Abstract
Purpose
This study compared the total antioxidant status (TAS) and superoxide dismutase (SOD) activity in the saliva of periodontally compromised patients before and after scaling and root planing (SRP) to assess their diagnostic utility.
Methods
Severe chronic periodontitis patient (test group) and subjects with no attachment loss, sites showing a 3 mm or more probing depth and a sulcus bleeding index < 10% (control group) were enrolled in this study. Saliva sampling and clinical examination were performed at one week, one month and 3 months after SRP. The TAS and SOD activity in each patient's saliva was measured for the comparative analysis between the groups.
Results
In the test group, the TAS decreased directly after SRP. With time, it increased slightly and was relatively unchanged compared to the baseline. In the control group, the TAS also decreased immediately after SRP but increased gradually with time until 3 months. The SOD activity in the test and control subjects decreased immediately after SRP until 1 month. At 3 months, the SOD activity had increased. Both groups had a similar profile of SOD activity. However, the SOD activity of the control group was significantly higher than that of the test group at each point in time (P < 0.05).
Conclusions
There was a significant difference in the total salivary antioxidant level between the periodontitis and healthy or gingivitis (control) group during the experiment period. The total antioxidant level in the saliva was higher in the patients with severe chronic periodontitis than the healthy or gingivitis control before SRP. The SOD activity of the periodontitis patients was lower than the control at each time point. These findings conclusively reveal the possible use of saliva as a diagnostic tool for periodontal health.
Keywords: Antioxidants, Periodontal disease, Saliva
INTRODUCTION
Periodontal disease can be caused by immune reactions between pathogenic bacteria and the host. Specific bacteria induce the release of cytokines, such as interleukin-6 and tumor necrosis factor-α, which increase the number of polymorphonuclear leukocytes and their activation [1]. Reactive oxygen species (ROS), such as superoxide anion, hydroxyl radical, nitrous oxide and hydrogen peroxide, can be produced via the bacteria-host mediated pathway inducing tissue injury. At the same time, polymorphonuclear leukocytes produce and increase the concentration of ROS, resulting in oxidative damage to the periodontal tissue [2]. These destructive mechanisms include DNA damage, lipid peroxidation, protein damage, and the oxidation of enzymes [3,4].
The host has the ability to eliminate ROS and inhibit the tissue destruction caused by inflammatory reactions by releasing defensive substances (e.g. antioxidants) in the humor and tissues.
Antioxidants include superoxide dismutase (SOD), uric acid, ascorbic acid, α-tocopherol, glutathione and albumin. One important antioxidant, SOD catalyzes the dismutation of the superoxide anion, defending the cells against the hazardous effects of ROS [5]. There are few reports on the relationship between SOD and inflammation in oral tissues, and the results that have been reported are controversial. SOD activity increases remarkably in irreversible pulpitis while it decreases significantly in the gingival tissue close to the deep periodontal pocket [6-8].
Saliva contains water, proteins, electrolytes, gingival sulcular fluid, serum, cells, bacteria, food residue, and organic molecules. It includes a range of antioxidants and is affected by inflammatory reactions. A weakened antioxidative defense system in the gingival crevicular fluid (GCF) accelerates the effects of periodontal disease [9,10]. Previous research focused on the immune mechanisms of the secretory immunoglobulin A and protein-enzymatic defense system [11]. Recently, research into various enzymatic salivary systems as antioxidants has clarified the importance of other defense systems in the saliva [10,12,13]. Chapple et al. [9] reported that patients with periodontal diseases had a low total antioxidant status (TAS). Liskmann et al. [12] reported that the TAS was lower in the peri-implant disease group than healthy group. On the other hand, Moore et al. [14] reported no significant difference in the TAS level in the saliva between patients with periodontal disease and a normal group. There have been no published reports on the relationship between periodontal disease and the antioxidants in saliva, or on the potential use of saliva as a diagnostic tool.
This study compared the TAS and SOD activity in the saliva of periodontally diseased patients before and after scaling and root planing (SRP) to determine its diagnostic utility.
MATERIALS AND METHODS
Study population
The test group included 14 patients diagnosed with severe chronic periodontitis in the Department of Periodontology, Chonnam National University Hospital from May to October in 2008. The patients diagnosed with severe chronic periodontitis had more than two areas with a 5 mm or more probing depth (PD) in the quadrant and bone loss > 30% of the root length on the radiographs. In a control group, 12 patients who did not have attachment loss or sites showing a PD of 3 mm or more with a sulcus bleeding index (SBI) < 10% were enrolled. Both the control and test groups had more than 20 functional teeth.
