Abstract
Background
Endometriosis, a dysplastic disease affecting approximately 5-10% of U.S. reproductive-age women, has been linked in epidemiologic studies to exposures indicating high circulating estrogen levels. One such exposure may be night shift work, which has been associated with menstrual disruption and increased risk of two other estrogen-influenced diseases, breast cancer and adverse coronary events.
Methods
In this population-based case-control study, 235 health maintenance organization enrollees aged 18-49 first diagnosed with surgically-confirmed endometriotic disease between 4/1/1996 and 3/31/2001 were frequency-matched on age with 545 randomly selected female GH enrollees without history of endometriosis. Participants were asked about night shift work in all paid fulltime or part-time jobs worked from age 18 to the reference date. Genotypes for T3111C hClock were determined for a subset of 218 cases and 456 controls.
Results
Any night shift work was associated with a 50% increase in risk of endometriosis (odds ratio (OR) 1.48, 95% confidence interval (CI) 0.96, 2.29), and working more than half of shifts on a job at night was associated with a nearly doubled disease risk (OR 1.98, 95% CI 1.01, 3.85.) Changing sleep patterns on days off was associated with further increases in disease risk. T3111C hClock polymorphism was unrelated to endometriosis status and did not modify the effect of shift work on endometriosis.
Conclusions
These findings suggest that some aspect of night shift work may influence the development of endometriosis.
Introduction
Endometriosis, the presence of functioning endometrial glands and stroma outside the uterine cavity, affects 5 -10% of United States women of reproductive age1 and is the third leading cause of gynecologic hospitalization in the United States. The stimulation of endometriosis tissue growth is thought to be related to estrogen synthesis and metabolism 2. Some evidence suggests that serum estrogen may have a circadian secretion pattern in premenopausal women after adjustment for the monthly ovarian rhythm 3, 4, raising the possibility that estrogen may be vulnerable to circadian disruption. One cause of circadian disruption, night shift work, has been found to affect estrogen secretion and metabolism and has been associated with increased risk of several estrogen-influenced conditions, including menstrual cycle changes 5, 6, breast cancer 7-9, and adverse coronary events 10. Sensitivity to circadian disruption may have genetic components in humans. A common single-nucleotide polymorphism (T3111C) in hClock, the human counterpart of a circadian rhythm gene (Circadian Locomotor Output Cycles Kaput) found in most mammals, has been associated with diurnal preference 11, insomnia in mood disorders 12, and seasonal affective disorder 13. A deletion mutation of the transcriptional activation region of the homologous gene in mice causes irregular, lengthy estrous cycles 14. We conducted this population-based case-control study to test these hypotheses: 1) that shift work is associated with endometriosis risk, and 2) that hClock T3111C polymorphism may modify the effect of shift work on endometriosis.
Methods and Materials
Parent study
In the parent study, Women’s Risk of Endometriosis (WREN), all women 18 to 49 years of age enrolled a large health-maintenance organization in Washington State (Group Health Cooperative (GH)) who were first diagnosed with endometriosis (International Classification of Disease 9th Revision diagnostic codes 617.0-617.5, 617.8, and 617.9, excluding 617.0, adenomyosis without endometriosis) between April 1, 1996 and March 31, 2001 (n = 467) were invited to participate as cases. The reference date was the month and year a woman first visited GH reporting symptoms leading to a diagnosis of endometriosis. Controls (n = 1016) were randomly selected from a list of women enrolled in GH during the same time period, frequencymatched on five-year age group and assigned a reference date to correspond to the distribution of case reference dates. Women who did not speak English or who reported a hysterectomy or bilateral oophorectomy at initial telephone eligibility screening were excluded from participation. Participants were interviewed in person using a structured questionnaire that included questions regarding demographics, employment, prior medical conditions, menstrual history, pregnancy history, contraceptive methods, hormone use, cigarettes and alcohol use, and family history of endometriosis. In-patient and out-patient medical records of cases were reviewed and abstracted for symptom type (e.g. pelvic pain, abnormal bleeding) and severity, and endometriosis lesion characteristics (e.g. location, dimensions.)
