Abstract
Advanced eusociality sometimes is given credit for the ecological success of termites, ants, some wasps, and some bees. Comprehensive study of bees fossilized in Baltic amber has revealed an unsuspected middle Eocene (ca. 45 million years ago) diversity of eusocial bee lineages. Advanced eusociality arose once in the bees with significant post-Eocene losses in diversity, leaving today only two advanced eusocial tribes comprising less than 2% of the total bee diversity, a trend analogous to that of hominid evolution. This pattern of changing diversity contradicts notions concerning the role of eusociality for evolutionary success in insects.
Keywords: Apidae, Baltic amber, Hominidae, Paleogene
The origin of sterile-worker castes in insects has long been an arena for debate in evolutionary biology and was cited by Darwin (1) as one of the more troubling biological phenomena for his “species theory.” Eusociality, the category of societies characterized by, among other features, nonreproducing individuals (i.e., workers) that cooperate to raise the brood of another female (i.e., queen), is a trait that has appeared in numerous lineages from mammals (2) to shrimp (3) but is best known and developed within the insects. Some eusocial insect societies possess a further level of complexity through morphological specialization of the worker caste. Advanced eusocial societies, those societies with a morphologically differentiated worker caste, are among the most dominant and conspicuous groups of insects in the world and include the termites, ants, social wasps (vespids), and some bees. It is significant that these insects, at least the first two, are arguably the most ecologically dominant groups of their class (4–8). The ecological success and diversity of the termites (≈2,700 spp.), ants (≈16,000 spp.), and social wasps (≈1,000 spp.) have been attributed to their advanced eusocial behavior as it has been also for the advanced eusocial bees (6, 7). The advanced eusocial insects are highly efficient foragers; the division of labor allows task specialization, rapid recruitment to both floral and nonfloral resources, mobilization of colony defense, and support for large colony sizes (6).
The bees (with ≈20,000 spp.; ref. 8) as a whole are more diverse than the other advanced eusocial lineages. They are known for their dominant role as pollinators of flowering plants (9–11), thereby sustaining major ecological systems worldwide. The great majority of bees, however, are solitary. Advanced eusocial behavior is found in only a single group of floral generalist bees: the corbiculate Apinae, so named for the modification of the hind tibia into a corbicula or “pollen basket.” The corbiculate bees comprise four living tribes and include the most commonly recognized of all bees: the orchid bees (Euglossini), the bumble bees (Bombini), the stingless bees (Meliponini), and the honey bees (Apini). Of these four, only the stingless bees and honey bees exhibit advanced eusocial behavior. Although the bumble bees are eusocial also, the only morphological difference between queen and worker is size. The orchid bees are not eusocial, although some species are communal.
Monophyly of a clade consisting of the two advanced eusocial tribes has been debated in recent years on the basis of morphological and molecular evidence (12–14). Associated with the conflict over corbiculate bee relationships have been various estimates for the number of times eusociality has arisen. The corbiculate bees are a derived clade of the family Apidae, itself the most derived group of the seven families of bees (15, 16). Based on the derived position of the corbiculate apines among other bees, some authors have suggested that complex social behavior in bees is a rather recent trait, having its origins and rapid diversification merely 30–40 million years ago (17, 18). A paleontological perspective is critical not only for understanding the past diversity of life on Earth, episodes of extinction and radiation, the origin of evolutionary novelties (i.e., synapomorphies), and paleoecologies but also for the reconstruction of hierarchical relationships among taxa (19–22). Thus, a study of the geological diversity of social bees potentially can provide insights into the evolution of this group of bees as well as resolve the contention over their phylogeny.
