three independent and reproducible animal experiments is shown.
Statistical significance was calculated with the unpaired two-tailed Students t test and p-values were recorded.
Reduction in bacterial load calculated as log10 CFU g−1.
Means ± SEM of five randomly chosen serum IgG titers were measured before staphylococcal infection by ELISA.
Histopathology of hematoxylin-eosin–stained thin-sectioned kidneys from 10 animals. The mean number of abscesses per kidney was recorded and averaged again for the final mean ± SEM.
SpAKKAA immunization promotes host antibody response to staphylococcal infection
After infection with virulent S. aureus Newman and clearance of the pathogen with antibiotic treatment, mice do not develop protective immunity against subsequent infection with the same strain (Fig. S3 S). The mean abundance of SpA-DKKAA–specific IgG in these animals was determined by dot blot as 0.20 µg ml−1 (±0.04) and 0.14 µg ml−1 (±0.01) for infections caused by S. aureus strains Newman and USA300 LAC, respectively (Fig. 4 A). A concentration of 4.05 µg ml−1 (±0.88) for SpA-specific IgG was estimated to confer disease protection in SpAKKAA-or SpA-DKKAA–immunized mice (P ≤ 0.05 log10 reduction in staphylococcal CFU g−1 renal tissue; unpublished data). The mean serum concentration of SpA-specific IgG in adult healthy human volunteers (n = 16) was 0.21 µg ml−1 (±0.02). Such antibody concentration may not be sufficient to generate protection against staphylococcal infection. By comparison, the mean serum concentration of IgG specific for diphtheria toxin in human volunteers, 0.68 µg ml−1 (± 0.20), is thought to be within range for protective immunity against diphtheria (Lagergård et al., 1992).
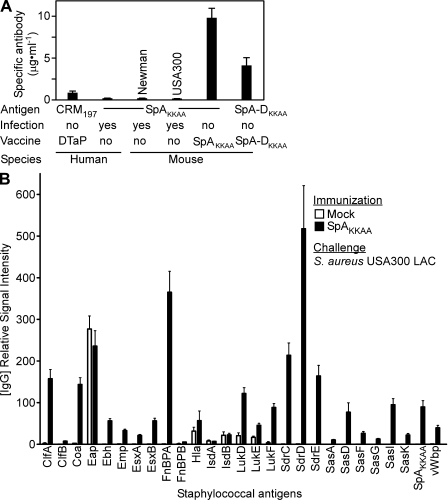
Figure 4.
SpAKKAA immunization modifies host immune responses to staphylococcal infection. (A) Human or mouse serum antibody titers to diphtheria toxoid (CRM197) and nontoxigenic SpAKKAA or SpA-DKKAA. Human volunteers with a history of diphtheria (DTaP) immunization and staphylococcal infection (n = 16), as well as mice (n = 20) that had been infected with S. aureus Newman or USA 300 LAC or immunized with SpAKKAA or SpA-DKKAA, were examined by quantitative immunoblot. (B) Cohorts of BALB/c mice (n = 15) were immunized with SpAKKAA or with PBS/adjuvant control (mock) and then challenged by intravenous inoculation with 5 × 106 CFU S. aureus USA300 LAC. 30 d after infection, animals were bled and serum samples were analyzed for antibody responses to staphylococcal antigens. 27 recombinant His6-tagged staphylococcal proteins were purified by Ni-NTA affinity chromatography and immobilized on nitrocellulose membrane at 2 µg. Signal intensities in sera from mice were quantified and normalized by infrared imaging. Data are the means and error bars represent ±SEM. Results in A and B are representative of three independent analyses.
