Abstract
The clam Lucina pectinalis supports its symbiotic bacteria by H2S transport in the open and accessible heme pocket of Lucina Hb I and by O2 transport in the narrow and crowded heme pocket of Lucina Hb II. Remarkably, air-equilibrated samples of Lucina Hb I were found to be more rapidly oxidized by nitrite than any previously studied Hb, while those of Lucina Hb II showed an unprecedented resistance to oxidation induced by nitrite. Nitrite-induced oxidation of Lucina Hb II was enabled only when O2 was removed from its active site. Structural analysis revealed that O2 clams up the active site by hydrogen bond formation to B10Tyr and other distal-side residues. Quaternary effects further restrict nitrite entry into the active site and stabilize the hydrogen-bonding network in oxygenated Lucina Hb II dimers. The dramatic differences in nitrite reactivities of the Lucina Hbs are not related to their O2 affinities or anaerobic redox potentials, which were found to be similar, but are instead a result of differences in accessibility of nitrite to their active sites; i.e. these differences are due to a kinetic rather than thermodynamic effect. Comparative studies revealed heme accessibility to be a factor in human Hb oxidation by nitrite as well, as evidenced by variations of rates of nitrite-induced oxidation that do not correlate with R and T state differences and inhibition of oxidation rate in the presence of O2. These results provide a dramatic illustration of how evolution of active sites with varied heme accessibility can moderate the rates of inner-sphere oxidative reactions of Hb and other heme proteins.
Keywords: hemoglobin, dynamics, adaptations, nitrite, heme oxidation, heme accessibility
11. INTRODUCTION
Nitrite is a unique Hb oxidant because of its ability to react with oxyHb in a complex reaction leading to formation of metHb and nitrate [1–5], and with deoxyHb in a reaction that generates metHb and NO [6–7]. Many recent studies have focused on the formation of bioactive NO from nitrite that is catalyzed by deoxy Hb because of the potential, but still controversial, role of this reaction in blood pressure regulation [8–14 ].
Work in our laboratories on the reactions of normal and cross-linked forms of Hb with nitrite led to some puzzling results. Conditions that stabilized human Hbs T state and reduced its intrinsic ease of oxidation had varied and sometimes opposing effects on rates of nitrite-induced oxidation. We were led to the conclusion that human Hbs reactions with nitrite are under the control of something other than the proteins quaternary equilibrium and associated oxygen affinity and redox potential [15–16]. We began to look for model systems to test the idea that evolutionary processes could have led to separate mechanisms of control for Hbs oxygen transport and for its reaction with heme oxidants.
Hbs of the clam Lucina pectinalis have characteristics that make them attractive model systems for our studies. Lucina Hb I, which transports H2S to the clams symbiotic bacteria, has a relatively open heme pocket, while Lucina Hb II, which transports O2 even in the presence of high levels of H2S, has a heme pocket that is narrow and crowded [17–20]. The rates of oxygen binding to the two Hbs reflect these differences in heme pocket architecture, with very fast binding to Lucina Hb I and slow binding to Lucina Hb II [1,21].
This report documents the results of extended studies of Lucina Hbs I and II. Surprisingly, although nitrite-induced oxidation is a common feature of all Hbs previously studied [22], differences between Lucina Hb I and II in their reactions with nitrite greatly exceeded any previously observed. Air-equilibrated samples of Lucina Hb I were more rapidly oxidized by nitrite than any previously studied Hb, while those of Lucina Hb II showed an unprecedented resistance to nitrite-induced oxidation. We carried out oxygen binding and redox measurements on the two clam Hbs and found that their O2-binding affinities and anaerobic redox potentials were very similar. Their differences in rates of reaction with nitrite were thus found to be independent of the thermodynamic driving forces for heme oxidation and heme O2-binding.
The extreme differences in rates of reaction of air-equilibrated forms of Lucina Hbs I and II with nitrite closely parallel their previously reported differences in reaction with H2S [17–20]. The clam can benefit from the structural differentiation of its Hbs via the protection provided against environmental nitrite, whose levels can vary appreciably in the black mud of mangrove swamps where it lives. It is also intriguing to consider that nitrite resistance may be protective against nitrite generated by nitrate-based respiration. Nitrite production by nitrate-based respiration is not found in higher organisms, but has been reported for many organisms containing symbiotic bacteria, including some lucinid clams where the respiration of nitrate to nitrite by the clams symbionts is coupled to the oxidation of elemental sulfur [23–24]. Internally generated nitrite is potentially more problematic than that encountered in the environment.
Our results provide a dramatic illustration of how evolution of active sites with varied heme accessibility can moderate inner-sphere oxidative reactions catalyzed by Hb. As will be discussed, active sites with varied heme accessibility may have evolved differently in the Hbs of varied organisms to meet specific environmental and physiological needs. Examination of heme accessibility in various Hbs under varied experimental conditions can thus provide a useful new vantage point for investigation of the molecular controls of the oxidative reactions catalyzed by Hb and whether these controls are based on thermodynamic or kinetic factors.
2. Materials and Methods
2.1 Isolation and Purification of Hbs and Mbs
Hbs were stripped of effectors by chromatographic procedures and studied at pH 7.4–7.5, 20°C, in 0.05 M bis-Tris. Anionic allosteric effectors were added as indicated in the text. Adult human hemoglobin (Hb A0) was isolated and purified from human blood using stripped Hb and fast-phase liquid chromatography as the final purification step [25]. The isolation of different types of Hbs from the clam Lucina pectinata was performed as previously described [26] with minor modifications [27]. The recombinant form of Lucina Hb I (Hb I Phe → B10Tyr) was prepared as previously described [26, 27]. Clam Hb samples were treated with dithionite to reduce all oxidized heme sites and subjected to Sephadex G-25 chromatography to remove the reductant prior to functional analysis.
2.2 Oxygen Binding Studies
Oxygen equilibria were measured using tonometric methods and UV-Vis spectrophotometry [28]. Deoxygenation of samples before air addition was achieved by repetitive cycles of exposure of 3 mL of Hb, held at 20°C in large volume tonometers, to N2and vacuum. To achieve full deoxygenation of the high affinity Lucina Hbs, these cycles were repeated for approximately one hour. A gastight syringe was used to inject measured volumes of room air through the rubber septum of the tonometer containing the Hb sample. After each addition the tonometers were rotated in a water bath for 10 min before an absorbance spectrum was measured. At each equilibration step the PO2was calculated and changes in the visible absorption spectrum, measured at three wavelengths on an HP-diode array spectrophotometer, were averaged and used to calculate the corresponding fractional O2saturation.
2.3 Anaerobic Oxidation Potential Determinations
Anaerobic oxidation potentials were determined under conditions like those used for oxygen binding determinations, making use of published spectoelectrochemical methods developed in our laboratories [29–30]. All potentials are reported versus NHE. UV-Vis spectra were taken on a CARY BIO 100 UV-Vis spectrophotometer and a cell temperature of 20°C was maintained using a circulating water bath. Applied potentials in spectroelectrochemical studies were controlled with an EG & G Princeton Applied Research model 363 potentiostat and samples were allowed to equilibrate for at least 15 min at each applied potential. Full oxidation and deoxygenation was ensured by exposure to large positive applied potentials for at least 1 hr and an anaerobic environment was maintained using argon.