The Baltic-amber fauna, recently dated approximately 45 million years in age (middle Eocene), is the earliest diverse fossil record of bees. Previously believed to consist of a few enigmatic taxa (23), Baltic amber actually harbors a remarkable array of taxa representing most higher clades of the Apoidea. Indeed, bee fossils are rare in amber with one specimen appearing for approximately every 5,000 inclusions examined; however, I was able to find 152 specimens representing 38 extinct species and include them in cladistic analyses with living families, tribes, and genera of corbiculate and noncorbiculate bees (16). In addition, fossil bees from all other deposits and ages were compared with the Baltic amber as well as Recent faunas to gain an insight into changes of the fauna and diversity through time. Compression fossils provide additional data on ages and locations, but preservation in amber has unique fidelity. These fossils are therefore more meaningful, because bees are typically identifiable on the basis of minute structures of the mouthparts, hairs, or leg spurs, characters that are frequently not preserved in compression fossils. Moreover, amber preserves internal skeletal features as well as tissues (24), making it possible to compare some internal characteristics of fossil and Recent bees. One result of this paleontological work was the discovery of a hitherto unexpected diversity of Eocene corbiculate bees. Although corbiculate bee fossils are known also from a variety of other deposits (e.g., refs. 25–27), their numbers in terms of species are dramatically less than those from Baltic amber. These amber fossils reveal interesting patterns of the overall temporal changes in the composition of bee faunas as well as compelling evidence for the monophyly of the advanced eusocial bee tribes and implications for their diversification. More recent corbiculate bee taxa (i.e., Oligocene or younger) preserved as either amber inclusions or compression fossils group cladistically not only within the four living tribes, but some are within living genera (e.g., refs. 25 and 27).
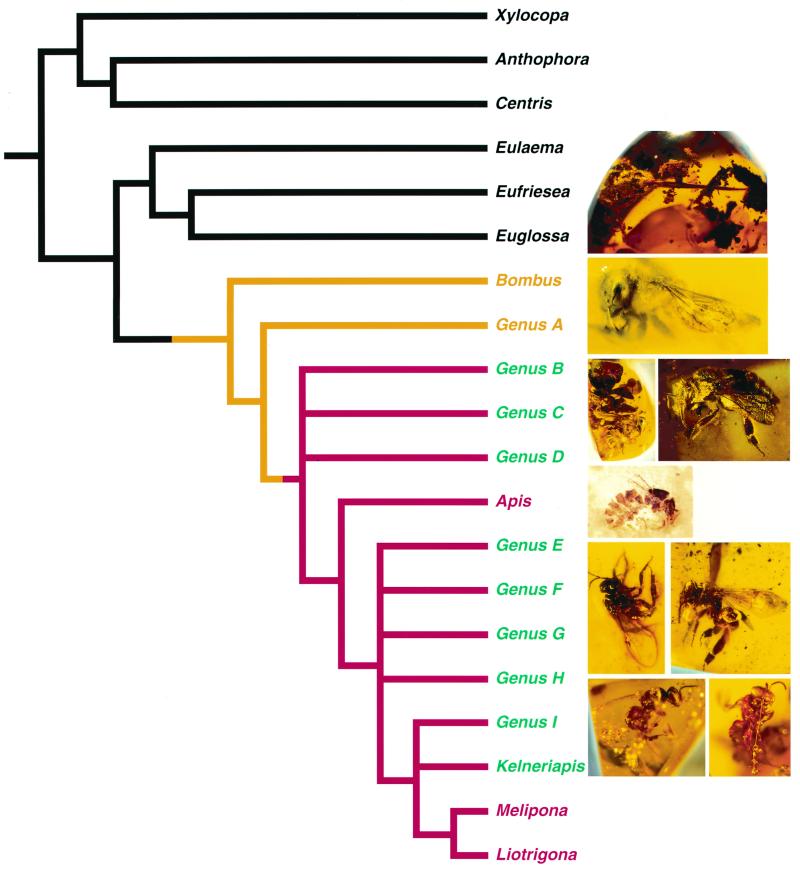
Cladistic analyses of Recent and fossil corbiculate bee taxa by using 51 characters of both external and internal morphology (tree length, 65; consistency index, 81; retention index, 93) (Fig. 1) indicate the presence in the Eocene of a previously unknown diversity of extinct corbiculate clades. The data matrix of characters and states on which the cladogram is based is available (16), and specimens are deposited in public institutions. Nearly all of these fossil lineages are represented by individuals exhibiting the reduced metasoma and to a lesser degree the barbed sting found only in the worker caste of advanced eusocial species, especially well developed in the Meliponini for the former character and Apini for the latter. Thus, although the fossils were coded and analyzed as unknown for their social-character state, all except one (Genus A in Fig. 1) apparently were advanced eusocial forms. The facts that these extinct taxa group cladistically with the honey bees and stingless bees on the basis of all other traits, and the primitively eusocial bumble bees form their sister group (Fig. 1), further support the interpretation that the fossils also were members of advanced eusocial societies (28). These fossils therefore reinforce the phylogenetic relationships of both monophyletic general-eusocial and advanced-eusocial clades (Fig. 1). Advanced eusociality thus originated only once within the bees, presumably sometime in the Late Cretaceous. The origin of eusociality is fairly ancient in the bees because an advanced eusocial corbiculate bee (a meliponine) is known from latest Cretaceous deposits, presumably Maastrichtian (11), from North America (26).