These results are in agreement with a model of immune evasion during S. aureus infection. Cell wall–anchored or secreted SpA (e.g., 20% of peptidoglycan and attached surface protein is released during bacterial division; Ton-That et al., 1999) activate B cells via IgM receptor cross-linking. Without stimuli from specific antigens, activated B cells undergo apoptosis, thereby hindering the production of antibody against staphylococcal antigens. If so, neutralizing antibodies directed against SpA may enable humoral immune responses against many different staphylococcal antigens. This was tested by immunizing BALB/c mice with SpAKKAA or an adjuvant (aluminum hydroxide) control, followed by intravenous challenge with a sublethal dose of MRSA strain USA300. Serum samples were withdrawn 30 d after MRSA challenge and then analyzed by immunoblotting with 27 staphylococcal antigens immobilized on a membrane filter (Fig. 4 B). Naive mice, which had not been infected with the MRSA strain USA300 LAC, did not harbor antibodies against staphylococcal antigens (unpublished data). Mock immunized mice (adjuvant only) that had been subjected to USA300 infection developed high-titer antibodies against the Eap protein as well as low-titer antibodies against Hla, IsdA, IsdB, LukD, LukE, and LukF (Fig. 4 B). In response to USA300 challenge, animals that had been immunized with SpAKKAA (IgG titer 2,907 [±357]; P < 0.001, SpAKKAA vs. mock) mounted humoral immune responses against every antigen examined (Fig. 4 B). With the exception of Eap, IsdA, and IsdB antibodies, the serum of SpAKKAA-immunized animals harbored higher antibody titers against staphylococcal antigens as compared with mice that had been naive at the time of challenge (Fig. 4 B).
In summary, S. aureus isolates express SpA, an essential virulence factor whose B cell superantigen activity and evasive attributes toward opsonophagocytic clearance are required for staphylococcal abscess formation and the establishment of lethal disease (Cheng et al., 2009). SpA can be thought of as a toxin that is essential for pathogenesis and whose molecular attributes must be neutralized to achieve protective immunity. By generating nontoxigenic variants unable to bind Igs via Fcγ or VH3-Fab domains, we identified SpA-neutralizing immune responses as a correlate for protective immunity against S. aureus infection. In contrast to many methicillin-sensitive strains, the CA-MRSA isolate USA300 LAC is significantly more virulent (Cheng et al., 2009). For example, immunization of experimental animals with the surface protein IsdB (Kuklin et al., 2006) raises antibodies that confer protection against S. aureus Newman (Kuklin et al., 2006) but not against USA300 challenge (Fig. S3 T). In contrast, neutralizing SpA antibody responses generate protection against strains of the current MRSA epidemic. These antibodies exert at least two functions. As shown in Fig. 3, the SpA antibodies enable phagocytic killing of staphylococci in blood. Moreover, by neutralizing B cell superantigen activity, SpA antibodies enable the development of humoral immune responses to many different antigens that, assuming synergism, may together contribute toward the establishment of immunity. In agreement with this, the SpAKKAA vaccine elicited greater protection against abscess formation, which monitors infected animals over a prolonged period of time (Fig. 4), as compared with the lethal challenge, when most animals die within 1–2 d (Fig. S1).
MATERIALS AND METHODS
Antibody isolation.
5 mg of protein was covalently linked to HiTrap NHS-activated HP and loaded with rabbit serum. Antibodies were eluted with 1 M glycine, pH 2.5, and 0.5 M NaCl, neutralized with 1 M Tris-HCl, pH 8.5, and dialyzed against PBS. Affinity-purified antibodies were mixed with 3 mg pepsin at 37°C for 30 min and quenched with 1 M Tris-HCl, pH 8.5. F(ab)2 fragments were again affinity purified, dialyzed against PBS at 4°C, separated by 15% SDS-PAGE, and visualized with Coomassie Blue.
Active and passive immunization.
The coding sequence for SpA was PCR amplified with two primers, 5′-GCTGCACATATGGCGCAACACGATGAAGCTCAAC-3′ and 5′-AGTGGATCCTTATGCTTGAGCTTTGTTAGCATCTGC-3′, using S. aureus Newman DNA. SpA-D was PCR amplified with two primers: 5′-AACATATGTTCAACAAAGATCAACAAAGC-3′ and 5′-AAGGATCCAGATTCGTTTAATTTTTTAGC-3′. The sequence for SpA-DKKAA was mutagenized with two sets of primers: 5′-CATATGTTCAACAAAGATAAAAAAAGCGCCTTCTATGAAATC-3′ and 5′-GATTTCATAGAAGGCGCTTTTTTTATCTTTGTTGAACATATG-3′ for Q9K and Q10K, and 5′-CTTCATTCAAAGTCTTAAAGCCGCCCCAAGCCAAAGCACTAAC-3′ and 5′-GTTAGTGCTTTGGCTTGGGGCGGCTTTAAGACTTTGAATGAAG-3′ for D36A and D37A. The sequence of SpAKKAA was synthesized by Integrated DNA Technologies, Inc. PCR products were cloned into pET-15b generating N-terminal His6-tagged recombinant protein. BALB/c mice were immunized by intramuscular injection and boosted with the same antigen after 11 d. On day 20, mice were bled to obtain serum for specific antibody titers. Affinity-purified antibodies were injected into the peritoneal cavity of BALB/c mice at 4–24 h, and blood was collected for specific antibody titers before challenge.
