Abstract
The prevalence and incidence of nephrolithiasis is reported to be increasing across the world. Herein, we review information regarding stone incidence and prevalence from a global perspective. A literature search using PubMed and Ovid was performed to identify peer-reviewed journal articles containing information on the incidence and prevalence of kidney stones. Key words used included kidney stone prevalence, incidence, and epidemiology. Data were collected from the identified literature and sorted by demographic factors and time period. A total of 75 articles were identified containing kidney stone-related incidence or prevalence data from 20 countries; 34 provided suitable information for review. Data regarding overall prevalence or incidence for more than a single time period were found for 7 countries (incidence data for 4 countries; prevalence data for 5 countries). These included 5 European countries (Italy, Germany, Scotland, Spain, and Sweden), Japan, and the United States. The body of evidence suggests that the incidence and prevalence of kidney stones is increasing globally. These increases are seen across sex, race, and age. Changes in dietary practices may be a key driving force. In addition, global warming may influence these trends.
Key words: Nephrolithiasis, Kidney stones, Stone incidence, Epidemiology
The prevalence and incidence of nephrolithiasis is reported to be increasing across the world. This article reviews information regarding stone incidence and prevalence from a global perspective.
Methods
A literature search using PubMed and Ovid was performed to identify peerreviewed journal articles containing information on the incidence and prevalence of kidney stones. Key words used included kidney stone prevalence incidence, and epidemiology. Data were collected from the identified literature and then sorted by demographic factors and time period.
Results
A total of 75 articles were identified containing kidney stone-related incidence or prevalence data from 20 countries; 34 articles provided suitable information for review. Data regarding overall prevalence or incidence for more than a single time period were found for 7 countries (incidence data for 4 countries; prevalence data for 5 countries). These included 5 European countries (Italy, Germany, Scotland, Spain, and Sweden), Japan, and the United States.
Prevalence
In the United States, overall stone prevalence has doubled since the 1964–1972 time period, and appears to have stabilized since the early 1980s.1–3 Other countries with documented increases in prevalence include Germany, Spain, and Italy.4–7 Regional reports from Milan, Italy, also document an increased prevalence. 8 Only Scotland had a slight decrease in prevalence from 3.83% in 1977 to 3.5% in 19879,10 (Table 1 and Table 2).
Table 1.
Reported Kidney Stone Prevalence by Country and Year
| Country | Year | Population | Prevalence |
| United States | 1964–1972 | All | 2.62% |
| 1976–1980 | All | 3.8% | |
| 1982 | All | 5.4% | |
| 1988-994 | All | 5.2% | |
| Italy | 1983 | All | 1.17% |
| 1993–1994 | All | 1.72% | |
| Scotland | 1977 | All | 3.83% |
| 1987 | All | 3.5% | |
| Spain | 1977 | All | 0.1% |
| 1979 | All | 3.0% | |
| 1984 | All | 4.16% | |
| 1987 | All | 2.0% | |
| 1991 | All | 10.0% | |
| Turkey | 1989 | All | 14.8% |
Table 2.
Reported Kidney Stone Incidence by Country and Year
| Country | Year | Population | Incidence (Affected Individuals/100,000) |
| United States | 1971 | All | 122 |
| 1977 | All | 208 | |
| 1978 | All | 164 | |
| 2000 | Age 18–65 y | 116 | |
| Germany | 1979 | Age >14 y | 120 |
| 2000 | Age >14 y | 720 | |
| Japan | 1965 | All | 54.2 |
| 1971 | All | 58.6 | |
| 1975 | All | 56.4 | |
| 1980 | All | 55.7 | |
| 1985 | All | 62 | |
| 1990 | All | 58.4 | |
| 1995 | All | 68.9 | |
| 2005 | All | 114.3 | |
| Spain | 1977 | All | 810 |
| 1980 | All | 500 | |
| 1984 | All | 270 | |
| Sweden | 1954 | All | 130 |
| 1969 | All | 200 |
Countries or regions reporting prevalence rates for 1 year only included Iceland; Buenos Aires, Argentina; Thebes, Greece; Northeast Thailand; Seoul, Korea; Balearic Islands, Spain; Hellin, Spain; Taiwan, China; and Eastern Tennessee (Table 3).4,11–19
Table 3.
