Abstract
Background
A review of current literature shows that the combined use of the cell permeable esterase-substrate fluorescein diacetate (FDA) and the cell impermeant nucleic acid stain propidium iodide (PI) to be one of the most common fluorescence-based methods to assess the viability of isolated islets of Langerhans, and it is currently used for islet product release prior to transplantation in humans. However, results from this assay do not correlate with islet viability and function or islet transplantation success in animals or humans (Eckhard et al. 2004; Ricordi et al. 2001). This may be in part attributed to considerable differences as well as discrepancies in the use of these reagents on islets.
We critically surveyed the literature and evaluated the impact of a number of variables associated with the use of FDA/PI to determine their reliability in assessing islet cell viability. In addition, we evaluated other fluorescent stains, such as SYTO®13, SYTO®24 and SYBR®14 as possible alternatives to FDA.
Results
We found that the stability of stains in storage and stock solutions, the number of islets stained, concentration of stains, staining incubation time, the buffer/media used, and the method of examining islets were significant in the final scoring of viability. For archival file photos, the exposure time and camera/software settings can also impact interpretation of viability.
Although our results show that FDA does detect intracellular esterase activity and staining with PI does assess cell membrane integrity, the results obtained from using these stains did not correlate directly with expected islet function and viability per transplantation into diabetic athymic nude mice (Papas et al. 2007). In addition, the use of two nucleic acid stains, such as SYTO®13 and PI, for live/dead scoring exhibited staining anomalies which limit their accuracy in assessing islet viability.
Conclusions
From a review of the literature and from our observations on the impact of reagent handling and various staining and imaging parameters used to visually evaluate islets, consistent interpretation of islet cell membrane integrity and viability is dependent upon a number of factors. We discuss the utility and limitations of these reagents in evaluating islet cell membrane integrity and viability.
Background
Since the first successful isolation and transplantation of the islets of Langerhans [1] further enhancements in islet isolation [2-6] have promoted substantial advances in clinical islet transplantation for the treatment of type I diabetes [7-9]. Ongoing research continues to improve methods of pancreas preservation, islet isolation, culture and shipment [10-13].
Current methods in the isolation of islets may cause significant damage to β-cells, either by inducing cells to undergo apoptosis [14-16] or cell destruction [17-19]. The timely and accurate assessment of islet quality is not only important in improving isolation methods, but also critical in predicting the success of subsequent xeno- and allo-transplantation [20, 21].
Viability assays based on the use of fluorescent stains and/or dye-conjugated substrates (Tables 1 and 2) are either based solely on membrane integrity using two nucleic acid stains differing in cell permeability or, based on intracellular enzyme activity plus membrane integrity [22-26]. The former method utilizes a cell permeable fluorescent nucleic stain to stain all cells, and a cell impermeant nucleic acid stain of another fluorescent color to stain all dead cells. The proprietary SYTO® and SYBR® dyes (Molecular Probes Inc, Eugene, OR) and acridine orange (AO) passively diffuse across the plasma membrane whereas propidium iodide (PI), ethidium bromide (EB) or ethidium homodimer-1 (EH-1) are excluded and can only enter cells that have compromised membranes [27]. Usually the dead cell stain is provided in a slight molar excess to competitively displace the ‘live’ stain from nucleic acids in dead cells to improve discrimination. In the latter method, live cells are enumerated by either the presence or absence of enzymatic activity by using a non-fluorescent, cell permeable enzyme substrate that is converted to a fluorescent product retained within the cells for a sufficient amount of time to assess cell response; dead cells would exhibit little or no fluorescence with these substrates and are more clearly enumerated by the use of a cell impermeant nucleic acid stain such as PI, EB or EH-1. Both methods score viable and dead cells with two color detection, usually green and red fluorescence, green for viable cells and red for dead.
Table 1.
Spectral characteristics of various nucleic acid stains used in viability assays. Excitation and emission maxima of commonly used nucleic acid stains (data of dye bound to nucleic acids in aqueous buffer).
| Nucleic Acid Stain | Permeability | Excitation (nm) |
Emission (nm) |
Molar Extinction Coefficient |
EC Solvent |
|---|---|---|---|---|---|
| Acridine Orange/DNA | Yes | 500 | 526 | 53,000 | H2O/DNA |
| Acridine Orange/RNA | Yes | 460 | 650 | 25,000 | H2O/RNA |
| 7-AAD | No | 546 | 647 | 25,000 | H2O/DNA |
| Ethidium bromide | No | 518 | 605 | 5,200 | H2O/DNA |
| Propidium iodide | No | 535 | 617 | 5,400 | H2O/DNA |
| SYTO® 13 | Yes | 488 | 509 | 74,000 | H2O/DNA |
| SYTO® 24 | Yes | 490 | 515 | 58,000 | H2O/DNA |
| SYBR® 14 | Yes | 487 | 517 | 50,500 | H2O/DNA |
Table 2.
Spectral characteristics of various cytoplasmic stains Excitation and emission maxima of commonly used cytoplasmic stains and their derivatives.
| Stain | Permeability | Excitation (nm) |
Emission (nm) |
Molar Extinction Coefficient |
EC Solvent |
|---|---|---|---|---|---|
| Calcein, AM | Yes | <300 | None | -- | Methanol |
| Calcein | No | 494 | 515 | 77,000 | KPO4/pH9 |
| Fluorescein diacetate | Yes | <300 | None | -- | Methanol |
| Fluorescein | No | 490 | 514 | 93,000 | KPO4/pH9 |
A comparison of fluorescence-based viability protocols for isolated islets used for the last twenty years is outlined in Table 3 [4, 28-52]. Although this is not a complete listing, as clearly noted from the table, the disparities in the stains employed, sample size (number of islets stained), final stain concentrations and incubation times are considerable. What is not noted on this table is the method of staining/viewing; some authors view single islets spread under a coverslip and others view one or more islets free floating in excess buffer/media in a microplate well or Petri dish. Also not noted on this table are the inconsistencies in the type of filter sets used for fluorescence detection. For dual color assays using green and red fluorescence, the authors listed used either a green/red filter set to detect each color under the appropriate filter, or only a single emission filter (assumed to be a FITC longpass filter) to detect both green and red fluorescence. Many other papers make no mention of what filter sets were used to detect green and red fluorescence and provide little or no information as to what modifications were performed when following a cited method.
Table 3.
Comparison of fluorescent viability assays used on isolated islets of Langerhans from various species.
