Abstract
Plants are under constant attack from insects, microbes, and other physical assaults that damage or remove body parts. Regeneration is one common strategy among plants to repair their body plan. How do organisms that are proficient at regeneration adapt their developmental programs for repatterning tissues? A new body of research employing high resolution imaging together with cell-fate markers has led to new insights into the tissues competent to regenerate and the mechanisms that re-establish pattern. In a parallel to new findings in metazoan systems, recent work in plants shows that regeneration programs commonly thought to rely on dedifferentiated cells do not need to reprogram to a ground state. Imaging studies that track the expression of regulators of the plant's proliferative centers, meristems, in conjunction with mutant analysis have shed new light on the earliest organizational cues during regenerative organ formation. One promise of plant regeneration studies is to reveal the common design attributes of programs that pattern similar organs in different developmental contexts.
Introduction
More than 50 years ago, plant researchers reared single differentiated cells into entire plants, demonstrating the totipotency of some adult plant cells [1]. Gurdon's classic nuclear transplantation experiments in frog showed the pluripotent potential of some differentiated metazoan cells [2]. By 2006, researchers achieved the long-sought goal of inducing an adult mammalian cell into a pluripotent state [3-4]. If important strides have been made in manipulating pluripotency, one immediate challenge in regeneration research is a better understanding of how specific developmental mechanisms are invoked during repatterning by pluripotent cells.
Few developmental pathways involved in patterning are likely to be shared across kingdoms [5]. However, the task of repatterning in plants and animals raises a set of parallel questions in regeneration, which is defined here as the replacement of lost or damaged parts. How are stereotypical developmental pathways adapted during regeneration? And, which cells are typically recruited and what triggers the activation of specific programs in them? The broadest possible comparisons may yield insights into common constraints and alternate solutions to regeneration.
Plants and animals demonstrate examples of in situ restoration of a damaged organ or appendage, such as in salamander limb or plant root tip regeneration (Fig. 1). A second mode of repair is de novo regeneration of organs or whole organisms often in an ectopic location. Examples include the appearance of shoots or roots from stem cuttings in plants or re-establishment of the complete body plan from tissue fragments in Planaria. In plants, one critical feature of regeneration is the reformation of meristems, the proliferative tissues that contain stem cells and ultimately lead to adult growth. Although we distinguish the activation of dormant axillary meristems and other existing growth centers from regeneration, one intriguing question is what is the role of embryonic and post-embryonic growth mechanisms in regeneration?
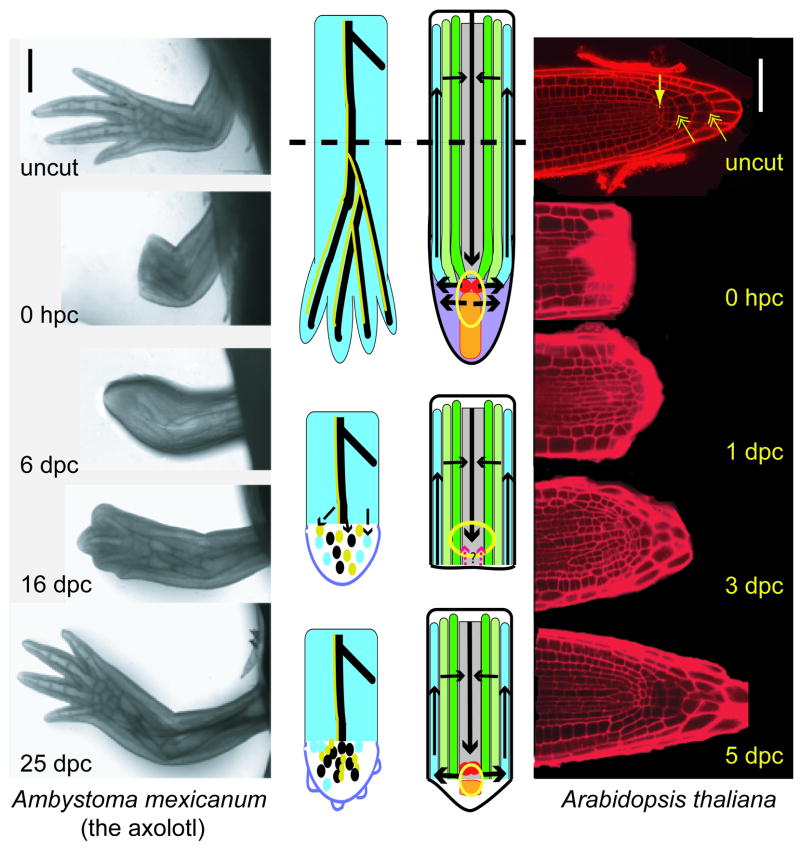
Figure 1. Two strategies for regeneration.