2.4 Nitrite-induced Reactions of Oxy and Deoxy Hbs
Rapid and manual mixing methods were employed to determine reaction kinetics of Hbs or Mbs with freshly prepared solutions of nitrite (Fisher Scientific, Rochester, NY). In the rapid-mixing mode, an Applied Photophysics SF-17 microvolume stopped-flow instrument was used to measure the reaction kinetics. The dead time of this instrument is 1.3 ms. The Hb or Mb solutions analyzed were rapidly mixed with equal volumes of nitrite and the absorbance changes were followed by a photodiode array detector. At least 200 spectra were collected at any given reaction time with a resolution of 2.38 ms per spectrum for each reaction. The whole set of spectral data were then subjected to global analysis and curve fitting routines included in the Applied Photophysics software. The spectra of major reaction species were reconstructed, and the reaction rate constants were calculated. Under some experimental conditions, the reaction time courses were also monitored at a single wavelength (577 nm) and fitted to exponential equations using non-linear least square regression to obtain the reaction rate constants.
In manual-mixing mode, 3 mL of deoxygenated or oxyHbs in large-volume tonometers were agitated after injection of deoxygenated or oxygenated nitrite solutions with a gas-tight Hamilton syringe. Changes in the visible spectrum following nitrite injection were recorded with an HP diode array spectro-photometer to determine the rate and extent of nitrite-induced heme oxidation and extent (if any) of HbNO formation. Spectral component analyses at varied times following nitrite addition were carried out as described previously [31] using spectral standards for Hb derivatives and an iterative program for fitting standards to observed spectra.
2.5 Structural Analysis
Molecular modeling of the heme pocket of Lucina Hb II was performed using Insight II (Biosym Technologies) on a Silicon Graphics Indigo workstation and crystal coordinates from the Brookhaven Protein Data Bank. Structural solutions and refinements of the Lucina crystal structures were carried out as described in earlier publications [18].
3. Results
3.1 Oxygen Binding Studies
Representative Hill plots of oxygen binding by Lucina Hb I, Lucina Hb II and Hb A0 are shown in Figure 1. The Log P50 values and other relevant parameters determined for the Lucina Hbs and for human Hb under varied conditions are listed in Table I.
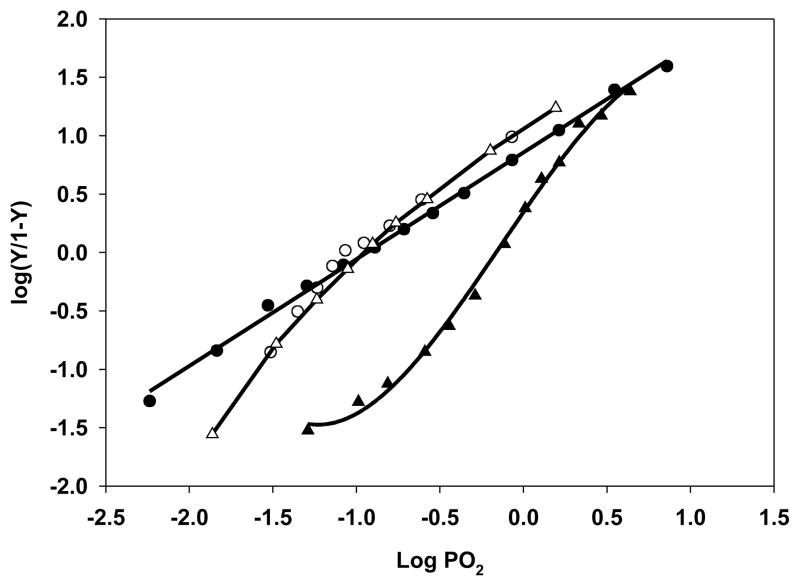
Fig. 1.
Hill plots of oxygen binding for Lucina Hb I (closed circles), Lucina Hb II (open circles and open triangles) and stripped Hb A0 (closed triangles). Hbs were all studied in 0.05 M bis-Tris, pH 7.5, 20°C. A representative Hill plot for Lucina Hb II in the presence of 0.2 M Cl− is also shown (open triangles).
Table I.
Comparison of clam (Lucina pectinata) Hbs and human Hb with regard to oxygenation and oxidation parameters, related to half-times for nitrite-induced oxidation of 60 μM (in heme) air-equilibrated solutions by 100-fold excess nitrite (over heme). Except as otherwise indicated, Hbs were in 0.05 M bis-Tris, pH 7.5, 20°C.
| Hb Type | Oxygen Affinity Log P50 | Oxygen Dissociation k (s-1) | H2O2 Oxidation (M−1s−1) | Redox Potential E1/2 (mV) | Nitrite Oxidation t1/2 (min) |
|---|---|---|---|---|---|
| Lucina Hb I | −0.9 | 92.2 ± 3.8 (c) | 403.2 ± 2.7 (c) | 52 | < 2 |
| Engineered Lucina Hb I with B10Tyr | −1.7 | 0.6 ± 0.02 (c) | 52.0 ± 4.7 (c) | 25 | 4 |
| Lucina Hb II | −1.0 | 0.15 ± 0.01 (c) | 64.0 ± 2.3 (c) | 8 | Very slow (> 2000) |
| Hb A0 | − 0.2 | 26.5 ± 0.5 | 22 (b) | 83 | 28 |
| + 0.2 M Cl− | 0.3 | 37.2 ± 0.6 | 122 | 18 | |
| + 0.7 M Cl− | 0.6 | 43.0 ± 1.5 | 85 | 18 | |
| + 150 uM IHP | 1.6 (a) | 66.5 ± 1.9 | 55 (b) | 135 | 50 |
| Hb-DBBF (Cross-linked) | 0.8(a) | 56.0 ± 0.6(c) | 120.5 ± 5.6 (c) | 125 (a) | 18 (a) |
Lucina Hbs I and II both showed much higher oxygen affinity than Hb A0. Lucina Hb I exhibited Hill plots with unity slopes, consistent with its monomeric nature.
Cooperative binding in the early stages of oxygenation of Lucina Hb II was observed. Hill plot slopes (n) at 20% saturation for Lucina Hb II were typically about 2, with lower values for partially oxidized samples. The slopes of Hill plots for Lucina Hb II were lower at 50% oxygenation and decreased to unity above 60% saturation. Essentially identical Hill plots were observed for Lucina Hb II without added effectors and in the presence of 0.2 M chloride (Fig. 1). The lowering of affinity below 50% saturation for Lucina Hb II may aid in O2 unloading to the clams symbiotic bacteria.
Although non-cooperative O2 binding by both Lucina Hbs I and II was previously reported [1], the earlier studies were done by other methods and under different experimental conditions. As is evident in Figure 1, the cooperativity exhibited by Lucina Hb II is most apparent below 50% oxygen saturation, with the result that its n50 value is not much greater than the previously reported value of unity.