| Reference | Species | No. of Islet/ Avg Size (μm) |
Islet Staining Media |
Live Stain/Stock Solvent |
Live Stain Stock Conc. (mM) |
Live Stain Final Conc. (μM) |
Incubation Time/Temp |
Dead Stain/Stock Solvent |
Dead Stain Stock Conc. (μM) |
Dead Stain Final Conc. (μM) |
Incubation Time/Temp |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deleers 1985 | Rat | 10/unk | HEPES+ | FDA/unk | 10 | 50 | 0 to 60 m/37°C | None | -- | -- | -- |
| Gray 1987 | Rat | 1/unk | HBSS | FDA/Acetone | 12 | 30 | Immed/RT | EB/HBSS | 500 | 250 | Immed/RT |
| Bank 1987 | Rat, Mou, Can., Hum |
unk | D-PBS | AO/D-PBS | unk | 0.67 | 60 m/unk | PI/D-PBS | unk | 75 | 60 m/unk |
| Bank 1988 | Rat | 1-50/80-350 | D-PBS | AO/D-PBS | 670 | 0.67 | 5 m-24 h/RT | PI/D-PBS | 750 | 75 | 5 m-24 h/RT |
| Vasir 1989 | Rat | unk | RPMI-1640 | FDA/Acetone | 12 | 60 | 2 m/RT | EB/HBSS-HEPES | 500 | 250 | 2 m/RT |
| London 1989 | Hum, Rat | unk | RPMI | FDA/Acetone | 0.024 | 0.67 | 105 m/RT | PI/PBS | 95 | 4 | 105 m/RT |
| London 1990 | Human | 1/150-450 | PBS | FDA/Acetone | 0.024 | 0.67 | 105 m/RT | PI/PBS | 95 | 4 | 105 m/RT |
| Rat | 1/90-380 | RPMI-1640 | FDA/Acetone | 0.024 | 0.67 | 105 m/RT | PI/PBS | 95 | 4 | 105 m/RT | |
| Merchant 1993a | Rat | unk | RPMI-1640 | AO/D-PBS | unk | 10 | 45 m/RT | PI/D-PBS | unk | 15 | 45 m/RT |
| Merchant 1993b | Rat | 50-75/unk | D-PBS | AO/D-PBS | unk | 10 | 45 m/RT | PI/D-PBS | unk | 15 | 45 m/RT |
| Merchant 1996 | Rat | unk | AO &FDA/unk | unk | 10 & unk | 45 m/RT | PI/D-PBS | unk | 15 | 45 m/RT | |
| Cruise 1998 | Pig | unk | M199 | FDA/Acetone | 12 | 150 | 5 m/RT | EB/HBSS | 500 | 500 | 5 m/RT |
| Olack 1999 | Human | 20-25/150 | KRBB | FDA/unk | unk | unk | unk | PI/unk | unk | unk | unk |
| Aiken 2000 | Canine | 100/150 | PBS | FDA/unk | unk | 0.67 | 30 m/RT | PI/unk | unk | 4 | 30 m /RT |
| Miyamoto 2000 | Canine | 100/>100 | RPMI-1640 | FDA/Acetone | 12 | 33 | 2 m/RT-37°C | EB/PBS | 500 | 278 | 2 m/RT-37°C |
| Schneider 2002 | Rat | 50/80-270 | RPMI | FDA/Acetone | unk | 0.67 | unk | PI/PBS | unk | 4 | unk |
| von Mach 2003 | Rat | unk | RPMI-1640 | FDA/Acetone | unk | 0.67 | unk | PI/PBS | unk | 4 | unk |
| Matsumoto 2003 | Macaca | 50 | CMRL-1066 | AO/unk | unk | 10 | unk | PI/unk | unk | 15 | unk |
| Human | unk | CMRL-1066 | |||||||||
| Barnett 2004 * | Human | 50/>50 | D-PBS | FDA/Acetone | 24 | 0.25 | 2 m/RT | PI/D-PBS | 750 | 15.7 | 2 m/RT |
| SYTO®13/DMSO* | 5 | 0.5 | EB/D-PBS | 50 | 1 | ||||||
| Calcein,AM/DMSO* | 4 | 17 | EH-1/D-PBS | 40 | 0.8 | ||||||
| Gurol 2004 | Rat | unk | unk | FDA/unk | unk | unk | unk | PI/unk | unk | unk | unk |
| Eckhoff 2004 | Rat | unk | unk | AO/unk | unk | 331 | unk | EB/unk | unk | 254 | unk |
| Boffa 2005 | Human | unk | HBSS | Hoechst & MitoTracker Red |
unk | 80 & 2.5 |
1 h + 1 h/unk | YOPRO-1/DMSO | 1,000 | 4 | 1 h/unk |
| Giovagnoli 2005 | Pig | unk | unk | FDA/Acetone | unk | 33 | 2 m/RT | EB/PBS | unk | 278 | 2 m/RT |
| Ichii 2005 | Human | 50/unk | PBS | FDA/unk | unk | 0.67 | unk | PI/unk | unk | 75 | unk |
| Yonekawa 2005 | Pig | unk | M199 | AO/unk | unk | 10 | unk | PI/unk | unk | 15 | unk |
| Pinske 2006 | Rat | 100/unk | RPMI-1640 | SYTO® ?/DMSO | unk | unk | 1 h/unk | Dead Red/DMSO | unk | unk | 1 h/unk |
KRBB = Kreb’s Ringers Bicarbonate Buffer; ‘unk’ = unknown, no information provided.
‘’ = final dye concentrations may be inaccurate due to discrepancies in protocol.
The use of these fluorescent substrates and stains on islets has been reviewed extensively [30-34, 41, 45]. The use of AO and EB has been refuted by London et al. (1989 and 1990) due to their low quantum yield [33, 34]. In addition, the green/red metachromasia of AO can complicate analysis due to the poor spectral separation from either EB or PI. Nevertheless, these stains are still utilized (Table 3).
Fluorescein diacetate (FDA) is an acetylated derivative of the green fluorescent dye fluorescein [22]. The attachment of acetyl groups on the xanthene group renders the dye non-fluorescent, but also confers the ability to passively diffuse through a phospholipid bilayer. Once FDA is in the cytoplasm, non-specific esterases de-acetylate the molecule to convert it to fluorescein and the by-products acetic acid/acetaldehyde. The charged groups on fluorescein promote retention within the cell. This retention time is an inherent property of the cell, and can vary by cell type, as well as culture conditions. Calcein is an iminodiacetic acid derivative of fluorescein [53], and functions in the same manner. Unlike fluorescein, calcein fluorescence is quenched by Mn2+, Fe2+, Co2+, Ni2+ and Cu2+ and other ions in the micromolar range [54], but greatly enhanced with Zn2+ [55] which is significant since β-cells have a high zinc content. The observations by Barnett et al. (2004) that islets stained with calcein, AM showed a ‘fragmentation artifact’ and the use of this stain was ‘detrimental to islet integrity’ [45] may be related to either the concentration of calcein, AM used and/or its binding affinity with zinc.
The fluorescent zinc indicator Newport Green (Molecular Probes, Inc., Eugene, OR) has been used to identify β-cells from other cells to assess purity and viability [56] as well as to gate on viable β-cells for flow cytometry [57], but a subsequent review of the literature indicates that the use of this stain has been limited.
Using FDA as a reagent for assessing viability poses a number of limitations in the accuracy in scoring of viable and damaged/dead islets. The presence of extracellular esterases and/or high concentrations of FDA can provide a high background. The green emission peak of the fluorescent product fluorescein extends into the red region of the spectrum and in the presence of an excess amount of fluorescein in the cytoplasm relative to the red dead cell stains, the red emission detected may not be from the nucleic acid stains PI, EB or EH-1. This bleedthrough of fluorescein emission in the red spectrum (Figure 1) can be minimized by using only as low a final working concentration of FDA as necessary for detection and may also require the use of color compensation by the imaging software.
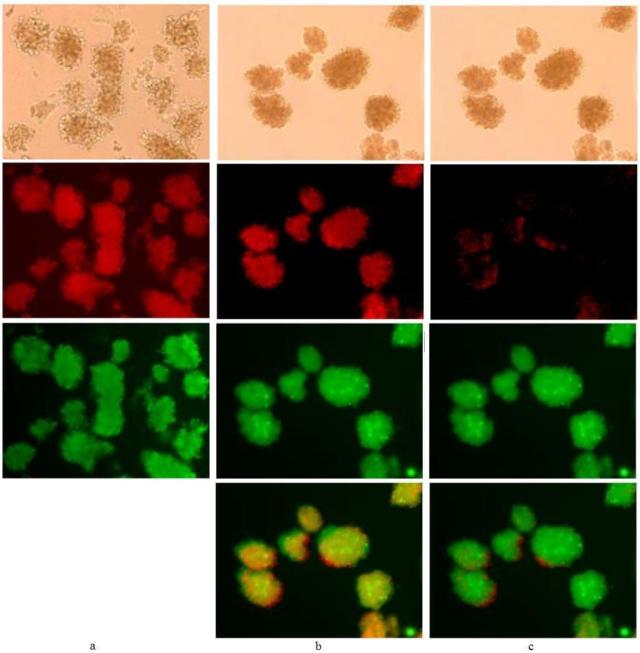
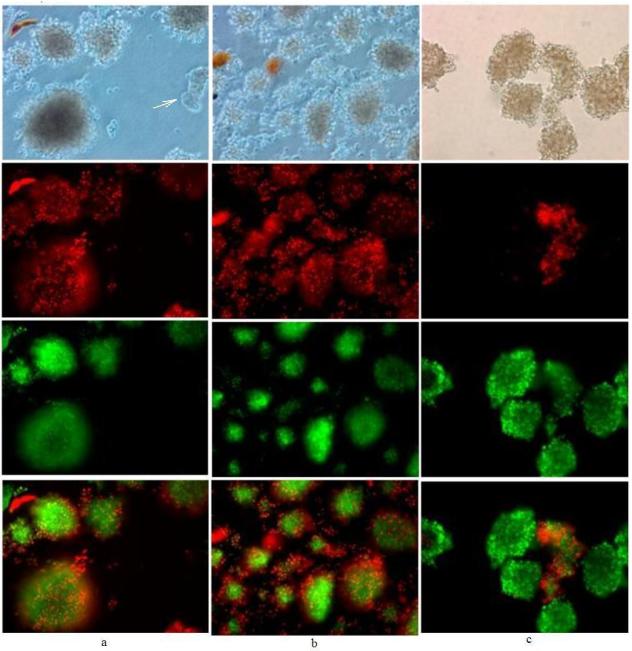
Figure 1. Example of bleedthrough and image subtraction.