Mouse infection.
Staphylococci were used to infect anesthetized mice by retroorbital injection (1 × 107 CFU S. aureus Newman, 5 × 106 CFU S. aureus USA300, or 3 × 107 CFU S. aureus Mu50). On day 4, 15, or 30, mice were killed, kidneys removed, and homogenized tissue spread on agar for colony formation. Animal experiments were performed in agreement with the institutional guidelines according to experimental protocol review and approval by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at the University of Chicago.
Bacterial survival in blood.
As previously described (Thammavongsa et al., 2009), whole blood was collected from BALB/c mice with 5 µg ml−1 of lepirudin anticoagulant. 50 µl of 5 × 104–6 CFU ml−1 S. aureus Newman were mixed with 950 µl of mouse blood. Samples were incubated at 37°C with slow rotation for 30 min and then incubated on ice with 1% saponin/PBS. Dilutions of staphylococci were plated on agar for colony formation.
SpA ligands.
1 µg ml−1 of purified SpA and its variants were coated onto ELISA plates in 0.1 M carbonate buffer, pH 9.5. Plates were incubated with peroxidase-conjugated human IgG, Fc, or F(ab)2 fragments, IgM (The Jackson Laboratory), and vWF (Thermo Fisher Scientific) and developed using OptEIA reagent. For inhibition, plates were incubated with either naive rabbit F(ab)2 fragments (The Jackson Laboratory) or 10 µg ml−1 of affinity-purified F(ab)2 fragments before ligand binding.
B cell apoptosis.
150 µg of purified protein was injected into the peritoneum of 6-wk-old BALB/c. 4 h after injection, animals were killed and spleens removed and homogenized. Red blood cells were lysed in ACK buffer. White blood cells were stained with R-PE–conjugated anti-CD19 (eBioscience). Cells were washed and fixed with formalin and analyzed by FACSCanto (BD).
Antibody quantification.
Nitrocellulose membrane was blotted with human/mouse IgG (The Jackson Laboratory), SpAKKAA, and CRM197, blocked, and incubated with either human or mouse sera. IRDye 700DX–conjugated anti–human/mouse IgG (Rockland Immunochemicals, Inc.) was used to quantify signal intensities from healthy human volunteers or mice using the Odyssey infrared imaging system (LI-COR Biosciences). Experiments with blood from human volunteers involved protocols that were reviewed, approved, and performed under regulatory supervision of The University of Chicago’s Institutional Review Board. For the staphylococcal antigen matrix, nitrocellulose membrane was blotted with 2 µg of a collection of Ni-NTA affinity-purified recombinant His6-tagged staphylococcal proteins. Signal intensities in mouse sera were quantified and normalized using anti-His6 antibody with the Odyssey.
Statistical Analysis.
Unpaired two-tailed Student’s t tests were performed to analyze the statistical significance of renal abscess, ELISA, and B cell superantigen data.
Online supplemental material.
Fig. S1 shows that SpA is a virulence factor for lethal infection after intraperitoneal injection of S. aureus Newman into BALB/c mice and that active or passive immunization with antibodies raised against SpAKKAA can protect against this disease. Fig. S2 shows that SpAKKAA, unlike wild-type SpA, does not induce B cell apoptosis in mice and that antibodies raised against SpAKKAA neutralize the B cell superantigen attributes of SpA. Fig. S3 shows that immunization of mice with SpAKKAA prevents multiple S. aureus strains from inducing renal abscess formation in mice and that prior infection with S. aureus does not elicit immunity to subsequent infection with the same strain. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20092514/DC1.