Reported Regional Kidney Stone Prevalence Rates per Country and Year
| Region | Year | Population | Prevalence |
| Buenos Aires, Argentina | 1998 | Age ≥ 19 y | 5.14% |
| 1998 | All | 3.96% | |
| Thebes, Greece | 2005 | Age ≥ 14 y | 15.2% |
| Iceland | 1991 | All | 3.9% |
| Milan, Italy | 1986 | Age ≥ 25 y | 5.9% |
| 1998 | Age ≥ 25 y | 9.0% | |
| Northeast Thailand | 1997 | Age 17–80 y | 16.9% |
| Seoul, Korea | 1998 | Age 40–79 y | 5.0% |
| Balearic Islands, Spain | 1990 | All | 14.3% |
| Hellin, Spain | 1996 | All | 0.26% |
| Taiwan, China | 2002 | All | 9.6% |
| Eastern Tennessee | 1986 | Uranium-exposed workers | 18.5% |
In countries reporting prevalence rates in the 1980s and 1990s, the nonweighted, average global prevalence was 3.25% in the 1980s and 5.64% in the 1990s.3–7,9,10 The highest prevalence rates across all reports were for uranium workers in eastern Tennessee (18.5%) and adults in Northeast Thailand (16.9%) (Table 3).15,19
Incidence
In the United States, overall incidence increased during 1971 to 1978.1,20 In the year 2000, an incidence of 1116 per 100,000 was reported for 18- to 64-year-old employees covered by 2 large insurance carriers.21 This incidence is significantly higher than those from the aforementioned periods. Studies performed in Rochester, MN, showed a steady incidence increase from the 1950s through 1990, with a drop somewhat in 2000.22,23
In Japan, the incidence of nephrolithiasis has doubled over a 40-year time period, both in men and women. These increases were most prominent in the last 10 to 20 years, with rates among men increasing sharply since the 1990s, and rates among women increasing more gradually since the 1980s.24–26
Countries or regions reporting incidence rates for only 1 year include Seoul, Korea, and 4 Spanish cities (Granada, Tudela, Marina Alta, Saragossa)4,16 (Table 4).
Table 4.
Reported Regional Kidney Stone Incidence Rates per Country and Year
| Region | Year | Population | Incidence (Affected Individuals/100,000) |
| Tajima, Japan | 1991 | All | 141 |
| 1993 | All | 93 | |
| Rochester, MN | 1950–1954 | All | 58.7 |
| 1955–1959 | All | 58.4 | |
| 1960–1964 | All | 70.3 | |
| 1965–1969 | All | 63.4 | |
| 1970–1974 | All | 73.4 | |
| 1970 | All | 98.7 | |
| 1980 | All | 116.5 | |
| 1990 | All | 117.1 | |
| 2000 | All | 85.1 | |
| Seoul, Korea | 1998 | 40–79 y | 900 |
| Granada, Spain | 1982 | All | 240 |
| Tudela, Spain | 1990 | All | 510 |
| Marina Alta, Spain | 1990 | All | 280 |
| Saragossa, Spain | 2002 | All | 350 |
Sex and Age
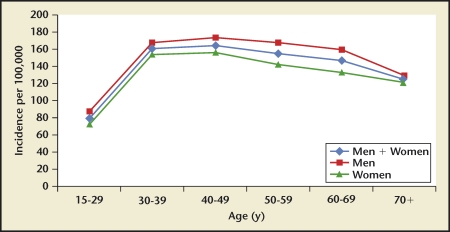
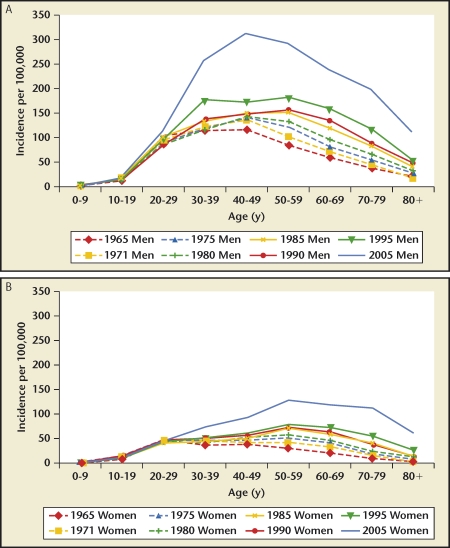
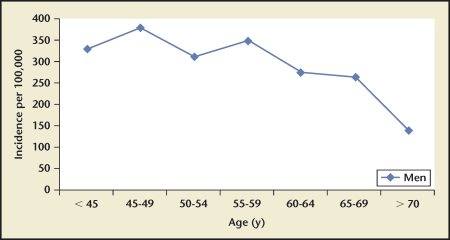
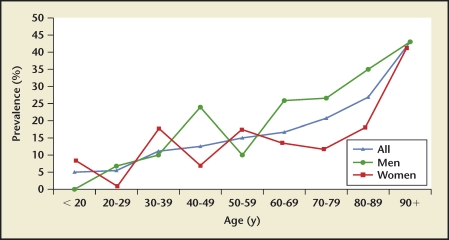
Iran, Japan, and the United States had stone incidence reports stratified by age22–24,27 (Figures 1–3). Incidence rates reported by age group consistently show a rise-and-fall pattern as a population ages. Age at peak incidence was similar among these 3 countries: Age at peak incidence was similar among these 3 countries, ranging from 40 to 49 years, except for Japanese women for whom the peak incidence occurred between ages 50 to 59 years. The actual incidence rate was similar for men age 40 to 49 years in the United States and Japan but lower in Iran.