Hill plots (not shown) for oxygen binding by genetically engineered Lucina Hb I with Phe(B10) → Tyr(B10) showed an increased oxygen affinity relative to native Lucina Hb I. The oxygen tension required for half-saturation of the engineered Hb is listed in Table I for comparison with the native Lucina Hbs.
3.2 Anaerobic Oxidation Potentials
The anaerobic oxidation potentials of the normal and engineered forms of Lucina Hbs were determined using spectroelectrochemical methods for obtaining accurate Nernst plots of the oxidation process. Results given in Table I list the redox properties of the Lucina clam Hbs under the same experimental conditions as used for studies of oxygen-binding and nitrite-induced oxidation.
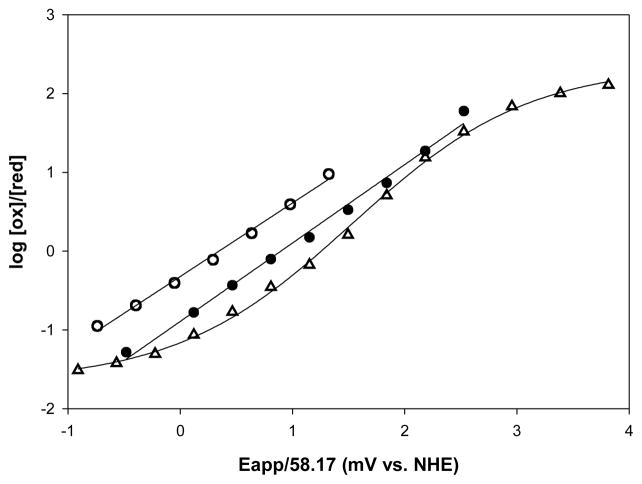
Nernst plots obtained for the Lucina Hbs are shown in Figure 2. Both Lucina Hb I and Lucina Hb II are more readily oxidized than adult human Hb. The redox potential of Lucina Hb I was similar to that of the R-state conformation of Hb A0. Under the experimental conditions of this study, Lucina Hb II was more easily oxidized than Lucina Hb I, consistent with Lucina Hb II having a more polar distal heme pocket. The Nernst plots for both Lucina Hbs I and II had midpoint slopes of near unity. This is consistent with the monomeric nature of Lucina Hb I and the unity slopes observed in its Hill plots of oxygen binding.
Fig. 2.
Representative Nernst plots of the anaerobic oxidation of Lucina Hb I (filled circles ), Lucina Hb II (open circles), and Hb A0 (triangles). Lucina Hbs were 0.1 mM (in heme) in 0.05M bis-Tris with no additional salt at pH 7.5 and 20°C. Hb A0 was 0.08 mM (in heme) in 0.05 M bis-Tris and 0.1 M KCl at pH 7.5 and 25°C.
Surprisingly, although cooperativity was observed in the initial stages of Hill plots of oxygen binding to Lucina Hb II, significant deviations from linearity were not apparent in the corresponding Nernst plots. The presence of an electrochemical mediator may have altered the extent of aggregation of Lucina Hb II, and thus its ability to show cooperative interactions in its oxidation curves.
Nernst plots obtained for the genetically engineered form of Lucina Hb I, with the substitution of Phe(B10) → Tyr(B10), indicated a shift in the redox potential to a value intermediate between that of Lucina Hb I and Lucina Hb II (Table 1). This result is consistent with an increase in the polarity of the heme cavity in the engineered form.
Small differences in our redox potential results obtained relative to earlier studies, done under other conditions and with different methods [1], were attributable to the anion-dependence of Hbs redox behavior. The reducing agent/mediators used in previous studies included some anions that may have shifted the measured reduction potentials positive relative to intrinsic (anion-free) values. As previously noted, the spectroelectrochemical technique employed in this study used a cationic mediator, which ensures that anionic effects are not introduced by the measurement itself [29, 32].
3.3 Kinetics of Nitrite Reactions with Clam Hbs
The reaction of oxyHb with nitrite typically results in formation of metHb and nitrate. Remarkably, air-equilibrated solutions of Lucina Hb II strongly resisted nitrite-induced oxidation. Spectral analysis 500 s after rapid mixing showed it to be less than 5% oxidized by 500 μM nitrite in 0.05 M Tris, pH 7.4, 20°C. This behavior was unprecedented. To our knowledge, all previously studied Hbs have become readily oxidized in the presence of excess nitrite.
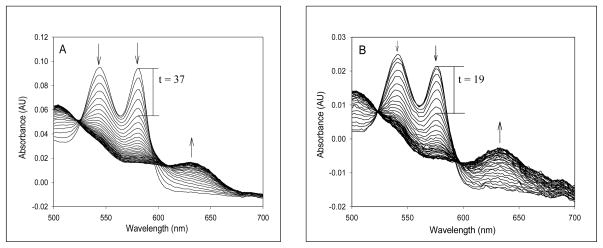
Unlike Lucina Hb II, air-equilibrated Lucina Hb I rapidly reacted with nitrite. Figure 3 shows the faster absorbance changes of air-equilibrated Lucina Hb I relative to those of horse Mb following rapid mixing with 500 μM nitrite in 0.05 M Tris, pH 7.4, 20°C. Under these reaction conditions Lucina Hb I had a half-time for nitrite-induced oxidation of 19 s, roughly ½ that for horse Mb (37 s), which was in turn ½ that for stripped Hb A0 under the same conditions (74 s). The genetically modified form of Lucina Hb I (Phe to Tyr at B10) had a half-time for nitrite-induced oxidation of 50 s, approximately 2.5 times longer than that of Lucina Hb I under these conditions.
Fig. 3.
Spectral changes as a function of time following addition of nitrite to air-equilibrated samples of (A) Mb (horse sketetal myogobin from Sigma Chemical Co) and (B) Lucina Hb I. The solutions containing 30 μM Hb or Mb were mixed with 500 μM nitrite in 0.05 M Tris buffer at pH 7.4 in a stopped-flow instrument at room temperature. The absorbance changes were monitored by a photodiode array detector over a reaction time of 500 seconds. The arrows indicate the directions of the absorbance changes with time, and the reaction time was labeled for the midpoint of total absorbance change.
The spectral changes shown in Figure 3 following nitrite addition to air-equilibrated Lucina Hb I were similar to those shown by Mb and were interpreted (see section 4.2 of Discussion) as being due to sequential formation of ferryl (Fe4+) and ferric (Fe3+.) forms during the reaction process. The rates of appearance of ferryl and ferric forms presented in Table II were determined by global analysis of the spectral changes based on a two consecutive step reaction, i.e. Fe2+ -> Fe4+ ->Fe3+. This analysis allows for the calculation of apparent rates k1 and k2, where d[ferrous]/dt = - k1[ferrous][nitrite], d[ferryl]/dt = k1[ferrous][nitrite] - k2[ferryl], and d[ferric]/dt = k2[ferryl].
Table II.