On outside panels, axolotl limb regeneration through blastema formation and Arabidopsis root meristem regeneration without formation of callus (hpc, hours post cut; dpc, days post cut). In the diagram of axolotl, cells migrate from different tissues in the stump to form a heterogeneous blastema. Colors in diagram represent cells from different lineages that retain a memory of the tissue of origin. The blastema regenerates the limb over 25 days with new tissues populated by cells descendent from the same lineage in many cases. In the confocal image of Arabidopsis, the arrow shows the position of the QC cells and the double arrow shows the position of the columella cells. In the diagram of Arabidopsis, distal (toward tip) cell identities, PIN domains for lateral auxin distribution, and the auxin maximum (arrows) are removed by the excision (red=QC; orange=columella, yellow circle=auxin maximum). In a potential model, auxin accumulates at the tip and induces PINs localized to redistribute auxin laterally. The auxin maximum determines the position of QC and then induces further patterning through a yet unknown mechanism. Arrows coming up from the cut site indicate the potential for other signals that induce cell identities or mediate auxin flux. Scale bars; axolotl=1mm; Arabidopsis=50μm.
Moreover, plants produce similar organs in very different developmental contexts. For example, plants produce roots in the embryo, in the adult body as continuously emerging lateral roots, and often as adventitious roots [6]. The initial morphogenesis of these organs is dramatically different but the developmental outcome is largely the same, if not identical in some cases. How do these different programs, which utilize the same genomic toolbox, reveal common organizing principles in plant organogenesis? Are any of these principles utilized during regeneration as well?
Potent reserves
After the demonstration of the totipotency of carrot phloem cells [1], a body of experiments repeated these results using other differentiated cell types, suggesting that virtually any plant cell could be a starting point for regeneration [7]. Recently, reliable microscopic imaging has begun to distinguish which cell types are typically recruited during regeneration from mixed tissue samples. In Arabidopsis, a regenerative tissue mass called callus can be induced in culture by treating tissue cuttings with the phytohormone auxin. Organ regeneration can then be induced from callus tissue by modulating the ratio between auxin and another phytohormone, cytokinin, in the culture medium. Using this system in combination with confocal imaging of cell-type specific markers, it was shown that callus typically arose from either proliferating and relatively undifferentiated meristematic cells or from the differentiated cell type pericycle, from which lateral roots naturally initiate [8**, 9*, 10*]. When pericycle cells were genetically ablated using cell-specific expression of diphtheria toxin, callus could no longer be induced from mature root zones [9]. Thus, despite widespread totipotency, certain cell types appear to be more likely recruits for callus formation, at least in Arabidopsis. More high-resolution cell tracking is needed in other species but previous work includes reports of “natural” callus emerging in planta from other tissues associated with post-embryonic growth, such as lateral meristems, which contribute to girth growth in adult plants [11, 12].
If callus is treated with a high ratio of cytokinin-to-auxin concentration, it will develop shoots. The inverse ratio will induce roots [13]. This organ-specification principle, first reported in 1957, has become an important tool for regeneration studies. In one recent study, Che et al. [9] showed that auxin pre-conditioning made explants competent to express hundreds of genes after the shoot-forming treatment, including meristem determinants like WUSCHEL (WUS). These studies reveal the role of auxin treatment in making cells competent to express patterning genes. Indeed, it has long been thought that the auxin treatment enables callus to reach a dedifferentiated state.