20× magnification of porcine islets viewed under light microscopy (top row) and with fluorescence settings under the rhodamine filter (second row), FITC filter (third row) and images overlaid (fourth row for b and c). Islets were either stained immediately after isolation (day 0) with (a) only FDA (45 nM for 30 min; elapsed time after incubation to image capture was 10 to 30 min) or stained after 48 h/37°C storage under nitrogen with FDA/PI (400 nM/3.7 μM, 30 min; elapsed time after incubation to imagecapture was 30 min) and viewed without subtraction (b) and with subtraction(c) under the rhodamine filter.
The spontaneous hydrolysis of FDA to fluorescein during storage is promoted by the presence of trace amounts of water in the solvent used to resolubilize the substrate to make stock solutions or by the gradual accumulation of moisture during storage. Other authors have stated solutions of FDA in acetone are stable from two weeks [41] to six months [34], when in fact, the stability of FDA in a stock solution made with acetone, or any other solvent, is dependent upon the quality of the solvent (purity and water content) and storage conditions. Stock solutions can vary in available cell permeable dye and thus provide discrepancies in staining intensities of samples performed over the storage life of the reagent. An absorbance scan of FDA diluted in methanol would show the extent of any spontaneous hydrolysis - FDA has no absorbance peak beyond 400 nm.
Viability assays using PI with FDA are scored on the assumption that viable cells have robust esterase activity within the cytoplasm and dead or dying cells show little or no esterase activity. Dead cells may exhibit residual esterase activity, complicating the evaluation of dead cells that show staining with both dyes.
The object of this work is to provide an overview, assess the utility and identify limitations in the use of nucleic acid stains for evaluating islet viability. In addition to evaluating the most commonly used FDA/PI staining, other cell permeable nucleic acid stains, SYTO®13, SYTO®24, and SYBR®14 (Molecular Probes, Inc., Eugene, OR) were examined as possible alternatives to FDA.
Porcine and human islets share significant anatomical and physiological similarities. The staining protocols in this work have been tested mostly on isolated porcine and, to a smaller extent, human islets. Unlike previous work [33, 34, 56, 57], viability was assessed using visualization/imaging of intact islets free floating in excess buffer with a fluorescence microscope equipped with filter sets to detect both green and red emission for optimal spectral discrimination. Examining intact islets eliminates any potential artifacts of handling from dissociating islets into individual β-cells required for flow cytometry [56, 57] or spreading islets under a coverslip [33, 34]. However, this approach is still limited in quantification and the identification of stained cells within the center, versus the periphery, of the islet due to the multicellular spheroidal nature of an intact islet.
Results
Figures 1 through 6 are images of either porcine or human islets taken at 20× magnification viewed under either light microscopy (top row) or with fluorescence settings under the rhodamine filter (second row), FITC filter (third row) and with the two color images overlaid (fourth row, except Figures 1a, 3 and 6).
Figure 6. Effects of buffer and media upon staining.
20× magnification of two day old porcine islets incubated for 10 minutes with SYTO®13/PI (500 nM/750 nM) in either (a) E199 medium with serum, (b) E199 medium without serum and (c) D-PBS+Ca+Mg, with another 240 minutes incubation prior to image capture for all samples (top, light microscopy, middle, rhodamine filter and bottom, FITC filter).
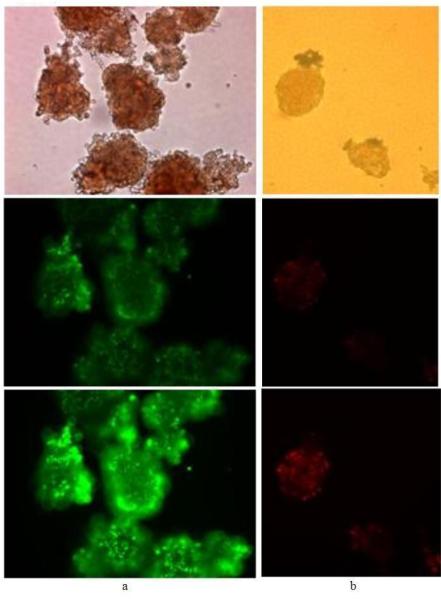
Figure 3. Effect of image capture exposure time and viability determination.
20× magnification of (a) seven day old porcine islets stained with SYBR®14/PI (500 nM/500 nM, 10 min) and (b) one day old human islets stained with SYBR®14/PI (250 nM/750 nM, 30 min), viewed under light microscopy (top) and with fluorescence settings under either only the FITC filter at 500 ms (a, middle) and 1,000 ms (a, bottom) or the rhodamine filter at 500 ms (b, middle) and 1,000 ms (b, bottom) with settings at bin 1, gain 1 and, gamma 1 for all images. Elapsed time after incubation to image capture was from 10 to 60 min.
These figures provide examples of staining discrepancies that are often overlooked, such as fluorescence bleedthrough (Figure 1), staining anomalies and sample contamination with particulates (Figure 2), and the effects of image capture parameters (Figure 3). A comparison between SYTO®13, SYBR®14 and FDA/PI staining (Figures 4 and 5) and the effects of the buffer or medium used during staining (Figure 6) highlight the differences in the utility of these dyes. SYTO®24 exhibited poor staining (low fluorescence intensity) relative to SYTO®13 and SYBR®14 for all final working concentrations and incubation times tested and was not pursued further.
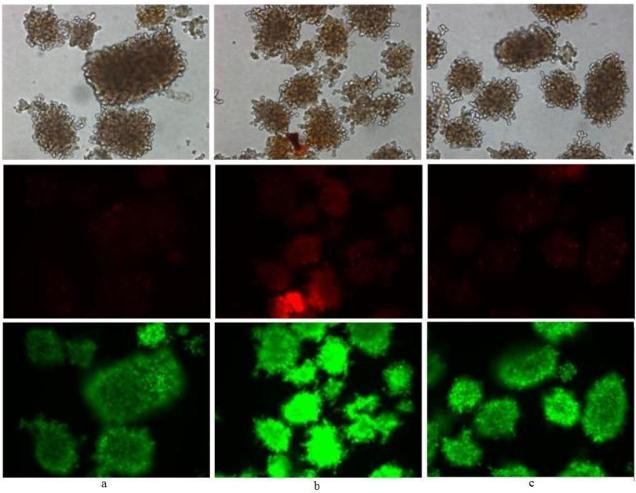
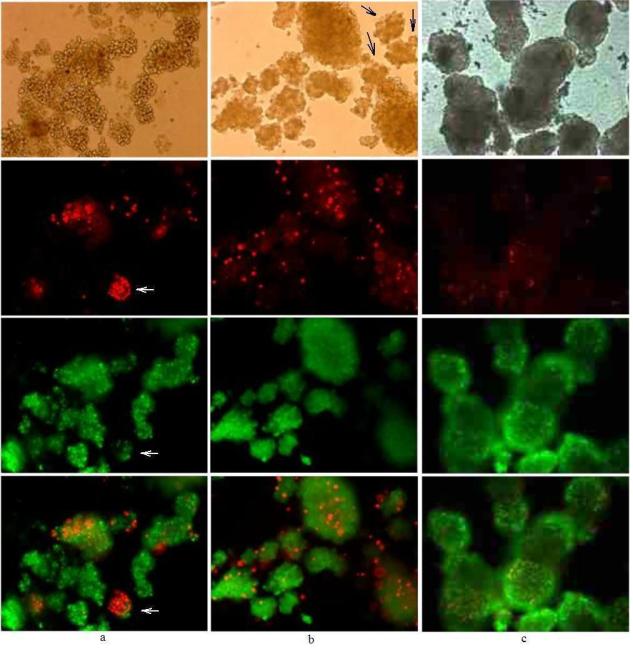
Figure 2. Comparison of FDA/PI, SYTO®13/PI and SYBR®14/PI staining.
20× magnification of porcine islets stained with either (a) FDA/PI (279 nM/75 nM for 30 min;); (b) SYBR®14/PI (1 μM/75 nM for 10 min) or (c) SYTO®13/PI (1 μM/3.7 μM for 30 min) with elapsed time after incubation to image capture from 10 to 60 min.