Figure 1.
2005 Iran kidney stone incidence by age group. A rise-and-fall pattern is observed for reported incidence rates in Iran during 2005. Peak incidence is observed in the 40- to 49-year-old age group.
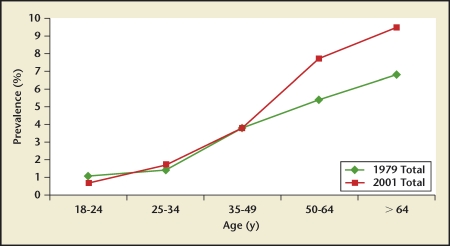
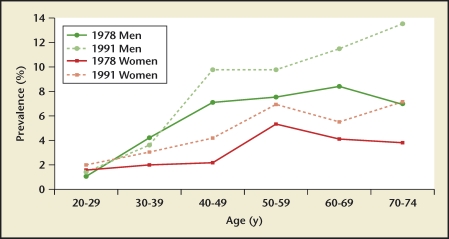
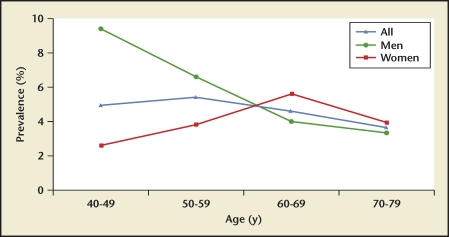
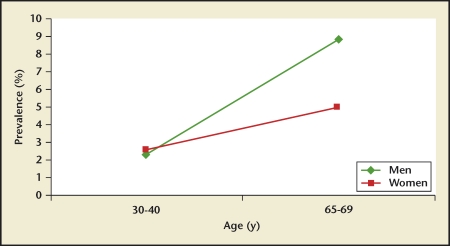
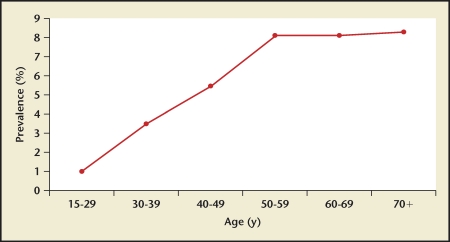
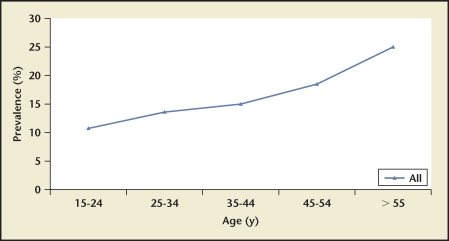
Stone prevalence increased with increasing age in Germany, Iceland, Iran, Italy, Greece, Turkey, and the United States (Figures 4–10), although there is a sharp decrease in prevalence in Italians, age > 60 years, living in Milan2,5,7,11,12,14,27(Figure 11). In Korea, prevalence rates decreased as men aged, but increased in women and peaked at age 60 to 69 years16 (Figure 12). In the United States, a study during the 1976 through 1980 time period showed that prevalence rates decreased in women over age 59 and men over age 69, but by 1991 prevalence rates continued increasing as the population got older among all age groups2 (Figure 10).
Figure 4.
Germany kidney stone prevalence by age group. An increasing prevalence is observed for Germans as they age. This trend is observed in both 1979 and 2001.
Figure 10.
US kidney stone prevalence by age group. In 1978, prevalence in US men and women demonstrates a rise-and-fall pattern as the population ages, with peak prevalence occurring between age 60 and 69 years in men, and between age 50 and 59 in women. In 1991, prevalence continues increasing with advancing age in men, although remaining stable in women age > 59 years.
Figure 11.
Milan, Italy, kidney stone prevalence by age group. An increasing prevalence is observed with increasing age among those living in Milan, but a prevalence decrease occurs after age > 60 years.
Figure 12.
1998 Korea kidney stone prevalence by age group. Korean men demonstrated a decrease in stone prevalence with increasing age. Korean women demonstrated a rise-and-fall pattern, with peak incidence occurring between age 60 and 69 years.
