Abstract
Recent studies have shown that a carbohydrate-binding protein, galectin-3, is a novel pro-angiogenic molecule. The mechanism by which galectin-3 promotes angiogenesis remains unknown. We demonstrate here that galectin-3 is a mediator of vascular endothelial growth factor (VEGF)- and basic fibroblast growth factor (bFGF)-mediated angiogenic response. Angiogenesis assays revealed that galectin-3 inhibitors, β-lactose and dominant-negative galectin-3, reduce VEGF- and bFGF-mediated angiogenesis in vitro and that VEGF- and bFGF-mediated angiogenic response is reduced in galectin-3 knockdown cells and Gal3−/− animals. Integrin αvβ3 was identified as the major galectin-3–binding protein and anti-αv, -β3, and -αvβ3 integrin function-blocking antibodies significantly inhibited the galectin-3–induced angiogenesis. Furthermore, galectin-3 promoted the clustering of integrin αvβ3 and activated focal adhesion kinase. Knockdown of GnTV, an enzyme that synthesizes high-affinity glycan ligands for galectin-3, substantially reduced: (a) complex N-glycans on αvβ3 integrins and (b) VEGF- and bFGF-mediated angiogenesis. Collectively, these data suggest that galectin-3 modulates VEGF- and bFGF-mediated angiogenesis by binding via its carbohydrate recognition domain, to the GnTV synthesized N-glycans of integrin αvβ3, and subsequently activating the signaling pathways that promote the growth of new blood vessels. These findings have broad implications for developing novel, carbohydrate-based therapeutic agents for inhibition of angiogenesis.
Angiogenesis, the formation of new blood vessels from preexisting vasculature, is a tightly regulated physiological process required for embryonic development (Breier, 2000), reproductive function (Fraser and Lunn, 2000), and wound healing (Tonnesen et al., 2000). Angiogenesis is also a key factor in the pathogenesis of several clinical conditions, including ocular diseases, such as age-related macular degeneration (Kulkarni and Kuppermann, 2005), diabetic retinopathy (Gardner et al., 2002), and corneal graft failure (Price et al., 2003), and nonocular diseases, such as cancer (Carmeliet and Jain, 2000) and rheumatoid arthritis (Koch, 1998). The process begins when the endothelial cells of a mature blood vessel wall are activated by angiogenic factors, that include, but are not limited to, basic fibroblast growth factor (bFGF) and the vascular endothelial growth factor (VEGF) families of cytokines (Cross and Claesson-Welsh, 2001). Activation promotes the loosening of endothelial cells from their basement membrane and the supporting periendothelial cells, thereby allowing them to migrate, proliferate, and ultimately form a capillary lumen, which is stabilized by pericytes and smooth muscle cells.
Galectin-3 is a member of the galectin family of mammalian lectins characterized by a conserved sequence within the carbohydrate recognition domain (CRD) that has affinity for β-galactoside structures. Extracellularly, the lectin is assumed to mediate cell–cell and cell–matrix interactions by binding to lactosamine-containing cell surface glycoconjugates via the CRD. Recently, galectin-3 has been implicated in the process of angiogenesis. Studies by Nangia-Makker et al. (2000) have shown that tumor angiogenesis, induced by subcutaneous injections with breast carcinoma cell in an animal model, is significantly greater when the carcinoma cells express galectin-3 as compared with galectin-3–null controls. These authors have further shown that exogenous galectin-3 promotes endothelial cell migration and capillary tubule formation in vitro. The mechanism by which galectin-3 influences angiogenesis, however, remains elusive. Binding assays predict two receptors on human umbilical vein endothelial cells (HUVEC), one of high affinity (Kd = 0.537 × 10−9) and the other of low affinity (Kd = 7.161 × 10−9; Nangia-Makker et al., 2000). The identity of these receptors, however, remains unknown. Yang et al. (2007), using a phage display biopanning method, identified galectin-3 as a binding partner for aminopeptidase N/CD13 (APN) in endothelial cells and suggested that the lectin may mediate angiogenesis via APN. However, because of its short cytoplasmic domain, APN is unlikely to singularly initiate galectin-3–mediated activation of endothelial cells (Yang et al., 2007). It is not known whether galectin-3 promotes angiogenesis independently of the action of angiogenic cytokines or whether galectin-3 contributes to the function of the known angiogenic molecules. One study has shown that modified citrus pectin (MCP), a galactose-rich polysaccharide that binds to galectin-3, and possibly also to other members of the galectin family, reduces bFGF-mediated migration of endothelial cells, suggesting that one or more members of the galectin family may participate in bFGF-mediated angiogenesis (Nangia-Makker et al., 2002). Thus far, more direct studies involving the use of galectin-3 knockout mice and cells have not been performed.
In this study, we investigate whether galectin-3 contributes to VEGF- and bFGF-mediated angiogenesis. We show that a decrease in the expression of galectin-3 by siRNA knockdown results in the reduction of angiogenic response to VEGF and bFGF in vitro and that VEGF- and bFGF-mediated angiogenesis in vivo is reduced in Gal3−/− mice. We further demonstrate that: (a) αvβ3 integrin is the major galectin-3–binding protein; (b) galectin-3 activates αvβ3 integrin signaling; and (c) carbohydrate-mediated interaction between galectin-3 and complex N-glycans on αvβ3 integrin plays a key role in galectin-3–mediated angiogenesis.
RESULTS
Galectin-3 promotes angiogenesis in vivo: the C-terminal carbohydrate recognition domain and the N-terminal self-association domain are required for galectin-3–mediated angiogenesis
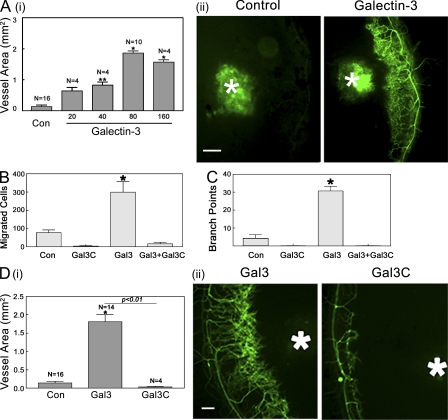
A previous study has shown that galectin-3 promotes migration and capillary tubule formation of HUVECs in vitro and that tumor angiogenesis induced by subcutaneous injections with breast carcinoma cells in mice is significantly greater when the carcinoma cells express galectin-3 compared with galectin-3–null cells (Nangia-Makker et al., 2000). Here, we show that galectin-3 directly promotes angiogenesis in vivo. Galectin-3–mediated angiogenesis in vivo was evaluated by the mouse corneal micropocket assay. The vessel area, representing the extent of angiogenesis, was calculated 5 d after pellets containing various concentrations of galectin-3 were implanted in the mouse corneas. In the concentration range tested (20–160 ng galectin-3/pellet), the extent of angiogenesis increased in a dose-dependent manner up to 80 ng/pellet. At concentrations >80 ng/pellet, there was no further increase in the degree of angiogenesis (Fig. 1 A; Vessel Area, Control [n = 16; 0.14 ± 0.051 mm2]; Galectin-3: 20 ng [n = 4; 0.64 ± 0.225 mm2], 40 ng [n = 4; 0.82 ± 0.195 mm2], 80 ng [n = 10; 1.86 ± 0.225 mm2], and 160 ng [n = 4; 1.57 ± 0.15 mm2]).
Figure 1.
Galectin-3 promotes angiogenesis in vivo in a dose-dependent manner. (A) Angiogenesis in vivo was evaluated using the mouse corneal micropocket assay. Sustained-release polymer pellets containing various doses of galectin-3 (20–160 ng/pellet) were implanted in the corneas of Gal3+/+ animals. (i) 5 d after surgery, the vessel area was calculated as described in the text. Data are expressed as mean ± SEM (n = 4 or more). *, P < 0.01 compared with control pellets (Con) without any protein. (ii) Representative fluorescence images of corneas are shown in A (i). White asterisks indicate pellets. (B–D) Full-length galectin-3 but not truncated galectin-3 (Gal3C) promotes angiogenesis in vitro and in vivo. (B) Modified Boyden Chamber Assay. The cells that migrated from the upper chamber to the lower chamber during the period of 6 h in response to the protein gradients created by full-length galectin-3 and/or Gal3C were counted in three nonoverlapping fields under a microscope (320×). Data are expressed as mean ± SEM (n = 3/group). *, P < 0.01 compared with cells incubated with media alone (Con). (C) Capillary tubule formation assay. HUVECs premixed with galectin-3 and/or Gal3C were plated on solidified matrigel (4.5 mg/ml) and after 6-h incubation, the extent of capillary tubule formation was evaluated by counting the number of branch points. Data are expressed as mean ± SEM (n = 3 or more/group). *, P < 0.01 compared with cells incubated with media alone (Con). (D) Angiogenesis in vivo was evaluated using the mouse corneal micropocket assay using pellets containing full-length (Gal3) and truncated galectin-3 (Gal3C; 80 ng/pellet). Data are expressed as mean ± SEM (n = 4 or more). *, P < 0.01 compared with control pellets (Con). Representative fluorescence images of corneas are shown in D (ii). White asterisks indicate pellets. Bars, 200 µm. Data are representative of three or more independent experiments.