The exclusion criteria were as follows: people with other medical conditions that could affect the results, people who had taken anti-inflammatory drugs or antibiotics within the previous 3 months, pregnant women, people who use mouth-washes regularly, and people who had taken vitamins in the previous 3 months.
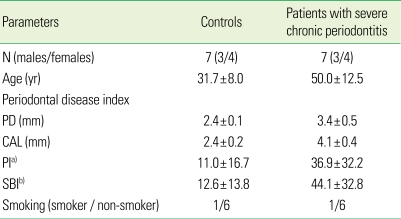
There were some drop-outs during the study resulting in a final test group and control group consisting of 7 subjects (3 males, 4 females; average age, 50.5 years [range, 31 to 66 years]) and 7 subjects (3 males, 4 females; average age, 31.7 years [range, 26 to 49 years]), respectively. All subjects in the test group were diagnosed with severe chronic periodontitis and there was one smoker. Their periodontal condition was good and there was only one smoker (Table 1). This study was approved by the Chonnam National University Hospital Institutional Review Board (IRB No. 1-2008-04-046), and a consent agreement was obtained from all the participants.
Table 1.
Clinical data at the baseline (mean ± SD).
PD: probing depth, CAL: clinical attachment level, PI: plaque index, SBI: sulcus bleeding index.
a)Sum of PI, b)Sum of SBI.
Methods
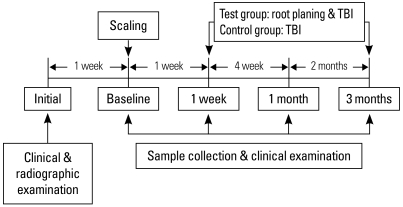
At the initial examination, the test and control group members were selected after a clinical and radiographic examination. After 1 week, saliva sampling, clinical examination, as well as SRP, were performed in each group. One week, one month and 3 months later saliva sampling and clinical examination were again performed.
The test group took tooth-brushing instruction (TBI) 1 week after scaling, and root planing was carried out for each quadrant under local anesthesia with a 1 week interval. In the control group, SRP was performed and TBI only was carried out after 1 week (Fig. 1).
Figure 1.
Experimental protocol scheme. TBI: tooth-brushing instruction.
Clinical examination
The clinical examinations were performed after taking the saliva samples. All procedures were carried out by a calibrated examiner. The clinical examinations were performed before treatment, and 1 week, 1 month and 3 months after SRP.
1) Plaque index
The plaque in the gingival margin was evaluated using the plaque index (PI) reported by Silness and Löe. The PIs were recorded on four tooth surfaces (mesial, distal, buccal, and lingual). The scores for the PI were:
0: no plaque in the gingival area.
1: a film of plaque adhering to the free gingival margin and adjacent area of the tooth. The plaque could be recognized only by running a probe across the tooth surface.
2: moderate accumulation of soft deposits within the gingival pocket and on the gingival margin and/or adjacent tooth surface that could be seen by the naked eye.
3: abundance of soft matter within the gingival pocket and/or on the gingival margin and adjacent tooth surface.
2) Sulcus bleeding index
The level of gingival inflammation was evaluated using the SBI reported by Mühlemann and Son. The SBI was recorded on six tooth surfaces (mesio-buccal, buccal, disto-buccal, mesio-longual/palatal, lingual/palatal, and disto-lingual/palatal. The scores for the SBI were:
0: no bleeding.
1: bleeding on probing with no change in color and no swelling.
2: bleeding on probing with a change in color and no swelling or macroscopic edema.
3: bleeding on probing with a change in color and edematous swelling.
4: bleeding on probing with a change in color due to inflammation, edematous swelling with ulceration.
5: spontaneous bleeding, changes in color and marked swelling with ulceration.
3) Probing depth and clinical attachment level
The PD was defined as the distance from the free gingival margin to the pocket base. The clinical attachment level (CAL) was defined as the sum of the distance from the cementenamel junction to the free gingival margin and pocket depth. All measurements were taken using a Williams probe (23 W, Hu-Friedy, Chicago, IL, USA) with 0.5 mm units. The measurements were recorded on six surfaces of each tooth (mesio-buccal, buccal, disto-buccal, mesio-longual/palatal, lingual/palatal, and disto-lingual/palatal). The average measurements of all surfaces were taken.