More men form stones than women. The sex ratios range from 2.5:1 in Japan to 1.15:1 in Iran.27,28 However, there are age ranges in some countries where this ratio is reversed. This occurrence was reported for 14- to 24-year-olds in Germany, 21- to 30-year-olds in Milan, Italy, 60- to 79-year-olds in Korea, 20- to 29-year-olds in the United States, and 3 age groups in Greece (age < 20, age 30 to 39 years, and age 50 to 59 years).2,7,8,14,16 Although women demonstrated higher prevalence rates in these instances, the difference between men and women was minimal.
Race
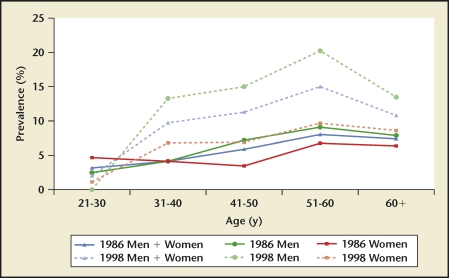
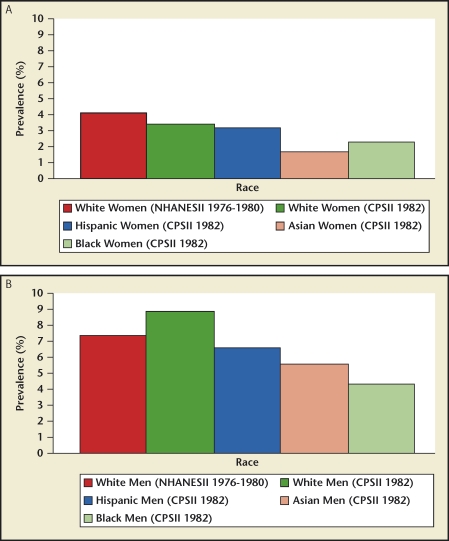
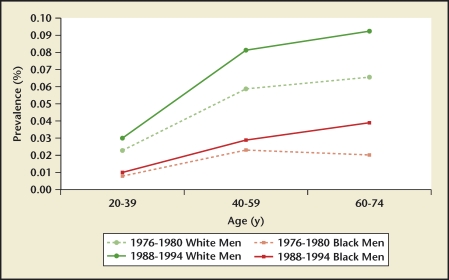
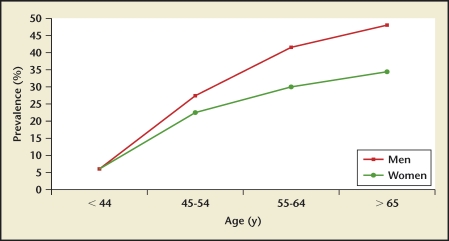
Data comparing stone disease differences between races within one country were available only for the United States.2 Prevalence and incidence rates were highest for whites, followed by Hispanics, blacks, and Asians (Figure 13). Of interest, stone disease rates have nearly doubled in US blacks in the 60- to 74-year old age group when comparing the 1976 through 1980 and 1988 through 1994 time periods (Figure 14). White men have the highest kidney stone incidence rate whereas Asian women have the lowest rate (Figure 13). Within individual races, men still have a higher disease burden when compared with women from the same race.
Figure 13.
US kidney stone prevalence rates by race. Data for kidney stone prevalence rates show rates being lowest in Asian women (A) and highest in white men (B). CPS, Cancer Prevention Study; NHANES, National Health and Nutrition Examination Survey.
Figure 14.
US kidney stone prevalence by race and age group. An increasing prevalence with increasing age is observed in US white and black men for both reporting periods. Prevalence has nearly doubled for black men in the 60- to 74-year-old age group between the 2 time periods.
Radiographic Studies
Three studies published between 1991 and 2003 examined asymptomatic stone prevalence rates by performing ultrasonography on randomly selected subjects.29–31 The stone rates in asymptomatic subjects were 3.0%, 2.1%, and 2.0% in Pakistan, Denmark, and Japan, respectively.
Discussion and Conclusions
Our review demonstrates that there has been an increase in the prevalence and incidence of kidney stones in the United States and other parts of the world. The cause of these changes is unclear. Kidney stone formation is usually due to genetic and environmental factors. Although genetic factors influence stone risk, changes in the gene pool occur at a slow rate. Therefore, it is unlikely to be the driving force for these trends. Environmental factors are also varied and complex, but their influence is more apparent as changes in these factors occur over much shorter intervals. We believe that changes in 2 of the most important environmental factors-diet and climate-have the most significant impact on these trends.