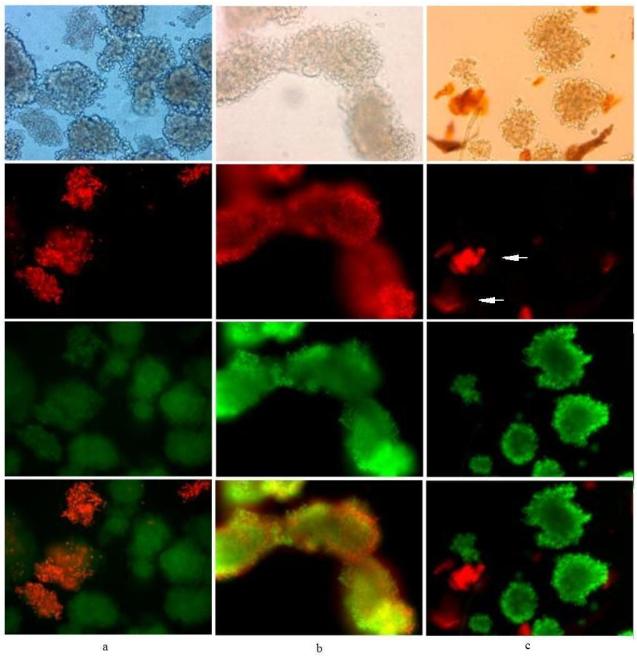
Figure 4. Comparison between SYTO®13 and SYBR®14 nucleic acid stains.
20× magnification of porcine islets stained with either (a and b) SYTO®13/PI (500 nM/187 nM; 2 day old islets) or (c) SYBR®14/PI (250 nM/1 μM; 7 day old islets) for 10 to 30 min; elapsed time after incubation to image capture was from 10 to 60 min.
Figure 5. Comparison of FDA/PI, SYTO®13/PI and SYBR®14/PI staining.
20× magnification of porcine islets stained with either (a) FDA/PI (335 nM/3.7 μM for 30 min) or (b) SYTO®13/PI (1 μM/3.7 μM for 30 min) after incubation for 24 h in 95% air, 5% CO2 prior to staining and (c) human islets stained with SYBR®14/PI (250 nM/750 nM for 45 min) after 2 days of processing and storage under optimal conditions.
Examples of bleedthrough and image subtraction
Islets stained with only FDA immediately after isolation (day 0) were examined under both the rhodamine and FITC filters (Figure 1a). The autofluorescence of porcine islets under the rhodamine filter was negligible (not shown) and the red fluorescence shown is solely from the bleedthrough of fluorescein emission into the red spectrum.
Islets stained with both FDA and PI after 48 h culturing under nitrogen were anticipated to exhibit extensive staining with PI due to cell death induced by oxygen starvation [58, 59] but Figure 1b, like 1a, shows excessive red fluorescence from fluorescein. The actual contribution of red fluorescence from PI was negligible when the image was corrected by subtracting for fluorescein bleedthrough under the rhodamine filter (Figure 1c). Although the green fluorescence from enzymatically hydrolyzed FDA is quite bright in Figure 1b and 1c, from these photos, the anticipated result of largely dead islets from anoxia was not evident from the lack of robust PI staining.
Cells that are recently dead may still exhibit robust esterase activity but, without subtracting the bleedthrough of green dye emission under the rhodamine filter viable islets could be misinterpreted as completely dead islets. The anomalous staining of these islets (Fig. 1b and 1c) exhibits the limitations of this method.
Performing a single dye control of cells stained with only FDA (Figure 1a) and examining under both filters proved the effects of green emission bleedthrough with the filters sets used and, provided the standard for green emission subtraction under the rhodamine filter. In most instances, staining islets with the lowest concentration of the green fluorescent dye, sufficient for signal detection, can eliminate the need for software color subtraction, as was done in subsequent images.
Examples of anomalous staining: FDA/PI, SYTO®13/PI and SYBR® 14/PI
Islets stained with FDA/PI show a low level of green fluorescence (Figure 2a) that may be interpreted as due to poor esterase activity, and yet, most of these islets still exclude PI (Figure 2a). The islets staining bright red from PI also show a low level of green fluorescence of similar or lower intensity as islets showing no PI staining.
Figure 2b shows untreated islets stained with SYBR®14/PI. The cell permeable dye SYBR®14 stains both live and dead cells and staining with PI yields a two-color overlay that shows color blending to a mostly yellow color. Although these islets were untreated, they can be considered mostly dead. This level of staining is the same observed with dead islet controls (not shown).
SYTO®13/PI stained porcine islets only stain green from SYTO®13, the red fluorescence was from non-islet cells or debris of unknown origin (Figure 2c, arrows), but it was not determined if this non-islet material was staining with PI or autofluoresced red. Without the light microscopy image, the red fluorescing particulates may be erroneously scored as dead islets or β-cells. This would be critical if a microplate or flow cytometry assay were employed; without visual inspection under light microscopy, these red particulates may be counted either as dead islets in a microplate assay or, if of appropriate size, gated as dead cells in a flow cytometry assay.
Effect of image capture exposure time and viability assessment
Figure 3 shows light microscopy and fluorescence images of porcine and human islets stained with SYBR®14/PI and viewed under one filter but at different exposure times with all other image parameters held the same. SYBR®14/PI stained porcine islets were examined under only the FITC filter (Figure 3a) and human islets were examined under only the rhodamine filter (Figure 3b). The porcine islets can exhibit low or high fluorescence that may be due to either too little or too much SYBR®14 used or, poor binding or extensive binding of the dye, yet they are images of the same islets taken sequentially and only differ by exposure time. The human islets can be rated as mostly viable by low PI staining or mostly dead by intense PI staining, but again, these are the same islets. The interpretation of viability by the images generated can be significantly altered by exposure time and also other camera/software settings. Recording an accurate image of a fluorescent viability assessment would be of great importance for any archival filing of images.
Comparison between nucleic acid stains, SYTO®13 and SYBR®14
Figure 4 shows porcine islets stained with either SYTO®13/PI (Figure 4a and b) or SYBR®14/PI (Figure 4c). Islets in Figure 4a show varying degrees of both SYTO®13 and PI staining intensity. Since SYTO®13 stains both live and dead cells, a slight molar excess of PI allows for competitive displacement of SYTO®13 off the nuclear DNA, but one islet did not stain (first row, arrow) as well as a few dissociated cells surrounding the islets, and a non-islet particle showed intense red fluorescence (crescent-shaped debris in upper left corner). Figure 4b shows greater dissociation of islets exposed to various stresses (protease cocktail, centrifugation, density gradient with calcium channel blockers), again with less green staining from SYTO®13 and more PI staining, but many of the dissociated cells do not appear stained with either dye as compared to the number of dissociated cells seen in the light microscopy image (Figure 4b, first row). Figure 4c shows excellent staining with SYBR® 14/PI after 7 days in culture, with only a single crescent-shaped islet in the center of the photo staining with PI.
Interpreting viability using SYBR®14 or other cell permeable nucleic acid stains with islets staining green and red with both dyes is complicated if competitive displacement is not complete within the targeted stain incubation time (≤60 min). One objective of this study was to evaluate optimal dye concentrations that provide excellent staining within a reasonable amount of time (under 1 h) to avoid artifacts from prolonged incubation times. Both the SYTO® and SYBR dyes and the acetyl moieties on FDA are unstable in aqueous solutions and prolonged incubations beyond 1 h risked either overloading cells with stain or increasing cell exposure to toxic by-products from dye decomposition in the extracellular medium.
Comparison of stains: FDA/PI, SYTO®13/PI and SYBR®14/PI
Porcine islets stained with either FDA/PI (Figure 5a) or SYTO®13/PI (Figure 5b) look quite similar in appearance even though the two staining strategies are unrelated. One islet in Figure 5a (white arrow) is barely visible under the FITC filter due to assumed poor esterase activity but stains intensely with PI, but a cluster of islets left of this islet do not stain commensurately with either FDA or PI relative to the cell mass seen in the light microscopy image (Figure 5a, first row).
Supposedly SYTO®13 will stain all cells live and dead and competitive displacement with PI would explain the lack of or less SYTO®13 intensity, but islets clearly visible under light microscopy (Figure 5b, first row, black arrows) are not detected with either SYTO®13 or PI under both filter sets. Lack of esterase activity and an intact membrane may explain the limited FDA/PI staining in Figure 5a, but does not explain the inconsistent staining with SYTO®13/PI with both stains in excess. The evaluation of islets with FDA/PI staining in Figure 5a is problematic, technically not dead (excludes PI) yet not quite robust due to poor FDA conversion.