Galectin-3 (∼30 kD) is made up of a C-terminal CRD, a collagen-like internal R-domain, and the N-terminal domain that promotes oligomerization of the lectin. Cleavage of the N-terminal domain by collagenase digestion of R-domain gives rise to a 21-kD C-terminal domain of galectin-3 (Gal3C) that contains the entire CRD and retains its ability to bind to lactosamine-containing glycans. Therefore, Gal3C competes with the carbohydrate-binding ability of the endogenous galectin-3 and acts as a dominant-negative inhibitor of full-length galectin-3. However, because Gal3C lacks the N-domain, it is unable to form dimers or higher order oligomers (Hirabayashi et al., 2002; John et al., 2003). To determine whether the angiogenic property of galectin-3 is dependent on its ability to oligomerize and act in a bi-/multi-valent manner, we tested whether Gal3C was able to promote angiogenesis in vitro and in vivo. Unlike full-length galectin-3, Gal3C failed to induce migration and capillary tubule formation of endothelial cells in vitro (Fig. 1, B and C) and angiogenesis in vivo (Fig. 1 D). Moreover, Gal3C effectively inhibited in vitro angiogenesis advanced by full-length galectin-3 (Fig. 1, B and C; percentage of inhibition: migration, 95.2 ± 0.003%; capillary tubule formation, 99.6 ± 0.0002%). These results suggest that multivalent galectin-3 assembly by the N-terminal domain and the carbohydrate recognition domain are both required for galectin-3–mediated angiogenesis.
Galectin-3 promotes angiogenesis by influencing the function of VEGF and bFGF in a carbohydrate-dependent manner
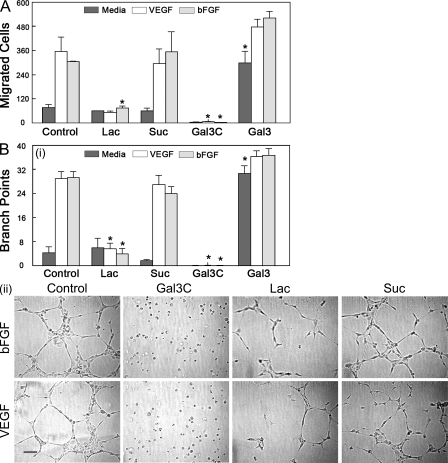
Multiple approaches were used to investigate whether galectin-3 influences the function of well-known angiogenic molecules, VEGF, and bFGF. First, we tested the effect of dominant-negative galectin-3, Gal3C, on VEGF- and bFGF-induced angiogenesis. Addition of Gal3C completely blocked VEGF- and bFGF-mediated endothelial cell migration (Fig. 2 A) and capillary tubule formation (Fig. 2 B). This suggests that not only is galectin-3 required for VEGF- and bFGF-mediated angiogenesis in vitro, but also that galectin-3 multimer formation is necessary for VEGF- and bFGF-mediated angiogenesis. Both VEGF- and bFGF-induced endothelial cell migration and capillary tubule formation were markedly reduced by a competing saccharide, β-lactose (percentage of reduction: cell migration, VEGF, 85.15 ± 1.89; bFGF, 75.53 ± 3.10; capillary tubule formation, VEGF, 80.46 ± 6.40; bFGF, 86.36 ± 5.91). In contrast, a noncompeting disaccharide, sucrose, had no effect. The inhibition was not a result of decreased cell viability. As assessed by trypan blue exclusion, cell viability was unchanged in the presence of saccharides (unpublished data). These data suggest galectin-3–mediated carbohydrate-based recognition also plays a role in the VEGF- and bFGF-induced angiogenesis.
Figure 2.
Galectin-3 inhibitors block VEGF- and bFGF-mediated angiogenesis in vitro. (A) Modified Boyden chamber assay. The effect of galectin-3 inhibitors on VEGF- and bFGF-induced cell migration was evaluated. The cells that migrated from the upper chamber to the lower chamber were counted as described in Fig. 1. (B) Capillary tubule formation assay. HUVEC suspensions premixed with cytokines were plated on solidified matrigel (4.5 mg/ml), and, after 6-h incubation, the extent of capillary tubule formation was evaluated by counting the number of branch points. Representative images from each group are shown in B (ii). Images of cells incubated in media alone (not depicted) were indistinguishable from those incubated in media containing VEGF or bFGF in the presence of β-lactose (Lac). The assays were performed in the absence (Control) and presence of truncated galectin-3 (Gal3C, a dominant-negative inhibitor of galectin-3), full-length galectin-3 (Gal3), 0.1 M β-lactose (Lac, a competing saccharide), and 0.1 M sucrose (Suc, a noncompeting control saccharide). Data are expressed as mean ± SEM (n = 3 or more/group), *, P < 0.01; **, P < 0.05 compared with respective controls incubated without galectin-3 inhibitors (Control) or with a noncompeting saccharide (Suc). Bar, 100 µm. Data are representative of three or more independent experiments.
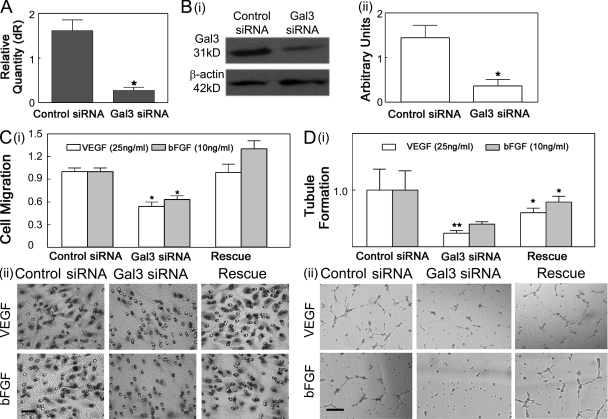
To further evaluate the role of galectin-3 in growth factor-mediated angiogenesis, expression of endogenous galectin-3 was reduced in HUVECs by siRNA-mediated knockdown. Galectin-3–specific siRNA reduced the expression of galectin-3 mRNA (Fig. 3 A) and protein (Fig. 3 B) by ∼70 and 64%, respectively. A nontargeting siRNA had no effect on galectin-3 mRNA and protein levels (Fig. 3, A and B). Neither galectin-3 nor nontargeting siRNA induced a nonspecific knockdown of β-actin (Fig. 3 B). Knockdown of endogenous galectin-3 markedly reduced the extent of VEGF- and bFGF-mediated cell migration (Fig. 3 C; percentage of reduction: VEGF, 46.3 ± 5.9; bFGF, 39.7 ± 4.1) and capillary tubule formation in vitro (Fig. 3 D; percentage of reduction: VEGF, 70.9 ± 5.4; bFGF, 50.12 ± 4.57). The addition of exogenous galectin-3 restored VEGF- and bFGF-mediated angiogenesis (Fig. 3, C and D, Rescue), supporting the hypothesis that galectin-3 is an important contributor in VEGF- and bFGF-mediated angiogenesis.
Figure 3.
Galectin-3 knockdown reduces VEGF- and bFGF-mediated angiogenesis in vitro. (A and B) HUVECs were transfected with siRNA duplexes directed against galectin-3 (Gal3 siRNA) or control nontargeting siRNA duplexes (Control siRNA). The knockdown of galectin-3 was evaluated by RT-qPCR (A) and Western blot analysis (Bi: a representative image, Bii: quantification of protein knockdown by ImageJ). (C and D) VEGF and bFGF induced cell migration and capillary tubule formation is reduced in galectin-3–knockdown cells. The ability of 25 ng/ml VEGF (open bars) and 10 ng/ml bFGF (solid bars) to promote chemotaxis (C) and capillary tubule formation (D) in galectin-3 siRNA (Gal3 siRNA) transfected and nontargeting siRNA-transfected (control siRNA) HUVECs, as well as galectin-3 siRNA transfected HUVECs in the presence of 10 µg/ml exogenous galectin-3 (Rescue) was compared. A value of 1.0 was assigned to cell migration and capillary tubule formation values of control cells. The values of all other cells were calculated as a fold change with respect to control cells. Data are expressed as mean ± SEM (n = 3/group). *, P < 0.01 and **, P < 0.05 as compared with nontargeting siRNA transfected HUVECs). Representative images of the cells that migrated from the upper chamber to the lower chamber and capillary tubule formation in response to VEGF and bFGF stimulation are shown in C (ii) and D (ii), respectively. Bars: (C, ii) 100 µm; (D; ii) 200 µm. Data are representative of two or more independent experiments.