There is historical evidence of the influence of diet on stone formation. The first documented increase in stone disease occurred during the 16th century when European Stein-Schneiders (stone cutters) found that their services were more in demand.32 During this period, there were improvements in food production and corn became a popular food staple.33 The increased consumption of starchy foods derived from corn promoted obesity, currently a known risk factor for stone formation.3,5,34
The impact of agricultural modernization remains today, and is reflected by the epidemic in obesity seen in many countries, especially the United States. The prevalence of obesity has been tracked in the United States since 1960. Obesity in adults has risen from 14.6% in the 1971 through 1974 time period to 35.2% in the 2005 through 2006 time period.35 Moreover, a similar trend is present for children, with 11% to 17.8% being in the overweight category in the 2005 through 2006 time period.35 The consumption of fast foods and high fructose corn syrup preparations has been thought to promote this epidemic. In the United States alone, the percentage of meals coming from fast-food eateries or restaurants rose from 9.6% to 23.5% during the timeframe of 1977 to 1996.36 These dietary changes have also been reported in many other countries including China, India, Egypt, Russia, and the Philippines. 36–39 High fructose consumption has been demonstrated to be a risk factor for stone formation.40
Other dietary risk factors for stone formation have been identified. There is strong evidence that diminished fluid and calcium consumption are risk factors.14,41–44 Increased oxalate consumption has also been demonstrated to promote stone formation. 45,46 Epidemiologic studies have demonstrated that increased sodium and animal protein intake have an equivocal impact on stone risk. However, a randomized prospective dietary intervention study demonstrated that reduction of sodium and animal protein and maintenance of normal dietary calcium intake attenuates stone activity in recurrent hypercalciuric stone formers.41 There is evidence that the consumption of animal protein has increased in a number of countries, paralleling the acceleration of stone disease.36–39 There are also studies that demonstrate an increased intake of sodium and sodium-rich foods in certain cohorts.47
Global climate change is another environmental factor that affects stone disease rates. For many years the concept of global warming has been debated, and today it is more accepted as a legitimate phenomenon. The general consensus is that average global temperatures have increased.48 In addition, studies have documented the association between increased environmental temperatures and increased kidney stone rates.49 Two recent studies have shown the temporal relationship between exposure to high temperatures and the subsequent development of kidney stones. Evans and Costabile50 compared the time of arrival of US soldiers to Kuwait and the time to development of acute renal colic at a US military hospital. Doumerc and colleagues51 recorded temperature and number of renal colic admissions at a French tertiary care center between 2002 and 2004. These 2 studies reported time delays between exposure to higher temperatures and clinical manifestation of symptoms of 93 days and 2 months, respectively. Imaging studies to identify stones prior to exposure to warmer temperatures were not done in these studies. Furthermore, epidemiologic studies in the United States have shown that regions with higher average temperatures have the highest stone rates.2,3,52 The correlation between increased environmental temperature and increased number of stone events supports the conclusion that global warming has an impact on the development of stones. This has been recently addressed in a study by Brikowski and associates.49 They examined how global warming alters regional distribution of kidney stones using a modeling technique. They predicted that, based on the effects of global warming, the percentage of people living in areas designated as high risk for kidney stone formation would increase from 40% in 2000 to 56% by 2050, and up to 70% by 2095. This would result in a significant “climate-related” increase in kidney stone events.
Our review demonstrated that there were decreases in stone prevalence among older age groups. This could be due to differences in sampling methods or subjects with stones dying at a younger age. The latter is certainly plausible as kidney stone formation has been linked to a number of medical comorbidities including obesity, diabetes mellitus, hypertension, chronic kidney disease, and cardiovascular problems.5,34,53–56
The body of evidence suggests that the incidence and prevalence of kidney stones is increasing globally. These increases are seen across sex, race, and age. Changes in dietary practices may be a key driving force. In addition, global warming may influence these trends.
Overall stone prevalence has doubled in the United States since the 1964 through 1972 time period, although it appears to have stabilized since the early 1980s. Other countries with documented increases in prevalence include Germany, Spain, and Italy. Only Scotland had a slight decrease in prevalence from 3.83% in 1977 to 3.5% in 1987.
Iran, Japan, and the United States had stone incidence reports stratified by age. Incidence rates reported by age group consistently show a rise-and-fall pattern as a population ages. Peak incidence was age 40 to 49 years for all 3 countries, but for Japanese women, peak incidence occurred at age 50 to 59 years. The actual incidence rate was similar for men age 40 to 49 years in the United States and Japan but lower in Iran.
The incidence and prevalence of kidney stones is increasing globally and is seen across sex, race, and age. Changes in dietary practices may be a key driving force influencing these trends as well as the effects of global warming.
Figure 2.
Japan kidney stone incidence by age group. Incidence data reported for Japanese men (A) and women (B) show a consistent rise-and-fall pattern in every year of reporting. Male peak incidence occurs between ages 40 and 49 years, whereas female peak incidence occurs between ages 50 and 59 years. One can also observe an increase in incidence over time in both men and women.