The route to cellular plasticity
Recent studies have challenged the role of dedifferentiation in regeneration. In experiments where the Arabidopsis root tip is cut to initiate regeneration, the global identity of the cells in the remnant stump was tracked in time, showing that many cell-type specific markers for lost cell types were expressed within 5 hours [14**]. The analysis also showed that newly restored columella cells, which sense gravity in the root, were functional within about 24 hours (Fig. 1). The speed of marker and cell-type recovery and lack of apparent cell dedifferentiation at the morphological and transcriptional level suggested the intriguing possibility that these cells traversed fates directly without entering a dedifferentiated state. Similarly, in cultured root explants, it was found that lateral roots initiating from the pericycle tissue could be re-specified directly into shoots without callus induction after transfer to cytokinin-rich media [10].
Two recent studies draw into question assumptions about the dedifferentiated state of callus. Surprisingly, it was shown that callus is not a homogenous mass but retains root meristem identity [10*, 15**]. Even callus generated in vitro from the pericycle of aerial organs, a layer that does not produce lateral roots in planta, passed through cellular states reminiscent of those found in the root meristem, as judged by marker expression and genomic profiling [15]. Moreover, there appears to be a functional requirement for such a root-like phase as even shoot-derived tissues from mutants in lateral root initiation, such as alf4, could not effectively form callus [15]. Thus, callus does not appear to be a dedifferentiated tissue after all.
In a parallel finding in metazoans, researchers probed the nature of the blastema, a regenerative tissue mass analogous to callus from which, for example, a limb can regenerate in salamander (Fig. 1) [16**]. Remarkably, cell lineage tracking showed that many blastema cells appear to retain a memory of their original identity and are restricted to their own lineage during regeneration [16]. Thus, in taxonomically diverse organisms, structures thought to represent dedifferentiated tissue that regenerate entire organs or limbs contain many cells that do not need to dedifferentiate to a ground state. In axolotls, the lineage memory of blastema cells may be one mechanism to help guide the appropriate limb program during regeneration. In contrast, meristematic Arabidopsis cells do not show evidence of being committed to their fate [17]. Indeed, these young but differentiated cells appear to be widely pluripotent, competent to translate positional cues directly into a wide variety of cell fates.
Rebooting: which program is executed?
Within root and shoot meristems, a canonical stem cell niche continuously generates new cells, which continue to divide until they differentiate [17]. In principle, regeneration could be orchestrated by the existence of a local patterning organizer such as the niche, or emerge as the consequence of global self-organizing properties of the meristem as a whole. In the first scenario, a central organizer like the niche would be necessary for tissue reorganization and would have to be established early in the process, while in the second case its appearance would be a mere consequence of repatterning. Experiments that have determined the order of cell type recovery in the meristem are providing important clues about the nature of reorganization mechanisms during regeneration.
Within the root stem cell niche, quiescent center (QC) cells are required for maintaining surrounding stem cells in an undifferentiated state [18]. The QC, upon which the cell files of the root physically converge, has long been thought to be an organizer of root development. Xu et al. [19**] showed that genes required for QC maintenance in adult roots, including PLETHORA1/PLETHORA2 (PLT1/2) and SCARECROW (SCR), were also critical in regenerating the QC and distal tip organization after QC ablation. These results are consistent with the QC and the functional stem cell niche acting as a central organizer. However, Sena et al. [14], who used a regeneration system in which the entire root tip is severed, found that plt1/2 and scr mutants could regenerate the root tip pattern, including re-specification of excised cell types, such as the columella. It is not clear why genes known to maintain the niche are necessary in the recovery from one type of injury and not the other, although it is possible that tip excision invokes different patterning mechanisms than those triggered by QC ablation. The scr and plt1/2 mutants do not completely eliminate QC identity [14], which may still play a role in repatterning. Nonetheless, the results from the complete root tip excision showed that a functional stem cell niche was not necessary for repatterning, opening the potential for a QC-independent patterning mechanism.
Similarly, in de novo regeneration of the shoot apical meristem (SAM) from callus, time-lapse confocal imaging of key regulators showed an early spatial partitioning of factors expressed in the central and peripheral regions of the shoot meristem, such as a REVOLUTA (REV) domain flanked by a FILAMENTOUS FLOWER (FIL) domain [8]. Evidence from both imaging analysis and global microarray studies, showed that broad regions of the meristem were specified early and many factors important for stem cell establishment and maintenance, such as CLAVATA3, were expressed later [8,20**]. Thus, temporally, it seems that domains that span the entire shoot meristem are patterned prior to the establishment of an active stem cell niche, as in the root tip excision experiments. Neither the shoot nor the root tip regeneration experiments rule out the existence of a central organizer but they suggest that at least part of meristem repatterning can be independent from the establishment of an active stem cell niche in plant apical meristems.