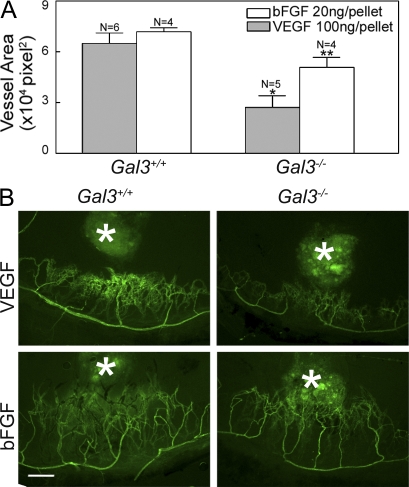
As a direct test of the role of galectin-3 in VEGF- and bFGF-mediated angiogenesis in vivo, we performed mouse corneal micropocket assays in Gal3−/− and Gal3+/+ animals. Pellets containing either 100 ng VEGF or 20 ng bFGF were implanted into mouse corneas and, 5 d after surgery, the animals were perfused with an endothelial cell marker, FITC-Bandeiraea simplicifolia lectin I (BS1), to visualize the vessels. Control pellets, which did not contain any protein, did not promote angiogenesis (unpublished data). In Gal3+/+ mice, both VEGF and bFGF induced robust corneal neovascularization (Fig. 4). The extent of vessel formation mediated by VEGF and bFGF was significantly reduced in Gal3−/− animals as compared with Gal3+/+ animals; specifically, fewer branching capillaries were seen in the Gal3−/− corneas (Fig. 4). Vessel density as assessed by quantifying the vessel-occupied area, was markedly lower in the Gal3−/− corneas as compared with the Gal3+/+ corneas (Fig. 4; percentage of reduction: VEGF, 58.2 ± 10.5; bFGF, 29.2 ± 7.3).
Figure 4.
VEGF- and bFGF-induced angiogenesis is reduced in Gal3−/− mice. Angiogenesis in vivo was evaluated using the mouse corneal micropocket assay as described in the text using VEGF and bFGF pellets. 5 d after surgery, the animals were perfused with FITC-BS1, and the extent of angiogenesis was evaluated by examining the flat mounts of corneas by fluorescence microscopy. Blood vessel area was calculated using ImageJ. (A) Vessel area of neovascularization expressed in pixel2 × 104. Data are expressed as mean ± SEM (n = >4/group). *, P < 0.05 compared with Gal3+/+ control animals. (B) Representative fluorescence images of corneas. White asterisks indicate pellets. Bar, 200 µm. Data are representative of two independent experiments.
Galectin-3 influences VEGF- and bFGF-mediated angiogenesis by modulating the activity of αvβ3 integrin
αvβ3 integrin is the major galectin-3–binding protein in endothelial cells.
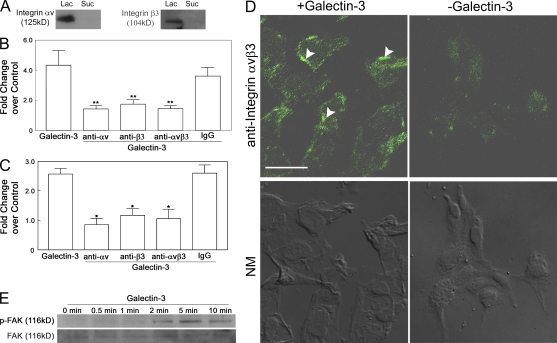
To define the molecular mechanism by which galectin-3 induces angiogenesis, information on its binding partners is prerequisite. To identify galectin-3–specific binding partners, HUVE cell lysates were chromatographed on a galectin-3–affinity column and bound proteins, eluted with β-lactose, were electrophoresed and characterized. Matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry analysis revealed that the fraction eluted from the galectin-3–affinity column contained several proteins including αv and β3 integrin subunits (number of peptides matched: integrin αv, 9 peptides [Fig. S1 A], integrin β3, 5 peptides [Fig. S1 B]) that have been shown to play a key role in VEGF- and bFGF-mediated angiogenesis (Brooks, 1994, 1996; Drake et al., 1995; Friedlander et al., 1995; Storgard et al., 1999; Hodivala-Dilke et al., 2003). Also, in Western blot analysis, 125-kD anti-αv and 104-kD anti-β3 integrin-reactive components were detected in the bound fraction eluted from the galectin-3 column by β-lactose but not in the fraction eluted with sucrose (Fig. 5 A). In an effort to characterize the role of αvβ3 integrin in the galectin-3–mediated angiogenesis, we further investigated: the effect of anti-αv and anti-β3 mAbs on galectin-3–induced angiogenesis; the ability of galectin-3 to promote αvβ3 integrin clustering and subsequent activation of signaling pathways; and the function of galectin-3 in the context of glycosylation pattern of αvβ3 integrin.
Figure 5.
Integrin αvβ3 serves as a galectin-3 counter-receptor. (A) HUVEC lysates were applied to a galectin-3 affinity chromatography column and bound proteins were sequentially eluted with a noncompeting saccharide, 0.1 M sucrose (Suc), and a competing saccharide, 0.1 β-lactose (Lac). Proteins eluted from the affinity column were identified by Western blot analysis. Both αv and β3 integrins were detected in the lactose eluate, but not in the sucrose eluate. (B and C) Integrin-blocking antibodies directed against integrins αv, β3, and αvβ3 inhibit galectin-3–induced cell migration (B) and capillary tubule formation (C). The ability of 10 µg/ml galectin-3 to promote chemotaxis (Boyden chamber assay) and capillary tubule formation in the presence and absence of 10 µg/ml αv, β3, and αvβ3 integrin-blocking antibodies or control mouse IgG was compared. Data are expressed as mean fold change over control cells incubated in media alone ± SEM (n = 3/group). **, P < 0.05; *, P < 0.01 as compared with cell migration and capillary tubule formation induced by galectin-3. (D) Galectin-3 promotes clustering of integrin αvβ3. HUVECs were serum starved overnight and treated with 10 µg/ml galectin-3 for 10 min. At the end of the incubation period, the distribution of integrin αvβ3 was evaluated by confocal microscopy after immunostaining with an anti–human integrin αvβ3 monoclonal antibody. Treatment with galectin-3 resulted in a marked redistribution of integrins into a punctate pattern, indicative of integrin clustering. Overlapping Normanski images are shown on the bottom (NM). Bar, 50 µm. (E) Galectin-3 mediates FAK activation. HUVECs were serum starved for 2 h and treated with 10 µg/ml of galectin-3 for various time periods (0, 0.5, 1, 2, 5, and 10 min). HUVEC lysates were then subjected to immunoblot analysis for pFAK (Y397; top) and FAK (bottom). Galectin-3 treatment promoted a time-dependent activation of FAK that started at 1 min and peaked at 5 min. Total FAK levels remained unchanged at all time periods. Data are representative of three or more independent experiments.
Anti-αvβ3 integrin antibodies inhibit galectin-3–mediated angiogenesis.
To determine whether αvβ3 integrin is involved in galectin-3–induced angiogenesis, HUVE cell migration and capillary tubule formation assays were performed using cells pretreated with, and in the presence of, function-blocking anti-αv (clone AV1), anti-β3 (clone B3A), or anti-αvβ3 (LM609) integrin mAbs. Addition of each of the three antibodies significantly inhibited galectin-3–mediated cell migration and capillary tubule formation (Fig. 5, B and C; percentage of reduction: migration, 67.1 ± 5.02% [αv], 59.8 ± 7.12% [β3], and 66.7 ± 4.71% [αvβ3]; capillary tubule formation, 63.1 ± 9.0% [αv], 62.7 ± 17.3% [β3], and 54.8 ± 13.5% [αvβ3]). These data suggest that integrin αvβ3 is a significant player in the cascade of events involved in galectin-3–induced angiogenesis.
Galectin-3 promotes clustering of integrin αvβ3 and activation of focal adhesion kinase (FAK).
To determine whether galectin-3 promotes clustering of integrin αvβ3, cells were treated with galectin-3 (10 µg/ml) for 10 min and then subjected to confocal microscopy. Cells exposed to galectin-3 demonstrated distinct punctuate staining throughout the cell body, which is indicative of integrin clustering (Fig. 5 D). In contrast, cells exposed to media alone exhibited weak, diffuse staining with occasional microclustering.
Because integrin clustering has been shown to trigger intracellular signaling via FAK activation (Parsons, 2003), and FAK signaling has been implicated in the process of angiogenesis (Ilic et al., 1995, 2004; Shen et al., 2005; Braren et al., 2006), it was of interest to determine whether stimulation of endothelial cells with galectin-3 would result in FAK activation. To investigate whether galectin-3 activated FAK, the phosphorylation status of FAK in HUVECs treated with 10 µg/ml galectin-3 for 30 s, 1, 2, 5, and 10 min was examined by immunoblot analysis. Treatment with galectin-3 induced time-dependent activation of phosphorylated FAK (pFAK). FAK became phosphorylated within 2 min after treatment with galectin-3 and remained active for the entire 10 min (Fig. 5 E). At the same time, the total FAK levels remained unchanged. These data lead us to conclude that galectin-3–induced αvβ3 integrin clustering promotes FAK activation, an event that most likely modulates galectin-3–mediated angiogenesis.
A reduction in β1,6GlcNAc-branched N-glycans on αvβ3 integrin abolishes the ability of galectin-3 to promote angiogenesis.
To evaluate the importance of a galectin-3–αvβ3 integrin glycan interaction in galectin-3–mediated angiogenesis, the expression of N-acetylglucosaminyltransferase V (GnTV), an enzyme that synthesizes N-glycan intermediates that are elongated with N-acetyllactosamines to create high-affinity ligands for galectin-3, was disrupted. The effect of GnTV knockdown was assessed on the following factors: binding of galectin-3 to cell surface glycoprotein receptors; αv and β3 integrin glycans; and galectin-3 and growth factor-induced angiogenesis.
