Abstract
In this issue of Cancer Cell, Yu and colleagues mapped the genomic binding sites of ERG and androgen receptor, two crucial transcription factors in prostate cancer. There is an extraordinary degree of overlap between binding sites, with the suggestion that ERG inhibits androgen receptor-mediated differentiation and promotes EZH2-mediated dedifferentiation.
The androgen receptor (AR) plays a critical role for both normal prostate and for prostate cancer development. AR inhibition represents the most effective systemic treatment of prostate cancer and resistance mechanisms predominantly involve reactivation of AR signaling, such as by AR overexpression, instead of bypassing the need for AR (Chen et al., 2004). Yet, the role of AR in prostate cancer is complicated. AR regulates transcriptional programs for both growth and differentiation and many prototypic AR regulated genes, such as prostate specific antigen (PSA), are markers of differentiation. Clinicians have long observed that patients with higher grade prostate cancer tend to manifest lower PSA relative to disease volume and several large scale gene expression datasets suggest that these AR targets are downregulated in both higher grade disease and at metastatic sites. On the other hand, expression of AR itself tends to increase with higher grade and is notably higher in castration resistant metastatic disease (Holzbeierlein et al., 2004). These observations suggest that in prostate cancer, AR may be co-opted to preferentially regulate growth genes instead of differentiation genes. Recent work showing that AR regulates a distinct set of cell growth genes in castration-resistant compared to hormone sensitive cells highlights the plasticity of AR (Wang et al., 2009). What governs AR specificity is a crucial question.
Tomlins and colleagues made the seminal discovery that ~50% of all prostate cancers harbor a fusion between the androgen regulated TMPRSS2 gene and ERG. Three other ETS family transcription factors, ETV1, ETV4, and ETV5, were also found fused to various highly expressed genes in another ~10% of prostate cancers. Mouse modeling and tissue reconstitution assays have confirmed the oncogenicity of these ETS factors in prostate, particularly in the context of cooperating lesions such as PTEN loss (King et al., 2009). Yet, despite intense study, the mechanisms by which ETS transcription factors mediate oncogenesis are largely unknown.
Gene regulation by ETS transcription factors is highly complex. There are 27 ETS factors in the human genome, with many co-expressed within any given cell type, all characterized by binding to a short GGA(A/T) core motif found in a large fraction of promoters. Despite this apparent redundancy, ETS factors clearly have unique functions since targeted deletion of single ETS family members in mice confers distinct phenotypes. Global analyses of ETS binding sites in various tissues suggest the following themes: (i) multiple ETS factors simultaneously bind the promoters of many “housekeeping” genes but no single ETS factor significantly regulates these genes; (ii) specific ETS factors bind enhancers of many tissue-specific genes and regulate their expression and (iii), most importantly, ETS factors function in concert with other transcription factors on cis-regulatory binding modules. Examples include co-regulation of Ras-responsive elements by ETS and JUN factors, endothelial specific genes by ETS and Forkhead factors, erythroid and megakaryocytic specific genes by ETS and GATA factors and T-cell specific genes by ETS and RUNX factors (Hollenhorst et al., 2009).
Does pathologic expression of ERG in prostate cancer follow a similar model? In this issue of Cancer Cell, Yu and colleagues have taken a first step in addressing this question by annotating ERG binding sites in the prostate cancer genome and find over 42,000 ERG binding sites mapping to 14,000 genes (Yu et al, Cancer Cell, this issue). While it may seem incredible that ERG maps to nearly half of all known genes, this number is consistent with recent ChIP-Seq studies of ETS family proteins in other tissues. In a parallel analysis, Yu et al report 13,000 AR binding sites mapping to over 6,000 genes, with an extraordinary degree of overlap between AR and ERG binding sites, particularly in enhancer regions. This finding confirms the prediction of an earlier study that showed enrichment of ETS motifs adjacent to AR binding sites (Massie et al., 2007). Collectively these data strongly suggest that ERG and AR cooperate at cis-regulatory elements to drive gene expression in TMPRSS2-ERG positive cancers (Figure 1). But unlike the established models of ETS cooperativity in normal tissues listed above, ERG is not expressed in the normal prostate and presumably activates an aberrant transcription program, perhaps by perturbing the normal AR network.
Figure 1. Coordinate regulation of target genes by androgen receptor and ERG.