Saliva sampling and evaluation of TAS
All the saliva samples were taken in the morning after an overnight fast. Before taking the samples, the subjects were asked not to drink any beverage except for water. Saliva samples were taken before treatment, and 1 week, 1 month and 3 months after treatment.
1) Saliva sampling
In order to take the stimulated saliva samples, the subjects were asked to chew a gum base for 1 minute to stimulate the saliva glands and spit into a cooled 50 mL disposable tube with a lowered head position. More than 5 mL of stimulated saliva was collected. The tubes were combined with 50 µL of Complete™ protease inhibitor cocktail (Rosche, Manheim, Germany) and centrifuged at 2,000 g for 15 minutes. After centrifugation, the supernatant solution was separated and placed in a 1 mL tube. The tubes were stored at -20℃. The samples were analyzed within 4 weeks.
2) Analysis of TAS
The TAS was analyzed using an ImAnOx (TAS) Kit (Immundiagnostik AG, Bensheim, Germany) according to the manufacturer's instructions, and measured at 450 nm using an ELISA reader (Model-680®, Bio-Rad, Osaka, Japan). The TAS was determined from the manufacturer's formula using the values from ELISA. The units of measurement were µM.
3) Analysis of SOD activity
The SOD activity was evaluated using an SOD Assay Kit - WST (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instruction, and an ELISA reader (Model-680®) at 450 nm. The SOD activity was obtained from the manufacturer's formula using the values from ELISA. The unit of measurement was %.
Statistical analysis
All measurements were recorded as the mean ± standard deviation. Statistical analysis was performed using SPSS (SPSS Inc., Chicago, IL, USA). Two-way ANOVA was performed to compare the TAS and SOD activity of the control and test group according to the elapsed postoperative time. P < 0.05 was considered significant.
RESULTS
Changes in clinical indices
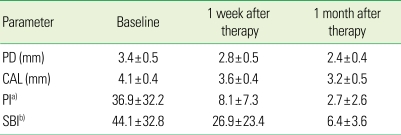
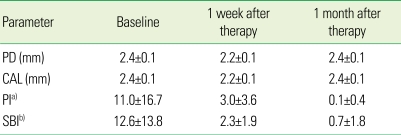
The mean PD in the test group was 3.4, 2.8, and 2.4 mm at the baseline, 1 week and 1 month after treatment, respectively, showing a decrease with time. The PD in the control group was 2.4, 2.2, and 2.4 mm at the baseline, 1 week, and 1 month after treatment, respectively. The PD in the control group decreased slightly 1 week after treatment but returned to the baseline at 1 month after treatment. The mean CAL in the test group was 4.1 mm, 3.6 mm, and 3.2 mm at baseline, 1 week and 1 month after treatment, respectively, whereas the mean CAL in the control group was 2.4 mm, 2.2 mm, and 2.4 mm, respectively. The average sum of the PI in the test group was 36.9, 8.1, 2.7 at the baseline, 1 week, and 1 month after treatment, respectively, showing a significant decrease, whereas the average sum of the PI in the control group was 11.0, 3.0, and 0.1, respectively. The mean SBI in the test group was 44.1, 26.9, and 6.4 at the baseline, 1 week, and 1 month after treatment, respectively, whereas that in the control group was 12.6, 2.3, and 0.7, respectively (Tables 2 and 3). Both groups showed a decrease but the test group showed a much larger reduction.
Table 2.
Changes in clinical parameters (mean ± SD) of patients with severe chronic periotontitis (n = 7) after a periodontal treatment.
PD: probing depth, CAL: clinical attachment level, PI: plaque index, SBI: sulcus bleeding index.
a)Sum of PI, b)Sum of SBI.
Table 3.
Changes in clinical parameters (mean ± SD) of the controls (n = 7) after periodontal treatment.
PD: probing depth, CAL: clinical attachment level, PI: plaque index, SBI: sulcus bleeding index.
a)Sum of PI, b)Sum of SBI.