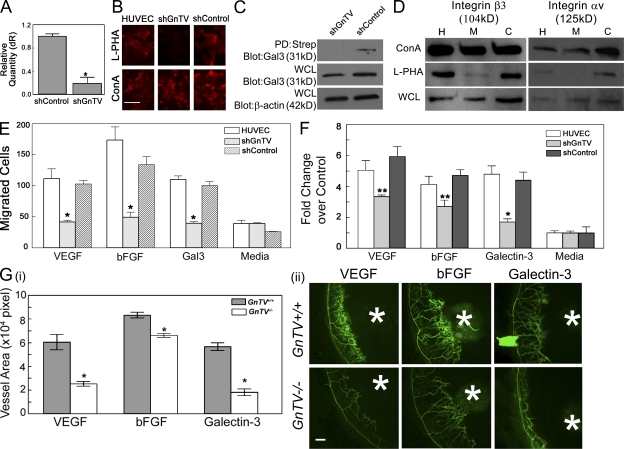
The expression of GnTV was knocked down by transfection of HUVECs with shRNA constructs directed against GnTV. As controls, HUVECs were transfected with constructs expressing a nontargeting sequence. The loss of N-glycan intermediates synthesized by GnTV was confirmed by the loss of the ability of cells to bind FITC-labeled Phaseolus vulgaris leukoagglutinin (L-PHA) lectin, which reacts specifically with core β1,6-branched products synthesized by GnTV (Cummings and Kornfeld, 1982). The knockdown of GnTV at the mRNA level was analyzed by RT-qPCR. Transfection of HUVECs with shRNA constructs directed against GnTV resulted in a substantial knockdown (>80%) of GnTV mRNA expression (Fig. 6 A) and synthesis of GnTV products (Fig. 6 B) as compared with untransfected cells and cells transfected with a nontargeting control construct. The morphology and growth pattern of noninfected control and GnTV shRNA expressing cells were similar. Although galectins lack transmembrane domains, secreted galectins associate with the cell surface by binding to plasma membrane glycoproteins. To determine whether GnTV knockdown resulted in the reduction of galectin-3 counterreceptors on the cell surface, the cell surface proteins of HUVECs transfected with lentiviral particles expressing shRNA directed against GnTV (shGnTV) or nontargeting shRNA control (shControl) were biotinylated and cross-linked in a single step. The biotinylated proteins were isolated by streptavidin pull-down, treated with Laemmli sample buffer to release individual proteins of linked complexes, electrophoresed in reducing conditions, and then probed with anti-galectin-3 mAb. In the streptavidin pull-down fraction (PD; Strep), a reduced level of the ∼30-kD anti-galectin-3–reactive component was detected in the GnTV knockdown cells compared with the control cells transfected with nontargeting lentiviral particles (Fig. 6 C). This suggests that GnTV knockdown reduces the expression of glycoprotein counterreceptors of galectin-3 on the endothelial cell surface.
Figure 6.
Disruption of GnTV reduces binding of galectin-3 to cell surface glycoprotein receptors with a concomitant reduction in galectin-3 and growth factor-mediated angiogenesis in vitro and in vivo. (A) HUVECs were transfected with lentiviral particles expressing shRNA directed against GnTV (shGnTV) or nontargeting shRNA control (shControl). The knockdown of endogenous GnTV was evaluated at the mRNA level by RT-qPCR. The value for HUVECs transfected with GnTV and control shRNA is expressed as the change in the expression level with respect to untransfected cells which served as a calibrator. *, P < 0.01 compared with control cells. (B) Expression of GnTV product, core β 1,6-branched saccharides, in HUVECs transfected with lentiviral particles was evaluated by staining with rhodamine-conjugated l-PHA, which specifically reacts with core β 1,6-branched saccharides. Bar, 100 µm. (C) The cell surface proteins of HUVECs transfected with lentiviral particles expressing shRNA directed against GnTV (shGnTV) or nontargeting shRNA control (shControl) were biotinylated and cross-linked in a single step, the biotinylated proteins were isolated by streptavidin pull-down, electrophoresed in reducing conditions and then probed with anti–galectin-3 mAb. Regardless of whether the Western blot analysis was performed using the streptavidin pull-down proteins (PD:Strep) or whole-cell lysates (WCL) of the labeled cells, reduced level of galecin-3 was detected in the GnTV knockdown cells compared with the cells transfected with control, nontargeting lentiviral particles. (D) GnTV modifies integrins αv and β3. RIPA buffer cell lysates (WCL) of untransfected HUVECs (H) and HUVECs transfected with control (C) and GnTV (M) targeting lentiviral particles were incubated with either l-PHA-agarose or ConA-agarose (1 h at 4°C). Bound proteins were eluted from the agarose beads by boiling in SDS-PAGE sample buffer, separated on SDS-PAGE, and immunostained to detect integrins αv and β3. Note that GnTV knockdown cell lysates show markedly reduced binding of integrins αv and β3 to l-PHA indicating that integrins αv and β3 are modified by GnTV. (E and F) The ability of galectin-3 (10 µg/ml), VEGF (25 ng/ml), and bFGF (10 ng/ml) to promote cell migration (E, Boyden chamber assay) and capillary tubule formation (F) is substantially reduced in GnTV knockdown cells. Data are expressed as mean fold change over control cells incubated in media alone ± SEM (n = 3/group), *, P < 0.01; **, P < 0.05 compared with untransfected controls. (G) VEGF- and bFGF-induced angiogenesis is reduced in GnTV−/− mice. Angiogenesis in vivo was evaluated by the mouse corneal micropocket assay using VEGF and bFGF pellets. On day 5 after surgery, the animals were perfused with FITC-BS1, and the extent of angiogenesis was evaluated by examining the flat mounts of corneas by fluorescence microscopy. Blood vessel area was calculated using ImageJ. (i) Vessel area of neovascularization expressed in pixel2 x 104. Data are expressed as mean ± SEM (n = at least 4/group). *, P < 0.05 compared with GnTV+/+ control animals. (ii) Representative fluorescence images of corneas. White asterisks indicate pellets. Bar, 100 µm. Control pellets, which did not contain any protein, did not promote angiogenesis [not depicted; images identical to that shown in Fig. 1 A (ii)]. Data are representative of two independent experiments each repeated in triplicate.
To determine whether β1,6GlcNAc-branched N-glycans on αv and β3 integrin subunits were reduced in GnTV shRNA–expressing cells, pull-down experiments were conducted using agarose-conjugated plant lectins (L-PHA and concanavalin A [ConA]) and the proteins bound to the lectins were analyzed for reactivity with anti-αv and anti-β3 integrin antibodies by Western blot analysis. Both αv and β3 integrins from GnTV shRNA–expressing cells showed a significant reduction in l-PHA binding (Fig. 6 D, lane M) compared with noninfected (Fig. 6 D, lane H) and non–target shRNA–expressing cells (Fig. 6 D, lane C). In contrast, the binding of integrins to a control lectin, ConA, was not affected (Fig. 6 D). These data indicate that the knockdown of GnTV gene expression leads to a significant reduction in β1,6GlCNAc-branched N-glycans on αv and β3 integrin subunits.
Next, we determined whether reduced GnTV enzyme product on N-glycans of αv and β3 integrins hinders galectin-3 and/or growth factor-induced angiogenesis in vitro. Indeed, galectin-3, VEGF, and bFGF failed to promote cell migration (Fig. 6 E) and capillary tubule formation (Fig. 6 F) of endothelial cells in which GnTV expression was reduced. In contrast, there was no difference in the extent of galectin-3 and growth factor–mediated migration and capillary tubule formation between noninfected and non–target shRNA–expressing cells. Galectin-3, and growth factors, also failed to advance angiogenesis in vivo in GnTV−/− animals, whereas it led to robust angiogenesis in GnTV+/+ animals (Fig. 6 G). These data support the concept that GnTV modified complex N-glycans on integrin αv and β3 play a role in galectin-3–mediated angiogenesis. Moreover, as expected, the decrease in VEGF- and FGF-induced cell migration and capillary tubule formation seen in the GnTV knockdown cells could not be rescued by exogenous galectin-3 (unpublished data). These data lead us to conclude that interactions between GnTV-modified glycans and galectin-3 are required for the growth factor–mediated angiogenesis.
