Abstract
Purpose
Thalamofrontal abnormalities have been identified in chronic primary generalized epilepsy and specifically in juvenile myoclonic epilepsy (JME). These regions also underlie executive functioning, although their relationship has yet to be examined in JME. This study examined the relationship between thalamic and frontal volumes and executive function in recent onset JME as compared to healthy control subjects and recent onset Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS), a syndrome not typically associated with thalamocortical or executive dysfunction.
Methods
20 children with recent onset JME were compared to 51 healthy controls and 12 children with BCECTS using quantitative MRI and measures of executive abilities. Quantitative thalamic and frontal volumes were obtained through semi-automated software. Subtests from the Delis-Kaplan Executive Function System (D-KEFS) and the Behavior Rating Inventory of Executive Function (BRIEF) were used to measure executive function.
Results
Executive functions were significantly impaired in JME subjects compared to control and BCECTS subjects. Subjects with JME had significantly smaller thalamic volumes and more frontal CSF than control and BCECTS subjects. Thalamic and frontal volumes were significantly related to executive functioning in the JME group, but not in the other two groups.
Discussion
Children with JME have significant executive dysfunction associated with significantly smaller thalami and more frontal CSF. Children with recent onset BCECTS do not display the same pattern. Frontal and thalamic volumes appear to mediate the relationship between executive functioning and brain structure in JME.
Keywords: Executive Function, Quantitative magnetic resonance imaging, Idiopathic Generalized Epilepsy
Juvenile myoclonic epilepsy (JME) is a generalized epilepsy syndrome characterized by myoclonic seizures, particularly early in the morning, with peak onset usually between 12 and 18 years (Duncan, 1997; Jallon and Latour, 2005; Nordli, 2005). Electrophysiological and functional neuroimaging (i.e., fMRI and proton magnetic resonance spectroscopy) studies suggest that fronto-thalamo-frontal circuitry dysfunction underlies JME neuropathophysiology (Moeller et al., 2007; Mory et al., 2003; Savic et al., 2004). The thalamus has been shown to play a significant role in generalized seizure modulation and propagation, with cortical structures providing the excitatory drive and the thalamus amplifying and synchronizing seizure activity (Bertram et al., 2001; Gotman et al., 2005; Guye et al., 2006).
By definition, individuals with idiopathic generalized epilepsies (IGEs) do not have a visually observable epileptogenic lesion on MRI. However, there is evidence of quantitative structural and functional abnormalities of the frontal lobes (Tae et al., 2006; Woermann et al., 1999) and thalamus in JME (Betting et al., 2006a; Kim et al., 2007). The anatomical connectivity between the thalamus and prefrontal cortex make it ideally situated to influence executive functions (Sandson et al., 1991; Carrera and Bogousslavsky, 2006; Maxwell et al., 2006; Van der Werf et al., 2003; Van der Werf et al., 2000). Predominant dysfunction of the frontal network has led some to conceptualize JME as a variant of frontal lobe epilepsy rather than a generalized syndrome (Craiu et al., 2006; Koepp, 2005).
Executive functions encompass skills necessary for purposeful, goal-directed activity that enable an individual to synthesize information, plan an appropriate strategy, and execute that strategy (Anderson, 2001; Klenberg et al., 2001; Samagano-Sprouse, 2007). A number of studies have provided evidence for executive dysfunction in JME. Specifically, impairments in concept formation, abstract reasoning, mental flexibility, cognitive speed, and planning have been reported (Devinsky et al., 1997; Pascalicchio et al., 2007; Piazzini et al., 2007; Sonmez et al., 2004). Converging evidence from neuroimaging and neuropsychological studies provide a strong basis to hypothesize that frontal lobe and thalamic dysfunction underlie executive impairments in JME (Devinsky et al., 1997; Woermann et al., 1999). However, there has not yet been a direct investigation of the structural brain correlates of executive dysfunction in JME, and it is unknown to what extent the thalamus and/or frontal lobes contribute to the reported executive deficits. Furthermore, most studies of thalamofrontal integrity or executive functioning have examined adults with a relatively longstanding duration of epilepsy. The timing or onset of these abnormalities and the neurodevelopemental course of JME remains largely unknown as there is a marked paucity of investigation of the integrity of thalamofrontal circuitry and its developmental trajectory in patients early in the course of their epilepsy.
In contrast to JME, thalamofrontal circuitry is not believed to play a significant a role in most localization related epilepsy (LRE) syndromes, with the exception of frontal lobe epilepsy (Crespel et al., 1998; Tekin and Cummings, 2002). Although executive impairments have been identified in temporal and frontal lobe epilepsies, there is presently no evidence to necessarily implicate thalamofrontal involvement. Direct comparison of patients with JME to recent onset LRE provides a basis to determine if thalamic, frontal lobe, or executive function abnormalities are a common feature of recent onset epilepsy or if they are specific to JME. Benign Childhood Epilepsy with Centrotemporal Spikes (BCECTS) is an idiopathic LRE syndrome where abnormalities in thalamofrontal circuitry and executive function have not been reported.
The aims of the current study were threefold: (1) determine the presence of thalamic and frontal lobe volumetric abnormalities in children with recent onset JME; (2) determine the relationship between thalamofrontal structure and executive function in children with JME., and (3) compare JME to LRE to determine if thalamofrontal and executive dysfunction are common features in recent onset childhood epilepsy, or if these features are syndrome specific. The examination of recent onset epilepsy in children provides an important time point of investigation, as findings will be relevant to proposals concerning the timing of documented brain structural and functional abnormalities in this group.
