Abstract
Overactive bladder (OAB) is a symptom complex of urinary frequency, urinary urgency, and nocturia, with or without urgency incontinence. This syndrome is idiopathic in most instances without clearly defined pathophysiology. Studies clearly show that OAB negatively impacts health-related quality of life and impairs daily functioning in a large proportion of patients. Despite recent advances in drug delivery and improved tolerability of antimuscarinic drug class, a large percentage of patients remain refractory to conventional pharmacological therapy for this chronic condition. There are several unique and effective treatments that are available for this difficult population. We review the various surgical options within the urological armamentarium to treat patients with refractory OAB.
Keywords: Overactive bladder syndrome, Urinary urgency incontinence, Muscarinic receptor-blocking agents, Posterior tibial nerve stimulation, Sacral neuromodulation, Botulinum neurotoxins
Overactive bladder (OAB) is a chronic medical condition that is estimated to affect 33 million adults in the United States and approximately 100 million worldwide. As such, the economic impact of OAB is tremendous, with estimated total costs approaching $26 billion. The negative psychosocial and quality-of-life impact of OAB and urinary urgency incontinence (UUI) has been well established through numerous epidemiologic studies. As the population continues to age, the prevalence of this condition will likely increase, placing a greater demand for health care resources directed toward this chronic condition.
Refractory OAB remains one the most complex and challenging clinical problems in urological practice. Initial patient management has become quite standardized and includes behavioral therapy with bladder retraining, dietary management with avoidance of bladder irritants and fluid modification, pelvic floor exercises with or without biofeedback, and pharmacological management with muscarinic receptor-blocking (MRB) agents. Despite improvements in drug therapy and delivery systems, the discontinuation rate for drug therapy is extremely high and related to lack of efficacy, adverse side effects, tolerability, and cost.1 Thus, there is demand for alternative therapeutic modalities that effectively treat medically refractory OAB.
The precise etiology of OAB remains elusive, although neuromuscular changes to the lower urinary tract and pelvic floor, age, hormonal status, and afferent neuropathic and myogenic mechanisms likely play important causative roles. Furthermore, chronic comorbid medical conditions and polypharmacy can play a substantial role in the elderly patient population.
In contradistinction to empirical pharmacological therapy for OAB, and while acknowledging that most OAB is idiopathic, it is important to consider complete evaluation of patients with cystoscopy, urodynamics, and laboratory studies including urine culture and urine cytology, to rule out serious underlying pathology prior to proceeding with invasive surgical treatment options for refractory OAB.
Peripheral Neuromodulation
Posterior Tibial Nerve Stimulation (PTNS)
PTNS is a minimally invasive, office-based procedure that involves percutaneous placement of a 34-gauge (ga) needle over the medial malleolus of the ankle with subchronic electrical stimulation of the posterior tibial nerve. The procedure is a 30-minute treatment session administered over a period of 12 weeks. Recently, Peters and colleagues2 reported data from a randomized, multicenter study comparing PTNS with pharmacological treatment with 4 mg/day tolterodine tartrate extended release (ER) capsules. One hundred patients with OAB were randomized to PTNS versus tolterodine tartrate ER. Patients were well matched in terms of OAB severity, and results demonstrated similar objective improvements in bladder diary variables (PTNS vs tolterodine tartrate ER %Δ: voids/d, 15.8% vs 17.7%; nocturia, 23.2% vs 10.2%; incontinence episodes [IE]/d, 52.6% vs 23.4%; voided volume, 35.2% vs 23.6%). Using a global response assessment, the researchers noted that a greater percentage of patients were cured/improved with PTNS (79.5% vs 54.8%; P < .01). Both treatments were well tolerated without serious treatment-related adverse events.
In a separate prospective, multicenter trial, Govier and colleagues3 assessed the efficacy and safety of PTNS for the treatment of refractory OAB. Patients were evaluated using 3-day voiding diaries and a number of standardized quality-of-life measures. The primary endpoint was the change in mean daytime frequency from baseline to 12 weeks. The treatment resulted in at least a 25% reduction in daytime frequency, a 21% reduction in nighttime voids, and a mean reduction of urgency incontinence episodes of 35%. Overall, 71% of patients were classified as a treatment success and were continued on long-term therapy. Improvements were noted in select quality-of-life measures and the procedure was very well tolerated.
PTNS has the potential to be an office-based therapy and first-line treatment option for patients with OAB due to the fact that it is minimally invasive and does not involve placement of a permanent implant. Limitations to more widespread use of this modality include response durability with the need for maintenance treatment sessions and third-party reimbursement concerns.
Sacral Neuromodulation
Historical Overview
Sacral neuromodulation (SNM) has been US Food and Drug Administration (FDA)-approved for treatment of refractory UUI and urinary urgency/frequency since 1997. Large-scale registration trials have shown the success of this treatment modality and numerous studies have shown long-term success in reducing several bladder diary variables including IE, voids per day, voided volume, and episodes of urgency.
The contemporary concept of neuromodulation of the sacral nerve roots for treatment of OAB evolved from the work of Schmidt and Tanagho at the University of California in San Francisco as they attempted to develop a “bladder pacemaker” for neuropathic voiding dysfunction. They studied a canine model due to similar neuroanatomy of the sacral nerves. This early work led to the observation that reflex inhibitory pathways exist between the pelvic floor and detrusor, most likely mediated through the pudendal nerve.4
In the 1990s, Schmidt devised an outpatient diagnostic test that involved percutaneous placement of a wire to stimulate the S3 nerve root and evaluate motor and sensory responses. 5 The innovative technique allowed for subchronic S3 nerve root stimulation and this peripheral nerve evaluation (PNE) served as the basis for future clinical applications of SNM.
Mechanism of Action
Although the mechanism of action of SNM is not completely understood, it is generally accepted that electrical stimulation of the sacral nerve roots serves to modulate efferent and afferent neural reflex pathways between the pelvic floor, bladder, and urethra. Furthermore, direct inhibition of detrusor preganglionic neurons and ascending afferent interneuronal transmission may play important roles. Thus, low threshold electrical stimulation serves to optimize bladder storage function and urethral closure pressure, leading to attenuation of urinary urgency, frequency, and urgency incontinence.6 Furthermore, research utilizing positron emission tomography scanning has implicated centers in the periventricular gray (which regulate the micturition reflex) that are influenced by the effects of SNM.7 More recently, Blok and colleagues8 demonstrated differences in sensorimotor learning areas of the cerebral cortex with acute versus chronic SNM. Studies such as these suggest that mechanisms operating centrally through projections into the pontine micturition center and cerebral cortex are responsive to SNM.
SNM Test Phase: PNE + 1-Stage Implant Versus 2-Stage Implant
SNM offers the patient who is refractory to pharmacological and behavioral therapy a unique, minimally invasive treatment option. Despite efforts to optimize patient selection by identifying predictive factors, currently the only way to select patients who will ultimately benefit from this therapy is to perform a test stimulation procedure. Thus, testing technique plays a critical role in determining which patients will undergo permanent device implantation.