DISCUSSION
This study was undertaken to examine the mechanism by which galectin-3 mediates angiogenesis. We demonstrate here that both the C-terminal CRD and the N-terminal self-associating domain of the lectin are required for galectin-3–mediated angiogenesis. In the presence of its multivalent saccharide ligands, galectin-3 forms multimer via the association of single galectin-3 molecules at the N terminus (Ahmad et al., 2004). This allows the lectin to function in a multivalent fashion and cross-link many cell surface and extracellular matrix (ECM) glycoproteins, such as integrins, growth factor receptors, laminins, and vitronectin (Ohannesian et al., 1995; Dong and Hughes, 1997; Matarrese et al., 2000; Partridge et al., 2004; Lagana et al., 2006; Stillman et al., 2006). Recent studies have suggested that cross-linking of galectin-3–binding glycans present on cell surface receptors (e.g., epidermal growth factors and α5β1 integrin) provides a potential mechanism by which galectin-3 mediates its action (Partridge et al., 2004; Lagana et al., 2006). That the multimeric form of the lectin is required for galectin-3–induced angiogenesis is demonstrated by our findings that, unlike full-length galectin-3, Gal3C, that is unable to form dimers or higher order oligomers (Hirabayashi et al., 2002; John et al., 2003), is not angiogenic. In our study Gal3C not only failed to induce angiogenesis, it effectively inhibited the angiogenesis induced by full-length galectin-3. This suggests that Gal3C which contains the entire CRD and retains the ability to bind to lactosamine-containing glycans effectively competed with endogenous full-length galectin-3 and that CRD and the carbohydrate binding property of the lectin is also required for the angiogenic activity of the lectin. These findings are consistent with the report of Nangia-Makker et al. (Nangia-Makker et al., 2000) showing that β-lactose and galactose-rich polysaccharide, MCP, also inhibit galectin-3–mediated-angiogenesis in vitro.
The major finding of the current study is that galectin-3 modulates VEGF- and bFGF-mediated angiogenesis. Our studies show that Gal3C, the dominant-negative inhibitor of galectin-3, and the saccharide inhibitor of galectin-3, lactose (but not a noncompeting disaccharide, sucrose), ameliorated VEGF and bFGF angiogenic activity. In addition, silencing of galectin-3 in HUVECs by siRNA and knockdown of high-affinity ligands of galectin-3 by silencing GnTV substantially reduced the growth factor–mediated angiogenesis in vitro. Furthermore, in the in vivo corneal micropocket assay, the extent of angiogenesis induced by VEGF and bFGF was significantly less in Gal3−/− mice compared with the Gal3+/+ animals. Together, these data conclusively establish that galectin-3 significantly influences VEGF- and bFGF-mediated angiogenesis. To our knowledge, this is the first demonstration of a defect in vascular response of Gal3−/− mice.
In this paper, we also demonstrate that galectin-3 promotes angiogenesis by interacting with complex N-glycans on αvβ3 integrin and activating integrin signaling pathways that influence VEGF and bFGF angiogenic activity. Transmembrane integrin receptors are well known for their role in modulating angiogenesis (Brooks, 1996; Eliceiri and Cheresh, 2001; Stupack and Cheresh, 2004; Mahabeleshwar et al., 2006). Integrin αvβ3, a major vitronectin receptor on endothelial cells, plays a particularly important role in the cascade of events leading to the angiogenic response. Normally expressed in low levels, the expression of integrin αvβ3 is up-regulated in angiogenic vessels (Brooks, 1994, 1996; Storgard et al., 1999). The disruption of the function of αvβ3 integrin with antibodies or peptide antagonists effectively inhibits VEGF- and bFGF-induced angiogenesis in vitro and in vivo (Brooks, 1994, 1996; Drake et al., 1995; Friedlander et al., 1995; Storgard et al., 1999). It is thought that integrin αvβ3 influences cytokine-mediated angiogenesis by arbitrating vitronectin-mediated cell–matrix interactions that promote the following: enhanced expression of cytokine receptors, including VEGFR; EC migration; and VEGF- and bFGF-induced signaling leading to angiogenesis. Integrin αvβ3 also directly associates with cytokine receptors to enhance pro-angiogenic action of VEGF and bFGF, through a mechanism that is not fully understood (Brooks et al., 1994; Soldi et al., 1999).
Traditionally, functions of most integrins have been investigated in the context of protein–protein interactions (Hynes, 2002; Guo and Giancotti, 2004; Lock et al., 2008). However, almost all integrins are glycosylated, and in recent years several studies have revealed that integrin glycans can modulate the transmembrane signaling (for reviews see Bellis, 2004; Gu et al., 2004). For example, β1,6GlcNAc-branched complex N-glycans synthesized by a glycosyltransferase, GnTV enzyme, have been shown to modulate α5β1 integrin-dependent “inside-out” and “outside-in” signaling during cancer cell migration (Guo et al., 2005; Lagana et al., 2006). To date, relatively few studies have focused on the role of galectin-3–mediated carbohydrate interactions in modulating specifically integrin signaling. Lagana et al. (2006) have reported that in carcinoma cells, galectin-3 interactions with GnTV-modified N-glycans stimulate α5β1-integrin activation and fibronectin-dependent tumor cell spreading and motility. Likewise, Goetz et al. (2008) have reported that galectin-3–mediated activation of β1 integrin recruits tyrosine-phosphorylated Cav1, which stabilizes focal adhesions and regulates cell motility. Recent studies in our laboratory have also suggested that galectin-3 promotes epithelial cell migration by cross-linking GnTV-modified complex N-glycans on α3β1 integrin and subsequently activating α3β1 integrin-Rac1 signaling that regulates lamellipodia formation (Saravanan et al., 2009). In an effort to characterize the molecular mechanism by which galectin-3 mediates angiogenesis, in the current study, we investigated the function of galectin-3 in the context of the glycosylation pattern of αvβ3 integrin. Using affinity chromatography on a galectin-3–Sepharose column, we identified αv and β3 integrin subunits as major galectin-3–binding proteins in HUVECs. We further demonstrated that function-blocking mAbs against αv, β3, and αvβ3-integrin markedly reduce the ability of the lectin to promote angiogenesis. That the angiogenic property of galectin-3 is dependent on CRD and the carbohydrate-mediated integrin signaling is suggested by our findings that binding of galectin-3 to αv and β3 integrin subunits and the stimulatory effect of galectin-3 on angiogenesis was inhibited by a competing sugar, β-lactose, but not by a noncompeting sugar, sucrose, and that knockdown of gene expression of a rate-limiting enzyme, GnTV, resulted in the loss of β1,6GlcNAc-branched complex N-glycans, the high-affinity ligands of galectin-3, on αv and β3 integrin subunits with a concomitant inhibition of galectin-3–, VEGF-, and FGF-induced angiogenesis in vitro as well as in vivo.
Integrin-mediated events, such as cell adhesion and migration, are regulated by integrin clustering and resulting activation of intracellular signaling pathways (Giancotti and Ruoslahti, 1999). Numerous studies have speculated that galectin-3 has the potential to cluster integrins. Here, we directly demonstrate that galectin-3–induces clustering of αvβ3 integrins. Furthermore, we show that galectin-3–mediated clustering of integrin αvβ3 promotes FAK activation, a key signaling step in the process of angiogenesis.
From these data, we propose the following model for galectin-3–mediated angiogenesis: galectin-3 CRD binds to GnTV-modified N-glycans on αvβ3 integrin, and as a multimer, it cross-links and clusters the integrin and activates FAK-mediated signaling pathways that modulate events such as endothelial cell migration in the angiogenesis cascade. How might the carbohydrate-based galectin-3–integrin interaction fit into the cascade of events in the growth factor–mediated angiogenesis? It is thought that during angiogenesis, the ECM in contact with endothelial cells provides the necessary support for cytokine-mediated migration and capillary lumen formation (Kalluri, 2003). This support stems from the synergistic effects of cytokines and endothelial cell surface integrins (Eliceiri, 2001). As described earlier, it is well established that growth factor-induced angiogenesis depends on specific integrins and antagonists of integrin αvβ3 effectively inhibit VEGF- and bFGF-mediated angiogenesis in vitro and in vivo (Brooks, 1994, 1996; Drake et al., 1995; Friedlander et al., 1995; Storgard et al., 1999). Accordingly, we propose that in a dynamic ECM of endothelial cells undergoing angiogenesis, galectin-3 promotes clustering and activation of integrins and, this in turn, facilitates VEGF/bFGF-induced angiogenesis. Regarding the mechanism that mediates cross-talk between growth factors and integrins, it is known that one form of such cross-talk involves physical interaction between the two classes of proteins. Specifically, it has been reported that VEGFR2 binds to αvβ3 integrin (Soldi et al., 1999; Borges et al., 2000; Eliceiri, 2001; Eliceiri and Cheresh, 2001), and this binding occurs via the extracellular domain of the integrin and enhances the activity of the growth factor receptor (Borges et al., 2000). The biochemical interactions that mediate the binding of VEGFR2 with the integrins have not been elucidated. It is tempting to speculate that oligomeric galectin-3 may promote such interactions by binding to GnTV-modified N-glycans on αvβ3 integrin and VEGFR2. In this respect, it is known that the presence of N-glycans on VEGFR is required for its function (Vaisman et al., 1990) and, in a recent preliminary study, we have observed that galectin-3 interacts with VEGFR2 (unpublished data). Thus, it is possible that galectin-3 may influence angiogenesis by alternative mechanisms involving clustering of VEGFR2 leading to the reduction in the receptor endocytosis and augmenting signaling or promoting interactions between the VEGFR2 and integrins. Studies to determine whether galectin-3 influences angiogenesis by cross-linking and modulating endocytosis of VEGFR2 and/or promoting interactions between VEGFR2 and integrins are currently underway in our laboratory.
In summary, the results of this study show that galectin-3 is an important contributor to VEGF- and bFGF- mediated angiogenesis. These findings have broad implications for developing novel, carbohydrate-based therapeutic agents for inhibition of angiogenesis.