Methods
Participants
Research participants included children with recent onset JME (n = 20), recent onset BCECTS (n = 12), and healthy first-degree cousin controls (n = 51) ages 8-18 years. Individuals with BCECTS were selected as a “control” epilepsy group to determine if thalamofrontal integrity and executive function were compromised in a homogeneous group of LRE patients. Study participants were recruited from pediatric neurology clinics at two large Midwestern medical centers (University of Wisconsin-Madison and Marshfield Clinic). Selection criteria for patients with epilepsy included: 1) diagnosis of epilepsy within past 12 months, 2) chronological age between 8-18 years, 3) no other developmental disabilities (e.g., autism, developmental delay), 4) no other neurological disorder, 5) normal clinical MRI (i.e., no structural lesions), and 6) normal neurological examination. All cases underwent preliminary review by the study coordinator to ensure that they met criteria for study inclusion. Epilepsy diagnostic classification was determined by a pediatric epileptologist who reviewed all available medical records, including EEGs and seizure semiology, and was blinded to results from neuropsychological evaluation and MRI. Patients were determined to have JME if the following criteria were present: 1) 4-6 Hz polyspike and slow-wave generalized discharges, 2) history of myoclonic jerks, and 3) history of generalized tonic-clonic seizures (GTCS). BCECTS was diagnosed by the presence of either simple partial seizures during the waking hours or tonic clonic convulsive seizures at night associated with the presence of centrotemporal spikes on EEG, occurring independently in the right and left centrotemporal regions. Activation of spike wave charges with sleep was also considered a feature suggestive of this syndrome.
Control participants were first-degree cousins with no history of: 1) any initial precipitating event (e.g., simple or complex febrile seizures), 2) any seizure of seizure-like episode, 3) diagnosed neurological disease, 4) loss of consciousness due to trauma greater than five minutes, or 5) other family history of first-degree relative with epilepsy or febrile convulsions. First degree cousins were used as controls rather than siblings for the following reasons: 1) first degree cousins are more genetically distant from the participants with epilepsy and thus less predisposed than siblings to shared genetic factors that may contribute to anomalies in brain structure and cognition, 2) a greater number of first degree cousins are available than siblings in the target age range, and 3) the family link was anticipated to facilitate participant recruitment and retention over time.
Neuropsychological Evaluation
On the day of study participation, families and children gave informed consent or assent for participation in comprehensive neuropsychological testing and completion of behaviorally-oriented questionnaires by parents. Executive functioning was measured by the Delis-Kaplan Executive Function System (D-KEFS; Delis, Kaplan, & Kramer, 2001) and Behavior Rating Inventory of Executive Function (BRIEF; Gioia, Isquith, Guy, & Kenworthy, 2000). For safety reasons, the research assistants were aware of each participant's epilepsy status.
D-KEFS
Three subtests, the Sorting Test (Confirmed Correct Sort), Verbal Fluency Test (Category Switching Accuracy), and the Color-Word Interference Test (Inhibition), were used to examine executive function. Psychometric findings (e.g., test-retest reliability, internal consistency, reliability) for the D-KEFS measures are considered to be very good (Delis et al., 2004). The Sorting Test is a multi-condition task in which individuals are presented with six mixed-up cards that display both stimulus words and perceptual features. Examinees are asked to sort the cards into two groups in as many different categorizations as possible, while simultaneously explaining their rationale. The second condition requires that the examiner sort the cards into two groups while the examinee identifies the categorization rule. The Verbal Fluency task consists of multiple 60-second trials in which examinees are asked to generate words that begin with a particular letter, generate words that belong to a designated semantic category, or alternate between two different semantic categories. The Color-Word Interference Test requires that examinees rapidly read words, read colors, or name the dissonant ink colors in which alternate color words are printed. This task is highly similar to the well-known Stroop Task.
BRIEF
The BRIEF is an 86-item parent questionnaire. Parents indicate the frequency which they believe their children to exhibit certain behaviors (e.g., “Interrupts others,” “Is not a self-starter,” “Has a messy closet”). Two core summary index scores, the Behavioral Regulation Index (BRI) and Metacognition Index (MCI), were examined. Reliability studies show high internal consistency and test-retest reliability. Convergent validity was established with other measures of inattention, impulsivity, and learning skills in clinical ADHD populations (Gioia et al., 2000). Table 1 provides a brief summary of the domains assessed by each of the D-KEFS and BRIEF measures.
Table 1. Overview of cognitive measures.
| Measures | Domains Assessed |
|---|---|
| D-KEFS | |
| Card Sorting (Confirmed Correct Sorts) | Concept formation, ability to initiate problem solving, cognitive flexibility, and perseveration |
| Verbal Fluency (Category Switching Accuracy) | Verbal set-shifting, phonemic and semantic fluency |
| Color-Word Interference (Inhibition) | Inhibition of a prepotent response |
| BRIEF | |
| Behavioral Regulation | Inhibition, shifting, and emotional control |
| Metacognition | Initiation, working memory, planning and organizing, and self-monitoring |
Rationale for measure selection
The D-KEFS and BRIEF were selected as measures of executive function because of their psychometric soundness in detecting changes in executive functioning. Several studies have demonstrated the sensitivity of the D-KEFS for evaluating executive function in a variety of both adult and child clinical populations including children with epilepsy (McDonald et al., 2005; Parrish et al., 2007). The BRIEF has been used to assess executive function in several pediatric samples, including traumatic brain injury, attention deficit hyperactivity disorder, and hydrocephalus (Jarratt et al., 2005; Mahone et al., 2002; Mangeot et al., 2002). Both measures have demonstrated that children with recent onset epilepsy show significant difficulties in executive functioning (Parrish et al., 2007).
MRI Procedures
Images were obtained on a 1.5 Tesla GE Signa MR scanner. Sequences acquired for each participant included: 1) T1-weighted, three-dimensional SPGR acquired with the following parameters: TE = 5, TR = 24, flip angle = 40, NEX = 2, FOV =26, slice thickness = 1.5 mm, slice plane = coronal, matrix = 256×192; 2) Proton Density (PD), and 3) T2-weighted images acquired with the following parameters: TE = 36 msec (for PD) or 96 msec (for T2), TR = 3000 msec, NEX = 1, FOV = 26, slice thickness = 3.0 mm, slice plane = coronal, matrix = 256×192, and an echo train length = 8.
MRIs were processed on Linux workstations using a semi-automated software package, Brain Research: Analysis of Images, Networks, and Systems (BRAINS2) (Harris et al., 1999; Magnotta et al., 2002). MR processing staff was blinded to all clinical and sociodemographic characteristics of the participants. MR preprocessing has been described elsewhere (Oyegbile et al., 2006; Seidenberg et al., 2005). Briefly, T1, T2, and PD images were realigned to a standard orientation, coregistered, and resampled to 1 mm3 voxels. This process establishes the horizontal axis of the brain to the anterior commissure-posterior commissure (ACPC) line and allows for voxel by voxel correspondence between the three images. An automated tissue classification algorithm (Harris et al., 1999) was employed to create a continuously segmented image in which voxels were classified as gray matter, white matter, cerebrospinal fluid, or blood. Brain images were then volume rendered using local utilities, producing tissue volumes for regions of interest (ROIs) within the brain (i.e., thalamus and frontal lobes).