Acknowledgments
We thank Matt Frankel and Vilasack Thammavongsa for experimental assistance and others members of our laboratory for discussion.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases, Infectious Diseases Branch (AI52474 to O. Schneewind and AI75258 to D.M. Missiakas) and by Novartis Vaccines and Diagnostics (Siena, Italy). A.G. Cheng was a trainee of the National Institutes of Health (NIH) Medical Scientist Training Program at The University of Chicago (GM07281). D.M. Missiakas and O. Schneewind acknowledge membership within and support from the Region V Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- MRSA
- methicillin-resistant S. aureus
- SpA
- staphylococcal protein A
- vWF
- von Willebrand factor
References
- Berger-Bächi B. 1994. Expression of resistance to methicillin. Trends Microbiol. 2:389–393 10.1016/0966-842X(94)90617-3 [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J., Palazzolo-Ballance A.M., Otto M., Schneewind O., DeLeo F.R. 2008. Panton-Valentine leukocidin is not a virulence determinant in murine models of community-associated methicillin-resistant Staphylococcus aureus disease. J. Infect. Dis. 198:1166–1170 10.1086/592053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Sievert D.M., Hageman J.C., Boulton M.L., Tenover F.C., Downes F.P., Shah S., Rudrik J.T., Pupp G.R., Brown W.J., et al. ; Vancomycin-Resistant Staphylococcus aureusInvestigative Team 2003. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348:1342–1347 10.1056/NEJMoa025025 [DOI] [PubMed] [Google Scholar]
- Cheng A.G., Kim H.K., Burts M.L., Krausz T., Schneewind O., Missiakas D.M. 2009. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23:3393–3404 10.1096/fj.09-135467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G.P., Tomlinson I.M. 1995. The human immunoglobulin VH repertoire. Immunol. Today. 16:237–242 10.1016/0167-5699(95)80166-9 [DOI] [PubMed] [Google Scholar]
- de Haas C.J., Veldkamp K.E., Peschel A., Weerkamp F., Van Wamel W.J., Heezius E.C., Poppelier M.J., Van Kessel K.P., van Strijp J.A. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199:687–695 10.1084/jem.20031636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. 1981. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 20:2361–2370 10.1021/bi00512a001 [DOI] [PubMed] [Google Scholar]
- Diep B.A., Gill S.R., Chang R.F., Phan T.H., Chen J.H., Davidson M.G., Lin F., Lin J., Carleton H.A., Mongodin E.F., et al. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 367:731–739 10.1016/S0140-6736(06)68231-7 [DOI] [PubMed] [Google Scholar]
- Forsgren A. 1970. Significance of protein a production by staphylococci. Infect. Immun. 2:672–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Quie P.G. 1974. Effects of staphylococcal protein A on heat labile opsonins. J. Immunol. 112:1177–1180 [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. 1976. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur. J. Immunol. 6:207–213 10.1002/eji.1830060312 [DOI] [PubMed] [Google Scholar]
- Gómez M.I., Lee A., Reddy B., Muir A., Soong G., Pitt A., Cheung A., Prince A. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10:842–848 10.1038/nm1079 [DOI] [PubMed] [Google Scholar]
- Gómez M.I., O’Seaghdha M., Magargee M., Foster T.J., Prince A.S. 2006. Staphylococcus aureus protein A activates TNFR1 signaling through conserved IgG binding domains. J. Biol. Chem. 281:20190–20196 10.1074/jbc.M601956200 [DOI] [PubMed] [Google Scholar]
- Goodyear C.S., Silverman G.J. 2003. Death by a B cell superantigen: In vivo VH-targeted apoptotic supraclonal B cell deletion by a Staphylococcal Toxin. J. Exp. Med. 197:1125–1139 10.1084/jem.20020552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear C.S., Silverman G.J. 2004. Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like B lymphocytes. Proc. Natl. Acad. Sci. USA. 101:11392–11397 10.1073/pnas.0404382101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda H., Shiraishi M., Takahashi H., Kato K., Torigoe H., Arata Y., Shimada I. 1998. NMR study of the interaction between the B domain of staphylococcal protein A and the Fc portion of immunoglobulin G. Biochemistry. 37:129–136 10.1021/bi970923f [DOI] [PubMed] [Google Scholar]
- Graille M., Stura E.A., Corper A.L., Sutton B.J., Taussig M.J., Charbonnier J.B., Silverman G.J. 2000. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibody: structural basis for recognition of B-cell receptors and superantigen activity. Proc. Natl. Acad. Sci. USA. 97:5399–5404 10.1073/pnas.97.10.5399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg D.P., Bayer A.S., Cheung A.L., Ward J.I. 1989. Protective efficacy of protein A-specific antibody against bacteremic infection due to Staphylococcus aureus in an infant rat model. Infect. Immun. 57:1113–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guss B., Uhlén M., Nilsson B., Lindberg M., Sjöquist J., Sjödahl J. 1984. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur. J. Biochem. 138:413–420 10.1111/j.1432-1033.1984.tb07931.x [DOI] [PubMed] [Google Scholar]