Because these islets were isolated from animal/donor pancreas, there is no sufficient positive control available; cultured hybrid β-cells cannot be considered comparable and thus, a suitable alternative positive control. Human islets stained with SYBR®14/PI (Figure 5c) exhibit staining that can be subjectively construed as being viable due to the excellent live cell staining with SYBR®14 showing no bleedthrough in the red fluorescent range and, little PI staining, yet transplantation of unstained aliquots of the islets into athymic nude mice treated with streptozotocin to induce diabetes failed to reverse hyperglycemia. This was also observed with human islets stained with FDA/PI immediately after isolation and the unstained aliquots transplanted in mice [60].
Islets that stain heavily with PI are not typically transplanted into immunodeficient mice that have been treated with streptozotocin. As a limited negative control, porcine islets obtained from three animals, held for 7 d in culture and under anoxic conditions for 6 h with nitrogen, stained with either FDA/PI (two samples) or SYTO®13/PI (one sample) exhibited extensive PI staining throughout all islets and did not reverse hyperglycemia upon transplantation into streptozotocin-treated athymic nude mice.
Effect of buffer and media upon staining
In optimizing the SYBR®14 and SYTO® dye staining of islets it was observed that the type of buffer or media used for staining significantly influenced incubation times required for adequate staining. Samples of porcine islets stained with the same amount of SYBR®14/PI (250 nM/750 nM) showed excellent staining in D-PBS+Ca+Mg (after 1 min) compared to E199 medium with serum (>30 min), whereas islets stained in E199 medium without serum required only from 10 to 30 min incubation time for excellent staining. Staining discrepancies were also seen with SYTO®13/PI (Figure 6); using the exact same capture settings and incubation times, more intense staining of both dyes was observed in E199 medium without serum (Figure 6b) than either E199 medium with serum (Figure 6a) or D-PBS+Ca+Mg (Figure 6c). The less efficient staining observed in E199 medium with serum may be due to serum proteins binding dye, making less dye available for cell staining; the discrepancy in staining properties between buffer and E199 medium without serum needs to be investigated further.
Using the same final concentrations of stains and incubating in either D-PBS (with or without added calcium and magnesium) or E199 medium without serum, islets stained more quickly and appeared brighter with SYBR®14 than with SYTO®13, with both used in conjunction with PI.
Discussion
The ability to quickly and reliably evaluate the viability of islets is crucial to the decision to continue with transplantation. In examining established methods for assaying the viability of isolatedand purified islets of Langerhans (Table 3), considerable discrepancies in the materials and protocols tested yielded inconsistent results in our hands, most notably, too much dye or substrate is generally used. We found that the stability of stains in storage and stock solutions, the number of islets stained, concentration of stains, staining incubation time, media used, and the method of examining islets were significant in the final scoring of viability. For archival file photos, the exposure time and camera/software settings can also impact interpretation of viability.
To prevent spontaneous hydrolysis of FDA during storage, small aliquots of the dye were stored in powder form and only reconstituted at the time of use. To assure the utility of FDA, an absorbance scan of FDA was used to compare the material held in storage against the absorbance scan provided by the manufacturer.
DMSO stock solutions of the SYBR®14 and the SYTO® nucleic acid stains were also stored desiccated and mixed into aqueous solution only at the time of use. Although these dyes are not converted in aqueous solutions in the same manner as FDA, they are unstable over time if stored in aqueous solutions. We suspect that high concentrations of stains and/or long incubation times reported in prior work may have been used to compensate for stain that was degraded during storage.
We found that the method of killing islets for a dead islet control affected staining. Fixing islets with formalin provided inconsistent staining results and this may be due to overfixation, which can render the cell membrane even less permeable than healthy membranes, and still allow the exclusion of PI. Killing islets by alcohol [30, 32] and subsequent equilibration into aqueous buffer or media prior to staining, proved to be more reliable.
The initial use of SYTO®13 resulted in unusual staining patterns, with staining only observed after hours of incubation with the dye. To determine if this behavior was inherently related to the physiology of β-cells/islets or due to the decomposition or precipitation of the dye, fresh epithelial cells were obtained from a buccal swab and stained immediately with the same dye solution, under the same conditions (2 mL buffer or media in microplate wells). The cheek epithelial cells stained quickly and easily, proving that the nucleic acid stains were not defective. Parallel staining of freshly harvested cheek epithelial cells also served as an excellent utility test for FDA as well.
In our evaluations we found that SYBR®14 to be a better stain than SYTO®13 proposed by Barnett [45]. No detectable fluorescence was observed after 10 min with 1 μM to 10 μM SYTO®13, yet 1 μM of SYBR®14 stained islets after 10 min incubation. SYTO®13 staining only appeared after 30 min incubation or after 10 min incubation using 50 μM. SYTO®24 did not provide any improved staining relative to either SYBR®14 or SYTO®13.
For the 10 to 50 islets stained, 75 nM PI was sufficient to provide bright red fluorescent staining of dead cells throughout whole islets. Concentrations up to 3.7 μM were employed to provide a molar excess of PI relative to the concentration of SYBR®14 or SYTO® dyes to examine the propensity for competitive displacement of the two stains. Higher concentrations of PI resulted in a diffuse red haze around the islets rendering image analysis difficult.
FDA staining exhibited a dual optimal stain concentration, 10 μM provided sufficient staining for direct visual assessment and only 55 nM was needed for image acquisition, both after 30 min incubation. The greater sensitivity of image acquisition with a CCD camera poses a challenge in recording an image that is realistic, as inaccurate evaluations can be derived merely by manipulating exposure time or other image capture parameters.
Islets staining with both FDA and PI (Figures 2a and 5a) and, islets not staining with either FDA or PI (Figure 5a) have confounded the interpretation of viability. Membrane integrity of islets can be inferred from excluding PI, however, it cannot be directly inferred that a good qualitative assessment of high percentage of islets with intact membranes correlates directly with functional, viable islets. In several instances, human and pig islets stained after isolation presented cells that excluded PI upon application of either FDA/PI or SYTO®13/PI did not function to rescue streptozotocin-treated mice from hyperglycemia. Membrane integrity did not show a correlation with viability and, per a review of the literature, none of the stains examined have been used as apoptosis indicators. Exclusion of PI from islets can be reliably taken that membranes of islet cells are intact, however, an inference on viability cannot be made unequivocably.
Viability assays based on membrane integrity alone using two nucleic acid stains, one cell permeable and the other cell impermeant, can also provide erroneous results. The use of high concentrations of the dyes or extended incubation times, discrepancies in sample size, or other parameters in detection/imaging can provide an excessive contribution of the cell permeable stain which stains both live, apoptotic and dead cells. Cells often stain with both colors with little discrimination from the red fluorescence of PI and, may also be complicated by bleedthrough of the green emission peak of the green cell permeable dyes (Figure 1). What is inexplicable, the observation of islets not staining with either of the dyes (Figures 4 and 5).
Conclusions
This work examined commonly used stains for viability determination of islets of Langerhans (FDA/PI), compared this staining regimen with three green fluorescent nucleic acid stains, SYBR®14, SYTO®13 and SYTO®24 with PI and found the results questionable for all dyes examined. All aspects of stain storage, use, and imaging influenced the final evaluation of viability and, even after optimization of these parameters, the results were not definitive and results from transplantation into mice showed no direct correlation of islet viability with these two staining methods.
The use of FDA and PI to assess islet viability was not consistent and is not appropriate for the assessment of islet function and viability [61]. Ichii et al. [50] reported that control islets and islets subjected to six hours hypoxia had similar numbers of islets stained with the cell impermeant dye 7-aminoactinomycin-D (7-AAD). After transplantation into diabetic immunodeficient rodents, control islets functioned to reverse hyperglycemia whereas islets exposed to six hours of hypoxia did not. Pisania et al. [62] found that islets with high ratio of oxygen consumption rate (‘OCR’) per cell number (as measured by DNA content, ‘OCR/DNA’) stained with FDA/PI exhibited a large number of cells that maintained membrane integrity, by excluding PI. However, islets that had high ratio of OCR/DNA also exhibited a large number of islet cells that also excluded PI. Papas et. al. [60] demonstrated that islets with high ratio of OCR/DNA had much higher chances of reversing diabetes in mice than islets with low OCR/DNA, despite the fact that islets in either category had excellent membrane integrity based on FDA/PI staining. Only PI staining of islet cells with compromised membranes accurately scores the number of dead cells, which tracked consistently with the loss of in vivo function. From our observations and from a review of the literature on the application of stains to evaluate islet viability, interpretations extending beyond membrane integrity to indicate islet function and viability are unsubstantiated.