Thalamus Guidelines
A BRAINS2 automated neural network and additional guidelines established at the University of Iowa were used to guide the thalamus trace (Ooteman & Crestinger, 2006). Images were traced in the coronal plane using a color-enhanced T1 with reference to the segmented image and unenhanced T1. The thalamus was traced rostral to caudal with the most anterior portion of the thalamus determined by the neural net and using the presence of the anterior commissure. The genu and posterior limb of the internal capsule served as the lateral border and cerebrospinal fluid (CSF) of the third ventricle served as the medial border. Both the left and right portions of the thalamus were traced separately, excluding the massa intermedia when present. The superior border was determined by the lateral ventricles throughout, and the fornix in more posterior slices. The traces extended caudally and included both the lateral and medial geniculate bodies. The thalamus protruded posteriorly until coming into contact with either the atrium of the lateral ventricle, the tail of the hippocampus, or both structures. Volumes were obtained by an automated process that sums the total amount of voxels included within the region of interest. An inter-rater reliability of .98 for tracing the thalamus in our lab has already been achieved.
Frontal Guidelines
Frontal lobe volumes (grey matter, white matter,, and CSF) were obtained through an automated process whereby BRAINS2 uses anatomical Talairach-based landmarks to partition the cortex into lobes. Anatomical boundaries of the frontal lobe included the central sulcus as the posterior boundary, the temporal lobes as the lateral boundary, and the mesial border as the corpus callosum. CSF volumes largely consisted of surface CSF (i.e., surrounding the brain), although a small portion of the anterior horn of the lateral ventricles was included in this measurement for some subjects. Figure 1 shows axial, coronal, and sagittal views of thalamic and frontal lobe regions of interest.
Figure 1.
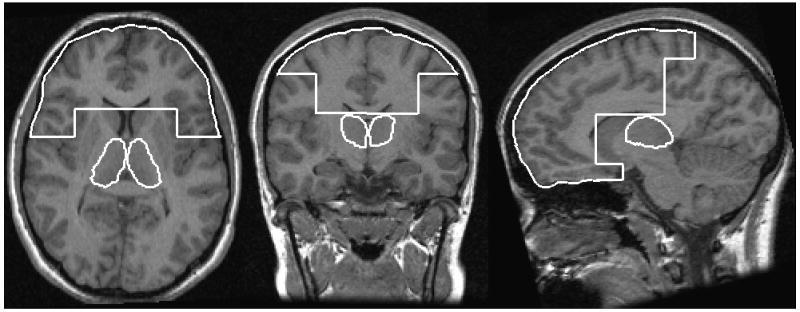
Axial, coronal, and sagittal views of thalamic and frontal lobe regions of interest.
Statistical Analyses
In order to adjust for head size volumetric differences, all thalamic volumes were adjusted for intracranial volume (ICV), which was calculated by summing total brain tissue volume and CSF. The three groups' ICVs did not significantly differ [F (2, 83) = 2.07, p = .13]. Additionally, to account for any age-related volume influences, analyses were also corrected for age. Correlations between age and volume for each group were first examined. What was found? A multiple analysis of covariance (MANCOVA), with ICV and age as covariates, was conducted to compare volumes between groups.
Age-corrected standardized scores on the D-KEFS and BRIEF were used (i.e., scaled scores with a mean of 10 and standard deviation of three or T scores with a mean of 50 and standard deviation of 10 were derived Thus, scores were rendered comparable across differing ages, and as such are intrinsically age-corrected. A multiple analysis of variance (MANOVA) was used to inspect group differences on executive function measures. Bonferroni corrections were used to correct for multiple comparisons in follow-up one-way ANCOVA comparisons.
Simultaneous regression was used to predict cognitive measures from brain volumes for each group. Cohen's d was used to report effect sizes. According to standard convention, small effect sizes are d < .50, medium effect sizes are d = .50-.79, and large effect sizes are d > .80 (Cohen, 1992). For the sake of consistency, negative effect sizes indicate greater impairment in the epilepsy group compared to the control group. Negative effect sizes in comparisons between the two epilepsy groups indicate greater impairment in the JME group.
Results
Demographic and Clinical Seizure Variables
Table 2 displays demographic, IQ, and clinical seizure information for all groups. The three groups differed significantly in age [F (2, 83) = 12.86, p < .001]. The JME group was significantly older than both the control and BCECTS groups, as would expected given the typical ages of onset of these seizure syndromes. Controls were also significantly older than the BCECTS group. Because education level was strongly correlated with age (r = .98, p < .001 for the combined group), the same pattern of group differences seen for age was also observed for education level [F (2, 83) = 13.88, p < .001]. As both epilepsy groups had epilepsy onset within one year of evaluation, the two groups did not differ on duration of epilepsy [t (30) = -1.23, p = .23]. The three groups did not differ from one another in gender makeup [χ2 (2) = .997, p = .61]. IQ scores did not differ between groups and were in the average range for all groups [F (6, 156) = .55, p = .77].