Historically, PNE followed by 1-stage implant was the primary method used to provide acute stimulation and subchronic home stimulation to identify patients responsive to this technology. Limitations of this approach include migration of the temporary wires and a suboptimal test phase, as well as the potential discrepancy in clinical response when the permanent quadripolar lead is implanted. Furthermore, some patients who do not respond to PNE may in fact have an excellent outcome when the permanent electrode and neurostimulator/implantable pulse generator (IPG) are implanted.9 Recently, PNE has become more popular among clinicians, which may reflect the convenience of an office-based procedure, although economic factors may also play a role.
A staged implant technique using the permanent quadripolar tined lead has been advocated as a means to improve patient selection for IPG implantation. An important study by Borawski and colleagues10 randomized 17 patients to staged implant and 13 patients to PNE. The staged implant group was significantly more likely to proceed to IPG implant than the PNE group (88% vs 46%; P = .02). Other nonrandomized studies have shown superior efficacy of a 2-stage implant technique versus PNE with an IPG implant rate of 70% to 80% versus 40% to 50%.11–13 Furthermore, increased response rate to SNM was noted when the testing period was extended from 5 to 7 days to 14 days per implanted electrode lead.14
Description of Technique
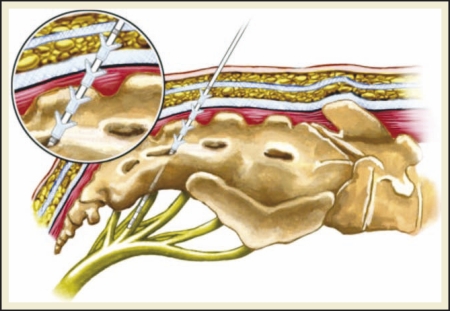
The field of neuromodulation was significantly advanced with the introduction of the quadripolar tined lead, which allowed for minimally invasive percutaneous electrode placement (Figure 1).11 This has replaced previously described fascial fixation techniques and contemporary SNM is performed exclusively with this method.
Figure 1.
The field of neuromodulation was significantly advanced with the introduction of the quadripolar tined lead, which allowed for minimally invasive percutaneous electrode placement.
The procedure is performed with the patient placed in the prone position using posterior-anterior and lateral fluoroscopy. A foramen needle is percutaneously inserted into the S3 foramen and confirmed by observing the typical S3 motor and sensory responses (Table 1). Once location is confirmed, a directional guide wire is placed through the foramen needle, followed by removal of the needle stylet. An introducer sheath and dilator are then passed over the guide wire into the S3 foramen and the guide wire and dilator are subsequently withdrawn, leaving the introducer sheath in position to facilitate insertion of the permanent quadripolar electrode. The lead and introducer sheath are then retracted as a unit until an S3 motor response is observed in a minimum of 3 of the 4 electrodes. The electrode is then tunneled subcutaneously (SC) toward the buttock, attached to a lead extension wire, and connected to an external pulse generator. A 7- to 14-day sub-chronic home test period is used to determine which patients meet criteria to have the IPG implanted. The IPG is implanted SC into the buttock, connected to the previously placed quadripolar electrode, and programmed to achieve an optimal clinical response.
Table 1.
Reflex Responses to Sacral Nerve Stimulation
| Nerve Root | Pelvic Floor Response | Ipsilateral Lower Extremity Response | Sensation |
| S2 | Anal sphincter contraction | Lateral leg rotation and “clamping” or contraction of toes and foot | Vaginal or proximal penile |
| S3 | “Bellows” response of pelvic floor and external anal sphincter | Plantar flexion of great toe | “Pulling” sensation in rectum and sensation in perineum, vagina, penis, or scrotum |
| S4 | “Bellows” response of pelvic floor and external anal sphincter | None | “Pulling” sensation in rectum |
Clinical Efficacy
For purposes of describing results from clinical studies, success is defined as > 50% in objective outcome variables as defined for the specific indication for SNM therapy. Success of SNM has been evaluated in prospective registration trials (Table 2).15,16 These studies showed sustained clinical efficacy with long-term follow-up > 12 months and therapy evaluation testing, during which stimulation was turned off, led to recrudescence of symptoms to baseline values.
Table 2.
Sacral Neuromodulation Study Results
| Study | Year | Study Design | Patients (n) | Technique | Efficacy |
| Schmidt RA et al5 | 1990 | RCT | 76 (34 SNM vs 42 control) | PNE | Mean IE/d (−7.1 vs+2.0; P < .0001) Mean pads/d (−5.1 vs +1.3; P < .0001) 47% completely continent and 29% improved at 6 mo; results maintained at 18 mo |
| Hassouna MM et al15 | 2000 | RCT | 51 (25 SNM vs 26 control) | PNE | Mean voids/d (−7.6 vs +0.5; P < .0001) Mean increase in volume per void (+ 108 mL vs −1 mL; P < .001) |
| Mean change degree of urgency prior to voiding (−0.6 vs −0.1; P = .01) | |||||
| Significant improvement in SF-36 subgroups in SNM group | |||||
| Sustained clinical benefit at 12- and 24-mo follow-up | |||||
| van Kerrebroeck PE et al18 | 2007 | Prospective, multicenter series | 96 urgency incontinence | PNE | 5-y urge incontinence data: IE/d (−5.7; P < .001); heavy IE/d (−1.8; P < .001); pads/d (−3.7; P < .001) |
| 25 urgency/frequency | 5-y urgency/frequency data: voids/d (−4.5; P < .001); voided volume (+72.9 mL; P < .001); urgency (−0.2; P = ns) | ||||
| 5-y success of SNM: 68% for urgency incontinence, 56% for urgency/frequency | |||||
| Sutherland SE et al19 | 2007 | Prospective, single-center series | 60 urgency incontinence | PNE and staged tined lead | Urgency incontinence: IE/d (−4.4; P < .0001) and pads/d (−2.3; P < .0001) |
| 23 urgency/frequency | Urgency/frequency: voids/d (−4.3; P < .0001) and voids/night (−1.0; P = .0091) | ||||
| 69% overall success rate with follow-up of 22 mo (range, 3–162 mo) | |||||
| Patient satisfaction: 23.4% delighted, 17.4% pleased, 19.8% mostly satisfied | |||||
| van Voskuilen AC et al20 | 2007 | Prospective | 39 OAB | Staged tined lead | Significant improvements in voids/d, urgency episodes, voided volume, and IE/d |
IE, incontinence episodes; ns, not significant; OAB, overactive bladder; PNE, peripheral nerve evaluation; SF, short form; SNM, sacral neuromodulation; RCT, randomized, controlled trial.
An excellent database review evaluated all studies of SNM for UUI between 1996 and 2003 and reported a success rate of 67% to 80% that was durable at 3 to 5 years.17 Several other studies of interest are presented in Table 2.18–20
Predictive Factors and Patient Selection
Several investigators have attempted to identify parameters that have predictive value in selecting those patients most likely to benefit from SNM therapy. Amundsen and colleagues21 reported on 105 patients with UUI who underwent PNE, of whom 55 (52%) went on to full implant with tined lead and IPG. Cure was defined as no IE following permanent implantation. Age > 55 years was associated with a significantly greater success rate (65% vs 37%; P < .05) despite similar baseline symptom severity between groups. Furthermore, they found that, regardless of age, the presence of more than 3 medical comorbidities and a neurologic condition associated with bladder dysfunction were similarly predictive of a poorer response to SNM.