Fluorescent reagents have been used extensively to develop simple and economical assays for viability detection, but a reliable and consistent determination of viability may be complicated by the cell or tissue type examined and the staining regimen selected. Although the commonly used assays examined in this work have been shown not to detect viability accurately or consistently for islets of Langerhans, other fluorescent reagents may be worth evaluating. Future work will examine fluorescent stains that are responsive to metabolic activity and/or mitochondrial membrane potential. Imaging by confocal microscopy will be used to assess the value of three-dimensional imaging and islet viability scoring compared to results observed with standard fluorescence microscopy. Staining optimization and imaging will also be correlated to a photostable in situ fluorescence standard, such as fluorescent polystyrene microspheres, as well as more extensive tracking of transplantation success or failure of islets into immunodeficient diabetic mice.
Methods
Pancreas procurement and islet isolation
Pancreata were harvested from adult market pigs weighing from 227 to 318 kg and from donor cadavers. Islet processing was performed in the University of Minnesota Molecular and Cellular Therapeutics Facility in compliance with federal regulations per the protocols outlined by Hering et al. [60, 63, 64]
Islet culturing, staining and imaging
Isolated islets were stained either immediately after isolation or after culturing in a 175 cm2 cell culture flask (Sarstedt Inc., Newton, NC, USA) in either 2 mL of E199 medium supplemented with glutamax, ITS premix, zinc sulfate, and ciproflaxin with 10% fetal bovine serum for porcine islets or with 2 mL of CMRL 1066 media supplemented with 10% fetal bovine serum (Mediatech, Hernandon, VA) for human islets. Islets were incubated in 95% air/5% CO2 at 37°C and, for longer islet incubation the respective media was changed every two days.
As specified in the figure legends, various islet samples were subjected to anoxic conditions by incubation under nitrogen at 37°C for up to 7 days; nitrogen was released into a chamber jacketed by water at flow rate less than 0.5 liters per minute.
Fluorescein diacetate was aliquoted by dissolving into absolute ethanol (10 mM), aliquoting 13.5 μL into 1.5 mL Eppendorf tubes and evaporating the ethanol by a refrigerated SpeedVac to provide 56 μg of dry FDA per tube. These smaller aliquots of dry FDA and the nucleic acid stains (either in DMSO stock solutions or in powder form) were stored at −20°C and kept desiccated in an air-tight RubberMaid® container with two 50 mL polypropylene screw-cap tubes (BD Biosciences, Franklin Lake, NJ) filled with KOH pellets as desiccant.
At the time of use, either 6 mL of Dulbecco’s phosphate buffered saline solution (D-PBS), E199 media (for pig islets) or CMRL 1066 complete media (for human islets) was added to 56 μg of FDA to make a 22.3 μM stock solution; for more concentrated stock solutions, 6 mL of buffer or media resolubilized the combined contents of two or more vials. Stock solutions were kept in a foil covered plastic tube and used immediately.
Propidium iodide was purchased in powder form (from either Sigma, St. Louis, MO or Invitrogen, Carlsbad, CA) and 3.74 mM stock solutions in ddH2O were stored frozen and protected from light.
SYBR®14, SYTO®13 and SYTO®24 were provided as concentrated DMSO stock solutions stored frozen, desiccated and protected from light. Volumes of ≤2 μL of the SYTO® or SYBR®14/DMSO stock solution were added directly to the 2 mL suspension of islets in buffer or media or prediluted in media to yield final staining concentrations from 25 nM to 1 μM. Islets stained with one or both dyes were exposed to ≤0.1% DMSO to minimize any artifact of solvent upon islet viability or membrane permeability [65, 66].
Using a 20 μL pipette, 10 to 50 porcine or human islets, each approximately 50 to 400 μm in size, were transferred into a single well filled with 2 mL of pre-warmed D-PBS or media/stain mixture (25°C or 37°C) on a 12-well microplate (BD Biosciences, Franklin Lake, NJ) and incubated at either 25°C or 37°C for 10 to 60 min for the nucleic acid stain and 30 min to 60 min for FDA. Stained islets were examined in the staining solution and the total time elapsed from initial exposure to the dyes and image capture usually ranged from 10 to ≤60 min, or longer as denoted. The staining solution was not exchanged for fresh buffer or media to minimize damage to the islets by excessive handling.
Final concentrations of the various dyes examined ranged from: (i) 45 nM to 50 μM of FDA; (ii) 25 nM to 1 μM SYTO®13; (iii) 62.5 nM to 1 μM of SYBR®14; (iv) 75 nM to 3.7 μM PI; and from (v) 500 nM to 1 μM of SYTO®24.
Optimal working concentrations and incubation times ranged from: (i) 10 μM FDA for direct viewing and 55 nM FDA for imaging, for 30 to 60 min; (ii) 500 nM to 1 μM SYTO®13 for 30 to 60 min, with best results after 60 min; (iii) 250 nM of SYBR®14 for 10 min; (iv) 75 nM of PI for 10 min and, (v) 1 μM of SYTO®24 for 30 to 60 min.
A dead islet control was prepared with 10 to 50 islets of similar size treated with either 10% formalin in D-PBS for 60 min and then stored in D-PBS at 25°C for a maximum of 4 h prior to staining or, following established methods, exposure to 50% - 70% ethanol for 5 minutes at 25°C [30, 32] and then stored in D-PBS or 0.9% Ringer’s physiological saline at 25°C for no more than four hours prior to staining.
Stained islets were examined within each microplate well free floating with a Nikon Eclipse TE 300 inverted light microscope (Nikon Inc., Tokyo, Japan) equipped with a Nikon high pressure mercury arc lamp. Green fluorescence was viewed under a FITC filter (excitation BP465-495, DM505, emission BP515-555; Nikon 96107M B-2E/C C12353, Tokyo, Japan) and red fluorescence under a rhodamine filter (excitation BP528-553, DM565, emission 590LP; Nikon DM575 G-2A, Tokyo, Japan). Images were photographed using a SPOT RT CCD camera (Diagnostic Instruments, Sterling Heights, MI) and either SPOT (version 4.1) or Metamorph imaging software (Molecular Devices Corp., Sunnyvale, CA).
Acknowledgements
The study was supported by grants from the National Center for Research Resources, National Institutes of Health (U42 RR016598-01), the Juvenile Diabetes Research Foundation (JDRF #4-1999-841), the Iacocca Foundation, the Schott Foundation, and the Carol Olson Memorial Diabetes Research Fund. We also wish to thank the ICR Basic Science Islet Distribution Program for providing human islets for some of the reported studies.
We also thank and acknowledge the invaluable contribution of Dr. Bernhard Hering and the members of our team: Dr. Stathis Avgoustiniatos, Eric Falde, DanielW. Fraga, Muhamad H. Abdulla, Thomas R. Gilmore, Jian Q. Hao, Phillip Rozak, Thomas Suszynski, Brad Weegman, and Gina M. Wildey.