Table 2. Demographic and clinical seizure characteristics.1.
| Groups | |||
|---|---|---|---|
| Controls (n = 51) |
BCECTS (n = 12) |
JME (n = 20) |
|
| Chronological age (years) | 13.24 (3.14) | 10.15 (1.48) | 15.48 (2.84) |
| Gender (M/F) | 22/29 | 7/5 | 10/10 |
| Education (years) | 7.10 (.05) | 4.00 (1.35) | 9.45 (2.93) |
| Full Scale IQ2 | 107.08 (12.97) | 108.42 (13.91) | 104.05 (13.52) |
| Verbal IQ | 106.57 (14.16) | 107.08 (10.74) | 102.85 (13.00) |
| Performance IQ | 105.90 (12.65) | 108.17 (16.48) | 103.15 (13.97) |
| Age of epilepsy onset (years) | __ | 9.24 (1.55) | 14.48 (3.03) |
| Duration of epilepsy (months) | __ | 7.00 (5.01) | 8.90 (3.73) |
| Antiepileptic Drugs (frequency) | __ | 4* | 20 |
| Carbamazepine | __ | 2 | __ |
| Oxcarbazepine | __ | 2 | 4 |
| Divalproex sodium | __ | __ | 16 |
All information is Mean (Standard Deviation)
As measured by the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III)
8 patients were not on AEDs
MRI Volumes
Linear and nonlinear correlational models were tested to determine the relationship between age and volumes (i.e., grey matter, white matter, and CSF). No statistically significant linear or nonlinear relationships were found between MR volume and age in either of the epilepsy groups (all p's > .05). However, significant correlations between age and CSF (r = .43, p = .002), grey matter (r = -.74, p < .001), and white matter (r = .60, p < .001) were found in the control group. Therefore, age remained as a covariate in all analyses. Thalamic volumes were not correlated with age in any group or as a combined group (all p's > .05).
MANCOVA (ICV and age as covariates) indicated a trend for an overall between-group significant difference in the linear combination of volumetric variables [F (10, 148) = 1.68, p = .09]. Examination of between-subjects effects for the specific volumes revealed significant group differences for frontal CSF (p = .003) and the right thalamus (p = .04). Follow-up one-way ANCOVAs indicated that the JME group had significantly smaller right thalami [F (1, 78) = 6.10, p = .02] and more frontal CSF [F (1, 78) = 11.58, p = .001] than the controls, with moderate to large effect sizes (d's = .66-.91). The JME group also had significantly more frontal CSF than the BCECTS group [F (1, 78] = 7.56, p = .007], also with a large effect size (d = 1.08). None of the groups significantly differed on left thalamic, frontal white matter, or frontal grey matter volumes, although the JME group had less volume than the other two groups in each of these instances. All other lobes' grey matter, white matter, and CSF were also tested for group differences to determine if frontal findings were region specific or more generalized [F (18, 140) = 1.64, p = .06). Although a trend towards significance was found for the linear combination of lobular volumes, no group differences were found for tissue type or CSF in any other lobe (all p's > .05). Table 3 shows mean frontal and thalamic volumes and effect sizes adjusted for age and ICV.
Table 3. Frontal and thalamic volumes (age and ICV adjusted) and effect sizes. 1.
| Groups | Effect Size2 | ||||||
|---|---|---|---|---|---|---|---|
| Controls (n = 51) |
BCECTS (n = 12) |
JME (n = 20) |
C vs. B | C vs. J | B vs. J | ||
| Frontal Lobe (cc3) | CSF | 21.75 (10.96) | 19.25 (12.12) | 32.13 (11.74) | -.21 | -.91 | -1.08 |
| Grey | 305.24 (14.95) | 308.87 (16.53) | 300.92 (16.01) | .23 | -.28 | -.49 | |
| White | 154.99 (13.75) | 156.94 (15.21) | 150.99 (14.74) | .14 | -.28 | -.40 | |
| Thalamus (cc3) | Left | 6.62 (.49) | 6.48 (.54) | 6.43 (.52) | -.28 | -.38 | -.10 |
| Right | 6.88 (.51) | 6.71 (.56) | 6.53 (.55) | -.32 | -.66 | -.33 | |
All information is Mean (Standard Deviation)
C = control group; B = BCECTS group; J = JME group
Executive Function Performance
As expected, age was not correlated with any of the standardized scores (all p's > .05) in the combined or three groups and was subsequently not further accounted for. MANOVA with age-adjusted standardized scores showed significant group differences in overall executive functioning performance [F (10,152) = 2.18, p = .02]. One-way post-hoc ANOVAs indicated that the JME group had significantly poorer performance than the control group on D-KEFS Inhibition (p = .01), and the Behavioral Regulation (p = .03) and Metacognition (p = .006) indexes from the BRIEF, with moderate to large effect sizes. Post-hoc comparisons showed no significant differences between the BCECTS and control groups or between BCECTS and JME groups on any executive function measures (all p's > .05). Table 4 shows mean scores and effect sizes for all three groups.
Table 4. Executive function measure scores. 1.
| Groups | Effect Size2 | ||||||
|---|---|---|---|---|---|---|---|
| Controls (n = 51) |
BCECTS (n = 12) |
JME (n = 20) |
C vs. B | C vs. J | B vs. J | ||
| D-KEFS (ScS)a | Category Switching Accuracy | 10.76 (2.70) | 12.58 (3.99) | 11.05 (2.95) | .53 | .10 | .44 |
| Inhibition | 11.43 (2.35) | 11.25 (2.09) | 9.30 (3.69) | -.08 | -.69 | -.65 | |
| Correct Card Sorts | 10.20 (2.21) | 10.25 (3.55) | 9.65 (2.54) | .02 | -.23 | -.19 | |
| BRIEF (T)b | Behavioral Regulation | 46.39 (8.62) | 48.33 (8.87) | 53.20 (12.86) | -.22 | -.62 | -.44 |
| Metacognition | 47.59 (8.57) | 50.58 (12.19) | 56.40 (13.19) | -.28 | -.90 | -.50 | |
All information is Mean (Standard Deviation)
C = control group; B = BCECTS group; J = JME group
ScS = Scaled Scores, Mean = 10 ± 3; lower scores indicate greater impairment
T = T scores, Mean = 50 ± 10; higher scores indicate greater impairment
Volume and Cognition Relationships
Standard simultaneous regression indicated that frontal tissue and thalamic volumes were the strongest and only statistically significant predictors of performance on D-KEFS measures. Age and ICV were entered in the first block to control for their effects on frontal and thalamic volumes. For the JME group, bilateral thalamic volumes significantly predicted Confirmed Correct Card Sorts (p's < .03), while frontal grey and white matter each individually predicted Category Switching Accuracy (p's ≤ .001) and frontal grey matter predicted performance on Inhibition (p = .05). Frontal white matter significantly predicted Confirmed Correct Card Sorts (p = .02) for the control group. No volumes were significant predictors in the BCECTS group, nor were volumes significant predictors of BRIEF ratings in any group (all p's < .05). Results of only the significant regression analyses are shown in Table 5. Effect sizes were calculated using t-values and degrees of freedom.