Apparent rate constants (see text) for reactions of horse skeletal Mb and Lucina clam Hbs with nitrite by global analysis of data shown in Figures 3.
| Horse Mb | Lucina Hb I | Lucina Hb I Tyr | Lucina Hb II | |
|---|---|---|---|---|
| k1 (s−1) oxy to ferryl | 0.02 | 0.037 | 0.014 | Very slow (not detected) |
| k2 (s−1) ferryl to met | 0.011 | 0.025 | 0.0071 |
As shown in Table II, both k1 and k2 for Lucina Hb I were about 2x larger than for horse Mb. The oxidation thus proceeds faster than for any Hb previously studied. The rates of spectral transitions for engineered Lucina Hb I (Phe to Tyr at B10) were only slightly slower than for Mb. In contrast, the reactions of air-equilibrated Lucina Hb II with nitrite were too slow for analysis by rapid mixing methods.
Manual mixing methods (Table I) confirmed the results of the rapid-mixing experiments. Air-equilibrated Lucina Hb I was oxidized rapidly after addition of nitrite. The reaction was too fast to measure adequately by manual mixing methods. In contrast, Lucina Hb II stayed in its ferrous (unoxidized) state for hours, even in the presence of 100-fold excess of nitrite.
3.4 Oxygen Slows Nitrite Reactions of Lucina Hb II
Spectral analysis showed that reactions of Lucina Hb II with nitrite in the deoxy state led to the production of HbNO and ferric Hb as reaction products. Nitrite-induced oxidation of Lucina Hb II was enabled only when O2 was removed from its active site. After one hour of incubation of a 60 μM (in heme) solution of air-equilbrated Lucina Hb II with 100-fold excess nitrite in a large volume tonometer, the Hb was still largely in its ferrous state, with less than 10% metHb.
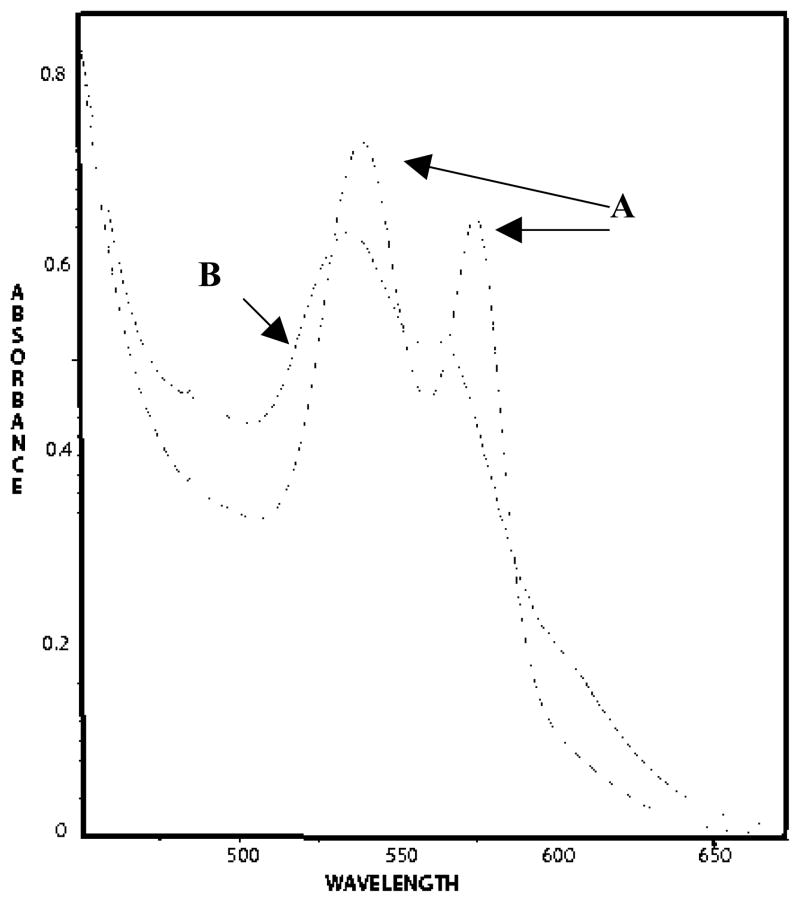
As shown in Figure 4, removal of O2 allowed nitrite-induced oxidation of Lucina Hb II to occur. After an hour of incubation of air-equilibrated Lucina Hb II with 100-fold excess nitrite, all O2 was removed by repetitive degassing and N2 flushing. The spectra of the mixture of Lucina Hb II and nitrite before and immediately after oxygen removal are shown in Figure 4. Deconvolution of the spectrum after oxygen removal revealed that reaction of nitrite with the deoxy Hb had generated a combination of metHb and HbNO. In separate studies it was shown that deoxygenation of the protein in the absence of nitrite did not induce oxidation.
Fig. 4.
Formation of metHb and HbNO upon deoxygenation of 60 μM Lucina Hb II in the presence of 100-fold excess nitrite. Spectra are shown (A- dashed line) for the air-equilibrated Hb one hour after nitrite addition, and (B - dotted line) for the same sample immediately after 1 hour of repetitive degassing and N2 flushing for removal of oxygen. The protein was in 0.05 M bis-Tris, pH 7.5 at 20°C.
These results show that Lucina Hb II reacts much more quickly in the deoxygenated state than when oxygenated. Oxygen removal in the presence of 100-fold excess nitrite took one hour. The protein was totally reacted with nitrite when examined immediately after deoxygenation. Its half-time for nitrite-induced oxidation when deoxygenated thus falls within the same range as that of deoxygenated human Hb, whose half-time for nitrite-induced oxidation under these conditions is about 10 minutes. The active site of Lucina Hb II was thus shown to be available for nitrite reaction when deoxygenated, and clammed up, essentially unreactive with nitrite, in its oxygenated state.
3.5 Structural Analysis
Our structural analysis showed that the sterically hindered heme pocket of Lucina Hb II provides an explanation for the proteins unusual resistance to nitrite-induced oxidation. Oxygen bound at the active site makes a network of hydrogen bonds to distal pocket residues as shown in Figure 5. The hydrogen-bonding network adds steric hindrance to the already crowded and narrow heme pocket of Lucina Hb II that was documented in previous studies [18–21]. As noted in section 4.4 of Discussion, structural constraints imposed by dimerization of Lucina Hb II may play a role in stabilizing distal pocket residues in a closed conformation that limits nitrite entry.
Fig. 5.