MATERIALS AND METHODS
Materials and cell culture.
HUVECs were provided by V. Sukhatme (Beth Israel Deaconess Medical Center, Boston, MA). The cells were maintained in endothelial cell growth medium (EGM-2; Lonza) and were used between passages 3 to 8). Purified full-length recombinant galectin-3 was prepared as described earlier (Hsu et al., 1992). Gal3C was prepared by collagenase treatment of full-length galectin-3 as described by John et al. (2003). Recombinant VEGF-A was obtained from Cell Sciences and R&D Systems. Growth factor–reduced matrigel, anti-integrin αv and β3 mAbs, and anti-FAK and pFAK were obtained from BD. Function-blocking mAbs directed against αv, β3, and αvβ3 were obtained from Millipore. All lectins and anti–mouse IgG and anti–rabbit IgG secondary antibodies were obtained from Vector Laboratories. FITC-anti–mouse IgG was obtained from Jackson Immunoresearch Laboratories. Hybridoma, M3/38, expressing monoclonal anti-galectin-3 antibody was obtained from American Type Culture Collection. RNeasy kit was purchased from QIAGEN. High Capacity cDNA Archive kit, qPCR master mix, and qPCR primers were purchased from Applied Biosystems. All siRNA duplexes were from Thermo Fisher Scientific. Oligofectamine and Opti-MEM were obtained from Invitrogen. EZ-Link-sulfo-NHS-LC-Biotin and 3,3′-Dithiobis(sulfosuccinimidylpropionate) were purchased from Thermo Fisher Scientific. All other reagents were obtained from Sigma-Aldrich.
Animals.
In this study, we used 6–8-wk-old Gal3+/+, Gal3−/−, GnTV−/−, and GnTV+/+ mice. Gal3−/− mice were generated by targeted interruption of the galectin-3 gene, as described previously (Hsu et al., 2000). Gal3+/+ and Gal3−/− mice were obtained by interbreeding Gal3+/− mice and carried as separate lineages. GnTV animals, generated by Dennis and collaborators (Granovsky et al., 2000), were obtained from the Consortium for Functional Glycomics (The Scripps Research Institute).
Corneal mouse micropocket angiogenesis assay.
All animal procedures were approved by the Institutional Animal Care and Use Committee at Tufts University. The investigation conformed to the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Vision Research and recommendations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The corneal micropocket angiogenesis assay was performed as described previously using implants containing a test agent, hydron, and sucralfate (Kenyon et al., 1996). Test agents included full-length galectin-3 (0.25–2 mg/ml), Gal3C (1 mg/ml), VEGF (2.5 mg/ml), and bFGF (0.25 mg/ml). Implants containing hydron and sucralfate alone served as negative controls. For micropocket assays, 6–8-wk-old mice were anesthetized by intraperitoneal injection of a cocktail of ketamine (90–120 mg/kg) and xylazine (10 mg/kg). The eyes were topically anesthetized with proparacaine and were gently proptosed with forceps. With a use of a corneal blade and a stereoscope, and intrastromal linear keratomy was performed ∼2 mm from the limbus. Using a von Graefe knife (Miltex), a pocket was extended toward the limbus, and the pellet was maneuvered into the pocket. The wound was coated with a veterinary ophthalmical ointment to prevent infection. To quantitate the extent of angiogenesis, on day 5 after surgery, the animals were anesthetized, corneas were photographed, and the vessel area was calculated using a formula PD × VL × CN × 0.628, where PD is the distance of the pellet from the limbal vein, VL is length of vessels, and CN is circumferential neovascularity. Alternatively, anesthetized animals were injected intracardially with 100 µl of 5 mg/ml of FITC-BS1 to visualize the newly formed vessels. After 30 min, the animals were sacrificed, the eyes were fixed with 0.1% paraformaldehyde, and the flat mounts of the dissected corneas were evaluated by fluorescence microscopy (Pola et al., 2001). The total area of corneal neovascularization was calculated using ImageJ (NIH; http://rsb.info.nih.gov/nih-imageJ).
Modified Boyden chamber migration assay.
The assay was performed as previously described (Leavesley et al., 1993). In brief, HUVECs were serum starved overnight in endothelial cell basal medium (EBM-2). Transwell inserts (8 µm pore size; Corning) were coated with 10 µg/ml of fibronectin and 5 × 104 HUVECs in 100 µl of EBM-2 were added to the upper chamber. The bottom chamber was filled with 600 µl of EBM-2 in the presence and absence of 10 ng/ml bFGF, 25 ng/ml VEGF, 10 µg/ml full-length galectin-3, or Gal3C. To assess the impact of CRD of galectin-3 on VEGF- and bFGF-mediated angiogenesis, the experiments were preformed in the presence of saccharides (0.1 M β-lactose and 0.1 M sucrose) and 10 µg/ml Gal3C. To determine the role of integrin αvβ3 in galectin-3–mediated angiogenesis, experiments were performed in the presence of galectin-3 and functional-blocking mAbs against human αv (clone AV1), β3 (clone B3A), and αvβ3 (clone LM609). After 6-h incubation at 37°C, in a CO2 incubator, the cells were fixed and stained using the Diff-Quik staining kit (Dade-Behring). The nonmigrating cells (upper chamber) were removed with a cotton swab, and the migrating cells (bottom chamber) were manually counted in three nonoverlapping fields. Assays performed with media alone in the bottom chamber served as a negative control. Cell viability in the presence of various substances was tested by a trypan blue exclusion test.
Capillary tubule formation assay.
The capillary tubule formation assay was performed as previously described (Nangia-Makker et al., 2000). In brief, HUVECs were plated on matrigel-coated 8 chamber slides (2 × 105 cells/chamber) in the presence and absence of various test substances described in the previous section for the cell migration assay. After 6 h of incubation in a CO2 incubator, cells were photographed. To quantitate the data, the number of branch points in four nonoverlapping fields was determined.
Transfection of HUVECs with siRNA.
Galectin-3 expression was reduced by transfection with siRNA duplexes directed against galectin-3 (NCBI Protein Database accession no. NM_002306). For transfection, 3 × 105 cells cultured in wells of a 6-well plate to 90% confluence were treated with 375 pmol of siRNA duplexes using 8 µl of Oligofectamine and OPTI-MEM (8 µl oligofectamine/200 µl Opti-MEM/well). After 18 h incubation with siRNA, gene silencing was examined by RT-qPCR and Western blotting. Control cells were treated with a nontargeting siRNA duplex (no. D-001210-02-05).
For RT-qPCR, RNeasy kit was used to isolate total RNA, and cDNA was synthesized using the High Capacity cDNA Archive kit. PCR was performed in triplicates according to the manufacturer’s instructions using cDNA, inventory gene-specific primers (Gal3, Hs00173587_m1; GAPDH, Hs99999905_m; Applied Biosystems Inc.), and master mix containing ROX as a passive reference. Reactions performed in the absence of template served as the negative control. Quantification data of each gene were normalized to the expression of GAPDH, which served as a positive amplification control. A value of 1.0 was assigned to the expression of each gene in the untransfected HUVECs which served as a calibrator. The expression values for galectin-3 knockdown HUVECs were calculated as change in expression level with respect to untransfected HUVECs.
For Western blot analysis, radioimmunoprecipitation (RIPA) buffer extracts of siRNA-transfected and control cell lysates were electrophoresed on a 4–20% SDS-PAGE gel. Protein blots of the gel were processed for immunostaining using monoclonal anti-galectin-3 (M3/38) and monoclonal β-actin (1:20,000) as primary antibodies; HRP-conjugated anti-rat IgG (1:50,000) and HRP-conjugated anti-mouse IgG (1:50,000) as secondary antibodies; and a chemiluminescence detection system. The intensity of the bands was quantified using ImageJ.
Isolation of galectin-3 binding proteins of HUVECs.
Galectin-3 binding proteins were isolated by chromatography of HUVEC cell extracts on a galectin-3 affinity column as described earlier (Saravanan et al., 2009). Proteins bound to the affinity column were eluted using PBS containing 0.1% Tween-20 (PBST) and 0.1 M β-lactose, and were resolved by electrophoresis in reducing 10% SDS-PAGE gels. Coomassie blue–stained components detected on the gel were subjected to peptide analysis using a prO-TOF 2000 MALDI-TOF mass spectrometer. In some experiments, to confirm sugar binding specificity of the galectin-3–binding proteins, before elution with β-lactose, the column was eluted with PBST containing 0.1 M sucrose. The identity of galectin-3–binding proteins of interest, specifically αv and β3 integrin subunits, was also confirmed by immunoblot analysis using monoclonal anti-integrin αv (1:250) and anti-integrin β3 (1:500) as primary antibodies and HRP-conjugated anti–mouse IgG (1:50,000) as a secondary antibody.
Integrin αvβ3 clustering.