Table 5. Regression analyses predicting executive functioning from volumes.
| Predictor | R | R2 | R2 change | Adjusted R2 | Standardized β | p (d) | |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| Correct Card Sorts | FW | .45 | .20 | .16 | .07 | -.67 | .02 (.75) |
| JME | |||||||
| Category Switching Accuracy | FG | .86 | .73 | .66 | .57 | -2.04 | .001 (2.55) |
| FW | 2.17 | < .001 (2.77) | |||||
| Inhibition | FW | .67 | .45 | .43 | .13 | 1.38 | .05 (1.23) |
| Correct Card Sorts | LTh | .78 | .61 | .45 | .39 | 1.62 | .02 (1.63) |
| RTh | -1.54 | .01 (1.79) | |||||
FW= frontal white matter; FG = frontal grey matter; LTh = left thalamus; RTh = right thalamus
Discussion
The present study provides evidence for structural abnormalities of the thalamus, and to a lesser extent, the frontal lobes, early in the course of recent onset JME. These findings were not observed in a sample of children with recent onset BCECTS. Our results also show that executive dysfunction, as measured by both cognitive and behavioral measures, is seen early in the course of JME, compared to healthy controls and the BCECTS. Finally, both thalamic and frontal volumes were significant predictors of cognitive performance only in the JME group. These findings demonstrate clinically significant disruption of thalamofrontal circuitry in JME related to dysregulated executive functioning.
The appearance of volumetric abnormalities within 12 months of seizure presentation in JME is noteworthy. Subjects with JME had a mean duration of epilepsy of less than nine months since diagnosis. As reported earlier, all studies to date examining brain morphology in JME have been conducted in adults, while studies of cognitive functioning have examined children or adults with chronic and well established JME (Betting et al., 2006a; Devinsky et al., 1997; Koepp, 2005; Pascalicchio et al., 2007; Woermann et al., 1999). The precise onset of the observed abnormalities remains to be determined. The assessment of children with JME at the time of diagnosis and prior to administration of AEDs would provide further information regarding the early developmental origin of these findings. JME is known to have a strong genetic inheritance, with a family history in as many as 50% of cases (Zupanc, 1996). We cannot rule out the possibility that genetic vulnerability may underlie, at least in part, the observed macrostructural abnormalities in the thalamus and frontal lobes or their related functioning and may be present at the time of, or even prior, to the first recognized seizure. Nevertheless, our findings suggest that these abnormalities are not simply the result of factors associated with chronic duration of seizures. It also has been suggested that thalamic dysfunction in IGE may be progressive (Bernasconi et al., 2003), although this remains to be determined because few studies have assessed individuals longitudinally. Prospective longitudinal study is needed to chart the course of thalamic and frontal development over the course of JME.
Comparisons between the BCECTS and JME groups also help distinguish between the shared effects of early onset epilepsy versus the unique effects associated with specific epilepsy syndromes. The greatest frontal and thalamic effects were seen for the JME group, with the BCECTS group evidencing only small effect sizes in comparison to the control group. In contrast to JME, thalamofrontal circuitry is not believed to play a significant a role in children with BCECTS, and MRI volumetric abnormalities of these regions have not been reported in this syndrome (Boxerman et al., 2007). Mild executive dysfunction in BCECTS is rare and has only been reported in individuals with focal spikes localized in central frontal regions, although the thalamus has not been implicated in this instance or in individuals without frontally localized spikes (Croona et al., 1999; Wolff et al., 2005). Contributions of the thalamus and frontal lobe in BCECTS appear minimal and suggest syndrome specific structural abnormalities in JME. The lack of group differences in the other lobes also indicates that reported thalamic volume loss is not a reflection of whole brain volume loss.
Structural neuroimaging studies in JME and other IGEs have shown that anterior and medial nuclei of the thalami demonstrate the greatest volumetric and density abnormalities (Betting et al., 2006a; Chan et al., 2006; Ciumas and Savic, 2006). The anterior and medial thalamus project to the dorsolateral prefrontal cortex via the anterior thalamic radiation (Masterman and Cummings, 1997; Radanovic et al., 2003), supporting other research that thalamofrontal dysfunction is a major mechanism in JME. The dorsolateral prefrontal circuit is most frequently associated with executive functions. The anterior thalami's connections to the frontal cortex are also implicated in the pathway underlying executive functions (Sandson et al., 1991; Carrera and Bogousslavsky, 2006; Maxwell et al., 2006; Van der Werf et al., 2003; Van der Werf et al., 2000). This circuit originates on the lateral surface of the anterior frontal lobes and projects to the dorsolateral head of the caudate nucleus, which then project to the mediodorsal globus pallidus interna and rostrolateral substantia nigra pars reticula. Fibers from the globus pallidus project to the parvocelluar portions of the ventral anterior and mediodorsal thalamus, projecting fibers back to the dorsolateral frontal cortex to complete the circuit (Tekin & Cummings, 2002). Gloor (1979) first suggested that an abnormal cortical response to thalamocortical afferents may disrupt normal mental activity in generalized epilepsy. To assess if the thalamofrontal circuit was related to the observed pattern of executive functioning, a series of regression analyses were conducted.
Results of the regression analyses indicated that segmented frontal tissue and thalamic volumes were significant predictors of D-KEFS performance only for the JME group, which was an entirely different pattern than was observed in normal controls, while frontal white matter was the only significant predictor in the control group. The Card Sorting Test of the D-KEFS involves multiple executive function components, such as problem solving and cognitive flexibility. It is possible that different aspects of executive functions (e.g., problem solving, working memory) underlie the relationships in each group. The varying underlying components that comprise these tasks could also explain the absence of group differences on Category Switching Accuracy and Correct Card Sorts from the D-KEFS. All three D-KEFS measures examined were significantly predicted by frontal tissue or thalamic volumes in the JME group, and none of the volumes significantly predicted performance in the BCECTS group. Notably absent from the results is the lack of significant predictors for the BRIEF in any group. A stronger relationship was found between volumes and cognitive measures than with parent report measures. Although the BRIEF is believed to be a valid measure of executive functions in pediatric samples (Jarratt et al., 2005; Mahone et al., 2002; Mangeot et al., 2002), there remains some debate as to the consistency of relationships between cognitive and behavioral measures of executive functioning. Small but significant correlations (r = .28 - .33) between D-KEFS subscales and the BRIEF's Metacognition Index have been reported in childhood epilepsy, while no significant D-KEFS-BRI correlations have been found (Parrish et al., 2007). It is possible that each measure is assessing different aspects of the multifaceted domain of executive functions. Table 1 demonstrates that the constructs that comprise the subscales of both measures share a number of similarities and differences.