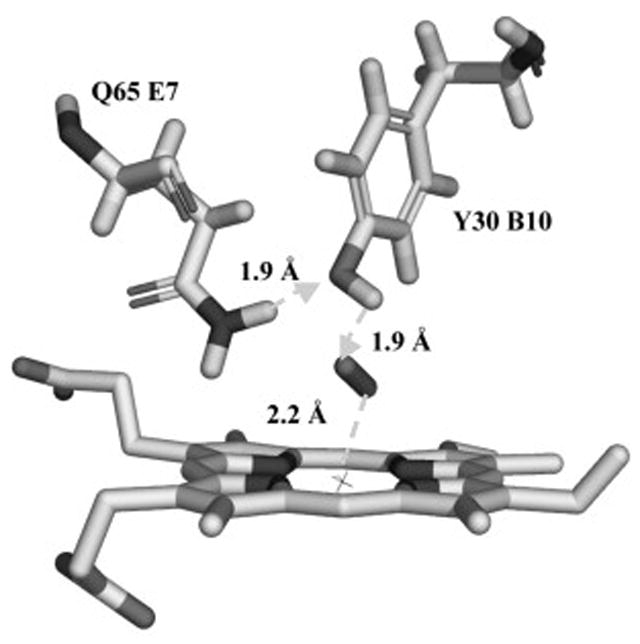
Hydrogen-bonding network in the active site of oxygenated Lucina Hb II. Bond interactions are shown with gray lines. The heme iron position is marked by X. Distances between residues are: Fe-O1 (2.2 Å), Tyr30 (OH)-O2 (1.9 Å), and Tyr30 (OH)-Gln65(NE2) (1.90Å). See reference 18 for details of methods used in structural analyses.
3.6 Oxygen Slows Kinetics of Nitrite Reactions with Human Hb
Studies of the oxygenation, oxidation and nitrite-induced reactions of purified forms of adult human Hb (Hb A0) and a cross-linked form of Hb (Hb-DBBF) were done side-by-side with those of the clam Hbs. Table I summarizes the results of these comparative studies.
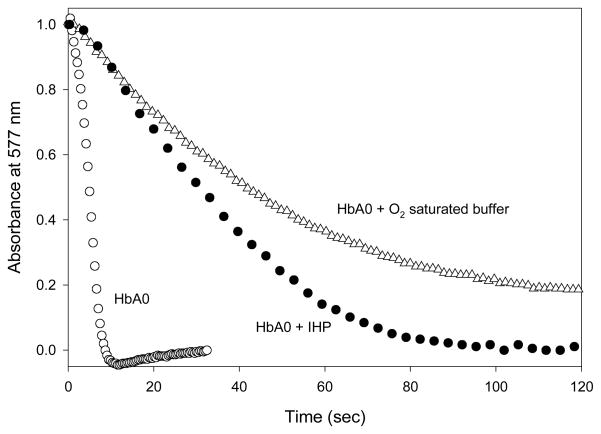
Representative time-courses shown in Figure 6 following rapid mixing of 200-fold excess nitrite with 30 μM Hb A0 illustrate the oxidation of stripped air-equilibrated human Hb (slow compared to Mb or Lucina Hb I) and the well-known inhibitory effect of inositol hexaphosphate (IHP) on the reaction, which increases the oxidation half-time from 5 s to 28 s under these reaction conditions.
Fig. 6.
Time courses of nitrite-induced oxidation of Hb A0 under varied conditions. Solutions containing 30 μM Hb were rapidly mixed with 200-fold excess nitrite in 0.05 M Tris buffer at pH 7.4 in a stopped-flow instrument at room temperature. The spectral changes were monitored by a photodiode array detector over time. The absorbance changes at 577 nm plotted versus time are shown for reaction of stripped Hb A0 in air-equilibrated buffer (open circles); in O2 saturated buffer (open triangles); and in the presence of IHP in air-equilibrated buffer (closed circles).
Also illustrated in Figure 6 is the strong inhibitory effect of oxygen on the reaction of human Hb with nitrite. When the level of O2 was increased from that of air-equilibration to a full atmosphere of O2 there was a 20x increase in the half-time for oxidation (from 5 s to 41 s for half-oxidation). The inhibitory action of oxygen on nitrites reactions with human Hb, clearly associated with decreased availability of deoxy sites, is a potentially important factor that merits further attention in regard to nitrites physiological effects.
4. Discussion
4.1 Evolution of Varied Functions in Invertebrate Hbs
Lucina pectinata is a large tropical clam that lives in the black sulfide-rich muds of mangrove swamps. The presence of high (mM) levels of functionally distinct Hbs in its gills provides adaptive advantages for the clam and its symbiotic bacteria [17–20]. The bacterial symbionts thrive and supply metabolic by-products to the clam only when supplied with both O2 and H2S. The clam contributes to the symbiosis by producing high levels of structurally distinct Hbs in its gills to perform both O2 and H2S transport functions [17].
Enormous diversity exists among invertebrate Hbs [33]. This report on the structurally and functionally diverse Hbs of a clam that hosts symbiotic bacteria adds another chapter to the story of how differences among invertebrate Hbs meet widely varying physiological demands. It also illustrates how evolution of active sites with varied heme accessibility can moderate the rates of inner-sphere oxidative reactions catalyzed by Hb and other heme proteins.
4.2 Extreme Differences in Rates of Nitrite-induced Oxidation of Lucina Hbs
Lucina Hbs I and II exhibited extreme differences in their rates of reaction for nitrite-induced oxidation. Air-equilibrated Lucina Hb I was very rapidly oxidized by nitrite. The half-time of the oxidative reaction with 500 μM nitrite was 19 s, half that for horse Mb under comparable experimental conditions. In striking contrast, air-equilibrated Lucina Hb II showed an unprecedented ability to resist nitrite-induced oxidation. Although oxy Hbs from varied organisms show variations in their rates of nitrite oxidation [22], none previously studied has shown a rate of nitrite oxidation as fast as that of Lucina Hb I or as slow as that of Lucina Hb II.
Nitrite is remarkable among heme oxidants because of its ability to react with oxyHb in a complex reaction leading to formation of metHb and nitrate [7–11]; and with deoxyHb in a reaction that generates metHb and NO [12–13]. Simplified forms of the relevant reactions are.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The reaction depicted by equation 1 is very complex. A slow initiation phase in the reaction of oxy Hb with nitrite produces both hydrogen peroxide and metHb. This is followed by a complex autocatalytic phase of heme oxidation that involves formation of NO2 radicals, ferric and ferryl heme iron, and protein-based free-radicals. The onset and rate of the autocatalytic phase is affected by the nature and oxidation state of the Hb, the ratio of nitrite to heme, and other experimental conditions such as choice of buffers and effectors [7–10; 14–17].
For our comparison of relative rates of the nitrite-induced oxidative reactions of Hbs, the rates of disappearance of Fe2+ and appearance of Fe4+ and Fe3+ forms of Hb were determined by global analysis of spectral changes of the iron during the reaction and modeled by a two consecutive-step reaction, i.e., Fe2+(ferrous Hb) → Fe4+(ferrylHb) → Fe3+(metHb), where k1 is for the first step of the oxidation and k2 is for the second step of the oxidation. As noted in section 3.3 of Results, both k1 and k2 for Lucina Hb I were about 2x larger than k1 and k2 for horse Mb. The rates of spectral transitions for engineered Lucina Hb I (Phe to Tyr at B10) were only slightly slower than for Mb. In contrast, the reactions of Lucina Hb II with nitrite were too slow for analysis by rapid mixing methods.