Figure 3.
1986 United States kidney stone incidence by age group. A rise-and-fall pattern is observed for reported incidence rates in the United States during 1986. Peak incidence is observed between ages 45 and 49 years.
Figure 5.
1996 Iceland kidney stone prevalence by age group. An increasing prevalence is observed in Iceland as the population ages. This trend is observed in both men and women.
Figure 6.
2005 Iran kidney stone prevalence by age group. Prevalence increases with increasing age among Iran’s population up until age 50 to 59 years, after which it remains stable.
Figure 7.
1993 Italian kidney stone prevalence by age group. An increasing prevalence with increasing age is observed in Italy for both men and women.
Figure 8.
2006 Thebes, Greece, kidney stone prevalence by age group. An increasing prevalence is observed with increasing age among those living in Thebes for both men and women.
Figure 9.
1989 Turkey kidney stone prevalence by age group. An increasing prevalence of kidney stones is observed as the population ages.
References
- 1.Hiatt RA, Dales LG, Friedman GD, Hunkeler EM. Frequency of urolithiasis in a prepaid medical care program. Am J Epidemiol. 1982;115:255–265. doi: 10.1093/oxfordjournals.aje.a113297. [DOI] [PubMed] [Google Scholar]
- 2.Stamatelou KK, Francis ME, Jones CA, et al. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63:1817–1823. doi: 10.1046/j.1523-1755.2003.00917.x. [DOI] [PubMed] [Google Scholar]
- 3.Soucie JM, Thun MJ, Coates RJ, et al. Demographic and geographic variability of kidney stones in the United States. Kidney Int. 1994;46:893–899. doi: 10.1038/ki.1994.347. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez-Martín FM, Millán Rodríguez F, Esquena Fernández S, et al. Incidence and prevalence of published studies about urolithiasis in Spain: a review [article in Spanish] Actas Urol Esp. 2007;31:511–520. doi: 10.1016/s0210-4806(07)73675-6. [DOI] [PubMed] [Google Scholar]
- 5.Amato M, Lusini ML, Nelli F. Epidemiology of nephrolithiasis today. Urol Int. 2004;72(suppl 1):1–5. doi: 10.1159/000076582. [DOI] [PubMed] [Google Scholar]
- 6.Serio A, Fraioli A. Epidemiology of nephrolithiasis. Nephron. 1999;81(suppl l):26–30. doi: 10.1159/000046295. [DOI] [PubMed] [Google Scholar]
- 7.Hesse A, Brändle E, Wilbert D, et al. Study on the prevalence and incidence of urolithiasis in Germany comparing the years 1979 vs. 2000. Eur Urol. 2003;44:709–713. doi: 10.1016/s0302-2838(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 8.Trinchieri A, Coppi F, Montanari E, et al. Increase in the prevalence of symptomatic urinary tract stones during the last ten years. Eur Urol. 2000;37:23–25. doi: 10.1159/000020094. [DOI] [PubMed] [Google Scholar]
- 9.Scott R, Freeland R, Mowat W, et al. The prevalence of calcified upper urinary tract stone disease in a random population-Cumbernauld Health Survey. Br J Urol. 1977;49:589–595. doi: 10.1111/j.1464-410x.1977.tb04536.x. [DOI] [PubMed] [Google Scholar]
- 10.Scott R. Prevalence of calcified upper urinary tract stone disease in a random population survey. Report of a combined study of general practitioners and hospital staff. Br J Urol. 1987;59:111–117. doi: 10.1111/j.1464-410x.1987.tb04799.x. [DOI] [PubMed] [Google Scholar]
- 11.Akinci M, Esen T, Tellaloğlu S. Urinary stone disease in Turkey: an updated epidemiological study. Eur Urol. 1991;20:200–203. doi: 10.1159/000471700. [DOI] [PubMed] [Google Scholar]
- 12.Indridson OS, Birgisson SJ, Edvardsson VO, et al. Epidemiology of kidney stones in Iceland: a population-based study. Scand J Urol Nephrol. 2006;40:215–220. doi: 10.1080/00365590600589898. [DOI] [PubMed] [Google Scholar]
- 13.Pinduli I, Spivacow R, del Valle E, et al. Prevalence of urolithiasis in the autonomous city of Buenos Aires, Argentina. Urol Res. 2006;34:8–11. doi: 10.1007/s00240-005-0003-7. [DOI] [PubMed] [Google Scholar]
- 14.Stamatiou KN, Karanasiou VI, Lacroix RE, et al. Prevalence of urolithiasis in rural Thebes, Greece. Rural Remote Health. 2006;6:610. [PubMed] [Google Scholar]