It is not clear if the observed volumetric abnormalities soon after seizure onset are specific to JME or if they are applicable to other IGEs. Thalamocortical circuitry is known to be a primary pathological factor in multiple IGE syndromes (Moeller et al., 2007), although the extent to which this circuit is involved or structurally affected may depend upon syndrome and seizure types. For example, individuals with chronic childhood absence epilepsy (mean duration of 11 years) showed increased thalamic grey matter volume loss relative to marginal white matter loss in the basal frontal lobe and cingulate compared to healthy controls (Chan et al., 2006). Similarly, we found greater volume loss in the thalamus than in the frontal lobes. It is possible that this is a typical pattern seen in IGEs in which the developmental trajectories of these structures differ slightly. A heterogenous IGE sample showed increased gray matter content in the anterior thalamus in patients with absence epilepsy (Betting et al., 2006a; Betting et al., 2006b). However, the effect of various combinations of seizure types is unknown. This may be an important factor, as myoclonic, absence, and generalized tonic-clonic seizure types are frequently seen in JME (Duncan, 1997; Zupanc, 1996).
A number of limitations of this study should be noted. The most apparent limitation was the lack of age-matched groups. The JME group was five years older than BCECTS and two years older than healthy controls. However, we do not feel that theses age differences significantly contributed to our findings for a number of reasons. Executive function standardized scores were derived from age-based normative data, thus rendering the scores directly comparable between age groups. Transformation to these standardized scores removed variance associated with age in all subjects. Furthermore, no significant correlations between age and standardized scores were found for the combined group or for any of the groups separately. To further be certain of the lack of influence of age on the executive measure scores, analyses were re-conducted using age as a covariate. The pattern of findings was exactly the same.
Volumes were also statistically corrected (i.e., covaried) for age in each analysis. Age was not significantly correlated with thalamic volumes, which was the only significant tissue difference found between groups. Similarly, there was no relationship between age and frontal CSF in the two epilepsy groups. The significant correlation between frontal CSF and age in the control suggests that caution should be used in interpreting these findings. However, tissue or CSF differences were not seen in any of the other lobes, suggesting that our findings are region-specific and do not merely reflect global atrophy or age-associated effects.
Second, our sample sizes in this study were relatively small, particularly for the BCECTS group. As a result, our study may have been underpowered to detect subtle volume loss or neuropsychological performance in this group. Greater caution should be used in the interpretation of results related to syndrome specific findings. However, we demonstrated moderate and even large effects sizes on volumetric and cognitive differences between the JME group and healthy controls. The present results must be replicated with a larger group of epilepsy subjects to confirm the findings. Also related to our sample is the issue of the use of patients' cousins as controls may have underestimated the current findings, as they obviously share genetic similarities (i.e., 1/8 of their genes).
Third, this study used only quantitative volumetric analyses of the whole thalamus which precluded the ability to determine which area or nuclei of the thalamus are primarily affected. It would be of benefit to determine if the anterior and medial nuclei, those with prefrontal projections, account for the volume loss.
Last, we were unable to determine the contribution of medications to brain volumes and cognitive functioning. All JME subjects in this study were on monotherapy (divalproex sodium or oxcarbazepine). Two recent studies reported that the use of oxcarabazepine and carbamazepine did not result in cognitive (including executive) decline from baseline (pre-AED) to six months in children with partial seizures (Donati et al., 2006; Donati et al., 2007). Divalproex sodium (the primary AED in the JME group) has been shown to have the fewest cognitive side effects compared to other first-line AEDs (e.g., phenytoin, carbamazepine), with one study indicating improved memory in children on valproate compared to the other AEDs (Hirsch, Schmitz, & Carreno, 2003; Forsythe, Butler, Berg, McGuire, 1991). Thus, the literature suggests that the medications used in this study do not adversely affect cognitive functioning. We report that intelligence was found to well within the average range for all three groups, with no significant group differences. Thus, epilepsy subjects in this study did not exhibit global cognitive deficits, further supporting the notion that AED effects did not significantly contribute to group differences in executive functions. However, further research pertaining to the effects of specific AEDs on executive functions is needed. An examination of patients prior to the introduction of AEDs, particularly divalproex sodium, would most effectively rule out potential treatment contributions to the volumetric and neuropsychological effects. There are also no known reports of the effects of these specific AEDs on brain volume in children.
In summary, the current study results point to a specific frontal-thalamic brain volume abnormality with important cognitive implications in recent onset pediatric JME. Longitudinal study is needed to determine the course of this relationship and its implications for neuropsychological functioning.
Acknowledgments
This work was supported by NIH grants NINDS RO1 44351 and MO1 RR 03186 (GCRC). This investigation was completed with the help of Michelle Szomi who was responsible for overall project coordination and Kevin Dabbs for coordinating the MR scan analyses. We sincerely thank Drs Fred Edelman, Carl Stafstrom, David Hsu, and Jason Dozier for referring patients to this investigation.
Footnotes
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
None of the authors has any conflict of interest to disclose.