4.3 Oxygen Can Slow Heme Oxidation
Oxygen levels determine the equilibrium between oxy and deoxy Hb (equation 5) and thus influence the nitrite-induced oxidative reactions at a fundamental level. The presence of oxygen greatly reduced the rate of reaction of nitrite with Lucina Hb II. The Hbs oxidation via equation 1 is dramatically slower compared to its oxidation via equation 2. Moreover, due to the proteins high oxygen affinity, there are very few deoxy sites available even at low oxygen levels, so that reaction via equation 2 is effectively precluded in air-equilibrated solutions. Complete gasometric oxygen removal from Lucina Hb II allowed the reaction of nitrite with deoxyHb (equation 2) to occur. These results show that it is only the oxygenated form of Lucina Hb II that strongly resists oxidation.
That oxygen can also clam-up the active site of Hb A0 and slow the initial stages of its reaction with nitrite was shown in Fig. 6. This was an unexpected finding, but one with many documented analogs. Oxygen at elevated levels also inhibits human Hbs autoxidation and inhibits its oxidation by ethyl or butyl nitrite, ferricyanide, hydroxylamine, chlorate, hydrogen peroxide and quinines [7, 22, 34]. Unlike the reaction of human Hb with inorganic nitrite, which can occur with either oxy or deoxy heme sites, reaction with ethyl or butyl nitrite occurs exclusively with deoxy heme [34]. Although the full story of how inhibition by oxygen occurs in all these instances may be debated, it is clear that oxygen binding to both human and clam Hbs can provide protection against oxidation.
4.4 Heme Pocket Geometries of the Lucina Hbs
The functional differences between Lucina Hbs I and II can be understood in terms of their very different heme pocket geometries [18–20]. Unlike most mammalian Hbs, Lucina Hbs I and II both have a distal Gln residue in the heme pocket. They have, in addition, significant differences in the stereochemistry of their heme pockets [19–20]. The differences in heme accessibility suggested by the crystal structures for the active sites of Lucina Hbs I and II are consistent with the large differences they exhibit in their reactions with hydrogen sulfide [17–18], with peroxide and nitric oxide [21] and with nitrite (this report).
With a large distal heme pocket and without the distal His that normally forms a protective gate, Lucina Hb I has a more accessible heme than most vertebrate Mbs and Hbs [18–20]. Increased heme accessibility is thus a reasonable explanation for the higher rate of nitrite-induced heme oxidation observed for air-equilibrated Lucina Hb I.
In contrast, the resistance of air-equilibrated Lucina Hb II to nitrite-induced oxidation can be understood in terms of decreased heme accessibility. Lucina Hb II has a sterically restricted active site brought about by a narrow heme pocket, a His97 trans-effect, and Gln and Tyr as distal-side residues in close proximity to the heme group that participate in a hydrogen bonding network [20]. The involvement of bound oxygen in the hydrogen-bonding network in the small and narrow distal heme pocket of Lucina Hb II is shown in Figure 5 [first reported in ref. 18]. The combination of these structural features clearly retards the rate of oxidation of air-equilibrated Lucina Hb II by nitrite.
Amino acid residue Tyr(B10) may play a particularly important role in clamming-up the active site of Lucina Hb II by formation of a hydrogen bond to heme-bound oxygen (Fig. 5). The absence of this residue is one feature that differentiates Lucina Hb I, which reacts readily with H2S and nitrite, from Lucina Hb II, which does not. An engineered form of Lucina Hb I with the substitution of Phe by Tyr at position B10 showed modified oxygen binding and redox properties as well as greatly reduced O2 dissociation rates compared to native Lucina Hb I (Table I), These alterations suggest that Try(B10) in the engineered Hb can form a hydrogen bond to O2 in the active site as does Try(B10) in Lucina Hb II. In spite of these major alterations, the engineered form failed to reproduce the unusual nitrite-resistant properties of oxygenated Lucina Hb II.
The data summarized above indicate that factors in addition to hydrogen-bonding to O2 in the active site contribute to the sterric hindrance restricting the active site of Lucina Hb II and underlying its resistance to nitrite-induced oxidation. In oxidized Lucina Hb II, distal pocket residue Tyr(B10) can exist in open and closed conformations, where the closed conformation would favor the hydrogen-bonding network of the oxy protein [18]. The high resolution structural information available for Lucina Hb II suggests the involvement of both tertiary and quaternary structural elements in stabilization of the closed conformation of Tyr(B10) that restricts entry of nitrite to the oxygenated active site. At the tertiary level, the sterically-restricted active site of Lucina Hb II is brought about by a narrow heme pocket, a His97 trans-effect, and Gln and Tyr as distal-side residues in close proximity to the heme group. At the quaternary level, dimer formation brings the active heme groups into close proximity and may greatly restrict pathways of nitrite access. Moreover, dimer formation appears to add a large measure of stability to the closed Tyr(B10) conformation for the oxyHb. Cooperative subunit interactions within the dimer lead to higher oxygen affinity at higher than 60% O2 saturation (Fig. 1), indicative of a switch to the closed conformation. The absence of appreciable resistance to nitrite-induced oxidation of the monomeric engineered form of Lucina Hb I (TyrB10) is additional supportive evidence of quaternary effects that contribute to the low heme accessibilty of Lucina Hb II dimers.
It will be of interest to determine if other multimeric Hbs with polar B10 and E7 residues are resistant to nitrite-induced oxidation. A generality that can be drawn from prior reports is that when these residues are polar, as they are in Lucina Hb II, a hydrogen-bonding network can form within the distal pocket [35]. This motif is found in other invertebrate Hbs and typically results in a high O2 affinity and reduced rate of autoxidation [36]. Since polar B10 and E7 residues are often found in high-O2 affinity invertebrate Hbs, it would not be surprising if constraints brought about by subunit associations in some of these Hbs confer resistance to nitrite-induced oxidation.
4.5 Heme Accessibility as a Control of Nitrite-induced Heme Oxidation
The extreme differences in nitrite reactivity exhibited by the Lucina Hbs suggest an unexpected regulatory mechanism for control of heme oxidation in which heme accessibility is the primary variable.
The reactivity of Hb A0 with nitrite has typically been discussed in terms of the proteins R- and T-state conformations. High reactivity toward nitrite is associated with Hbs easily oxidized, high O2 affinity (R-state) conformation. Low nitrite reactivity is associated with Hbs difficult-to-oxidize, low O2 affinity (T-state) conformation. The association of high reactivity toward nitrite with Hbs R-state conformation is based on the large inhibitory effects of IHP on rates of nitrite-induced oxidation (shown in Fig. 6); studies with conformationally stabilized Hbs in sol-gels [37]; and autocatalytic increases in the rate of reaction of nitrite with deoxyHb [7–9].