Embryonic programs would seem to be a good source of whole meristem repatterning. In both shoot and root regeneration studies, regulators that play critical roles during embryogenesis appear early in regeneration [8-10, 14, 19, 20]. However, the changes in gene expression during regeneration appear to be a gradual refinement to adult expression patterns more than a recapitulation of embryogenesis [8, 14]. For example, in the embryo, WOX5 is normally expressed in the lens-shaped cell, which eventually gives rise to the QC [21]. In contrast, early in root regeneration WOX5 expressed in a u-shaped pattern, similar to other QC markers in adult roots treated with auxin [22]. Ultimately, its expression pattern refines to a group of cells with an adult QC morphology with no intervening lens-shaped stage [14, 19]. In fact, many other morphogenesis events in root regeneration differ from embryogenesis or lateral root formation but it is not clear if these differences represent a truly distinct developmental program or the adaptation of stereotypical organogenesis mechanisms onto the disorganized morphologies of regenerating tissues. Parallel questions have arisen in axolotl limb regeneration, as expression of HOX genes do not always follow the patterns of early development. The aberrant patterns have raised the question of whether they reflect a novel dedifferentiation stage, embryogenesis, late limb development, or something entirely unique to regeneration [23].
Interestingly, in Kalanchoe, vegetative plantlets form on the margins of leaves in a type of regeneration incorporated into normal development. Recent studies suggest that both adult organogenesis and embryonic developmental programs are co-opted into the process [24*]. This shows that plants can use a mix-and-match strategy to patterning.
Order from disorder
Unlike most normal development, regeneration often proceeds through unpredictable and seemingly disorganized morphologies. However, recurring patterns common to different realizations of regeneration could help identify critical spatial organizers, such as adjacent domains of key regulators that could set up tissue boundaries. For example, Gordon et al. [8] frequently observed that shoot inducing treatments (a high ratio of cytokinin-to-auxin concentration) led to the first signs of regulatory organization in callus, initiating the partition of mutually exclusive auxin and cytokinin response domains. These domains appeared to subsequently induce spatially distinct expression patterns for the meristem initiation genes CUP-SHAPED COTYLEDON2 (CUC2) and WUS. The results help explain the mechanisms behind the organ-specification principle in which hormone ratios induced organ identity [13]. The authors speculate that, after the establishment of hormone response and gene expression domains, interactions between hormones and regulatory cues progressively establish more complex patterning typical of the shoot meristem. In this view, the primary difference between in vitro regeneration and meristem initiation in planta is merely how the initial distribution of auxin and cytokinin is set up [8,25].
What triggers early events in regeneration when no exogenous hormones are applied? The question is important because the cues that re-establish order can reveal insights into the nature of the patterning mechanism. In the QC ablation experiments, Xu et al. [19] present a model in which QC ablation caused a disruption of the auxin flow that triggered regeneration. The model was supported by computational simulations showing that, given a specific distribution and polarity of PINs, an auxin maximum could be re-established a few cells proximal to its original position [26*]. The model is based on the fact that QC and a few neighboring cell types express several members of the auxin efflux carrier family, named after the PIN-FORMED 1 (PIN1) mutant. The cellular polarity and expression domain of PINs results in the directional transport of auxin within the tissue. The most distal PINs of the root move auxin laterally and contribute to a circulation system that establishes a concentration maximum at the tip (Fig. 1 [27]). Auxin is known to be a critical cue for root organization, because auxin flux has been shown to be necessary for root regeneration and perturbations in auxin distribution that change the location of the auxin maximum are sufficient to switch the polarity of the root [19, 14, 22].
Other studies have shown that auxin can influence its own movement by inducing expression of PIN genes [28]. Such canalization models posit that auxin flux can feedback on PIN polarity to reinforce the direction of auxin flow, such that auxin can self-organize its own movement [29-31]. Indeed, it has been shown that, in wound healing, auxin flux reinforces polarization of PINs leading to vascular strand formation [32*] and the model was similarly corroborated recently in leaf vein formation [33].