Footnotes
Authors’ contributions VB cultured, stained and imaged all islet samples and helped to draft the manuscript. OMC participated in the design of the study, compiled and reviewed all literature and drafted the manuscript. KKP conceived of the study, participated in the design of the study and interpretation of results and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Lacy PE, Kostianovsky M. A method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967;16:35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- 2.Ricordi C, Gray DWR, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJM, Socci C, Alejandro R, Zeng Y, Scharp DW, Viviani G, Falqui L, Tzakis A, Bretzel RG, Federlin K, Pozza G, James RFL, Rajotte RV, Di Carlo V, Morris PJ, Sutherland DER, Starzl TE, Mintz DH, Lacy PE. Islet isolation assessment in man and large animals. Acta Diabetologica Latina. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 3.Olack BJ, Swanson CJ, Howard TK, Mohanakumar T. Improved method for the isolation and purification of human islets of Langerhans using Liberase® enzyme blend. Human Immunology. 1999;60:1303–1309. doi: 10.1016/s0198-8859(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 4.Yonekawa Y, Matsumoto S, Okitsu T, Arata T, Iwanaga Y, Noguchi H, Nagata H, O’Neil JJ, Tanaka K. Effective Islet Isolation Method with Extremely High Islet Yields From Adult Pigs. Cell Transplantation. 2005;14:757–762. doi: 10.3727/000000005783982512. [DOI] [PubMed] [Google Scholar]
- 5.Kin T, Johnson PRV, Shapiro AMJ, Lake JRT. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation. 2007;83:7–12. doi: 10.1097/01.tp.0000243169.09644.e6. [DOI] [PubMed] [Google Scholar]
- 6.Balamurugan AN, He J, Guo F, Stolz DB, Bertera S, Geng X, Ge X, Trucco M, Bottino R. Harmful delayed effects of exogenous isolation enzymes on isolated human islets: relevance to clinical transplantation. J Transplant. 2005;5:2671–2681. doi: 10.1111/j.1600-6143.2005.01078.x. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro AM, Lakey JR, Ryna EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a gluococorticoid-free immunosuppressive regimen. Engl. J. Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 8.Shamblott MJ, Clark GO. Cell therapies for type 1 diabetes mellitus. Expert Opinion on Biological Therapy. 2004;4:269–277. doi: 10.1517/14712598.4.3.269. [DOI] [PubMed] [Google Scholar]
- 9.O’Connell PJ, Hawthorne WJ, Holmes-Walker DJ, Nankivell BJ, Gunton JE, Patel AT, Walters SN, Pleass HC, Allen RD, Chapman JR. Clinical islet transplantation in type 1 diabetes mellitus: results of Australia’s first trial. Medical Journal of Australia. 2006;184:221–225. doi: 10.5694/j.1326-5377.2006.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 10.Avgoustiniatos ES, Hering BJ, Papas KK. The Rat Pancreas is not an Appropriate Model for Testing the Preservation of the Human Pancreas with the Two-Layer Method. Transplantation. 2006;81(10):1471–1472. doi: 10.1097/01.tp.0000215389.64186.3f. [DOI] [PubMed] [Google Scholar]
- 11.Papas KK, Hering BJ, Gunther L, Rappel MJ, Colton CK, Avgoustiniatos ES. Pancreas oxygenation is limited during preservation with the two-layer method. Transplant Proc. 2005;37:3501–3504. doi: 10.1016/j.transproceed.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 12.Papas KK, Avgoustiniatos ES, Tempelman LA, Weir GC, Colton CK, Pisania A, Rappel MJ, Friberg AS, Bauer AC, Hering BJ. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37:3412–3414. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 13.Ichii H, Sakuma Y, Pileggi A, Fraker C, Alvarez A, Montelongo J, Szust J, Khan A, Inverardi L, Naziruddin B, Levy MF, Klintmalm GB, Goss JA, Alejandro R, Ricordi C. Shipment of human islets for transplantation. Am J Transplant. 2007;7(4):1010–1020. doi: 10.1111/j.1600-6143.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 14.Paraskevas S, Duguid WP, Maysinger D, Feldman L, Agapitos D, Rosenberg L. Apoptosis occurs in freshly isolated human islets under standard culture conditions. Transplant. Proc. 1997;29:750–752. doi: 10.1016/s0041-1345(96)00452-6. [DOI] [PubMed] [Google Scholar]
- 15.Rosenberg L, Wang R, Paraskevas S, Maysinger D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 1999;126:393–398. [PubMed] [Google Scholar]
- 16.Paraskevas S, Maysinger D, Wang R, Duguid TP, Rosenberg L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 2000;20:270–276. doi: 10.1097/00006676-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wang RN, Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of islet-matrix relationship. J. Endocrinol. 1999;163:181–190. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 18.Thomas FT, Contreras JL, Bilbao G, Ricordi C, Curiel D, Thomas JM. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 1999;126:299–304. [PubMed] [Google Scholar]
- 19.Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatis islet cell survival following islet isolation: The role of cellular interactions in the pancreas. J. Endocrinol. 1999;161:357–364. doi: 10.1677/joe.0.1610357. [DOI] [PubMed] [Google Scholar]
- 20.Eckhard D, Brandhorst D, Winter C, Jaeger C, Jahr H, Bretzel R, Brendel M. The role of current product release criteria for identification of human islet preparations suitable for clinical transplantation. Transplant Proc. 2004;36(5):1528–1531. doi: 10.1016/j.transproceed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Ricordi C, Lakey JR, Hering BJ. Challenges toward standardization of islet isolation technology. Transplant Proc. 2001;33(1-2):1709. doi: 10.1016/s0041-1345(00)02651-8. [DOI] [PubMed] [Google Scholar]
- 22.Kramer DN, Guilbault GG. A substrate for the fluorometric determination of lipase activity. Anal. Chem. 1963;35:588–589. [Google Scholar]
- 23.Guilbault GG, Kramer DN. Fluorometric determination of lipase, acylase, alpha- and gamma-chymotrypsin and inhibitors of these enzymes. Anal. Chem. 1964;36:409–412. [Google Scholar]
- 24.Rotman B, Papermaster BW. Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. PNAS. 1966;55:134–141. doi: 10.1073/pnas.55.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crissman HA, Steinkamp JA. Rapid, simultaneous measurement of DNA, protein, and cell volume in single cells from large mammalian cell populations. J Cell Biol. 1973;59:766–771. doi: 10.1083/jcb.59.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengbusch GV, Couwenbergs C, Kuhner J, Muller U. Fluorogenic substrate turnover in single living cells. Histochemical J. 1976;8:341–350. doi: 10.1007/BF01003822. [DOI] [PubMed] [Google Scholar]
- 27.Fluorescence microscopy of living cells in culture. Part B. Quantitative fluorescence microscopy--imaging and spectroscopy. Methods in Cell Biology. 1989;30:1–498. [PubMed] [Google Scholar]
- 28.Deleers M, Lebrun P, Malaisse WJ. Effects of cations, ionophores and hypoglycemic sulfonylureas on the fluorescence of fluorescein-labeled pancreatic islets. Research Commununications in Chemical Pathology and Pharmacology. 1984;44:83–92. [PubMed] [Google Scholar]
- 29.Deleers M, Lebrun P, Malaisse WJ. Nutrient-induced changes in the pH of Pancreatic Islet Cells. Horm. Metabol. Res. 1985;17:391–395. doi: 10.1055/s-2007-1013555. [DOI] [PubMed] [Google Scholar]
- 30.Gray DW, Morris PJ. The use of fluorescein diacetate and ethidium bromide as a viability stain for isolated islets of Langerhans. Stain Technology. 1987;62:373–381. doi: 10.3109/10520298709108028. [DOI] [PubMed] [Google Scholar]
- 31.Bank HL. Assessment of islet cell viability using fluorescent dyes. Diabet. 1987;30:812–816. doi: 10.1007/BF00275748. [DOI] [PubMed] [Google Scholar]
- 32.Bank HL. Rapid assessment of islet viability with acridine orange and propidium iodide. In Vitro Cellular Developmental Biology. 1988;24:266–273. doi: 10.1007/BF02628826. [DOI] [PubMed] [Google Scholar]
- 33.London NJ, Contractor H, Lake SP, Aucott GC, Bell PR, James RFL. A microfluorometric viability assay for isolated human and rat islets of Langerhans. Diabetes Research. 1989;12:141–149. [PubMed] [Google Scholar]
- 34.London NJ, Contractor H, Lake SP, Aucott GC, Bell PR, James RF. A fluorometric viability assay for single human and rat islets. Horm. Metab. Res. Supp. 1990;25:82–87. [PubMed] [Google Scholar]