- 15.Yanagawa M, Kawamura J, Onishi T, et al. Incidence of urolithiasis in northeast Thailand. Int J Urol. 1997;4:537–540. doi: 10.1111/j.1442-2042.1997.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Jo MK, Kwak CK, et al. Prevalence and epidemiologic characteristics of urolithiasis in Seoul, Korea. Urology. 2002;594:517–521. doi: 10.1016/s0090-4295(01)01606-5. [DOI] [PubMed] [Google Scholar]
- 17.Grases F, Conte A, March JG, et al. Epidemiology of urinary stone disease in the Balearic Islands Community. Int Urol Nephrol. 1994;26:145–150. doi: 10.1007/BF02768277. [DOI] [PubMed] [Google Scholar]
- 18.Lee YH, Huang WC, Tsai JY, et al. Epidemiological studies on the prevalence of upper urinary calculi in Taiwan. Urol Int. 2002;68:172–177. doi: 10.1159/000048445. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Schober S. Urolithiasis in Tennessee: an occupational window into a regional problem. Am J Public Health. 1991;81:587–591. doi: 10.2105/ajph.81.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sierakowski R, Finlayson B, Landes RR, et al. The frequency of urolithiasis in hospital discharge diagnoses in the United States. Invest Urol. 1978;15:438–441. [PubMed] [Google Scholar]
- 21.Saigal CS, Joyce G, Timilsina AR. Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int. 2005;68:1808–1814. doi: 10.1111/j.1523-1755.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 22.Johnson CM, Wilson DM, O’Fallon WM, et al. Renal stone epidemiology: a 25-year study in Rochester, Minnesota. Kidney Int. 1979;16:624–631. doi: 10.1038/ki.1979.173. [DOI] [PubMed] [Google Scholar]
- 23.Lieske JC, Peña de la Vega JS, Slezak JM, et al. Renal stone epidemiology in Rochester, Minnesota: an update. Kidney Int. 2006;69:760–764. doi: 10.1038/sj.ki.5000150. [DOI] [PubMed] [Google Scholar]
- 24.Yasui T, Iguchi M, Suzuki S, Kohri K. Prevalence and epidemiological characteristics of urolithiasis in Japan: national trends between 1965 and 2005. Urology. 2008;71:209–213. doi: 10.1016/j.urology.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida O, Terai A, Ohkawa T, Okada Y. National trend of the incidence of urolithiasis in Japan from 1965 to 1995. Kidney Int. 1999;56:1899–1904. doi: 10.1046/j.1523-1755.1999.00754.x. [DOI] [PubMed] [Google Scholar]
- 26.Iguchi M, Umekawa T, Katoh Y, et al. Prevalence of urolithiasis in Kaizuka City, Japan-an epidemiologic study of urinary stones. Int J Urol. 1996;3:175–179. doi: 10.1111/j.1442-2042.1996.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 27.Safarinejad MR. Adult urolithiasis in a population-based study in Iran: prevalence, incidence, and associated risk factors. Urol Res. 2007;35:73–82. doi: 10.1007/s00240-007-0084-6. [DOI] [PubMed] [Google Scholar]
- 28.Fujita K. Epidemiology of urinary stone colic. Eur Urol. 1979;5:26–28. doi: 10.1159/000473055. [DOI] [PubMed] [Google Scholar]
- 29.Buchholz N, Abbas F, Afzal M, et al. The prevalence of silent kidney stones—an ultrasonographic screening study. J Pak Med Assoc. 2003;53:24–25. [PubMed] [Google Scholar]
- 30.Emamian SA, Nielsen MB, Pedersen JF, Ytte L. Sonographic evaluation of renal appearance in 665 adult volunteers. Correlation with age and obesity. Acta Radiol. 1993;34:482–485. [PubMed] [Google Scholar]
- 31.Oshibuchi M, Nishi F, Sato M, et al. Frequency of abnormalities detected by abdominal ultrasound among Japanese adults. J Gastroenterol Hepatol. 1991;6:165–168. doi: 10.1111/j.1440-1746.1991.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 32.López M, Hoppe B. History, epidemiology and regional diversities of urolithiasis. Pediatr Nephrol. 2010;25:49–59. doi: 10.1007/s00467-008-0960-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen MN. Health and Rise of Civilization. New Haven, CT: Yale University Press; 1991. [Google Scholar]
- 34.Taylor EN, Stampfer M, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 35.National Center for Health Statistics. Health, United States, 2009: With Special Feature on Medical Technology. Hyattsville, MD: National Center for Health Statistics; 2010. p. 27. [PubMed] [Google Scholar]