Changes in TAS
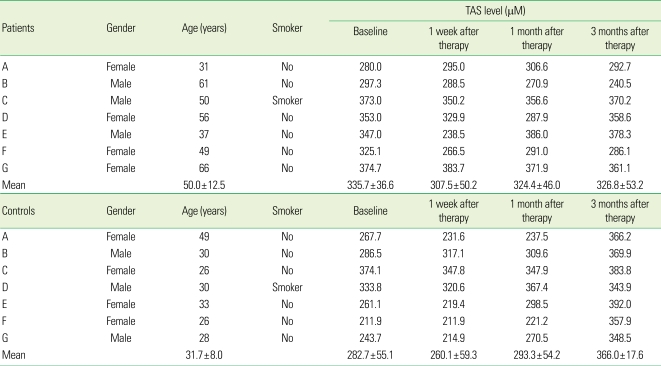
The mean TAS in the test group was 335.7 ± 33.6 µM, 307.5 ± 50.2 µM, 324.4 ± 46.0 µM and 326.8 ± 53.2 µM at the baseline, 1 week, 1 month, and 3 months after treatment, respectively. On the other hand, the mean TAS in the control group at the same time points was 282.7 ± 55.1 µM, 260.1 ± 59.3 µM, 293.3 ± 54.2 µM, and 366.0 ± 17.6 µM, respectively (Table 4).
Table 4.
TAS in patients with severe chronic periodontitis and controls.
TAS: total antioxidant status.
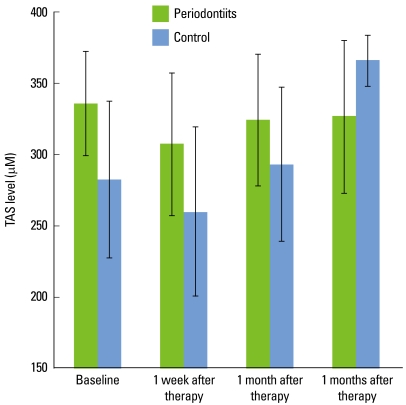
TAS of the test group decreased immediately after scaling and remained relatively constant with time. TAS in the control group decreased immediately after scaling but increased gradually with time (Fig. 2).
Figure 2.
Total antioxidant status (TAS) in severe chronic periodontitis patient group and control subject group. In the test group, the TAS decreased immediately after scaling and root planing (SRP). With time, there was little change. In the control group, the TAS was also decreased immediately after SRP but increased gradually thereafter. The TAS level of the control group increased 3 months after SRP. Error bars show standard deviation.
Changes in SOD activity
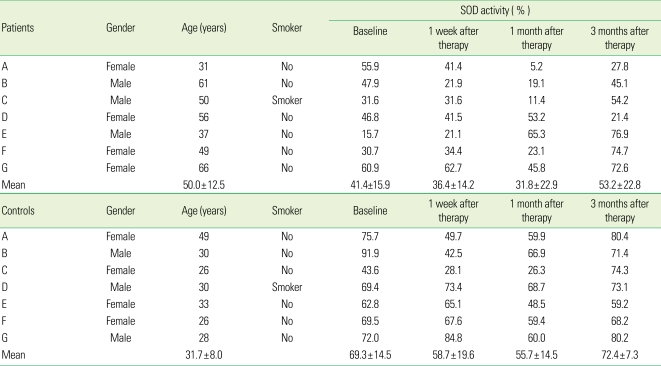
The mean SOD activity of the test group was 41.4 ± 15.9%, 36.4 ± 14.2%, 31.8 ± 22.9% and 53.2 ± 22.8% at the baseline, 1 week, 1 month and 3 months after treatment, respectively. The corresponding values in the control group were 69.3 ± 14.5%, 58.7 ± 19.6%, 55.7 ± 14.5%, and 72.4 ± 7.3%, respectively (Table 5).
Table 5.
SOD activity in the patients with severe chronic periodontitis and controls.
SOD: superoxide dismutase.
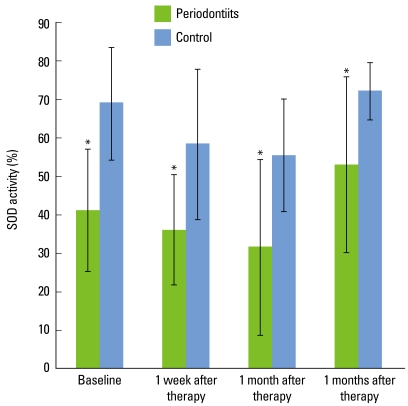
SOD activity in the severe chronic periodontitis patients and controls decreased immediately after SRP until 1 month but had increased again at 3 months. Both groups showed a similar SOD activity profile, but the SOD activity of the control group was higher than that of the test group at each time point (P < 0.05) (Fig. 3).