- 35.Vasir BS, Gray DWR, Morris PJ. Normalization of Hyperglycemia in Diabetic Rats by Intraportal Transplantation of Cryopreserved Islets from Four Donors. Diabetes. 1989;38:185–188. doi: 10.2337/diab.38.1.s185. [DOI] [PubMed] [Google Scholar]
- 36.Merchant FA, Aggarwal SJ, Diller KR, Bartels KA, Bovik AC. Three-dimensional distribution of damaged cells in cryopreserved pancreatic islets as determined by laser scanning confocal microscopy. J Microscopy. 1993a;169:329–338. doi: 10.1111/j.1365-2818.1993.tb03309.x. [DOI] [PubMed] [Google Scholar]
- 37.Merchant FA, Aggarwal SJ, Diller KR, Bartels KA, Bovik AC. Analysis of volumetric changes in rat pancreatic islets under osmotic stress using laser scanning confocal microscopy. Biomed Sci Instrum. 1993b;29:111–119. [PubMed] [Google Scholar]
- 38.Merchant FA, Diller KR, Aggarwal SJ, Bovik AC. Viability Analysis of Cryopreserved Rat Pancreatic Islets Using Laser Scanning Confocal Microscopy. Cryobiology. 1996;33:236–252. doi: 10.1006/cryo.1996.0024. [DOI] [PubMed] [Google Scholar]
- 39.Cruise GM, Hegre OD, Scharp DS, Hubbell JA. A sensitivity study of the key parameters in the interfacial photopolymerization of Poly(ethyleneglycol) diacrylate upon porcine islets. Biotech. Bioeng. 1998;57:655–665. doi: 10.1002/(sici)1097-0290(19980320)57:6<655::aid-bit3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 40.Aiken R, Rosenberg L, Maysinger D. Phosphatidylinositol 3-kinase signaling to Akt mediates survival in isolated canine islets of Langerhans. Biochem. Biophys. Res. Comm. 2000;277:455–461. doi: 10.1006/bbrc.2000.3664. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto M, Morimoto Y, Nozawa Y, Balamurugan AN, Xu B, Inoue K. Establishment of fluorescein diacetate and ethidium bromide (FDAEB) assay for quality assessment of isolated islets. Cell Transplantat. 2000;9:681–686. doi: 10.1177/096368970000900514. [DOI] [PubMed] [Google Scholar]
- 42.Schneider S, von Mach M-A, Kraus O, Kann P, Feilen PJ. Intraportal transplantation of allogenic pancreatic islets encapsulated in barium alginate beads in diabetic rats. Artif Organs. 2002;27:1053–1056. doi: 10.1046/j.1525-1594.2003.07159.x. [DOI] [PubMed] [Google Scholar]
- 43.von Mach M-A, Schlosser J, Weiland M, Feilen PJ, Ringel M, Hengstler JG, Seilemann S, Beyer J, Kann P, Weber MM, Schneider S. Cryopreservation of islets of Langerhans: Optimization of protocols using rat pancreatic tissue. EXCLI Journal. 2003;2:6–21. [Google Scholar]
- 44.Matsumoto S, Rigley TH, Reems J, Kuroda Y, Steve RB. Improved islet yields from Macaca nemestrina and marginal human pancreata after two-layer method preservation and endogenous trypsin inhibition. Am J Transplant. 2003;3:53–63. doi: 10.1034/j.1600-6143.2003.30110.x. [DOI] [PubMed] [Google Scholar]
- 45.Barnett MJ, McGhee-Wilson D, Shapiro AMJ, Lakey JRT. Variation in human islet viability based on different membrane integrity stains. Cell Transplant. 2004;13:481–488. doi: 10.3727/000000004783983701. [DOI] [PubMed] [Google Scholar]
- 46.Gurol AO, Yillar G, Kursun AO, Kiran B, Aktas E, Salman S, Deniz G, Yilmaz MT. Effect of human somatotropin hormone on cultured rat islets. Transplant. Proc. 2004;36:1613–1614. doi: 10.1016/j.transproceed.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 47.Eckhoff DE, Eckstein C, Smyth CA, Vilatoba M, Bilbao G, Rahemtulla FG, Young CJ, Thompson JA, Chaudry IH, Contreras JL. Enhanced isolated pancreatic islet recovery and functionality in rats by 17β-estradiol treatment of brain death donors. Surgery. 2004;136:336–345. doi: 10.1016/j.surg.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Giovagnoli S, Luca G, Casaburi I, Blasi P, Macchiarulo G, Ricci M, Calvitte M, Basta G, Calafiore R, Rossi C. Long-term delivery of superoxide dismutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: In vitro effects on isolated neonatal porcine pancreatic cell clusters. J Controlled Release. 2005;107:65–77. doi: 10.1016/j.jconrel.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 49.Boffa DJ, Waka J, Thomas D, Suh S, Curran K, Sharma VK, Besada M, Muthukumar T, Yang H, Suthanthiran M, Manova K. Measurement of apoptosis of intact human islets by confocal optical sectioning and stereologic analysis of YO-PRO-1-stained islets. Transplant. 2005;79:842–845. doi: 10.1097/01.tp.0000155175.24802.73. [DOI] [PubMed] [Google Scholar]
- 50.Ichii H, Pileggi A, Molano RD, Baidal DA, Khan A, Kuroda Y, Inverardi L, Goss JA, Alejandro R, Ricordi C. Rescue purification maximizes the use of human islet preparation for transplantation. Am J. Transplant. 2005;5:21–30. doi: 10.1111/j.1600-6143.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 51.Yonekawa Y, Matsumoto S, Okitsu T, Arata T, Iwanage Y, Noguchi H, Nagata H, O’Neill JJ, Tanaka K. Effective islet isolation method with extremely high islet yields from adult pigs. Cell Transplant. 2005;14:757–762. doi: 10.3727/000000005783982512. [DOI] [PubMed] [Google Scholar]
- 52.Pinske GGM, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, de Heer E. Integrin signaling via RGD peptides and anti-β1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes. 2006;55:312–317. doi: 10.2337/diabetes.55.02.06.db04-0195. [DOI] [PubMed] [Google Scholar]
- 53.Diehl H, Ellingboe JL. Indicator for Titration of Calcium in Presence of Magnesium Using Disodium Dihydrogen Ethylenediamine Tetraacetate. Anal. Chem. 1956;28:882. [Google Scholar]
- 54.Wallach DF Hoelzl, Surgenor DM, Soderberg J, Delano E. Preparation and Properties of 3,6-Dihydroxy-2,4-bis-[N,N’-di-carboxymethyl)-aminomethyl] fluoran. Anal. Chem. 1959;31:456–460. [Google Scholar]
- 55.Haugland RP. Handbook of Fluorescent Probes and Research Chemicals. 10th Ed Molecular Probes Inc.; Eugene, OR: 1996. Figure 19.77. [Google Scholar]
- 56.Lukowiak B, Vandewalle B, Riachy R, Kerr-Conte J, Gmyr V, Belaich S, Lefebvre J, Pattou F. Identification and purification of functional human beta cells by a new specific zinc-fluorescent probe. J Histochem. Cytochem. 2001;49:519–527. doi: 10.1177/002215540104900412. [DOI] [PubMed] [Google Scholar]
- 57.Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren P-O, Ricordi C. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am. J Transplant. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 58.Papas KK, Colton CK, Gounarides JS, Roos ES, Jarema MAC, Shapiro MJ, Cheng LL, Cline GW, Shulman GI, Wu H, Bonner-Weir S, Weir GC. NMR Spectroscopy in β-cell Engineering and Islet Transplantation. Ann NY Acad Sci. 2001;944:96–119. doi: 10.1111/j.1749-6632.2001.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 59.Colton CK, Papas KK, Pisania A, Rappel MJ, Powers DE, O’Neil JJ, Omer A, Weir G, Bonner-Weir S. Characterization of Islet Preparations. In: Halberstadt Craig, Emerich Dwaine F., editors. Cell Transplantation from Laboratory to Clinic. Elsevier, Inc.; New York: 2006. In Press. [Google Scholar]
- 60.Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE, III, Pisania A, Weir GC, Hering BJ. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant. 2007;7:38–47. doi: 10.1111/j.1600-6143.2006.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pisania A, Papas KK, Bonner-Weir S, Weir GC, Colton CK. Assessment of islet quality. The 2006 Annual Meeting; San Francisco California number 182f. 2006. [Google Scholar]
- 63.Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala P, Murtaugh MP, Kirchhof N, Schuurman HJ. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301–303. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 64.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, Paraskevas S, Eckman PM, Sageshima J, Nakano M, Sawada T, Matsumoto I, Zhang HJ, Sutherland DE, Bluestone JA. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4(3):390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 65.Levy J, Herchuelz A, Sener A, Malaisse-Lagae F, Malaisse WJ. Cytochalasin B-induced impairment of glucose metabolism in islets of Langerhans. Endocrinology. 1976;98:429–437. doi: 10.1210/endo-98-2-429. [DOI] [PubMed] [Google Scholar]
- 66.Crawford JM, Braunwald NS. Toxicity in vital fluorescence microscopy: Effect of dimethylsulfoxide, Rhodamine-123, and DiI-Low Density Lipoprotein on fibroblast growth in vitro. In Vitro Cell. Dev. Biol. 1991;27A:633–638. doi: 10.1007/BF02631106. [DOI] [PubMed] [Google Scholar]