Figure 1.
Phylogeny of the corbiculate bees (Apinae) as well as representative fossils from a variety of Tertiary deposits based on analysis of 51 adult morphological characters of external and internal skeletal features. Topology presented is a strict consensus of two most parsimonious trees: tree length, 65; consistency index, 81; and retention index, 93 (16). From top to bottom (and left to right), the fossils are Euglossa moronei Engel, Genus A, Genus B, Genus D, Apis henshawi Cockerell, Genus E, Genus G, Genus I, and Nogueirapis silacea (Wille). Eocene fossil taxa on the cladogram include genera A–I and the meliponine Kelneriapis. Black lines indicate noneusocial (solitary or communal) species; yellow indicates primitively eusocial lineages (i.e., lacking a morphologically specialized worker caste); red indicates advanced eusocial lineages. Fossils represented by individuals that are morphologically workers are indicated in green. Most fossil genera are not monotypic (e.g., Genus B has five species, Genus C has four species) (16).
Another significant aspect of the diversity of Eocene advanced eusocial bees is their extinction, not the simple loss of a few species but the apparent loss of several higher clades of advanced eusocial bees (Fig. 1). The success and diversification of the advanced eusocial insects frequently is attributed to their sociality (6); a general overview of the modern diversity of the ants, termites, and wasps would seem to support this notion. The advanced eusocial bees, however, comprise ≈380 spp., less than 2% of the total diversity of bees worldwide (8). This fact, plus the status of advanced eusocial bees as seeming to be derived phylogenetically recently (15), suggests that on the basis of the extant fauna alone, bee eusociality is in nascent stages of evolution. Indeed, advanced eusociality in bees would seem to have had the same general effect that it has had in the ants, termites, and wasps; that is, social cooperation has led to ecological dominance. Honey bees (Apis), in particular, are aggressive foragers and sometimes outcompete other native-bee species foraging for the same floral resources (29–33). In areas that the western honey bee, Apis mellifera, has been introduced, the diversity of native bees has become diminished somewhat, with some species competitively excluded, including other advanced eusocial lineages (29, 30, 32, 34–36). In fact, local extinction of stingless-bee colonies was one of the predicted likely outcomes of the competitive interactions between introduced Apis and native Meliponini (36). Apis mellifera introduced into Australia has been shown to have a negative impact not only on other bee species but also on some nectar-feeding bird populations (37). Interestingly, in the most ecologically dominant and aggressive advanced-eusocial lineage (i.e., the honey bees, genus Apis), there are only seven extant species (38). The generally less aggressive stingless bees are more diverse, and their maximal diversity occurs in areas where Apis species are not native (i.e., South and Central America; refs. 8 and 39).
An epoch-by-epoch overview of the geological history of the corbiculate bees including all known amber and compression fossils throughout the world shows a marked decrease in diversity of clades between the Eocene and more recent epochs (16). Today there are only two tribal-level clades of advanced eusocial bees. In the Eocene, there were at least three in central Europe alone, and the cladogram suggests that the lineage that eventually gave rise to the Apini in the Oligocene was present as well (Fig. 1). There was at least a 50% loss of suprageneric clades among the advanced eusocial bees. At infratribal levels, a 30–33% loss of diversity can be documented between the Eocene and subsequent faunas. This loss is borne out not only by the Eocene Baltic-amber fauna but also by Eocene compression fossils of similar taxa in other deposits (e.g., ref. 40) and unknown in post-Eocene sediments. Beginning in the Oligocene, compression and amber fossil bees are distinctly modern in appearance (16, 23, 25, 27). Amber potentially can give a biased picture of a particular fauna by capturing primarily those taxa that collect resin for nest construction. The picture of corbiculate bee Eocene diversity in the Baltic area is likely quite accurate because living corbiculate bees collect resins and are therefore more likely to be preserved in amber than are bees of other lineages (e.g., sweat bees, digger bees). Thus, considering the corbiculate bees alone, if any bias is indeed present, then it is oversampling the corbiculate bee fauna. The observed diversity can be only an underestimate of the actual diversity, thereby making these estimates of changes in the corbiculate bee fauna probably conservative.