In a separate study, Foster and colleagues22 reported long-term quality-of-life data in a group of urge incontinent patients who were at least 12 months remote from SNM implantation. Fifty-two patients were followed with mean follow-up of 27.2 months (range, 12–52 months). Satisfied patients had a greater reduction in 24-hour pad weight (−84.5% vs −60.6% pad weight; P = .002) during test stimulation, but no difference in daily pad usage (−4.5 vs −3.4 pads/d; P = .19) compared with patients who were dissatisfied following SNM. Using a multiple logistic regression model (P = .009 with concordance index of 0.84), the authors concluded that reduction in 24-hour pad weight best predicted long-term patient satisfaction with SNM therapy.
There is a strong correlation between emotional disorders and neuropsychological dysfunction and the OAB symptom complex. Several studies have shown that these patients are far more likely to respond poorly to test stimulation, have symptom recrudescence following permanent implant, and have a higher incidence of reoperations.13,23
Urodynamic studies have consistently been unable to predict response to test stimulation, as well as response following permanent implantation. South and colleagues24 recently reported that the presence or absence of detrusor overactivity on urodynamic investigations did not correlate with the likelihood of responding to test stimulation. Thus, urodynamics may not add much value over clinical history and voiding diary data. This finding should be confirmed in a prospective, randomized fashion.
SNM Complications and Troubleshooting
The very nature of this mode of therapy mandates a 100% reoperation to replace the IPG due to the limited longevity of the neurostimulator. Following IPG implant, patients need lifelong surveillance to manage device-related issues that may arise. Hijaz and colleagues25 reported an excellent review of complication management and implant troubleshooting strategy from the Cleveland Clinic database of 214 tine lead implants. One hundred and sixty-one patients (75.5%) had placement of the IPG and complications were stratified into infectious, mechanical, and responserelated problems. Seventeen patients (10.5%) had the device completely removed for infection (n = 8) and failure of clinical response (n = 9). Twenty-six patients (16.1%) underwent device revision due to attenuation of response (n = 17), infection (n = 4), pain at IPG site (n = 4), and lead migration (n = 1). The majority of patients with revision due to poor response had abnormal impedance measurements, with equalization of impedance in 2 leads being the most common finding. Thus, the authors strongly advocate IPG interrogation with impedance testing to completely evaluate patients with response-related dysfunction.
Future Considerations
SNM remains an excellent treatment modality for refractory OAB. Technological advances have made this a truly minimally invasive procedure with improved patient safety. More information is required to identify how IPG stimulation parameters influence and optimize clinical response, as this is largely a trial-and-error process. The lack of a dual-chamber neurostimulator in the United States has made it difficult to assess the effects of bilateral stimulation. Furthermore, more research is necessary to determine mechanism of action, development of neuroplasticity, and the longterm cortical effects of SNM.
Botulinum Toxin for the Treatment of Refractory Idiopathic OAB
Structure and Physiology
Botulinum neurotoxins (BoNTs) have become well established as the most potent naturally occurring neuromuscular toxins known to man. These neurotoxins are derived from the gram-negative anaerobic bacterium Clostridium botulinum of which 7 distinct serotypes have been identified (A, B, C, D, E, F, and G). The various serologic forms of toxin all share a common molecular structure with a molecular weight of 150 kDa. The serotypes are composed of a heavy chain of 100 kDa and a light chain of 50 kDa joined by a disulfide bond.
The end-organ effects of BoNTs are mediated at the parasympathetic presynaptic nerve terminal (Figure 2).26 The heavy chain moiety of the toxin binds to a synaptic vesical (SV) receptor protein on the nerve terminal (SV2) and is internalized via receptormediated endocytosis. Once within the cytosol, an endopeptidase enzyme cleaves the disulfide bond, allowing the light chain moiety to translocate from the endocytotic vesical to the cytosol, where it can exert its clinical effects via inhibition of neurotransmitter release.
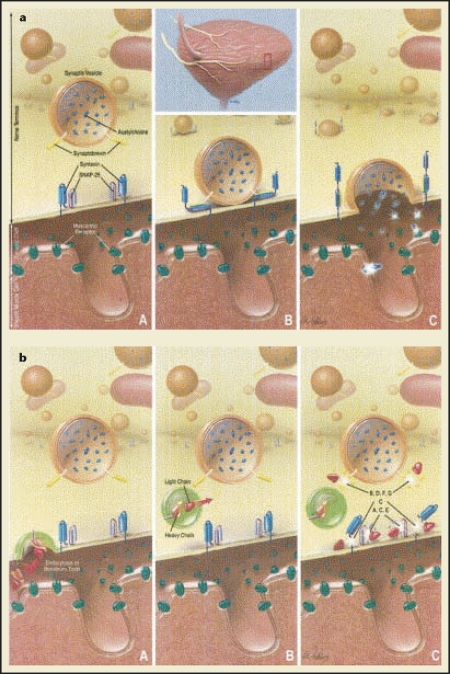
Figure 2.
(a) Normal fusion and release of acetylcholine (ACh) from nerve terminals via interaction of vesicle and membrane bound N-ethylmaleimide-sensitive factor attachment protein receptor complex (SNARE) proteins. (A) Parasympathetic nerves innervating bladder with nerve terminal in unactivated state displaying numerous vesicles containing neurotransmitter ACh. SNAP-25, synaptosomal associated membrane protein. (B) Following nerve activation, assembly of SNARE protein complex (eg, synaptobrevin, SNAP-25, and syntaxin) occurs, which leads to (C) release of ACh and activation of postjunctional muscarinic receptors, resulting in bladder contraction. (b) Parasympathetic nerve terminal. (A) Binding of toxin heavy chain to as yet unidentified receptor and internalization of toxin within nerve terminal. (B) Translocation of light chain into cytosol. (C) Inhibition of neurotransmitter release by cleavage of specific SNARE proteins. A to G, botulinum toxin serotypes. Reprinted from The Journal of Urology, Vol. 171, Smith CP, Chancellor MB, “Emerging role of botulinum toxin in the management of voiding dysfunction,” pp. 2128–2137, Copyright 2004, with permission from Elsevier.
The process of neurotransmitter release is extremely complex. It involves the adenosine diphosphate (ADP)-dependent transport of neurotransmitter-containing vesicles to the plasma membrane and release of the neurotransmitter. Exocytosis of the neurotransmitter is triggered by membrane depolarization and intracellular calcium influx, and is facilitated by the interaction of several key proteins known as the soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex (SNARE complex). These SNARE proteins include synaptosomal-associated protein (25 kDa; SNAP-25), synaptobrevin, and syntaxin, all of which are targeted by specific clostridia neurotoxins. For example, BoNT type A (BoNT-A) specifically targets SNAP-25, thus blocking vesicle fusion with the presynaptic plasma membrane.
Mechanism of Action of BoNT
Much of the clinical efficacy of BoNT is mediated by direct inhibition of acetylcholine and adenosine-5′- triphosphate (ATP) release from presynaptic cholinergic nerve terminals, resulting in reversible chemodenervation and flaccid muscle paralysis. The onset of action typically occurs within 48 to 72 hours and can last between 6 and 9 months. This reversibility of clinical response is secondary to axonal regeneration and nerve sprouting, which leads to attenuation of clinical effect and the need for repeated injections.