References
- Anderson V. Assessing executive functions in children: biological, psychological, and developmental considerations. Pediatr Rehabil. 2001;4:119–136. doi: 10.1080/13638490110091347. [DOI] [PubMed] [Google Scholar]
- Bernasconi A, Bernasconi N, Natsume J, Antel SB, Andermann F, Arnold DL. Magnetic resonance spectroscopy and imaging of the thalamus in idiopathic generalized epilepsy. Brain. 2003;126:2447–2454. doi: 10.1093/brain/awg249. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Li LM, Lopes-Cendes I, Guerreiro MM, Guerreiro CA, Cendes F. Voxel-based morphometry in patients with idiopathic generalized epilepsies. Neuroimage. 2006a;32:498–502. doi: 10.1016/j.neuroimage.2006.04.174. [DOI] [PubMed] [Google Scholar]
- Betting LE, Mory SB, Lopes-Cendes I, Li LM, Guerreiro MM, Guerreiro CA, Cendes F. MRI volumetry shows increased anterior thalamic volumes in patients with absence seizures. Epilepsy Behav. 2006b;8:575–580. doi: 10.1016/j.yebeh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Boxerman JL, Hawash K, Bali B, Clarke T, Rogg J, Pal DK. Is Rolandic epilepsy associated with abnormal findings on cranial MRI? Epilepsy Res. 2007;75:180–185. doi: 10.1016/j.eplepsyres.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera E, Bogousslavsky J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. 2006;66:1817–1823. doi: 10.1212/01.wnl.0000219679.95223.4c. [DOI] [PubMed] [Google Scholar]
- Chan CH, Briellmann RS, Pell GS, Scheffer IE, Abbott DF, Jackson GD. Thalamic atrophy in childhood absence epilepsy. Epilepsia. 2006;47:399–405. doi: 10.1111/j.1528-1167.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67:683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Craiu D, Magureanu S, van Emde Boas W. Are absences truly generalized seizures or partial seizures originating from or predominantly involving the pre-motor areas? Some clinical and theoretical observations and their implications for seizure classification. Epilepsy Res. 2006;70:S141–S155. doi: 10.1016/j.eplepsyres.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Crespel A, Baldy-Moulinier M, Coubes P. The relationship between sleep and epilepsy in frontal and temporal lobe epilepsies: practical and physiopathologic considerations. Epilepsia. 1998;39:150–157. doi: 10.1111/j.1528-1157.1998.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Croona C, Kihlgren M, Lundberg S, Eeg-Olofsson O, Eeg-Olofsson KE. Neuropsychological findings in children with benign childhood epilepsy with centrotemporal spikes. Dev Med Child Neurol. 1999;41:813–818. doi: 10.1017/s0012162299001620. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. The Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10:301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Gershengorn J, Brown E, Perrine K, Vazquez B, Luciano D. Frontal functions in juvenile myoclonic epilepsy. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:243–246. [PubMed] [Google Scholar]
- Donati F, Gobbi G, Campistol J, Rapatz G, Daehler M, Sturm Y, Aldenkamp AP. Effects of oxcarbazepine on cognitive function in children and adolescents with partial seizures. Neurology. 2006;67:679–682. doi: 10.1212/01.wnl.0000230138.46508.5b. [DOI] [PubMed] [Google Scholar]
- Donati F, Gobbi G, Campistol J, Rapatz G, Daehler M, Sturm Y, Aldenkamp AP. The cognitive effects of oxcarbazepine versus carbamazepine or valproate in newly diagnosed children with partial seizures. Seizure. 2007;16:670–679. doi: 10.1016/j.seizure.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Idiopathic generalized epilepsies with typical absences. J Neurol. 1997;244:403–411. doi: 10.1007/s004150050113. [DOI] [PubMed] [Google Scholar]
- Forsythe I, Butler R, Berg I, McGuire R. Cognitive impairment in new cases of epilepsy randomly assigned in carbamazepine, phenytoin and sodium valproate. Dev Med Child Neurol. 1991;33:524–534. doi: 10.1111/j.1469-8749.1991.tb14917.x. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child Neuropsychol. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Gloor P, Pellegrini A, Kostopoulos GK. Effects of changes in cortical excitability upon the epileptic bursts in generalized penicillin epilepsy of the cat. Electroencephalogr Clin Neurophysiol. 1979;46:274–289. doi: 10.1016/0013-4694(79)90202-5. [DOI] [PubMed] [Google Scholar]
- Gotman J, Grova C, Bagshaw A, Kobayashi E, Aghakhani Y, Dubeau F. Generalized epileptic discharges show thalamocortical activation and suspension of the default state of the brain. Proc Natl Acad Sci U S A. 2005;102:15236–15240. doi: 10.1073/pnas.0504935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, Bartolomei F. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. Journal of Comput Assist Tomogr. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Hessen E, Lossius MI, Reinvang I, Gjerstad L. Influence of major antiepileptic drugs on attention, reaction time, and speed of information processing: results from a randomized, double-blind, placebo-controlled withdrawal study of seizure-free epilepsy patients receiving monotherapy. Epilepsia. 2006;47:2038–2045. doi: 10.1111/j.1528-1167.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Schmitz B, Carreno M. Epilepsy, antiepiletic drugs (AEDs), and cognition. Acta Neurol Scand Suppl. 2003;180:23–32. doi: 10.1034/j.1600-0404.108.s180.4.x. [DOI] [PubMed] [Google Scholar]
- Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46:10–14. doi: 10.1111/j.1528-1167.2005.00309.x. [DOI] [PubMed] [Google Scholar]
- Jarratt KP, Riccio CA, Siekierski BM. Assessment of attention deficit hyperactivity disorder (ADHD) using the BASC and BRIEF. Appl Neuropsychol. 2005;12:83–93. doi: 10.1207/s15324826an1202_4. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee JK, Koh SB, Lee SA, Lee JM, Kim SI, Kang JK. Regional grey matter abnormalities in juvenile myoclonic epilepsy: a voxel-based morphometry study. Neuroimage. 2007;37:1132–1137. doi: 10.1016/j.neuroimage.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Klenberg L, Korkman M, Lahti-Nuuttila P. Differential development of attention and executive function in 3- to 12-year-old Finnish children. DevNeuropsychol. 2001;20:407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- Koepp MJ. Juvenile myoclonic epilepsy--a generalized epilepsy syndrome? Acta Neurol Scand Suppl. 2005;181:57–62. doi: 10.1111/j.1600-0404.2005.00511.x. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Zabel TA, Levey E, Verda M, Kinsman S. Parent and self-report ratings of executive function in adolescents with myelomeningocele and hydrocephalus. Child Neuropsychol. 2002;8:258–270. doi: 10.1076/chin.8.4.258.13510. [DOI] [PubMed] [Google Scholar]