It seems that advanced eusociality has not fueled post-Eocene diversification of the corbiculate bees. In fact, it is possible that over geological expanses of time, aggressively foraging advanced-eusocial species had a negative impact not only on nonsocial species but also and especially on other advanced eusocial lineages. A similar situation is reported from another highly competitive, social species (albeit not eusocial) for which the fossil record indicates aggressive exclusion of related social genera and species that may have caused their extinction—namely Homo sapiens (41). During the Pleistocene, a diversity of related genera and now extinct species of Homo existed but perhaps were competitively excluded by a single aggressive species that is the only survivor of a prior hominid radiation (41, 42). As for the human example as well, competition perhaps is not the sole explanation for the declining diversity of advanced eusocial bees. Clearly the global climate during the Eocene was warmer than that of today (e.g., tropical-like bee taxa in the Baltic region). Such conditions may have been favorable for the proliferation of these kinds of bees, although some advanced eusocial bees do occur in cold-temperate habitats today. The interaction of such factors across geological time may have led to their decline.
Eusociality also seems not to have been correlated directly with species diversity and abundance in the ants and termites. Both of these groups originated in the Cretaceous, during which time they remained rare and primitive while also being advanced eusocial (43, 44). The fact that ants and termites became abundant and diverse 50 million years or more after their origins indicates that, as for the bees, eusociality alone cannot account for their current success.
The pervasive factor in the success of the bees as a whole probably has been their mutualistic association with flowering plants, a relationship cultivated since the early mid-Cretaceous (8–11, 16, 26). Advanced eusocial behavior is not in its nascent stages in the bees but presently is a rudiment of what it once was and seems to have been unimportant in the greater diversification of bees. Eusociality in this context, despite its ecological importance and the evolutionary interest associated with it, has done little to inspire the diversity of one of its most highly celebrated examples—the bees.
Acknowledgments
I thank David A. Grimaldi, Kumar Krishna, Valerie Krishna, L. Krishtalka, Charles D. Michener, Molly G. Rightmyer, Jerome G. Rozen, Jr., and Thomas N. Taylor for encouragement and David A. Grimaldi, Charles D. Michener, Molly G. Rightmyer, Jerome G. Rozen, Jr., and four anonymous reviewers for reading earlier versions of the text. Steve Thurston kindly prepared the color cladogram. Support for this research was provided generously by Robert G. Goelet.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041600198.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041600198
References
- 1.Darwin C R. On the Origin of Species by Means of Natural Selection or the Preservation of Favored Races in the Struggle for Life. London: John Murray; 1859. [Google Scholar]
- 2.Sherman P W, Jarvis J U M, Alexander R D. The Biology of the Naked Mole Rat. Princeton: Princeton Univ. Press; 1991. [Google Scholar]
- 3.Duffy J E. Nature (London) 1996;381:512–514. [Google Scholar]
- 4.Krishna K, Weesner F M. Biology of Termites. Vol. 1. New York: Academic; 1969. [Google Scholar]
- 5.Krishna K, Weesner F M. Biology of Termites. Vol. 2. New York: Academic; 1970. [Google Scholar]
- 6.Wilson E O. The Insect Societies. Cambridge, U.K.: Belknap; 1971. [Google Scholar]
- 7.Hölldobler B, Wilson E O. The Ants. Cambridge, U.K.: Belknap; 1990. [Google Scholar]
- 8.Michener C D. The Bees of the World. Baltimore: Johns Hopkins Univ. Press; 2000. [Google Scholar]
- 9.Barth F G. Insects and Flowers: The Biology of a Partnership. Princeton: Princeton Univ. Press; 1985. [Google Scholar]
- 10.O'Toole C, Raw A. Bees of the World. London: Blandford; 1991. [Google Scholar]
- 11.Grimaldi D. Ann Mo Bot Gard. 1999;86:373–406. [Google Scholar]
- 12.Schultz T R, Engel M S, Prentice M. Univ Kans Nat Hist Mus Spec. 1999;24:125–138. [Google Scholar]
- 13.Mardulyn P, Cameron S A. Mol Phylogenet Evol. 1999;12:168–176. doi: 10.1006/mpev.1998.0606. [DOI] [PubMed] [Google Scholar]
- 14.Koulianos S, Schmid-Hempel R, Roubik D W, Schmid-Hempel P. J Evol Biol. 1999;12:380–384. [Google Scholar]
- 15.Roig-Alsina A, Michener C D. Univ Kans Sci Bull. 1993;55:123–162. [Google Scholar]
- 16.Engel, M. S. (2001) Bull. Am. Mus. Nat. Hist., in press.