In addition to the efferent effect of BoNT just described, there is also evidence supporting an afferent nerve effect, as well as the ability of BoNT to modulate the expression of key urothelial growth factors, peptides, and receptors. Basic science research has suggested the ability of BoNT-A to block a variety of substances (acetylcholine, substance P, ATP, calcitonin gene-related peptide, and glutamate) from afferent nerve terminals. These effects may influence treatment of bladder sensory disorders, including the OAB symptom complex. Furthermore, laboratory studies have shown that several growth factors/ receptors that are known to be mediators of bladder hyperactivity (TRPV1 [capsaicin sensitive], P2 × 3 [purinergic], and nerve growth factor) could be inhibited or downregulated by BoNT-A therapy. Thus, the clinical effects of BoNT-A in the treatment of medically refractory OAB appear to be multifactorial.27
Clinical Applications
Currently, the specific availability of BoNT depends on geographic location. In the United States, commercially available botulinum toxins include type A (Botox®; Allergan, Irvine, CA) and type B (Myobloc®; Elan Pharmaceuticals, Inc., San Francisco, CA). Two other formulations of BoNT-A are available in Europe and include Dysport® (abobotulinum toxin A; Ipsen Ltd., Slough, UK) and Xeomin® (Clostridium botulinum type-A; Merz Pharma GmbH, Frankfurt am Main, Germany). Doses of BoNT are referenced in mouse units with 1 unit of toxin equating to the amount necessary to kill 50% of a group of female mice (LD50). Formulations and dosages are not interchangeable and converting toxin dosages between 2 formulations is not currently recommended. As such a preponderance of the scientific literature is derived from studies investigating Botox, unless otherwise specified, BoNT-A will refer to the Botox formulation.
Intradetrusor Injection Technique
Almost all studies have injected BoNT directly into the detrusor muscle. Interestingly, recent data have demonstrated that there may be a role for intravesical instillation of BoNT. There are numerous variables that deserve consideration with respect to intradetrusor injection of BoNT. These factors include the amount of toxin, dilution volume, location, depth and number of injections, anesthetic technique, and endoscopic instrumentation. A comprehensive discussion of these issues is beyond the scope of this review.
Most studies commonly use between 100 to 300 U of BoNT-A with a dilution of 10 U/mL, injecting 1 mL over 10 to 30 sites in the bladder. Using a larger volume has been shown to achieve better distribution of toxin throughout the bladder. The solution can be combined with 0.1 mL indigo carmine to allow the physician to map the injection template. Although some investigators have recommended a trigone-sparing approach for fear of vesicoureteral reflux, this has not been problematic in studies where the trigone was injected. Earlier studies were typically performed under general or regional anesthesia using a rigid cystoscope. Less invasive techniques utilizing flexible cystoscopy and a 25-ga flexible injection needle have become more popular, allowing for a truly minimally invasive inoffice procedure.
Clinical Results of BoNT-A
In 2000, Schurch and colleagues28 first reported on the success of BoNT-A in the treatment of patients with refractory neurogenic detrusor overactivity. Although the use of BoNT-A is not currently approved by the FDA, its use in off-label and investigational protocols continues to accumulate, providing growing support as an indication for drug refractory OAB.29
Randomized Clinical Trials
Brubaker and colleagues30 reported data in a population of women with refractory idiopathic UUI randomized to 200 U of BoNT-A versus placebo. This was a well-designed, multicenter, randomized, clinical trial with a defined patient population and validated outcome measures. Females with 6 UUI episodes on a 3-day bladder diary and documented detrusor overactivity who were refractory to 2 anticholinergic medications and behavioral therapy were randomized in a 2:1 ratio. Patient Global Impression of Improvement (PGI-I) scores at 2 months were significantly better in the treatment group (2.7 vs 4.0; P = .003) and 60% of the women achieved a clinical response based on PGI-I with a median duration of response of 373 days versus 62 days in the placebo group (P < .0001). There was a highly significant reduction in daily IE in the BoNT-A group (P < .0001) and 72% of the women who completed a follow-up bladder diary experienced a 75% reduction in incontinence events. Furthermore, perception of bladder control was greater in the BoNT-A cohort (P = .0001), as was patient symptom bother measured with the Urogenital Distress Inventory (UDI-6; P = .003).
Of note, 12 patients (43%) in the BoNT-A arm developed increased postvoid residual urine (PVR) > 200 mL and began a program of intermittent self-catheterization. Nine of these 12 patients (75%) developed urinary tract infection (UTI) and were treated with antimicrobial therapy. Due to the high rate of increased PVR, the study was abruptly stopped and no further patients were recruited into the protocol. All patients with increased PVR were started on a program of self-catheterization, regardless of whether they were experiencing symptoms. The authors admit in their discussion that meaningful interpretation of the consequences of increased PVR cannot be extrapolated from the study. One would think that if patients’ symptoms were improved following BoNT-A injection due to increased functional bladder capacity and decreased IE, and they were not experiencing ill effects from elevated PVR, then the vast majority of patients may not need any treatment other than careful observation. Obviously, this deserves further study.
Sahai and colleagues31 randomized 16 patients to 200 U of BoNT-A and 18 patients to placebo and noted a significant improvement in the primary outcome measure of maximum cystometric capacity at 4 weeks (Δ + 144.69 mL; 95% confidence interval [CI], 100.95–215.75; P < .0001) and 12 weeks (Δ + 95.71 mL; 95% CI, 47.47–172.45; P < .001). There were also significant improvements in secondary outcome endpoints at 4 and 12 weeks, including 3-day bladder diary variables of urinary frequency (P < .0001 and P = .003) and IE/day (P = .03 and P = .008), as well as Incontinence Impact Questionnaire (IIQ-7) scores (P = .03 and P = .006) and UDI-6 (P = .0003 and P < .0001). During an open-label extension phase, all primary and secondary outcome measures maintained significant improvements at 24 weeks compared with baseline, sustaining efficacy for a duration of 10 months. A subsequent follow-up analysis from the same group showed significant improvement in quality-of-life parameters using the King’s Health Questionnaire (KHQ) in the BoNT-A group.32
Recently, Flynn and colleagues33 reported preliminary data from an ongoing double-blind, placebo-controlled trial comparing 2 doses of BoNT-A (200 U and 300 U) with placebo. As the study is ongoing and the investigators were blinded to dose of toxin, the data from the 2 BoNT-A groups were combined for the report. At 6 weeks (endpoint for the placebo-controlled phase) there were significant improvements in primary outcome measures of daily IE (Δ −57.5% vs +9.3%; P < .01) and scores on the IIQ-7 (Δ −67.3% vs 0.0; P < .01) and UDI-6 (Δ −37.5% vs +7.4%; P < .01). Six of 7 placebo-treated patients went on to therapy with BoNT-A. Ongoing follow-up will determine any differences in outcomes based on toxin dose.
Nonrandomized Studies
The largest study reporting on idiopathic OAB was a prospective, nonrandomized study by Schmid and colleagues.34 One hundred patients were injected with 100 U of BoNT-A at 30 sites sparing the trigone. Clinical, urodynamic, and quality-of-life assessments were made at 4, 12, and 36 weeks following injection. Ninety-two patients were considered responders to BoNT-A based on significant improvements in clinical and urodynamic parameters. Poor bladder compliance and maximal cystometric capacity (MCC) > 100 mL due to bladder fibrosis was felt to be predictive of a poor outcome. There were no differences in treatment outcome when patients with urodynamic detrusor overactivity incontinence were compared with patients with urgency/ frequency alone. Quality of life measured at 4 and 12 weeks showed improvement in urge-related items on the KHQ, including ability to travel, sleep, participate in social life, and accomplish household tasks.