- Hartleib J., Köhler N., Dickinson R.B., Chhatwal G.S., Sixma J.J., Hartford O.M., Foster T.J., Peters G., Kehrel B.E., Herrmann M. 2000. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood. 96:2149–2156 [PubMed] [Google Scholar]
- Jensen K. 1958. A normally occurring staphylococcus antibody in human serum. Acta Pathol. Microbiol. Scand. 44:421–428 [DOI] [PubMed] [Google Scholar]
- Klevens R.M., Morrison M.A., Nadle J., Petit S., Gershman K., Ray S., Harrison L.H., Lynfield R., Dumyati G., Townes J.M., et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 298:1763–1771 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- Klevens R.M., Edwards J.R., Gaynes R.P., System N.N.I.S.; National Nosocomial Infections Surveillance System 2008. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin. Infect. Dis. 47:927–930 10.1086/591698 [DOI] [PubMed] [Google Scholar]
- Kuklin N.A., Clark D.J., Secore S., Cook J., Cope L.D., McNeely T., Noble L., Brown M.J., Zorman J.K., Wang X.M., et al. 2006. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect. Immun. 74:2215–2223 10.1128/IAI.74.4.2215-2223.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagergård T., Trollfors B., Claesson B.A., Karlberg J., Taranger J. 1992. Determination of neutralizing antibodies and specific immunoglobulin isotype levels in infants after vaccination against diphtheria. Eur. J. Clin. Microbiol. Infect. Dis. 11:341–345 10.1007/BF01962074 [DOI] [PubMed] [Google Scholar]
- Lindmark R., Thorén-Tolling K., Sjöquist J. 1983. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J. Immunol. Methods. 62:1–13 10.1016/0022-1759(83)90104-7 [DOI] [PubMed] [Google Scholar]
- Lowy F.D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- Mazmanian S.K., Liu G., Ton-That H., Schneewind O. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science. 285:760–763 10.1126/science.285.5428.760 [DOI] [PubMed] [Google Scholar]
- Palmqvist N., Foster T.J., Tarkowski A., Josefsson E. 2002. Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death. Microb. Pathog. 33:239–249 10.1006/mpat.2002.0533 [DOI] [PubMed] [Google Scholar]
- Peterson P.K., Verhoef J., Sabath L.D., Quie P.G. 1977. Effect of protein A on staphylococcal opsonization. Infect. Immun. 15:760–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooijakkers S.H., Ruyken M., Roos A., Daha M.R., Presanis J.S., Sim R.B., van Wamel W.J., van Kessel K.P., van Strijp J.A. 2005. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 6:920–927 10.1038/ni1235 [DOI] [PubMed] [Google Scholar]
- Sasso E.H., Silverman G.J., Mannik M. 1989. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J. Immunol. 142:2778–2783 [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V.A. 1992. Sorting of protein A to the staphylococcal cell wall. Cell. 70:267–281 10.1016/0092-8674(92)90101-H [DOI] [PubMed] [Google Scholar]
- Schneewind O., Fowler A., Faull K.F. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 268:103–106 10.1126/science.7701329 [DOI] [PubMed] [Google Scholar]
- Shopsin B., Gomez M., Montgomery S.O., Smith D.H., Waddington M., Dodge D.E., Bost D.A., Riehman M., Naidich S., Kreiswirth B.N. 1999. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J. Clin. Microbiol. 37:3556–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman G.J., Goodyear C.S. 2006. Confounding B-cell defences: lessons from a staphylococcal superantigen. Nat. Rev. Immunol. 6:465–475 10.1038/nri1853 [DOI] [PubMed] [Google Scholar]
- Sjödahl J. 1977. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur. J. Biochem. 73:343–351 10.1111/j.1432-1033.1977.tb11324.x [DOI] [PubMed] [Google Scholar]
- Sjöquist J., Meloun B., Hjelm H. 1972. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur. J. Biochem. 29:572–578 10.1111/j.1432-1033.1972.tb02023.x [DOI] [PubMed] [Google Scholar]
- Thammavongsa V., Kern J.W., Missiakas D.M., Schneewind O. 2009. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 206:2417–2427 10.1084/jem.20090097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton-That H., Liu G., Mazmanian S.K., Faull K.F., Schneewind O. 1999. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl. Acad. Sci. USA. 96:12424–12429 10.1073/pnas.96.22.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]