- 17.Seeley T D. Honeybee Ecology. Princeton: Princeton Univ. Press; 1985. [Google Scholar]
- 18.Winston M. The Biology of the Honey Bee. Cambridge, MA: Harvard Univ. Press; 1987. [Google Scholar]
- 19.Donoghue M J, Doyle J A, Gauthier J, Kluge A G, Rowe T. Annu Rev Ecol Syst. 1989;20:431–460. [Google Scholar]
- 20.Gauthier J, Kluge A G, Rowe T. Cladistics. 1989;4:105–209. doi: 10.1111/j.1096-0031.1988.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 21.Novacek M J. In: Extinction and Phylogeny. Novacek M J, Wheeler Q D, editors. New York: Columbia Univ. Press; 1992. pp. 46–88. [Google Scholar]
- 22.Grimaldi D, Cumming J. Bull Am Mus Nat Hist. 1999;239:1–124. [Google Scholar]
- 23.Zeuner F E, Manning F J. Bull Br Mus Nat Hist Geol. 1976;27:149–268. [Google Scholar]
- 24.Grimaldi D, Bonwich E, Delannoy M, Doberstein S. Am Mus Novit. 1994;3097:1–13. [Google Scholar]
- 25.Engel M S. Am Mus Novit. 1999;3272:1–14. [Google Scholar]
- 26.Engel M S. Am Mus Novit. 2000;3296:1–11. [Google Scholar]
- 27.Camargo J M F, Grimaldi D A, Pedro S R M. Am Mus Novit. 2000;3293:1–24. [Google Scholar]
- 28.Schultz T R, Cocroft R B, Churchill G A. Evolution (Lawrence, Kans) 1996;50:504–511. doi: 10.1111/j.1558-5646.1996.tb03863.x. [DOI] [PubMed] [Google Scholar]
- 29.Roubik D W. Science. 1978;201:1030–1032. doi: 10.1126/science.201.4360.1030. [DOI] [PubMed] [Google Scholar]
- 30.Roubik D W. Ecology. 1983;64:971–978. [Google Scholar]
- 31.Eickwort G C, Ginsberg H S. Annu Rev Entomol. 1980;25:421–446. [Google Scholar]
- 32.Buchmann S L. In: The Conservation of Bees. Matheson A, Buchmann S L, O'Toole C, Westrich P, Williams I H, editors. New York: Academic; 1996. pp. 125–142. [Google Scholar]
- 33.Sugden E A, Thorp R W, Buchmann S L. Bee World. 1996;77:26–44. [Google Scholar]
- 34.Gross C L, MacKay D. Biol Conserv. 1998;86:169–178. [Google Scholar]
- 35.Sakagami S F. J Anim Ecol. 1959;28:51–68. [Google Scholar]
- 36.Roubik D W, Moreno J E, Vergara C, Wittmann D. J Trop Ecol. 1986;2:97–111. [Google Scholar]
- 37.Paton D C. Bioscience. 1993;43:95–103. [Google Scholar]
- 38.Engel M S. J Hymenoptera Res. 1999;8:165–196. [Google Scholar]
- 39.Michener C D. Univ Kans Sci Bull. 1990;54:75–164. [Google Scholar]
- 40.Lutz H. Mainzer Naturwiss Arch. 1993;31:177–199. [Google Scholar]
- 41.Tattersall I. Sci Am. 2000;282:56–62. [Google Scholar]
- 42.Tattersall I, Schwartz J H. Extinct Humans. New York: Westview; 2000. [Google Scholar]
- 43.Thorne B L, Grimaldi D, Krishna K. In: Termites. Evolution, Sociality, Symbiosis, and Ecology. Abe T, Bignell D, Higashi M, editors. Dordrecht, The Netherlands: Kluwer; 2000. pp. 45–60. [Google Scholar]
- 44.Grimaldi D, Agosti D. Proc Natl Acad Sci USA. 2000;97:13678–13683. doi: 10.1073/pnas.240452097. . (First Published November 14, 2000; 10.1073/pnas.240452097) [DOI] [PMC free article] [PubMed] [Google Scholar]