A unique study by White and colleagues35 sought to evaluate the clinical effects of BoNT-A injection in elderly patients age ≥75 years (mean age, 81.2 years; range, 75–92 years) with both idiopathic and neurogenic detrusor overactivity with UUI. Success was demonstrated in 16 of the 21 patients (76%) who noted a > 50% improvement in voids per day (11.4 ± 1.67 vs 5.19 ± 0.83; P < .001) and number of pads per day (4.0 ± 0.89 vs 1.3 ± 0.60; P < .001) at 1 month. Efficacy was maintained for 7.12 months without treatment-related adverse effects, specifically elevated PVR and urinary retention.
Several other nonrandomized studies have demonstrated the safety and efficacy of BoNT-A injection (Table 3).36–40
Table 3.
Botulinum Neurotoxin Study Results
| Study | Year | Condition | Study Design | Patients (N) | Toxin | Units | Dilution | Injections | Trigone injected | Anesthesia | Cystoscopy | Efficacy | PVR (mL) Criteria to Start CIC | Adverse Events |
| Brubaker L et al30 | 2008 | IDO | Multicenter RCT | BoNT-A = 28 | BoNT-A | 200 | 100 U/3 mL | 15–20 | No | Local | Rigid | 60% clinical response on PGI-I | > 200 | Increased PVR and UTI in BoNT-A group |
| Placebo = 15 | 6 mL normal saline | Significant reduction in IE | 43% CIC | |||||||||||
| Improvement in UDI urge subscale Time to deterioration: 373 d | ||||||||||||||
| White WM et al35 | 2008 | IDO | Observational cohort | 21 | BoNT-A | 200 | 200 U/10 mL | 20 | No | General | Rigid | 16 patients > 50% reduction in voids/d (P < .001) and pads/d (P < .001) | Not defined | No increased PVR |
| 14 IDO | Time to deterioration: 7.12 mo | 2 UTI | ||||||||||||
| 7 NDO | 2/5 failures had > 50% improvement with repeat injection at 3 mo | |||||||||||||
| Sahai A et al31 | 2007 | IDO | Single-center RCT | 34 | BoNT-A | 200 | 200 U/ | 20 | No | Local | Flexible | Increased maximum cystometric capacity | > 150 | Increased PVR; 6 required CIC (37.5%) |
| BoNT-A = 16 | 20 mL 20 mL normal saline | Reduced urinary frequency and urgency incontinence episodes | 7 UTI (6 performing CIC) | |||||||||||
| Placebo = 18 | Improved quality of life on IIQ-7 and UDI-6 Beneficial effects maintained for 24 wk | |||||||||||||
| Flynn MK er al33 | 2009 | IDO | Single-center RCT | 22 | BoNT-A | 200,300 | 200–300 U/3 mL | 8–10 | No | Local | Rigid | Significant reductions in IE/d, 24-h pad weight, and pads/d | Not defined | PVR > 200 mL (n = 4, 26.6%); 1 required CIC |
| BoNT-A = 15 | Significant improvements in UDI-6 and IIQ-7 quality-of-life scores | UTI: 2 BoNT-A (13%) and 2 placebo (28%) | ||||||||||||
| Placebo = 7 | No change in voids/d, nocturia, MCC, Qmax, or Pdet | Gross hematuria requiring CBI (n = 1) | ||||||||||||
| Schmid DM et al34 | 2006 | IDO | Multicenter, prospective, open label | 100 | BoNT-A | 100 | 100 U/10 mL | 30 | No | General or local | Rigid | Resolution of urgency at 4 wk (72%) and 12 wk (66%) | > 400 | PVR > 400 mL requiring CIC (n = 4) |
| Resolution of incontinence at 4 wk (74%) and 12 wk (80%) | PVR 150-200 mL, no CIC (n = 15) | |||||||||||||
| Significantly decreased urinary frequency and nocturia | UTI (n = 10) | |||||||||||||
| Improved urodynamic parameters: resolution DO (74%), increased MCC and compliance Time to deterioration: 6 ± 2 mo | ||||||||||||||
| Rapp DE et al39 | 2004 | IDO | Single center, observational | 35 | BoNT-A | 300 | 100 U/1 mL | 30 | Yes | IV sedation | Rigid | 34% “symptom resolution” and 26% “slight improvement” based on IIQ-7 and UDI-6 | Not defined | 7 patients with mild dysuria/hematuria < 3 d |
| 0 patients requiring CIC | ||||||||||||||
| Werner M et al40 | 2005 | IDO | Prospective | 26 | BoNT-A | 100 | 100 U/30 mL | 20 | No | General/spinal | Rigid | 60% improvement/success | ||
| −35% reduction in frequency; +58% MCC; −60% reduction in DO | > 100 | 8% patients with PVR > 100 mL performing CIC | ||||||||||||
| Kuo HC36 | 2005 | IDO | Prospective | 20 | BoNT-A | 200 | 100 U/10 mL | 40 | No | General | Rigid | 45% continent, 40% improved, 15% failure at 3-mo endpoint | > 250 | 30%–50% performing CIC |
| At 6 mo, 35% of patients remained continent | 35% treated for UTI | |||||||||||||
| Popat R et al38 | 2005 | IDO + NDO | Prospective, open-label | NDO = 44 | BoNT-A | NDO = 300 | 100 U/10 mL | NDO = 30 | No | Local | Flexible | Significant improvements (P < .0001) in MCC, Pdet.max, voids/d, IE/d, and urgency/d in both groups | > 100 | 19% (IDO) performing CIC |
| IDO = 31 | ID0 = 200 | IDO = 20 | No statistically signficant differences in outcomes in NDO vs IDO patients | 69% (NDO) performing CIC | ||||||||||
| Kessler TM et al37 | 2005 | IDO + NDO | Prospective | NDO = 11 | BoNT-A | 300 | 100 U/10 mL | 30 | No | Not reported | Not reported | No significant difference in clinical or urodynamic parameters in NDO vs IDO patients | > 150 | 36% (IDO) performing CIC |
| IDO = 11 | IDO clinical variables: voids/d (11 to 4; P = .004); nocturia (3 to 1; P = .004); pads/d (5 to 0; P = .001) | 45% (NDO) performing CIC | ||||||||||||
| IDO urodynamic variables: MCC (220 to 340 mL; P = .001); Pdet.max (45 to 29 cm; P = .002); Pdet.max (30 to 14 cm: P = .001) |
BoNT-A, botulinum neurotoxin type A; CBI, continuous bladder irrigation; CIC, clean intermittent catheterization; DO, detrusor overactivity; IDO, idiopathic detrusor overactivity; IE, incontinence episodes; IIQ, Incontinence Impact Questionnaire; IV, intravenous; MCC, maximal cystometric capacity; NDO, neurogenic detrusor overactivity; PGI-I, Patient Global Impression of Improvement scale; PVR, postvoid residual volume; RCT, randomized, controlled trial; UDI, Urogenital Distress Inventory; UTI, urinary tract infection.