We note several difficulties with assigning differences in rates of nitrite-induced Hb oxidation solely to shifts between R and T states. Notably, T-state stabilized Hb A0 in the presence of IHP has greatly decreased rates of nitrite-induced oxidation. In contrast, Hbs such as Hb-DBBF, stabilized in the T-state by chemical cross-linking, have lower oxygen affinities and reduced intrinsic propensities for oxidation, but have increased, rather than decreased, rates of nitrite-induced oxidation in both aerobic and anaerobic conditions [15–16]. Secondly, as shown in Table I, effector-induced shifts toward Hbs T-state are not uniformly accompanied by decreased rates of nitrite-induced oxidation. Notably, IHP decreases the rate of reaction of air-equilibrated Hb with nitrite while inorganic chloride has the opposite effect. This indicates that anionic effectors can induce heme pocket alterations that either hinder or facilitate oxidative reactions as they favor Hbs T-state conformation.
Both chloride addition and cross-linking of Hb increase rates of reaction with nitrite, apparently via more open heme pockets with enhanced heme accessibility. Taken together with the remarkable differences in nitrite-induced oxidation for Lucina Hbs I and II, these findings indicate that heme accessibility can vary appreciably under varied conditions and may play a large and previously unappreciated role in Hbs oxidative reactions.
4.6 Physiological and Clinical Implications
The strong and unprecedented resistance of Lucina Hb II to nitrite documented in this report may be protective against environmental nitrite or against nitrite generated by nitrate-based respiration.
The molecular controls of the complex reactions of human Hb with nitrite are still not well understood. These reactions merit further investigation because of their physiological and clinical significance. Traditionally, differences in Hbs reactivity with nitrite under varied conditions have been viewed in terms of differences between Hbs R and T conformations. However, we show in this report that rate differences in nitrite-induced oxidation of Hb may also arise as a result of alterations of heme accessibility that do not correlate in a straightforward manner with R or T quaternary states. The rate differences observed are evidently less determined by thermodynamic factors (as expressed by anaerobic redox potential and O2 affinity) than by kinetic factors that are expressed through heme accessibility. Examination of heme accessibility as a function of experimental conditions is thus a useful vantage point for investigation of how the rates of Hbs oxidative reactions are controlled.
In summary, the evolution of structurally distinct Hbs with differences in accessibility to their active sites is an intriguing molecular adaptation that allows Lucina pectinalis to live in the sulfide-rich muds of mangrove swamps. The Hbs of the clam support its symbiotic bacteria by H2S transport in the open and accessible heme pocket of Lucina Hb I and by O2 transport in the narrow and crowded heme pocket of Lucina Hb II. The sterically-hindered active site of Lucina Hb II restricts access to hydrogen sulfide [17–20]; to hydrogen peroxide and NO [21] and renders the oxygenated protein remarkably insensitive to nitrite (this report). We hypothesize that incorporation of elements that similarly restrict heme access in human Hb could lead to the design of new forms of cell-free Hbs that have reduced oxidative toxicity when used in vivo as therapeutic supplements to normal oxygen uptake and delivery.
Acknowledgments
CB thanks Giulia Ferruzi for excellent technical assistance and NIH for financial support (5PO1-HL-071064-04). ALC thanks NSF for support (CHE-0809466). JLG gratefully acknowledges partial support from NSF (MCB-0843608) and NIH (NIGMS MBRS-SCORE 2 S06GM08103- 34). The opinions and assertions contained herein are the scientific views of the author and are not to be construed as policy of the United States Food and Drug Administration.
Footnotes
Abbreviations: Hb A0 is adult human Hb; Hb-DBBF, generated by reaction of deoxy Hb A0 with bis(3,5-dibromosalicyl)fumarate, has a single intra-tetrameric cross-link between the α chains at 99Lys; IHP is inositol hexaphosphate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keszler A, Piknova B, Schechter AN, Hogg N. The reaction between nitrite and oxyhemoglobin: a mechanistic study. J Biol Chem. 2008;283:9615–9622. doi: 10.1074/jbc.M705630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosaka H, Imaizumi K, Tyuma I. Mechanism of autocatalytic oxidation of oxyhemoglobin by nitrite. An intermediate detected by electron spin resonance. Biochim Biophys Acta. 1982;702:237–241. doi: 10.1016/0167-4838(82)90508-8. [DOI] [PubMed] [Google Scholar]
- 3.Kosaka H, Tyuma I. Production of superoxide anion by N, N-bis(2-hydroxyethyl)-iminotris(hydroxymethyl)methane buffer during oxidation of oxyhemoglobin by nitrite and effect of inositol hexaphosphate on the oxidation. Biochim Biophys Acta. 1982;709:187–193. doi: 10.1016/0167-4838(82)90460-5. [DOI] [PubMed] [Google Scholar]
- 4.Doyle MP, Pickering RA, Dykstra RL, Nelson CL, Boyer RF. Involvement of peroxide and superoxide in the oxidation of hemoglobin by nitrite. Biochem Biophys Res Commun. 1982;105:127–132. doi: 10.1016/s0006-291x(82)80020-x. [DOI] [PubMed] [Google Scholar]
- 5.Lissi E. Autocatalytic oxidation of hemoglobin by nitrite: a possible mechanism. Free Radical Biol Med. 1988;24:1535–1536. doi: 10.1016/s0891-5849(98)00004-5. [DOI] [PubMed] [Google Scholar]
- 6.Brooks J. Action of nitrite on hemoglobin in the absence of oxygen. Proc R Soc Med. 1937;123:368–382. [Google Scholar]
- 7.Doyle MP, Pickering RA, DeWeert TM, Hoekstra JW, Pater D. Kinetics and mechanism of the oxidation of human deoxyhemoglobin by nitrites. J Biol Chem. 1981;256:12393–12398. [PubMed] [Google Scholar]
- 8.Gladwin MT, Schechter AN, Kim-Shapiro DB, Patel RP, Hogg N, Shiva S, Cannon RO, III, Kelm M, Wink DA, Espey MG, Oldfield EH, Pluta RM, Freeman BA, Lancaster JR, Jr, Feelisch M, Lundberg JO. The emerging biology of the nitrite anion. Nat Chem Biol. 2005;1:308–314. doi: 10.1038/nchembio1105-308. [DOI] [PubMed] [Google Scholar]