- Mangeot S, Armstrong K, Colvin AN, Yeates KO, Taylor HG. Long-term executive function deficits in children with traumatic brain injuries: assessment using the Behavior Rating Inventory of Executive Function (BRIEF) Child Neuropsychol. 2002;8:271–284. doi: 10.1076/chin.8.4.271.13503. [DOI] [PubMed] [Google Scholar]
- Masterman DL, Cummings JL. Frontal-subcortical circuits: the anatomic basis of executive, social and motivated behaviors. J Psychopharmacol. 1997;11:107–114. doi: 10.1177/026988119701100203. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, MacKinnon MA, Smith DH, McIntosh TK, Graham DI. Thalamic nuclei after human blunt head injury. J Neuropathol Exp Neurol. 2006;65:478–488. doi: 10.1097/01.jnen.0000229241.28619.75. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Delis DC, Norman MA, Tecoma ES, Iragui VJ. Discriminating patients with frontal-lobe epilepsy and temporal-lobe epilepsy: utility of a multilevel design fluency test. Neuropsychology. 2005;19:806–813. doi: 10.1037/0894-4105.19.6.806. [DOI] [PubMed] [Google Scholar]
- Moeller F, Siebner HR, Wolff S, Muhle H, Boor R, Granert O, Jansen O, Stephani U, Siniatchkin M. Changes in activity of striato-thalamo-cortical network precede generalized spike wave discharges. Neuroimage. 2007;39:1839–1849. doi: 10.1016/j.neuroimage.2007.10.058. [DOI] [PubMed] [Google Scholar]
- Mory SB, Li LM, Guerreiro CA, Cendes F. Thalamic dysfunction in juvenile myoclonic epilepsy: a proton MRS study. Epilepsia. 2003;44:1402–1405. doi: 10.1046/j.1528-1157.2003.67702.x. [DOI] [PubMed] [Google Scholar]
- Nordli DR., Jr Idiopathic generalized epilepsies recognized by the International League Against Epilepsy. Epilepsia. 2005;46:45–56. doi: 10.1111/j.1528-1167.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Crestinger K, Vol. 2006 University of Iowa, Mental Health Clinical Research Center.
- Oyegbile TO, Bhattacharya A, Seidenberg M, Hermann BP. Quantitative MRI biomarkers of cognitive morbidity in temporal lobe epilepsy. Epilepsia. 2006;47:143–152. doi: 10.1111/j.1528-1167.2006.00380.x. [DOI] [PubMed] [Google Scholar]
- Parrish J, Geary E, Jones J, Seth R, Hermann B, Seidenberg M. Executive functioning in childhood epilepsy: parent-report and cognitive assessment. Dev Med Child Neurol. 2007;49:412–416. doi: 10.1111/j.1469-8749.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- Pascalicchio TF, de Araujo Filho GM, da Silva Noffs MH, Lin K, Caboclo LO, Vidal-Dourado M, Ferreira Guilhoto LM, Yacubian EM. Neuropsychological profile of patients with juvenile myoclonic epilepsy: a controlled study of 50 patients. Epilepsy Behav. 2007;10:263–267. doi: 10.1016/j.yebeh.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Piazzini A, Turner K, Vignoli A, Canger R, Canevini MP. Frontal cognitive dysfunction in juvenile myoclonic epilepsy. Epilepsia. 2007 doi: 10.1111/j.1528-1167.2007.01482.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Radanovic M, Azambuja M, Mansur LL, Porto CS, Scaff M. Thalamus and language: interface with attention, memory and executive functions. Arq Neuropsiquiatr. 2003;61:34–42. doi: 10.1590/s0004-282x2003000100006. [DOI] [PubMed] [Google Scholar]
- Samagano-Sprouse C. In: The Human Frontal Lobes. Miller BL, Cummings JL, editors. The Guilford Press; New York: 2007. pp. 576–596. [Google Scholar]
- Sandson TA, Daffner KR, Carvalho PA, Mesulam MM. Frontal lobe dysfunction following infarction of the left-sided medial thalamus. Arch Neurol. 1991;48:1300–1303. doi: 10.1001/archneur.1991.00530240106031. [DOI] [PubMed] [Google Scholar]
- Savic I, Osterman Y, Helms G. MRS shows syndrome differentiated metabolite changes in human-generalized epilepsies. Neuroimage. 2004;21:63–172. doi: 10.1016/j.neuroimage.2003.08.034. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Kelly KG, Parrish J, Geary E, Dow C, Rutecki P, H B. Ipsilateral and contralateral MRI volumetric abnormalities in chronic unilateral temporal lobe epilepsy and their clinical correlates. Epilepsia. 2005;46:420–430. doi: 10.1111/j.0013-9580.2005.27004.x. [DOI] [PubMed] [Google Scholar]
- Sonmez F, Atakli D, Sari H, Atay T, Arpaci B. Cognitive function in juvenile myoclonic epilepsy. Epilepsy Behav. 2004;5:329–336. doi: 10.1016/j.yebeh.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Tae WS, Hong SB, Joo EY, Han SJ, Cho JW, Seo DW, Lee JM, Kim IY, Byun HS, Kim SI. Structural brain abnormalities in juvenile myoclonic epilepsy patients: volumetry and voxel-based morphometry. Korean J Radiol. 2006;7:62–172. doi: 10.3348/kjr.2006.7.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HB, Jolles J. Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia. 2003;41:1330–1344. doi: 10.1016/s0028-3932(03)00059-9. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Witter MP, Uylings HB, Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Free SL, Koepp MJ, Sisodiya SM, Duncan JS. Abnormal cerebral structure in juvenile myoclonic epilepsy demonstrated with voxel-based analysis of MRI. Brain. 1999;122:2101–2108. doi: 10.1093/brain/122.11.2101. [DOI] [PubMed] [Google Scholar]
- Wolff M, Weiskopf N, Serra E, Preissl H, Birbaumer N, Kraegeloh-Mann I. Benign partial epilepsy in childhood: selective cognitive deficits are related to the location of focal spikes determined by combined EEG/MEG. Epilepsia. 2005;46:1661–1667. doi: 10.1111/j.1528-1167.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- Zupanc ML. Update on epilepsy in pediatric patients. Mayo Clin Proc. 1996;71:899–916. doi: 10.4065/71.9.899. [DOI] [PubMed] [Google Scholar]