Adverse Effects of BoNT-A
Local side effects of BoNT-A injection are relatively uncommon and include gross hematuria, injection site pain, and UTI. Transient muscle weakness is exceedingly rare, but has been described. Far and away, the most concerning adverse event following BoNT-A injection is the development of clinically significant PVR and urinary retention (Table 3). As previously discussed, clinically significant PVR following therapy has not been consistently defined. Furthermore, populations at increased risk for this complication have not been well phenotyped and may include patients with detrusor hyperactivity with impaired contractility. Clearly, patients with a hypocontractile detrusor or atonic detrusor should be considered at higher risk for clinically significant increased PVR. Two well-done studies have recently addressed this concept. Khan and colleagues41 prospectively evaluated 81 consecutive patients injected with 200 U of BoNT-A for idiopathic detrusor overactivity. They defined PVR as more than 100 mL in association with lower urinary tract symptoms as the indication to initiate clean intermittent self-catheterization. Thirty-five patients (43%) required clean intermittent bladder catheterization (CIC) after injection and there was no significant difference in the rate of CIC in patients who had repeated injections (P = .87). If patients required CIC following BoNT-A injections, it was universally required after subsequent injections. Furthermore, they found that some patients required CIC after subsequent injections when CIC was not required during initial injections. Thus, the authors concluded that a willingness to perform CIC should be mandatory in patients considering this off-label therapy.
Despite a rate of CIC approaching 40% in some studies, the following question deserves further discussion: does this outweigh the potential benefits of therapy? Kessler and colleagues42 reported short-term quality-of-life data in patients performing CIC following BoNT-A injection. The authors evaluated IIQ-7 and UDI-6 scores 4 weeks following injection. Forty-three percent of patients required CIC following BoNT-A. There were no significant differences in IIQ-7 or UDI-6 scores among patients who were, or were not, performing CIC (IIQ-7, 62-30 vs 64-25; P < .001, and UDI-6, 61-33 vs 60-28, P < .001). At least in this short-term study, the benefits of BoNT-A therapy seem to outweigh the burden associated with CIC.
Enterocystoplasty and Urinary Diversion Techniques for Refractory OAB
Enlargement or augmentation cystoplasty is a useful technique in the urologist’s surgical armamentarium for medically refractory OAB. Generally speaking, all other modalities of treatment have been exhausted or contraindicated including pharmacological therapies, behavioral therapy, CIC, SNM, and BoNT-A injection. Virtually all bowel segments have been used for the purposes of augmenting the bladder with decreased functional and/or organic capacity. The common goal of this procedure is to promote urinary drainage into a low-pressure urinary reservoir with adequate capacity. Routine performance of this technique for neurologically intact patients with idiopathic, nonneurogenic detrusor overactivity is limited by the acute morbidity of the operation, potential for long-term metabolic and bowel-related complications, and the need for CIC.
Medical Evaluation
A thorough preoperative evaluation is performed with attention to the presence of neurologic disease, gastrointestinal disease, and previous abdominal surgery. Patients with risk factors or advanced age should have colonoscopy performed if colon is to be used in the reconstruction. Determination of renal function and creatinine clearance is routinely performed due to the potential for metabolic acidosis and deterioration in renal function. Upper tract imaging is also performed to confirm normal renal anatomy and rule out significant obstruction. Finally, urodynamic testing should be performed with emphasis on bladder compliance, detrusor overactivity, maximum cystometric capacity, and urethral sphincter function (severe urethral dysfunction may require concomitant bladder neck procedure at the time of reconstruction).
Surgical Technique
A clear liquid diet is begun 48 hours preoperatively and mechanical bowel preparation the day prior to surgery. Prophylactic antibiotics are administered, as is deep vein thrombosis prophylaxis with sequential compression devices ± lower molecular weight heparin. A midline abdominal incision is made and the peritoneum is entered and explored. A healthy segment of ileum is based on a broad, well-vascularized mesenteric pedicle and isolated using a stapling device. Typically, 20 to 40 cm of ileum or 20 cm of colon is needed to complete the reconstruction. The bowel is then reconstituted using a side-to-side, functional end-to-end stapled anastomosis and the mesenteric defect is closed with interrupted suture.
The bladder is then mobilized and a generous vertical cystotomy is made just above the bladder neck to the posterior bladder base. Alternatively, a transverse cystotomy and an anterior bladder flap have also been described for purposes of augmentation. The bowel is then detubularized on its antimesenteric border and can be reconfigured into a U-, S-, or W-shaped pouch and anastomosis to the bladder is performed with running 2-0 absorbable suture. If a continent catheterizable channel is to be performed simultaneously this can be done using the Mitrofanoff or Monti technique. If a right colon and cecal augment is performed, the ileocecal valve can be incorporated with a limb of terminal ileum, as is commonly done with an Indiana pouch during bladder replacement surgery. A suprapubic and urethral catheter are left for 2 to 3 weeks and removed once cystography confirms the absence of urine leak. The suprapubic catheter can then be used for a short time to measure PVRs during the follow-up period.
Follow-Up and Surveillance
All patients require routine follow-up with periodic assessment of the upper tracts with ultrasound or intravenous urography and regular assessment of serum electrolytes, blood urea nitrogen, and creatinine. Furthermore, all patients should incorporate a daily irrigation protocol to reduce inspissated mucous and stone formation. Routine cystoscopy should be performed annually after 5 years to evaluate for the development of carcinoma, although the cost effectiveness and evidence base for this practice has not been determined.
Complications
Complications related to augmentation cystoplasty can be divided into early versus late, as well as whether they are attributable to the bowel, bladder, or are metabolic/infectious in nature. Early complications are related to postoperative ileus, anastomotic leak, gastrointestinal fistula, urine leak, and abdominal abscess formation. Late complications include bladder perforation, bowel obstruction, bladder calculi, UTI and pyelonephritis, and metabolic acidosis (with potential osteopenia/osteoporosis secondary to bone resorption). Furthermore, there is the potential for development of adenocarcinoma and transitional cell carcinoma that requires endoscopic surveillance.
Efficacy
Most data on enterocystoplasty are derived from large series including pediatric patients with neuropathic bladder dysfunction and adult populations with spinal cord injury and other forms of neurogenic bladder. Successful continence outcomes have consistently been > 80%, with resolution of spontaneous detrusor contractions and increased maximum cystometric capacity.43,44 Neurologically intact patients may be able to void spontaneously but specific data on this endpoint are sparse, and therefore all patients should be prepared to perform CIC postoperatively. Several authors have recently shown that augmentation cystoplasty can be performed laparoscopically with excellent functional results.45,46
Which Therapy? Which Patient?
Although all of the therapeutic options discussed in this review have demonstrated safety and reasonable efficacy in the management of a very difficult patient population, the fact remains that patients still need individualized treatment options tailored to their pathophysiology, urodynamic parameters, medical comorbidities, and goals and expectations. Patients with elevated PVR or difficulty emptying may be better suited with SNM versus BoNT-A. Patients who do not possess the cognitive functioning to use the patient-programming device may not be good SNM candidates. Poor surgical candidates with numerous comorbid medical conditions may elect PTNS. In our opinion, due to the invasive and irreversible nature of augmentation cystoplasty, this procedure should only be performed in reasonably healthy and highly motivated patients who are prepared to perform CIC.