- 9.Crawford JT, Scott IT, Huang Z, Shiva S, Chacko B, Schechter AN, Darley-Usmar V, Kerby J, Lang J, Kraus D, Ho C, Gladwin MT, Patel R. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, III, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 11.Angelo M, Singel DJ, Stamler JS. An S-nitrosothiol (SNO) synthase function of hemoglobin that utilizes nitrite as a substrate. Proc Natl Acad Sci. 2006;103:8366–8371. doi: 10.1073/pnas.0600942103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Faassen EE, Bahrami S, Feelisch M, Hogg N, Kelm M, Kim-Shapiro DB, Kozlov AV, Li H, Lundberg JO, Mason R, Nohl H, Rassaf T, Samouilov A, Slama-Schwok A, Shiva S, Vanin AF, Weitzberg E, Zweier J, Gladwin MT. Nitrite as regulator of hypoxic signaling in mammalian physiology. Med Res Reviews. 2009;29:683–741. doi: 10.1002/med.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem. 2003;278:46349–46356. doi: 10.1074/jbc.M307572200. [DOI] [PubMed] [Google Scholar]
- 14.Salgado MT, Nagababu E, Rifkind JM. Quantification of intermediates formed during the reduction of nitrite by deoxyhemoglobin. J Biol Chem. 2009;284:12710–12718. doi: 10.1074/jbc.M808647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonaventura C, Henkens R, Alayash AI, Crumbliss AL. Allosteric effects on oxidative and nitrosative reactions of cell-free hemoglobins. IUBMB Life. 2007;59:498–506. doi: 10.1080/15216540601188546. [DOI] [PubMed] [Google Scholar]
- 16.Bonaventura C, Henkens R, Weaver KD, Alayash AI, Crumbliss AL. In: Protein Reviews. Bolognesi M, di Prisco G, Verde C, editors. Vol. 9. Springer; Verlag: 2008. Chapter 9. [Google Scholar]
- 17.Kraus DW, Wittenberg JB. Hemoglobins of the Lucina pectinata/bacteria symbiosis. I. Molecular properties, kinetics and equilibria of reactions with ligands. J Biol Chem. 1990;265:16043–16053. [PubMed] [Google Scholar]
- 18.Gavira JA, Camara-Artigas A, De Jesús-Bonilla W, López-Garriga J, Lewis A, Pietri R, Yeh SR, Cadilla CL, García-Ruiz JM. Structure and ligand selection of hemoglobin II from Lucina pectinata. J Biol Chem. 2008;283:9414–9423. doi: 10.1074/jbc.M705026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizzi M, Wittenberg J, Coda A, Ascenzi P, Bolognesi M. Structural bases for sulfide recognition in Lucina pectinata hemoglobin. J Mol Biol. 1996;258:1–5. doi: 10.1006/jmbi.1996.0228. [DOI] [PubMed] [Google Scholar]
- 20.Pietri R, Granell L, Cruz A, De Jesús-Bonilla W, Lewis A, Leon R, Cadilla CL, López-Garriga J. Tyrosine B10 and heme-ligand interactions of Lucina pectinata hemoglobin II: control of heme reactivity. Biochim Biophys Acta. 2005;1747:195–203. doi: 10.1016/j.bbapap.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 21.De Jesús-Bonilla W, Jia Y, Alayash AI, López-Garriga J. The heme pocket geometry of Lucina pectinata hemoglobin II restricts nitric oxide and peroxide entry: model of ligand control for the design of a stable oxygen carrier. Biochem. 2007;46:10451–10460. doi: 10.1021/bi7003262. [DOI] [PubMed] [Google Scholar]
- 22.Kiese M. Methemoglobinemias: A Comprehensive Treatise. CRC Press Inc; Cleveland: 1974. [Google Scholar]
- 23.Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988;52:190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hentschel U, Hand SC, Felbeck H. The contribution of nitrate respiration to the energy budget of the symbiont-containing clam Lucinoma aequizonata: a calorimetric study. J Exp Biol. 1996;199:427–433. doi: 10.1242/jeb.199.2.427. [DOI] [PubMed] [Google Scholar]
- 25.Bonaventura C, Ferruzzi G, Tesh S, Stevens RD. Effects of s-nitrosation on oxygen binding by normal and sickle cell hemoglobin. J Biol Chem. 1999;274:24742–24748. doi: 10.1074/jbc.274.35.24742. [DOI] [PubMed] [Google Scholar]
- 26.Pietri R, Lewis A, Leon RG, Casabona G, Kiger L, Yeh S, Fernandez-Alberti S, Marden MC, Cadilla CL, Lopez-Garriga J. Factors Controlling the Reactivity of Hydrogen Sulfide with Hemeproteins. Biochem. 2009;48:4881–4894. doi: 10.1021/bi801738j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon RG, Munier-Lehmann H, Barzu O, Baudin-Creuza V, Pietri R, López-Garriga J, Cadilla CL. High-level production of recombinant sulfide-reactive hemoglobin I from Lucina pectinata in Escherichia coli. Protein Expression Purif. 2004;38:184–195. doi: 10.1016/j.pep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 28.Riggs AF, Wolbach RA. Sulfhydryl groups and the structure of hemoglobin. J Gen Physiol. 1956;39:585–605. doi: 10.1085/jgp.39.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faulkner KM, Bonaventura C, Crumbliss AL. A spectroelectrochemical method for evaluating factors which regulate the redox potential of hemoglobins. Inorg Chim Acta. 1994;226:187–194. [Google Scholar]
- 30.Taboy CH, Bonaventura C, Crumbliss AL. Anaerobic oxidations of myoglobin and hemoglobin by spectroelectrochemistry. In: Sen CK, Packer L, editors. Methods in enzymology: redox cell biology and genetics Part B. Vol. 353. Academic Press; New York: 2002. pp. 187–209. [DOI] [PubMed] [Google Scholar]
- 31.Fago A, Crumbliss AL, Peterson J, Pearce JL, Bonaventura C. The case of the missing NO-hemoglobin: spectral changes suggestive of heme redox reactions reflect changes in NO-heme geometry. Proc Natl Acad Sci. 2003;100:12087–12092. doi: 10.1073/pnas.2032603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taboy CH, Faulkner KM, Kraiter D, Bonaventura C, Crumbliss AL. Concentration-dependent effects of anions on the anaerobic oxidation of hemoglobin and myoglobin. J Biol Chem. 2000;275:39048–39054. doi: 10.1074/jbc.M004547200. [DOI] [PubMed] [Google Scholar]
- 33.Weber RE, Vinogradov SN. Non-vertebrate hemoglobins: functions and molecular adaptations. Physiol Rev. 2001;81:596–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- 34.Doyle MP, Lepoire DM, Pickering RA. Oxidation of hemoglobin and myoglobin by alkyl nitrites: inhibition by oxygen. J Biol Chem. 1981;256:12399–12404. [PubMed] [Google Scholar]
- 35.Gow AJ, Payson AP, Bonaventura J. Invertebrate hemoglobins and nitric oxide: how heme pocket structure controls reactivity. J Inorg Biochem. 2005;99:903–911. doi: 10.1016/j.jinorgbio.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Crawford MJ, Goldberg DE. Role for the salmonella flavohemoglobin in protection from nitric oxide. J Biol Chem. 1998;273:12543–12547. doi: 10.1074/jbc.273.20.12543. [DOI] [PubMed] [Google Scholar]
- 37.Roche CJ, Dantsker D, Samuni U, Friedman JM. Nitrite reductase activity of sol-gel-encapsulated deoxyhemoglobin: influence of quaternary and tertiary structure. J Biol Chem. 2006;281:38757–38768. doi: 10.1074/jbc.M603914200. [DOI] [PubMed] [Google Scholar]
- 38.Rogers MS, Brocknor B, Cashon RE, Alayash AI. Effects of polymerization on the oxygen carrying and redox properties of diaspirin cross-linked hemoglobin. Biochim Biopys Acta. 1995;1248:135–142. doi: 10.1016/0167-4838(95)00017-o. [DOI] [PubMed] [Google Scholar]