- 36.Adair LS, Popkin BM. Are child eating patterns being transformed globally? Obes Res. 2005;13:1281–1299. doi: 10.1038/oby.2005.153. [DOI] [PubMed] [Google Scholar]
- 37.Zhai F, Wang H, Du S, et al. Lifespan nutrition and changing socio-economic conditions in China. Asia Pac J Clin Nutr. 2007;16(suppl l):374–382. [PubMed] [Google Scholar]
- 38.Shetty PS. Nutrition transition in India. Public Health Nutr. 2002;5(1A):175–182. doi: 10.1079/PHN2001291. [DOI] [PubMed] [Google Scholar]
- 39.Galal OM. The nutrition transition in Egypt: obesity, undernutrition and the food consumption context. Public Health Nutr. 2002;5(1A):141–148. doi: 10.1079/PHN2001286. [DOI] [PubMed] [Google Scholar]
- 40.Taylor EN, Curhan GC. Fructose consumption and the risk of kidney stones. Kidney Int. 2008;73:207–212. doi: 10.1038/sj.ki.5002588. [DOI] [PubMed] [Google Scholar]
- 41.Borghi L, Ferretti PP, Elia GF, et al. Epidemiological study of urinary tract stones in a northern Italian city. Br J Urol. 1990;65:231–235. doi: 10.1111/j.1464-410x.1990.tb14716.x. [DOI] [PubMed] [Google Scholar]
- 42.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Beverage use and risk for kidney stones in women. Ann Intern Med. 1998;128:534–540. doi: 10.7326/0003-4819-128-7-199804010-00003. [DOI] [PubMed] [Google Scholar]
- 43.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. Family history and risk of kidney stones. J Am Soc Nephrol. 1997;8:1568–1573. doi: 10.1681/ASN.V8101568. [DOI] [PubMed] [Google Scholar]
- 44.Hirvonen T, Pietinen P, Virtanen M, et al. Nutrient intake and use of beverages and the risk of kidney stones among male smokers. Am J Epidemiol. 1999;150:187–194. doi: 10.1093/oxfordjournals.aje.a009979. [DOI] [PubMed] [Google Scholar]
- 45.Meschi T, Maggiore U, Fiaccadori E, et al. The effect of fruits and vegetables on urinary stone risk factors. Kidney Int. 2004;66:2402–2410. doi: 10.1111/j.1523-1755.2004.66029.x. [DOI] [PubMed] [Google Scholar]
- 46.Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol. 2007;18:2198–2204. doi: 10.1681/ASN.2007020219. [DOI] [PubMed] [Google Scholar]
- 47.Engstrom A, Tobelmann RC, Albertson AM. Sodium intake trends and food choices. Am J Clin Nutr. 1997;65(suppl):704S–707S. doi: 10.1093/ajcn/65.2.704S. [DOI] [PubMed] [Google Scholar]
- 48.Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri RK, Reisinger A, eds.] Geneva, Switzerland: IPCC; 1977. Intergovernmental Panel on Climate Change. Climate Change 2007: Synthesis Report. [Google Scholar]
- 49.Brikowski TH, Lotan Y, Pearle MS. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci USA. 2008;105:9841–9846. doi: 10.1073/pnas.0709652105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans K, Costabile RA. Time to development of symptomatic urinary calculi in a high risk environment. J Urol. 2005;173:858–861. doi: 10.1097/01.ju.0000152578.07262.1c. [DOI] [PubMed] [Google Scholar]
- 51.Doumerc N, Game X, Mouzin M, et al. Suggestion of a two-month delay between extreme temperatures and renal colic. J Urol. 2008;179(suppl):481. [Google Scholar]
- 52.Curhan GC, Rimm EB, Willett WC, Stampfer MJ. Regional variation in nephrolithiasis incidence and prevalence among United States men. J Urol. 1994;151:838–841. doi: 10.1016/s0022-5347(17)35101-7. [DOI] [PubMed] [Google Scholar]
- 53.Stoller ML, Meng MV, Abrahams HM, Kane JP. The primary stone event: a new hypothesis involving a vascular etiology. J Urol. 2004;171:1920–1924. doi: 10.1097/01.ju.0000120291.90839.49. [DOI] [PubMed] [Google Scholar]
- 54.Rule AD, Bergstralh EJ, Melton LJ, 3rd, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:804–811. doi: 10.2215/CJN.05811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68:1230–1235. doi: 10.1111/j.1523-1755.2005.00516.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamano S, Nakatsu H, Suzuki N, et al. Kidney stone disease and risk factors for coronary heart disease. Int J Urol. 2005;12:859–863. doi: 10.1111/j.1442-2042.2005.01160.x. [DOI] [PubMed] [Google Scholar]