Conclusions
Refractory OAB is a complex, chronic medical condition with a profound impact on quality of life in affected patients. Traditional treatments that include behavioral interventions in conjunction with pharmacological therapy fail in a significant proportion of patients. PTNS, SNM, BoNT-A, and bladder augmentation are all effective treatments with a unique position in the continuum of care of the refractory OAB patient. Although each can be effective, each therapy has potential limits that must be taken into account prior to implementing therapy. Judicious consideration of each surgical option and a thorough understanding of these therapeutic limits, in conjunction with reasonable expectations on the part of the patient, are ultimately what lead to successful patient care.
Main Points.
Refractory overactive bladder (OAB) is a complex and challenging clinical problem in urological practice. Traditional treatments that include behavioral interventions in conjunction with pharmacological therapy fail in a significant portion of patients.
Posterior tibial nerve stimulation (PTNS), sacral neuromodulation (SNM), botulinum neurotoxin A (BoNT-A), and bladder augmentation are all effective treatments for the OAB patient, although each therapy has potential limits that must be taken into account prior to initiating therapy.
PTNS has the potential to be an office-based therapy and first-line treatment option for patients with OAB because it is minimally invasive and does not involve placement of a permanent implant. Limitations to its more widespread use include response durability with the need for maintenance treatment sessions and third-party reimbursement concerns.
SNM is an excellent treatment modality for refractory OAB. Technological advances have made this a truly minimally invasive procedure with improved patient safety. More information is required to identify how implantable pulse generator stimulation parameters influence and optimize clinical response, as this is largely a trial-and-error process.
Although the use of BoNT-A is not currently approved by the FDA, its use in off-label and investigational protocols continues to accumulate, providing growing support as an indication for drug refractory OAB.
Enlargement or augmentation cystoplasty is a useful technique if all other treatments have been exhausted. This procedure aims to promote urinary drainage into a low-pressure urinary reservoir with adequate capacity. Routine performance of this technique for neurologically intact patients with idiopathic, nonneurogenic detrusor overactivity is limited by its acute morbidity, potential for long-term metabolic and bowel-related complications, and the need for clean intermittent bladder catheterization.
References
- 1.Shaya FT, Blume S, Gu A, et al. Persistence with overactive bladder pharmacotherapy in a Medicaid population. Am J Manag Care. 2005;11(suppl 4):S121–S129. [PubMed] [Google Scholar]
- 2.Peters KM, Leong FC, Shobeiri SA, et al. A randomized multicenter study comparing percutaneous tibial nerve stimulation with pharmaceutical therapy for the treatment of overactive bladder. Poster presented at the American Urological Association Annual Meeting; April 25–30, 2009; Chicago. [Google Scholar]
- 3.Govier FE, Litwiller S, Nitti V, et al. Percutaneous afferent neuromodulation for the refractory overactive bladder: results of a multicenter study. J Urol. 2001;165:1193–1198. [PubMed] [Google Scholar]
- 4.Tanagho EA, Schmidt RA. Electrical stimulation in the clinical management of the neurogenic bladder. J Urol. 1988;140:1331–1339. doi: 10.1016/s0022-5347(17)42038-6. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt RA, Senn E, Tanagho EA. Functional evaluation of sacral nerve root integrity. Report of a technique. Urology. 1990;35:388–392. doi: 10.1016/0090-4295(90)80078-2. [DOI] [PubMed] [Google Scholar]
- 6.Leng WW, Chancellor MB. How sacral nerve stimulation neuromodulation works. Urol Clin North Am. 2005;32:11–18. doi: 10.1016/j.ucl.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Blok BF. Central pathways controlling micturition and urinary continence. Urology. 2002;59(suppl 5):13–17. doi: 10.1016/s0090-4295(01)01633-8. [DOI] [PubMed] [Google Scholar]
- 8.Blok BF, Groen J, Bosch JL, et al. Different brain effects during chronic and acute sacral neuromodulation in urge incontinent patients with implanted neurostimulators. BJU Int. 2006;98:1238–1243. doi: 10.1111/j.1464-410X.2006.06521.x. [DOI] [PubMed] [Google Scholar]
- 9.Janknegt RA, Weil EH, Eerdmans PH. Improving neuromodulation technique for refractory voiding dysfunctions: two-stage implant. Urology. 1997;49:358–362. doi: 10.1016/S0090-4295(96)00506-7. [DOI] [PubMed] [Google Scholar]
- 10.Borawski KM, Foster RT, Webster GD, Amundsen CL. Predicting implantation with a neuromodulator using two different test stimulation techniques: a prospective randomized study in urge incontinent women. Neurourol Urodyn. 2007;26:14–18. doi: 10.1002/nau.20332. [DOI] [PubMed] [Google Scholar]
- 11.Spinelli M, Giardiello G, Arduini A, van den Hombergh U. New percutaneous technique of sacral nerve stimulation has high initial success rate: preliminary results. Eur Urol. 2003;43:70–74. doi: 10.1016/s0302-2838(02)00442-6. [DOI] [PubMed] [Google Scholar]
- 12.Scheepens WA, Van Koeveringe GA, De Bie RA, et al. Long-term efficacy and safety results of the two-stage implantation technique in sacral neuromodulation. BJU Int. 2002;90:840–845. doi: 10.1046/j.1464-410x.2002.03028.x. [DOI] [PubMed] [Google Scholar]
- 13.Everaert K, Kerckhaert W, Caluwaerts H, et al. A prospective randomized trial comparing the 1-stage with the 2-stage implantation of a pulse generator in patients with pelvic floor dysfunction selected for sacral nerve stimulation. Eur Urol. 2004;45:649–654. doi: 10.1016/j.eururo.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Kessler TM, Madersbacher H, Kiss G. Prolonged sacral neuromodulation testing using permanent leads: a more reliable patient selection method? Eur Urol. 2005;47:660–665. doi: 10.1016/j.eururo.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Hassouna MM, Siegel SW, Nÿeholt AA, et al. Sacral neuromodulation in the treatment of urgency-frequency symptoms: a multicenter study on efficacy and safety. J Urol. 2000;163:1849–1854. [PubMed] [Google Scholar]
- 16.Schmidt RA, Jonas U, Oleson KA, et al. Sacral nerve stimulation for treatment of refractory urinary urge incontinence. Sacral Nerve Stimulation Study Group. J Urol. 1999;162:352–357. [PubMed] [Google Scholar]
- 17.Brazzelli M, Murray A, Fraser C. Efficacy and safety of sacral nerve stimulation for urinary urge incontinence: a systematic review. J Urol. 2006;175:835–841. doi: 10.1016/S0022-5347(05)00326-5. [DOI] [PubMed] [Google Scholar]