Figure 3.
Superoxide dismutase (SOD) activities in severe chronic periodontitis patient group and control subject group. The SOD activity in both groups decreased immediately after scaling and root planing until 1 month, and increased at 3 months. The SOD activity of the control group was higher than that of the test group at each time point. Error bars show standard deviation (*P < 0.05).
DISCUSSION
Recently, there has been increasing interest in ROS, lipid peroxidation products, and antioxidant system in the etiology of periodontitis. Several studies in the periodontal field have examined saliva, GCF, and antioxidant conditions in the serum of periodontal patients.
Antioxidant agents can be classified into three groups according to their function. First, preventive anti-oxidants inhibit the formation of free radicals, including SOD, carotenoids, catalase, glutathione peroxidase, transferrin, albumin, and haptoglobin. Secondly, radical-scavenging anti-oxidants, such as vitamin A, vitamin E, uric acid, ascorbic acid, albumin, and bilirubin, eliminate free radicals to inhibit the initiation and propagation of free radical damage. Subsequently, DNA repair enzymes, protease, transferase, lipase, and repair and de novo enzymes heal injuries and reorganize the structures of the membrane [15].
Among the many intrinsic antioxidants, SOD is a primary defending substance that protects cells from ROS by converting O2- to H2O2. The SOD variants found in the human body can be divided into copper/zinc-SOD (Cu/Zn-SOD, SOD 1), manganese SOD (Mn-SOD, SOD 2) and extracellular SOD (EC-SOD, SOD 3) according to the essential metal elements for their catalytic function [13,15]. Many studies have examined SOD and periodontitis. Na et al. [13] reported the expression of the SOD isoform associated with inflammatory gingival tissue. They carried out histological analysis on 33 chronic periodontitis patients and 20 periodontally healthy subjects. The results indicated that the increase in SOD activity in the inflammatory periodontal tissue is associated with oxidative stimulation in periodontitis patients [13].
Recently, there have been reports on the relationship between the TAS and periodontal diseases associated with the saliva, GCF, serum, and gingival tissue. Sculley and Langley-Evans [16] reported on the antioxidants in the saliva associated with periodontal conditions. They examined the antioxidant conditions in the saliva associated with an increase in injury due to oxidation and reported that gingivitis and periodontitis are associated with a decreased antioxidant level in the saliva and increased oxidative injury. They also reported that low concentrations of antioxidants in GCF increase the neutrophil-induced injuries in the gingiva and adjacent structures. Brock et al. [17] measured the local and systemic total concentrations of antioxidants in patients with periodontitis and periodontally healthy subjects. They concluded that the concentration of antioxidants in the GCF of patients with periodontitis is significantly lower than those in the control group. Cutando et al. [18] examined the relationship between the severity of periodontitis and melatonin, which is one of the antioxidants in saliva. They reported that the level of melatonin in the saliva decreased with increasing severity of periodontitis. Pussinen et al. [19] studied the relationship between periodontitis and the concentration of vitamin C in serum. They reported that patients with periodontitis, who were infected by Porphyromonas gingivalis, showed a lower vitamin C level in the serum than those not infected.
Meanwhile, Moore et al. [14] measured the amount of total antioxidant in the saliva of both periodontitis patients and periodontally healthy subjects, and reported no significant differences between the two groups. Similarly, Chapple et al. [9] evaluated the serum and saliva samples of a diseased group and control group, and found a similar serum antioxidant level in both groups. Tsai et al. [20] reported that patients diagnosed with chronic periodontitis showed high levels of lipid peroxidation in the GCF, which indicates the destruction of periodontal tissue by increased ROS. Canakci et al. [21] examined the TAS and antioxidants in the serum, saliva, and GCF of women with periodontitis and preeclampsia. They reported that the SOD level and the glutathione peroxidase activity in the GCF and serum, and TAS in the saliva, GCF, and serum of female patients with preeclampsia and periodontitis were lower than those of the control group. However, they showed high concentrations of malondialdehyde in the GCF and serum. Ozmeric et al. [22] studied the level of arginase, one of the antioxidants in the saliva in periodontitis patients. They reported that periodontitis patients showed high arginase activity of glutathione peroxidase in the saliva.