One of the missing pieces of the puzzle is what triggers regeneration. For example, how is an auxin maximum re-established once it is removed by the excision of the root tip? One possibility is that a net downward flux of auxin is present in the root stump, which retains its proximal-distal orientation of auxin efflux carriers [14]. These remaining PINs would transport auxin toward the root tip, where auxin would induce new PIN expression that stabilizes the auxin flux. Indeed, the expression pattern of PIN7 (lateral distribution of auxin), whose distal domain was completely lost after tip excision, was detected in the root stump only 24 hours after the cut along with evidence of a new auxin maximum [14].
Alternatively, other signals could initiate regeneration by triggering cell fate or other local changes. In animal systems, apoptotic cells at wound sites release Wnt3 in Hydra, which is necessary to induce head regeneration [34]. In a parallel scenario, early regeneration events may be independent of auxin. For example, Xu et al. [19] found that cell fate and QC identity preceded re-orientation of PINs during regeneration. It will be interesting to see if potential signals induced by dying cells or new edge cells can trigger repatterning either by inducing cell fate re-specification or by helping re-establish the appropriate auxin flux.
The triggers described above may establish a local organizer. Alternatively, auxin flux in the meristem may act as a non-localized, self-repairing system where initial positional cues are specified by the remnant flow of auxin in the stump. This auxin circulation system could be both independent from a local organizer and necessary to instruct further patterning. Yet another possibility is that the reorganization of a vast field of differentiated cells in plants could in fact be obtained independently of auxin as an emergent property of the whole system, simply based on other kinds of cell-cell interactions acting on intrinsic cell fate plasticity. Thus, in root tip regeneration, it is not yet clear if auxin alone can re-organize its own flow, if it requires other inputs, or if it is simply a necessary intermediate signaling event during re-organization.
Alternatively, one possible model is that auxin flux in the meristem resembles a non-localized, self-repairing system that specifies cellular identities and patterning throughout the meristem. Another possibility is that the reorganization of a vast field of differentiated cells in plants can be obtained independently of auxin as an emergent property of the whole system, simply based on other kinds of cell-cell interactions acting on intrinsic cell fate plasticity. Thus, in root tip regeneration, it is not yet clear if auxin alone can re-organize its own flow, if it requires other inputs, or if it is simply a necessary intermediate signaling event during re-organization.
Conclusion
The plant's ability to initiate growth into adulthood from many cell types means that highly potent cells are dispersed throughout the plant body. During regeneration, the plant appears to preferentially recruit what are often partially differentiated but uncommitted cells, such as pericycle. Repatterning of these cells during regeneration can be remarkably rapid without an obvious de-differentiation phase. In addition, distinguishing causal factors that initiate patterning in regeneration has profound implications for understanding how the known patterning mechanisms operate within an organizing system. Does a signaling center like the niche, or another region of the meristem, act as a necessary and sufficient local signaling source instructing root patterning? Recent work that shows a functional niche is not necessary for patterning together with results on the timing of de novo shoot regeneration open the possibility that critical patterning cues are positioned independently of a local organizer. Is the source of meristem patterning a local organizer such the QC, a canalizing auxin flux, or a self-organizing system depending on other kinds of local cell interactions. Further modeling at these early stages may help generate hypotheses about how organization is established. These important questions about the fundamental organization of meristems in plants are still open and need to be resolved.