- 18.van Kerrebroeck PE, van Voskuilen AC, Heesakkers JP, et al. Results of sacral neuromodulation therapy for urinary voiding dysfunction: outcomes of a prospective, worldwide clinical study. J Urol. 2007;178:2029–2034. doi: 10.1016/j.juro.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland SE, Lavers A, Carlson A, et al. Sacral nerve stimulation for voiding dysfunction: one institution’s 11-year experience. Neurourol Urodyn. 2007;26:19–28. doi: 10.1002/nau.20345. [DOI] [PubMed] [Google Scholar]
- 20.van Voskuilen AC, Oerlemans DJ, Weil EH, et al. Medium-term experience of sacral neuromodulation by tined lead implantation. BJU Int. 2007;99:107–110. doi: 10.1111/j.1464-410X.2006.06508.x. [DOI] [PubMed] [Google Scholar]
- 21.Amundsen CL, Romero AA, Jamison MG, et al. Sacral neuromodulation for intractable urge incontinence: are there factors associated with cure? Urology. 2005;66:746–750. doi: 10.1016/j.urology.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 22.Foster RT, Sr, Anoia EJ, Webster GD, Amundsen CL. In patients undergoing neuromodulation for intractable urge incontinence a reduction in 24-hr pad weight after the initial test stimulation best predicts long-term patient satisfaction. Neurourol Urodyn. 2007;26:213–217. doi: 10.1002/nau.20330. [DOI] [PubMed] [Google Scholar]
- 23.Weil EH, Ruiz-Cerdá JL, Eerdmans PH, et al. Clinical results of sacral neuromodulation for chronic voiding dysfunction using unilateral sacral foramen electrodes. World J Urol. 1998;16:313–321. doi: 10.1007/s003450050074. [DOI] [PubMed] [Google Scholar]
- 24.South MM, Romero AA, Jamison MG, et al. Detrusor overactivity does not predict outcome of sacral neuromodulation test stimulation. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:1395–1398. doi: 10.1007/s00192-007-0351-7. [DOI] [PubMed] [Google Scholar]
- 25.Hijaz A, Vasavada SP, Daneshgari F, et al. Complications and troubleshooting of two-stage sacral neuromodulation therapy: a single-institution experience. Urology. 2006;68:533–537. doi: 10.1016/j.urology.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Smith CP, Chancellor MB. Emerging role of botulinum toxin in the management of voiding dysfunction. J Urol. 2004;171:2128–2137. doi: 10.1097/01.ju.0000127725.48479.89. [DOI] [PubMed] [Google Scholar]
- 27.Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 28.Schurch B, Stöhrer M, Kramer G, et al. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: a new alternative to anticholinergic drugs? Preliminary results. J Urol. 2000;164:692–697. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 29.US National Institutes of Health. Current studies on BoNT-A. [Accessed May 4, 2010]. Available at: www.clinicaltrials. gov/ct2/results?term=BoNT-A.
- 30.Brubaker L, Richter HE, Visco A, et al. Refractory idiopathic urge urinary incontinence and botulinum A injection. J Urol. 2008;180:217–222. doi: 10.1016/j.juro.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahai A, Khan MS, Dasgupta P. Efficacy of botulinum toxin-A for treating idiopathic detrusor overactivity: results from a single center, randomized, double-blind, placebo controlled trial. J Urol. 2007;177:2231–2236. doi: 10.1016/j.juro.2007.01.130. [DOI] [PubMed] [Google Scholar]
- 32.Sahai A, Dowson C, Khan MS, Dasgupta P. Improvement in quality of life after botulinum toxin-A injections for idiopathic detrusor overactivity: results from a randomized double-blind placebo-controlled trial. BJU Int. 2009;103:1509–1515. doi: 10.1111/j.1464-410X.2009.08402.x. [DOI] [PubMed] [Google Scholar]
- 33.Flynn MK, Amundsen CL, Perevich M, et al. Outcome of a randomized, double-blind, placebo controlled trial of botulinum A toxin for refractory overactive bladder. J Urol. 2009;181:2608–2615. doi: 10.1016/j.juro.2009.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmid DM, Sauermann P, Werner M, et al. Experience with 100 cases treated with botulinum-A toxin injections in the detrusor muscle for idiopathic overactive bladder syndrome refractory to anticholinergics. J Urol. 2006;176:177–185. doi: 10.1016/S0022-5347(06)00590-8. [DOI] [PubMed] [Google Scholar]
- 35.White WM, Pickens RB, Doggweiler R, et al. Short-term efficacy of botulinum toxin a for refractory overactive bladder in the elderly population. J Urol. 2008;180:2522–2526. doi: 10.1016/j.juro.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Kuo HC. Clinical effects of suburothelial injection of botulinum A toxin on patients with nonneurogenic detrusor overactivity refractory to anticholinergics. Urology. 2005;66:94–98. doi: 10.1016/j.urology.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Kessler TM, Danuser H, Schumacher M, et al. Botulinum A toxin injections into the detrusor: an effective treatment in idiopathic and neurogenic detrusor overactivity? Neurourol Urodyn. 2005;24:231–236. doi: 10.1002/nau.20105. [DOI] [PubMed] [Google Scholar]
- 38.Popat R, Apostolidis A, Kalsi V, et al. A comparison between the response of patients with idiopathic detrusor overactivity and neurogenic detrusor overactivity to the first intradetrusor injection of botulinum-A toxin. J Urol. 2005;174:984–989. doi: 10.1097/01.ju.0000169480.43557.31. [DOI] [PubMed] [Google Scholar]
- 39.Rapp DE, Lucioni A, Katz EE, et al. Use of botulinum-A toxin for the treatment of refractory overactive bladder symptoms: an initial experience. Urology. 2004;63:1071–1075. doi: 10.1016/j.urology.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 40.Werner M, Schmid DM, Schüssler B. Efficacy of botulinum-A toxin in the treatment of detrusor overactivity incontinence: a prospective nonrandomized study. Am J Obstet Gynecol. 2005;192:1735–1740. doi: 10.1016/j.ajog.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 41.Khan S, Kessler TM, Apostolidis A, et al. What a patient with refractory idiopathic detrusor overactivity should know about botulinum neurotoxin type a injection. J Urol. 2009;181:1773–1778. doi: 10.1016/j.juro.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 42.Kessler TM, Khan S, Panicker J, et al. Clean intermittent self-catheterization after botulinum neurotoxin type A injections: short-term effect on quality of life. Obstet Gynecol. 2009;113:1046–1051. doi: 10.1097/AOG.0b013e3181a1f5ea. [DOI] [PubMed] [Google Scholar]
- 43.Reyblat P, Chan KG, Josephson DY, et al. Comparison of extraperitoneal and intraperitoneal augmentation enterocystoplasty for neurogenic bladder in spinal cord injury patients. World J Urol. 2009;27:63–68. doi: 10.1007/s00345-008-0351-3. [DOI] [PubMed] [Google Scholar]
- 44.Hasan ST, Marshall C, Robson WA, et al. Clinical outcome and quality of life following enterocystoplasty for idiopathic detrusor instability and neurogenic bladder dysfunction. Br J Urol. 1995;76:551–557. doi: 10.1111/j.1464-410x.1995.tb07777.x. [DOI] [PubMed] [Google Scholar]
- 45.Rackley RR, Abdelmalak JB. Laparoscopic augmentation cystoplasty. Surgical technique. Urol Clin North Am. 2001;28:663–670. doi: 10.1016/s0094-0143(05)70170-1. [DOI] [PubMed] [Google Scholar]
- 46.El-Feel A, Abdel-Hakim MA, Abouel-Fettouh H, Abdel-Hakim AM. Laparoscopic augmentation ileocystoplasty: results and outcome. Eur Urol. 2009;55:721–727. doi: 10.1016/j.eururo.2008.03.102. [DOI] [PubMed] [Google Scholar]