In this study, although the changes in the TAS level and SOD activity were different among individuals, the measurements in both groups decreased immediately after non-surgical treatment. The TAS level was higher in the test group than in the control group at the baseline. The TAS level of the control group increased 3 months after SRP, but the level in the severe chronic periodontitis patients had not changed after 3 months. The SOD activity in both groups showed a similar profile, a decrease until one month after SRP and an increase after 3 months. The SOD activity of the control group was higher than that of the severe chronic periodontitis patients at each time point.
In this study, before SRP, the TAS level in the test group was higher than that in the control group. These results are in contrast to those reported by Sculley and Langley-Evans [16]. Sculley and Langely-Evans [16] reported that gingivitis and periodontitis are associated with a decreased antioxidant level in the saliva and increased oxidative injury. It is possible that the TAS level in the saliva under chronic periodontitis is adapted to this pathological condition and is higher than under healthy conditions, and the test group was older than the control group. Therefore, when periodontitis is in the active state, the immune defense mechanism is activated and the ROS released from the polymorphonuclear leukocytes increases, resulting in the release of antioxidants in the body with a concomitant increase in the antioxidant level in saliva. Interestingly, the TAS of the severe chronic periodontitis patients was not changed after treatment until 3 months, while the TAS of the healthy control group increased after treatment until 3 months. It was assumed that nonsurgical therapy did not improve the TAS in severe chronic periodontitis patients.
Although a small number of subjects were included in this study, this study did not show identical patterns between TAS and SOD activity after SRP. SOD is a component of the TAS in all aspects. In addition, the constitution and proportions of other antioxidants due to an increasing or decreasing tendency toward inflammation can complicate the changing antioxidant patterns in the saliva. Previous studies selected simple periodontitis patients as test subjects without classifying them according to the different forms and severity of periodontitis specifically. The pathologic mechanisms were mixed with other periodontal conditions making it possible for an irregular antioxidative tendency to exist in the saliva. For this reason, in patients with severe chronic periodontitis, it is possible that the test group would show different patterns of total antioxidants in the saliva. Moreover, there was a difference in the average age between the two groups. The TAS level and SOD activity can be affected by age. In addition, some smokers were included in both groups and there were different gender ratios. The effects of smoking and the gender on the antioxidant level might complicate the changing antioxidant pattern in the saliva.
Therefore, a longer-term study with more periodontitis patients and periodontally healthy subjects is required. In addition, more research will be needed to determine the effects of the types of periodontitis with different pathologic mechanisms, severity, age, smoking or non-smoking, and saliva flow rates in men and women on the antioxidant level.
In conclusion, the periodontitis subjects showed a different profile of the salivary total antioxidant level than the healthy or gingivitis group. The total antioxidant level in the saliva was higher in the patients with severe chronic periodontitis than the healthy or gingivitis control before SRP. The profile of the SOD activity of the periodontitis patients was lower than the control at each time point. The salivary TAS and SOD activity were changed by SRP. These results suggest that the SOD level in the saliva can be used as a diagnostic marker of oxidative injury in severe chronic periodontitis patients.
ACKNOWLEDGMENTS
This study was supported by Chonnam National University Hospital Clinical Research Center (2008, CR108024-1).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Forrest JL, Hujoel PP, Lieberman MB. Host modulation. In: Newman MG, Takei HH, Carranza FA, editors. Carranza's clinical periodontology. 10th ed. St. Louis: Saunders/Elsevier; 2006. pp. 275–282. [Google Scholar]