Acknowledgments
We thank Tal Nawy and Bastiaan Bargmann for critical comments, Elly Tanaka for images and acknowledge Lihua Shen for the idea for Figure 1. K.D.B. is supported by grants from the National Institutes of Health (R01 GM078279) and the National Science Foundation (MCB-0929338).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steward FC, Mapes MO, Mears K. Growth and Organized Development of cultured Cells. II. Organization in Cultures Growtn from Freely Suspended Cells. American Journal of Botany. 1958;45:705–708. [Google Scholar]
- 2.Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelial cells of feeding tadpoles. J Embryol Exp Morphol. 1962;10:622–640. [PubMed] [Google Scholar]
- 3.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Meyerowitz EM. Plants compared to animals: the broadest comparative study of development. Science. 2002;295:1482–1485. doi: 10.1126/science.1066609. [DOI] [PubMed] [Google Scholar]
- 6.Esau K. Anatomy of seed plants. 2d. New York: Wiley; 1977. [Google Scholar]
- 7.Halperin W. Attainment and Retention of morphogenetic Capacity in Vitro. In: Vasil IK, editor. Cell culture and Somatic Cell Genetics of Plants. Vol. 3. 1986. p. 4. Plant Regeneration and Genetic Variability. [Google Scholar]
- **8.Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development. 2007;134:3539–3548. doi: 10.1242/dev.010298. [DOI] [PubMed] [Google Scholar]; A comprehensive analysis of key regulators of meristem organization during de novo regeneration of shoot apical meristem from callus. This research uses high resolution confocal microscropy to provide an uprecendented view of the formation shoot organogenesis. The work shows that the earliest steps in regenerating the shoot is setting up an opposing cytokinin and auxin domains that appears to then lay out the spatial patterns of early meristem initiation genes.
- *9.Che P, Lall S, Howell SH. Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta. 2007;226:1183–1194. doi: 10.1007/s00425-007-0565-4. [DOI] [PubMed] [Google Scholar]; The Howell lab's ongoing analysis of the developmental changes in callus tissue as it gains competence to repattern the shoot includes this detailed analysis of some of the cellular and molecular aspects of the process. The report includes an analysis of the role of pericycle cells in contributing to callus, including pericycle marker studies under confocal microscopy and genetic ablation of pericycle.
- *10.Atta R, Laurens L, Boucheron-Dubuisson E, Guivarc'h A, Carnero E, Giraudat-Pautot V, Rech P, Chriqui D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009;57:626–644. doi: 10.1111/j.1365-313X.2008.03715.x. [DOI] [PubMed] [Google Scholar]; A high resolution view of organogenesis from callus generated from pericycle. This report showed that callus formation from root explants resembled lateral root formation. Another intriguing and surprising part of this work was that shoot formation could occur directly from lateral root primordia after the typical shoot inducing treatment for callus without a callus phase. This demonstrated a surprising pluripotency for lateral root primordia and provided another case of how profilerative cells are relatively open in their developmental trajectory.
- 11.Steeves TA, Sussex IM. Patterns in plant development. 2nd. Cambridge England; New York: Cambridge University Press; 1989. [Google Scholar]
- 12.Sinnott EW. Plant Morphogenesis. 1960:244. [Google Scholar]
- 13.Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;54:118–130. [PubMed] [Google Scholar]
- **14.Sena G, Wang X, Liu HY, Hofhuis H, Birnbaum KD. Organ regeneration does not require a functional stem cell niche in plants. Nature. 2009 doi: 10.1038/nature07597. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research adapted root tip excision techniques practiced for over 100 years to the Arabidopsis model system. What the system permitted was a view of the order of events during meristem recovery and the overall re-organization of the meristem since it removed not only the QC but also differentiated cell types at the tip of the root. In general, the work showed that growth associated with the functional stem cell niche and the re-organization of pattern in the root were independent, providing a view of how the overall meristem is organized.
- **15.Sugimoto K, Jiao Y, Meyerowitz EM. Arabidopsis Regeneration from Multiple Tissues Occurs via a Root Development Pathway. Dev Cell. 2010;18:463–471. doi: 10.1016/j.devcel.2010.02.004. [DOI] [PubMed] [Google Scholar]; Callus was long-thought to be one of the prime examples of de-differentiated tissue in plants. The first unexpected finding of this paper was that callus actually retained the expression and loose patterning of the root meristem, even when it arose from shoot tissue. The second unexpected finding was that the pericycle layer in shoots was the layer that formed callus and was competent to induce the root meristem. This extends the developmental plasticity of plants and shows the wide competence of meristematic tissue.
- **16.Kragl M, Knapp D, Nacu E, Khattak S, Maden M, Epperlein HH, Tanaka EM. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]; A surprising finding on the nature of the blastema, a structure thought to be a quintesential example of a de-differentiated tissue that becomes pluripotent for regeneration. In the axololt limb, the Tanaka group shows that most blastema cells derived from specific lineages remain in the same lineage after the blastema reforms the excised limb. This has profound implications on the role of pluripotency during regeneration.