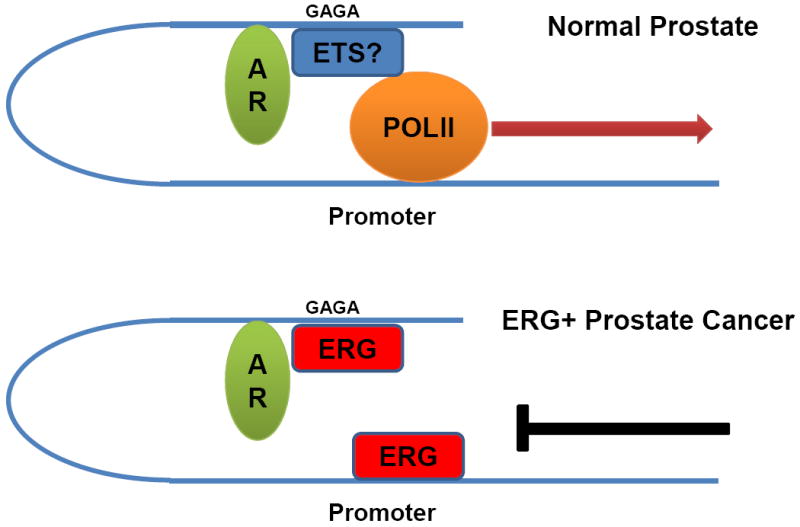
AR binding at enhancers of AR target gene causes looping and gene activation. The “GGAA” ETS motif is highly enriched at AR binding sites raising the possibility that an unidentified ETS protein (not ERG) may coordinately regulate transcription in normal prostate cells (top). In ERG+ prostate cancer, ERG now localizes at these sites, perhaps displacing an “endogenous” ETS factor, as well as gene promoters and inhibits AR mediates transcriptional activation.
The authors pursue this line of investigation and, surprisingly, find that ERG suppresses AR function – through a decrease in AR levels (ERG-mediated transcriptional suppression of AR) as well as inhibition of AR transcriptional activity on canonical target genes. At first glance this result seems paradoxical. AR activation is central to prostate cancer progression, even at the castration-resistant stage based on recent clinical success of next generation AR pathway antagonists such as abiraterone and MDV3100 (Scher et al., 2010). Further, expression of the oncogenic ERG fusion protein is controlled by the AR-dependent gene TMPRSS2; hence, ERG could extinguish its own expression! This is clearly not the case in human tumors; therefore, ERG and AR must function in a negative autoregulatory loop tightly controlling levels of both transcription factors. Too much AR activity increases ERG protein through TMPRSS2 regulatory regions, which turns down AR and ERG, and vice versa. Another possibility is that ERG shifts the balance of AR target gene regulation to those that primarily drive growth rather than differentiation programs.
While intriguing, more work is needed to clarify certain aspects of the model. Others have recently shown that the other ETS family fusion partner ETV1 also binds the PSA enhancer cooperatively with AR but exerts the opposite effect – an increase in AR target gene expression (Shin et al., 2009). Since ETV1 and ERG appear to have similar functions in prostate oncogenesis, it is difficult to reconcile opposing effects on AR output as central to their transforming activity. Indeed, the AR target genes downregulated by ERG, such as PSA, are markers of differentiation but there is little evidence to suggest that they are mediators of differentiation. More detailed characterization of AR target genes across a larger panel of ERG-and ETV1-positive cancers is required to fully flesh out the model. In addition, recent studies of nuclear receptor (NR) cistromes reveal cell-type specificity defined in part by “pioneer” transcription factors such as FOXA1 that bind in advance and modify chromatin for optimal NR binding (Lupien et al., 2008). Since AR expression almost certainly precedes ERG expression in fusion-positive prostate cancers, ERG is unlikely to pioneer AR binding but the relationship between the two needs further dissection – i.e., do they bind independently? cooperatively? does dual binding substantially alter chromatin landscape?
The authors also implicate the histone methyltransferase and polycomb group protein EZH2 as another ERG target gene independent of effects on AR. ERG binds to the EZH2 promoter and induces expression. This result is compelling since EZH2 was recently shown to enhance metastasis in an orthotopic prostate cancer model (Min et al., 2010). EZH2 overexpression is correlated with increased tumor grade in many tumor types and may reflect a state of dedifferentiation. Whether EZH2 upregulation in prostate cancer is exclusively linked to ERG fusion positive cancers requires further study. Taken together, this study unambiguously links AR and ERG as co-regulators of prostate cancer gene expression and proposes new models of prostate cancer progression. In light of this work, further characterization of ERG and AR signaling in murine models and human samples will be of great interest.
References
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Hollenhorst PC, Chandler KJ, Poulsen RL, Johnson WE, Speck NA, Graves BJ. DNA specificity determinants associate with distinct transcription factor functions. PLoS Genet. 2009;5:e1000778. doi: 10.1371/journal.pgen.1000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JC, Xu J, Wongvipat J, Hieronymus H, Carver BS, Leung DH, Taylor BS, Sander C, Cardiff RD, Couto SS, et al. Cooperativity of TMPRSS2-ERG with PI3-kinase pathway activation in prostate oncogenesis. Nat Genet. 2009;41:524–526. doi: 10.1038/ng.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132:958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8:871–878. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, Guney I, Strochlic DE, Macconaill LE, Beroukhim R, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16:286–294. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Kim TD, Jin F, van Deursen JM, Dehm SM, Tindall DJ, Grande JP, Munz JM, Vasmatzis G, Janknecht R. Induction of prostatic intraepithelial neoplasia and modulation of androgen receptor by ETS variant 1/ETS-related protein 81. Cancer Res. 2009;69:8102–8110. doi: 10.1158/0008-5472.CAN-09-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, et al. Cancer Cell. This issue. [Google Scholar]