- 2.Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24:287–296. doi: 10.1111/j.1600-051x.1997.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 3.Bartold PM, Wiebkin OW, Thonard JC. The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodontal Res. 1984;19:390–400. doi: 10.1111/j.1600-0765.1984.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 4.Varani J, Fligiel SE, Till GO, Kunkel RG, Ryan US, Ward PA. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985;53:656–663. [PubMed] [Google Scholar]
- 5.Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med. 1991;91:14S–22S. doi: 10.1016/0002-9343(91)90279-7. [DOI] [PubMed] [Google Scholar]
- 6.Akalin FA, Toklu E, Renda N. Analysis of superoxide dismutase activity levels in gingiva and gingival crevicular fluid in patients with chronic periodontitis and periodontally healthy controls. J Clin Periodontol. 2005;32:238–243. doi: 10.1111/j.1600-051X.2005.00669.x. [DOI] [PubMed] [Google Scholar]
- 7.Ellis SD, Tucci MA, Serio FG, Johnson RB. Factors for progression of periodontal diseases. J Oral Pathol Med. 1998;27:101–105. doi: 10.1111/j.1600-0714.1998.tb01923.x. [DOI] [PubMed] [Google Scholar]
- 8.Tulunoglu O, Alacam A, Bastug M, Yavuzer S. Superoxide dismutase activity in healthy and inflamed pulp tissues of permanent teeth in children. J Clin Pediatr Dent. 1998;22:341–345. [PubMed] [Google Scholar]
- 9.Chapple IL, Mason GI, Garner I, Matthews JB, Thorpe GH, Maxwell SR, et al. Enhanced chemiluminescent assay for measuring the total antioxidant capacity of serum, saliva and crevicular fluid. Ann Clin Biochem. 1997;34(Pt 4):412–421. doi: 10.1177/000456329703400413. [DOI] [PubMed] [Google Scholar]
- 10.Chapple IL, Brock G, Eftimiadi C, Matthews JB. Glutathione in gingival crevicular fluid and its relation to local antioxidant capacity in periodontal health and disease. Mol Pathol. 2002;55:367–373. doi: 10.1136/mp.55.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao RK, Thomas DW, Pepperl S, Porreca F. Salivary epidermal growth factor plays a role in protection of ileal mucosal integrity. Dig Dis Sci. 1997;42:2175–2181. doi: 10.1023/a:1018855525989. [DOI] [PubMed] [Google Scholar]
- 12.Liskmann S, Vihalemm T, Salum O, Zilmer K, Fischer K, Zilmer M. Characterization of the antioxidant profile of human saliva in peri-implant health and disease. Clin Oral Implants Res. 2007;18:27–33. doi: 10.1111/j.1600-0501.2006.01296.x. [DOI] [PubMed] [Google Scholar]
- 13.Na HJ, Kim OS, Park BJ. Expression of superoxide dismutase isoforms in inflamed gingiva. J Korean Acad Periodontol. 2006;36:97–112. [Google Scholar]
- 14.Moore S, Calder KA, Miller NJ, Rice-Evans CA. Antioxidant activity of saliva and periodontal disease. Free Radic Res. 1994;21:417–425. doi: 10.3109/10715769409056594. [DOI] [PubMed] [Google Scholar]
- 15.Battino M, Bullon P, Wilson M, Newman H. Oxidative injury and inflammatory periodontal diseases: the challenge of anti-oxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–476. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]
- 16.Sculley DV, Langley-Evans SC. Periodontal disease is associated with lower antioxidant capacity in whole saliva and evidence of increased protein oxidation. Clin Sci (Lond) 2003;105:167–172. doi: 10.1042/CS20030031. [DOI] [PubMed] [Google Scholar]
- 17.Brock GR, Butterworth CJ, Matthews JB, Chapple IL. Local and systemic total antioxidant capacity in periodontitis and health. J Clin Periodontol. 2004;31:515–521. doi: 10.1111/j.1600-051X.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 18.Cutando A, Galindo P, Gomez-Moreno G, Arana C, Bolanos J, Acuna-Castroviejo D, et al. Relationship between salivary melatonin and severity of periodontal disease. J Periodontol. 2006;77:1533–1538. doi: 10.1902/jop.2006.050287. [DOI] [PubMed] [Google Scholar]
- 19.Pussinen PJ, Laatikainen T, Alfthan G, Asikainen S, Jousilahti P. Periodontitis is associated with a low concentration of vitamin C in plasma. Clin Diagn Lab Immunol. 2003;10:897–902. doi: 10.1128/CDLI.10.5.897-902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai CC, Chen HS, Chen SL, Ho YP, Ho KY, Wu YM, et al. Lipid peroxidation: a possible role in the induction and progression of chronic periodontitis. J Periodontal Res. 2005;40:378–384. doi: 10.1111/j.1600-0765.2005.00818.x. [DOI] [PubMed] [Google Scholar]
- 21.Canakci V, Yildirim A, Canakci CF, Eltas A, Cicek Y, Canakci H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J Periodontol. 2007;78:1602–1611. doi: 10.1902/jop.2007.060469. [DOI] [PubMed] [Google Scholar]
- 22.Ozmeric N, Elgun S, Uraz A. Salivary arginase in patients with adult periodontitis. Clin Oral Investig. 2000;4:21–24. doi: 10.1007/s007840050108. [DOI] [PubMed] [Google Scholar]