HUVECs were plated in 8-chamber slides coated with 10 µg/ml fibronectin at the density of 104 cells/chamber. The cells were serum starved overnight in EBM-2, and then treated with 10 µg/ml galectin-3 for 10 min. Subsequently, the cells were fixed with 4% paraformaldehyde in PBS (10 min at 37°C), treated with 1% BSA in PBS (1 h at 37°C) to block nonspecific binding sites, and then sequentially incubated with 10 µg/ml anti-integrin αvβ3 mAb in blocking buffer (1 h at room temperature) and FITC-anti–mouse IgG (1:200; 1 h at room temperature). The slides were mounted using Vectashield mounting medium (Vector Laboratories) and viewed with a 20× objective lens (Carl Zeiss, Inc.) on a DMR confocal laser fluorescence microscope (Leica). Images were generated using the Leica Confocal Software at a resolution of 1024 × 1024 pixels.
FAK activation.
HUVECs (70% confluence) were serum starved for 2 h in EBM-2 basal media and were then treated with 10 µg/ml galectin-3 for 30 s, 1, 2, 5, and 10 min. RIPA buffer lysates of the lectin-treated cells were separated on 4–20% SDS-PAGE gels, and protein blots of the gels were subjected to Western blot analysis using rabbit anti-pFAK mAb (1:1,000; clone 141–9; 2 h at room temperature) and HRP-conjugated anti–rabbit IgG secondary antibody (1:20,000; 1 h at room temperature). For detection of total FAK, the blots were sequentially incubated with mouse anti-FAK mAb (1:1,000; clone 77; 2 h at room temperature) and HRP-conjugated anti–mouse IgG secondary antibody (1:50,000, 1 h at room temperature). Immunostained components were then visualized by a chemiluminescence detection system. All antibodies were diluted in the blocking buffer.
Silencing of GnTV Gene Expression in HUVECs.
Stable short hairpin RNA (shRNA) cell lines targeting GnTV were generated according to our recently published procedure (Saravanan et al., 2009). The clone selected was: TRCN0000036061. The extent of GnTV knockdown at the mRNA level was assessed by RT-qPCR as described above using inventory gene-specific primers (GnTV: Hs00159136_m1 and GAPDH: Hs99999905_m). The knockdown of β1–6GlcNAc-branched chains, the product of GnTV, was detected by staining with rhodamine-conjugated l-PHA lectin.
Analysis of galectin-3 interaction with GnTV glycans on endothelial cell surface.
Both control and GnTV knockdown HUVECs were lifted from the plate by incubation with 5mM EDTA and resuspended in PBS (25 × 106 cell/ml). Cell surface proteins were biotinylated and cross-linked in one-step procedure by incubation with 0.5mg EZ-Link-sulfo-NHS-LC-Biotin and 0.1mg 3,3′-Dithiobis(sulfosuccinimidylpropionate), as described by Altin et al. (Altin and Pagler, 1995). After incubation, the cells were washed with cold PBS and lysed with TNTE lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton-X100, 1 mM EDTA). Aliquots of cell lysates (0.5mg of protein) were precleared with 25 µg packed Sepharose 4B beads (1 h at 4°C) and incubated with streptavidin agarose CL-4B (1 h at 4°C). The streptavidin bound proteins were released by boiling the beads for 5 min in the Laemmli sample buffer, separated on 4–20% SDS-PAGE gels in reducing conditions, transferred onto a nitrocellulose membrane and probed for galectin-3 as described earlier.
Analysis of αvβ3 integrin glycans.
To determine whether β1,6GlcNAc-branched glycans are present on αv and β3 integrin subunits and, if so, whether they are reduced in the GnTV knockdown cells, RIPA buffer lysates of untreated, control shRNA- and GnTV shRNA–transfected HUVECs were incubated with 20 µl of unconjugated Sepharose 4B (1 h at 4°C) to reduce nonspecific binding. Unbound proteins were then incubated with 30 µl agarose-conjugated l-PHA or Con A for 1 h at 4°C. The beads were subsequently washed and the proteins bound to the beads were released by boiling in SDS sample buffer and separated on 12% SDS-PAGE gel. Protein blots of the gels were processed for immunostaining using anti-human αv and β3 integrin mAbs as described above.
Statistical analyses.
Data are expressed as mean ± SEM. Statistical significance was determined using a two-tailed Student’s t-test.
Online supplemental material.
Fig. S1 shows MALDI-TOF MS analysis peptide matches for integrin αv and β3 in the fraction eluted with lactose from the galectin-3 affinity column. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20090121/DC1.
Acknowledgments
The authors thank Dr. Marsha Moses for helpful comments and Catherine Butterfield for help with the mouse corneal micropocket assay. MALDI-TOF mass spectrometry was performed by Dr. Ian Rawe (The Schepens Eye Research Institute, Boston, MA). We also thank Dr. Hakon Leffler for galectin-3 constructs.
This work was supported by the National Eye Institute Grant EY007088 (N. Panjwani), R01AI20958 (F.T. Liu), the New England Corneal Transplant Fund, the Mass Lions Eye Research fund, and a challenge grant from Research to Prevent Blindness. GnTV animals were kindly provided by the Consortium for Functional Glycomics (La Jolla, California; grant number National Institutes of Health GM62116). Confocal microscopy was performed at the Tufts Center for Neuroscience Research Core facility supported by NIH grant P30 NS047243).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- bFGF
- basic fibroblast growth factor
- BS1
- Bandeiraea simplicifolia lectin I
- ConA
- concanavalin A
- CRD
- carbohydrate recognition domain
- EBM-2
- endothelial cell basal medium 2
- ECM
- extracellular matrix
- FAK
- focal adhesion kinase
- HUVEC
- human umbilical vein endothelial cells
- l-PHA
- Phaseolus vulgaris leukoagglutinin
- MALDI-TOF
- matrix-assisted laser desorption ionization time of flight
- MCP
- modified citrus pectin
- pFAK
- phosphorylated FAK
- VEGF
- vascular endothelial growth factor
References
- Ahmad N., Gabius H.J., André S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C.F. 2004. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 279:10841–10847 10.1074/jbc.M312834200 [DOI] [PubMed] [Google Scholar]
- Altin J.G., Pagler E.B. 1995. A one-step procedure for biotinylation and chemical cross-linking of lymphocyte surface and intracellular membrane-associated molecules. Anal. Biochem. 224:382–389 10.1006/abio.1995.1054 [DOI] [PubMed] [Google Scholar]
- Bellis S.L. 2004. Variant glycosylation: an underappreciated regulatory mechanism for beta1 integrins. Biochim. Biophys. Acta. 1663:52–60 10.1016/j.bbamem.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Borges E., Jan Y., Ruoslahti E. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867–39873 10.1074/jbc.M007040200 [DOI] [PubMed] [Google Scholar]
- Braren R., Hu H., Kim Y.H., Beggs H.E., Reichardt L.F., Wang R. 2006. Endothelial FAK is essential for vascular network stability, cell survival, and lamellipodial formation. J. Cell Biol. 172:151–162 10.1083/jcb.200506184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier G. 2000. Angiogenesis in embryonic development—a review. Placenta. 21:S11–S15 10.1053/plac.1999.0525 [DOI] [PubMed] [Google Scholar]
- Brooks P.C. 1996. Role of integrins in angiogenesis. Eur. J. Cancer. 32:2423–2429 10.1016/S0959-8049(96)00381-4 [DOI] [PubMed] [Google Scholar]
- Brooks P.C., Clark R.A., Cheresh D.A. 1994. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 264:569–571 10.1126/science.7512751 [DOI] [PubMed] [Google Scholar]
- Carmeliet P., Jain R.K. 2000. Angiogenesis in cancer and other diseases. Nature. 407:249–257 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- Cross M.J., Claesson-Welsh L. 2001. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 22:201–207 10.1016/S0165-6147(00)01676-X [DOI] [PubMed] [Google Scholar]
- Cummings R.D., Kornfeld S. 1982. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J. Biol. Chem. 257:11230–11234 [PubMed] [Google Scholar]
- Dong S., Hughes R.C. 1997. Macrophage surface glycoproteins binding to galectin-3 (Mac-2-antigen). Glycoconj. J. 14:267–274 10.1023/A:1018554124545 [DOI] [PubMed] [Google Scholar]
- Drake C.J., Cheresh D.A., Little C.D. 1995. An antagonist of integrin alpha v beta 3 prevents maturation of blood vessels during embryonic neovascularization. J. Cell Sci. 108:2655–2661 [DOI] [PubMed] [Google Scholar]
- Eliceiri B.P. 2001. Integrin and growth factor receptor crosstalk. Circ. Res. 89:1104–1110 10.1161/hh2401.101084 [DOI] [PubMed] [Google Scholar]
- Eliceiri B.P., Cheresh D.A. 2001. Adhesion events in angiogenesis. Curr. Opin. Cell Biol. 13:563–568 10.1016/S0955-0674(00)00252-0 [DOI] [PubMed] [Google Scholar]
- Fraser H.M., Lunn S.F. 2000. Angiogenesis and its control in the female reproductive system. Br. Med. Bull. 56:787–797 10.1258/0007142001903364 [DOI] [PubMed] [Google Scholar]