- 17.Dinneny JR, Benfey PN. Plant stem cell niches: standing the test of time. Cell. 2008;132:553–557. doi: 10.1016/j.cell.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 18.van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature. 1997;390:287–289. doi: 10.1038/36856. [DOI] [PubMed] [Google Scholar]
- **19.Xu J, Hofhuis H, Heidstra R, Sauer M, Friml J, Scheres B. A molecular framework for plant regeneration. Science. 2006;311:385–388. doi: 10.1126/science.1121790. [DOI] [PubMed] [Google Scholar]; This report provided a high resolution analysis of the genetic regulators of QC and auxin regulation after ablation of the QC. This was a first look of the molecular orchestration of how the plant restores the critical cell type that mediates adult growth in the root, finding an essential role for the genes that establish and maintain the root stem cell niche during regeneration.
- **20.Che P, Lall S, Nettleton D, Howell SH. Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol. 2006;141:620–637. doi: 10.1104/pp.106.081240. [DOI] [PMC free article] [PubMed] [Google Scholar]; The 1957 experiment that led to the organ-specification model meets modern transcriptional analysis of the phenomenon in this report. The authors undertake a comprehensive genomic analysis of tissue states during the acquisition of competence to generate roots and shoots and the conditioning steps. A nice aspect of the study is the comparison of cytokinin treatments with and without auxin conditioning. This is a valuable dataset on the study of dedifferentiation and the molecular underpinnings of developmental competence.
- 21.Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 2004;131:657–668. doi: 10.1242/dev.00963. [DOI] [PubMed] [Google Scholar]
- 22.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka EM. Cell differentiation and cell fate during urodele tail and limb regeneration. Curr Opin Genet Dev. 2003;13:497–501. doi: 10.1016/j.gde.2003.08.003. [DOI] [PubMed] [Google Scholar]
- *24.Garces HM, Champagne CE, Townsley BT, Park S, Malho R, Pedroso MC, Harada JJ, Sinha NR. Evolution of asexual reproduction in leaves of the genus Kalanchoe. Proc Natl Acad Sci U S A. 2007;104:15578–15583. doi: 10.1073/pnas.0704105104. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important study on how development occurs outside of Arabidopsis in an organism that demonstrates one of hallmark's of plant development, its plasticity. Here the Sinha group has developed Kalanchoe as a model to study its unusual habit of non-sexual reproduction by producing plantlets on its leaf margins, which other species can do under stress conditions. The authors show how typical developmental pathways are co-opted in this unusual form of vegetative reproduction.
- 25.Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- *26.Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]; The authors use a cell-based model of auxin transport using known parameters to show that the arrangement of PIN carriers in the root creates a robust auxin maximum. The important aspect of the work was that it showed that, given the observed pattern of PIN expression and polarity, the auxin efflux system created a robust mechanism to position the auxin maximum, regardless of the source of auxin. It also showed that a difficult to detect but postulated auxin gradient in the proximo-distal scale of the root was theoretically plausible.
- 27.Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 28.Vieten A, Vanneste S, Wisniewska J, Benkova E, Benjamins R, Beeckman T, Luschnig C, Friml J. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development. 2005;132:4521–4531. doi: 10.1242/dev.02027. [DOI] [PubMed] [Google Scholar]
- 29.Berleth T, Sachs T. Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol. 2001;4:57–62. doi: 10.1016/s1369-5266(00)00136-9. [DOI] [PubMed] [Google Scholar]
- 30.Sachs T. Polarity and the induction of organized vascular tissues. Annals of Botany. 1969;33:263–275. [Google Scholar]
- 31.Sachs T. Cell polarity and tissue patterning in plants. Development. 1991;1:83–93. [Google Scholar]
- *32.Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]; An test of Sach's canalization model that demonstrates the plausibility that auxin can influence its own transport in wound repair. Here the authors visualize the recovery of polar PIN localization around wound sites, showing that it responds to and organizes around auxin cues, where localized application can lead to a chain of auxin transport that influences cell identity.
- 33.Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev. 2009;23:373–384. doi: 10.1101/gad.497009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou JC, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]