- Friedlander M., Brooks P.C., Shaffer R.W., Kincaid C.M., Varner J.A., Cheresh D.A. 1995. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 270:1500–1502 10.1126/science.270.5241.1500 [DOI] [PubMed] [Google Scholar]
- Gardner T.W., Antonetti D.A., Barber A.J., LaNoue K.F., Levison S.W. 2002. Diabetic retinopathy: more than meets the eye. Surv. Ophthalmol. 47:S253–S262 10.1016/S0039-6257(02)00387-9 [DOI] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. 1999. Integrin signaling. Science. 285:1028–1032 10.1126/science.285.5430.1028 [DOI] [PubMed] [Google Scholar]
- Goetz J.G., Joshi B., Lajoie P., Strugnell S.S., Scudamore T., Kojic L.D., Nabi I.R. 2008. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine-phosphorylated caveolin-1. J. Cell. Biol. 180:1261–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovsky M., Fata J., Pawling J., Muller W.J., Khokha R., Dennis J.W. 2000. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6:306–312 10.1038/73163 [DOI] [PubMed] [Google Scholar]
- Gu Y.C., Bauer T.R., Jr., Ackermann M.R., Smith C.W., Kehrli M.E., Jr., Starost M.F., Hickstein D.D. 2004. The genetic immunodeficiency disease, leukocyte adhesion deficiency, in humans, dogs, cattle, and mice. Comp. Med. 54:363–372 [PubMed] [Google Scholar]
- Guo W., Giancotti F.G. 2004. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5:816–826 10.1038/nrm1490 [DOI] [PubMed] [Google Scholar]
- Guo H.B., Lee I., Bryan B.T., Pierce M. 2005. Deletion of mouse embryo fibroblast N-acetylglucosaminyltransferase V stimulates alpha5beta1 integrin expression mediated by the protein kinase C signaling pathway. J. Biol. Chem. 280:8332–8342 10.1074/jbc.M413532200 [DOI] [PubMed] [Google Scholar]
- Hirabayashi J., Hashidate T., Arata Y., Nishi N., Nakamura T., Hirashima M., Urashima T., Oka T., Futai M., Muller W.E., et al. 2002. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochim. Biophys. Acta. 1572:232–254 [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke K.M., Reynolds A.R., Reynolds L.E. 2003. Integrins in angiogenesis: multitalented molecules in a balancing act. Cell Tissue Res. 314:131–144 10.1007/s00441-003-0774-5 [DOI] [PubMed] [Google Scholar]
- Hsu D.K., Zuberi R.I., Liu F.T. 1992. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J. Biol. Chem. 267:14167–14174 [PubMed] [Google Scholar]
- Hsu D.K., Yang R.Y., Pan Z., Yu L., Salomon D.R., Fung-Leung W.P., Liu F.T. 2000. Targeted disruption of the galectin-3 gene results in attenuated peritoneal inflammatory responses. Am. J. Pathol. 156:1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R.O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell. 110:673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. 1995. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 377:539–544 10.1038/377539a0 [DOI] [PubMed] [Google Scholar]
- Ilic D., Kovacic B., Johkura K., Schlaepfer D.D., Tomasević N., Han Q., Kim J.B., Howerton K., Baumbusch C., Ogiwara N., et al. 2004. FAK promotes organization of fibronectin matrix and fibrillar adhesions. J. Cell Sci. 117:177–187 10.1242/jcs.00845 [DOI] [PubMed] [Google Scholar]
- John C.M., Leffler H., Kahl-Knutsson B., Svensson I., Jarvis G.A. 2003. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin. Cancer Res. 9:2374–2383 [PubMed] [Google Scholar]
- Kalluri R. 2003. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer. 3:422–433 10.1038/nrc1094 [DOI] [PubMed] [Google Scholar]
- Kenyon B.M., Voest E.E., Chen C.C., Flynn E., Folkman J., D’Amato R.J. 1996. A model of angiogenesis in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 37:1625–1632 [PubMed] [Google Scholar]
- Koch A.E. 1998. Review: angiogenesis: implications for rheumatoid arthritis. Arthritis Rheum. 41:951–962 [DOI] [PubMed] [Google Scholar]
- Kulkarni A.D., Kuppermann B.D. 2005. Wet age-related macular degeneration. Adv. Drug Deliv. Rev. 57:1994–2009 10.1016/j.addr.2005.09.003 [DOI] [PubMed] [Google Scholar]
- Lagana A., Goetz J.G., Cheung P., Raz A., Dennis J.W., Nabi I.R. 2006. Galectin binding to Mgat5-modified N-glycans regulates fibronectin matrix remodeling in tumor cells. Mol. Cell. Biol. 26:3181–3193 10.1128/MCB.26.8.3181-3193.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavesley D.I., Schwartz M.A., Rosenfeld M., Cheresh D.A. 1993. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J. Cell Biol. 121:163–170 10.1083/jcb.121.1.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J.G., Wehrle-Haller B., Strömblad S. 2008. Cell-matrix adhesion complexes: master control machinery of cell migration. Semin. Cancer Biol. 18:65–76 10.1016/j.semcancer.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Mahabeleshwar G.H., Feng W., Phillips D.R., Byzova T.V. 2006. Integrin signaling is critical for pathological angiogenesis. J. Exp. Med. 203:2495–2507 10.1084/jem.20060807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarrese P., Fusco O., Tinari N., Natoli C., Liu F.T., Semeraro M.L., Malorni W., Iacobelli S. 2000. Galectin-3 overexpression protects from apoptosis by improving cell adhesion properties. Int. J. Cancer. 85:545–554 [DOI] [PubMed] [Google Scholar]
- Nangia-Makker P., Honjo Y., Sarvis R., Akahani S., Hogan V., Pienta K.J., Raz A. 2000. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am. J. Pathol. 156:899–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P., Hogan V., Honjo Y., Baccarini S., Tait L., Bresalier R., Raz A. 2002. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl. Cancer Inst. 94:1854–1862 [DOI] [PubMed] [Google Scholar]
- Ohannesian D.W., Lotan D., Thomas P., Jessup J.M., Fukuda M., Gabius H.J., Lotan R. 1995. Carcinoembryonic antigen and other glycoconjugates act as ligands for galectin-3 in human colon carcinoma cells. Cancer Res. 55:2191–2199 [PubMed] [Google Scholar]
- Parsons J.T. 2003. Focal adhesion kinase: the first ten years. J. Cell Sci. 116:1409–1416 10.1242/jcs.00373 [DOI] [PubMed] [Google Scholar]
- Partridge E.A., Le Roy C., Di Guglielmo G.M., Pawling J., Cheung P., Granovsky M., Nabi I.R., Wrana J.L., Dennis J.W. 2004. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 306:120–124 10.1126/science.1102109 [DOI] [PubMed] [Google Scholar]
- Pola R., Ling L.E., Silver M., Corbley M.J., Kearney M., Blake Pepinsky R., Shapiro R., Taylor F.R., Baker D.P., Asahara T., Isner J.M. 2001. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat. Med. 7:706–711 10.1038/89083 [DOI] [PubMed] [Google Scholar]
- Price M.O., Thompson R.W., Jr., Price F.W., Jr 2003. Risk factors for various causes of failure in initial corneal grafts. Arch. Ophthalmol. 121:1087–1092 10.1001/archopht.121.8.1087 [DOI] [PubMed] [Google Scholar]
- Saravanan C., Liu F.T., Gipson I.K., Panjwani N. 2009. Galectin-3 promotes lamellipodia formation in epithelial cells by interacting with complex N-glycans on alpha3beta1 integrin. J. Cell Sci. 122:3684–3693 10.1242/jcs.045674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T.L., Park A.Y., Alcaraz A., Peng X., Jang I., Koni P., Flavell R.A., Gu H., Guan J.L. 2005. Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. J. Cell Biol. 169:941–952 10.1083/jcb.200411155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldi R., Mitola S., Strasly M., Defilippi P., Tarone G., Bussolino F. 1999. Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J. 18:882–892 10.1093/emboj/18.4.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B.N., Hsu D.K., Pang M., Brewer C.F., Johnson P., Liu F.T., Baum L.G. 2006. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176:778–789 [DOI] [PubMed] [Google Scholar]
- Storgard C.M., Stupack D.G., Jonczyk A., Goodman S.L., Fox R.I., Cheresh D.A. 1999. Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist. J. Clin. Invest. 103:47–54 10.1172/JCI3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupack D.G., Cheresh D.A. 2004. Integrins and angiogenesis. Curr. Top. Dev. Biol. 64:207–238 10.1016/S0070-2153(04)64009-9 [DOI] [PubMed] [Google Scholar]
- Tonnesen M.G., Feng X., Clark R.A. 2000. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 5:40–46 10.1046/j.1087-0024.2000.00014.x [DOI] [PubMed] [Google Scholar]
- Vaisman N., Gospodarowicz D., Neufeld G. 1990. Characterization of the receptors for vascular endothelial growth factor. J. Biol. Chem. 265:19461–19466 [PubMed] [Google Scholar]
- Yang E., Shim J.S., Woo H.J., Kim K.W., Kwon H.J. 2007. Aminopeptidase N/CD13 induces angiogenesis through interaction with a pro-angiogenic protein, galectin-3. Biochem. Biophys. Res. Commun. 363:336–341 10.1016/j.bbrc.2007.08.179 [DOI] [PubMed] [Google Scholar]