This study was approved by the institutional review boards of Fred Hutchinson Cancer Research Center and GH. After exclusions, 341 (73.0%) cases and 742 (73.0%) controls participated in the study (see Figure 1). Each participant gave written informed consent to participate in the study, and was compensated $20 for her time.
FIGURE 1.
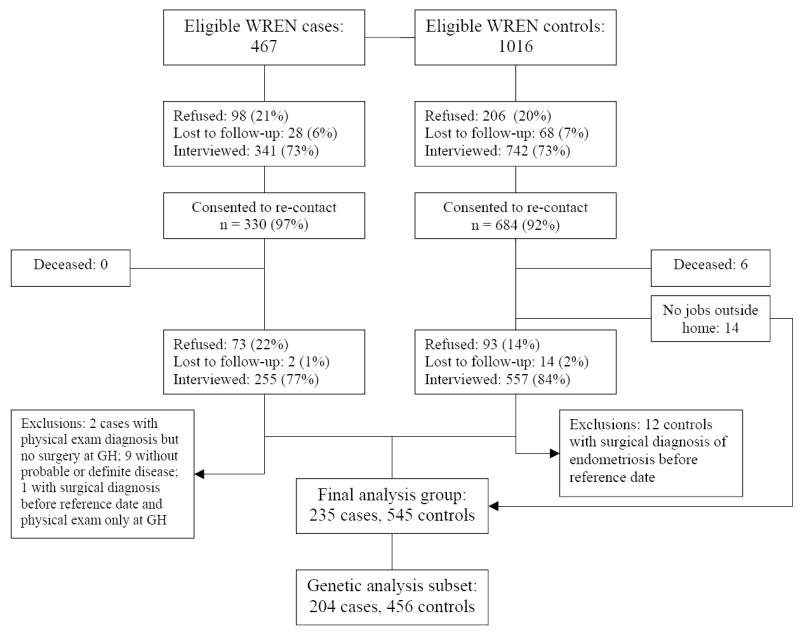
Flow chart of women enrolled in the Women’s Risk of Endometriosis Study who were included in this analysis.
Participants were asked about every paid job held for six months or longer from age 18 to the reference date. Occupational information collected included: job title and duties and industry (subsequently coded using 1980 Census-Occupation codes); hours worked per week; calendar years job started and ended; and detailed chemical and radiation exposures.
Follow-up study
At the time of the parent study, 330 cases and 684 controls consented to be re-contacted for future studies and provided contact information (see Figure 1 for full participation details.) In a follow-up study of disease recurrence (WRENSYR), cases who granted permission to be re-contacted were interviewed by telephone with a detailed questionnaire updating the items from the parent study to the present date, and inquiring about shift work in jobs worked before the original reference date as well as jobs between the reference date and the present date. WRENSYR participants were compensated $20 for their time. Controls granting consent for recontact and reporting jobs in WREN were interviewed by telephone, and were asked only about shift work in jobs before the original reference date. Fourteen controls reported no jobs during the parent study, and therefore did not require re-contact. Control participants were not compensated for the 10-minute interview. All participants gave verbal consent to be interviewed. This study was approved by the institutional review boards of Fred Hutchinson Cancer Research Center and GH.
After medical record review, nine cases whose disease did not meet the Holt-Weiss standard for definite or possible disease 15 were excluded from the study. The final analysis group consisted of 235 cases and 545 controls.
Assessment of shift working status
A modification of the shift-work questions asked by Davis et al. in a study of breast cancer etiology 7 was used. For each job the participant reported in WREN, she was asked if she worked anything other than the day shift. If so, she was asked the percentage of the time she worked day shift, evening shift, or night shift, and whether, on days off, she usually got up and went to bed at roughly the same time, within 2 hours, as on workdays. Finally, she was asked whether, during a 4-week period at this job, she usually worked the same shift or rotated shifts. Day shifts were defined as starting after 05:00 and ending before 19:00; evening shifts as starting after 12:00 and ending before 02:00; and night shifts as starting after 19:00 and ending before 09:00.
Genetic assessment
Leukocytic DNA from venous blood samples (204 cases and 456 controls in this analysis) and buccal epithelium genomic DNA from oral rinses (14 cases in this analysis) was extracted by salt precipitation. The T to C transition at position 3111 of the 3’ untranslated region of hClock was amplified using the primers described by Katzenberg et al. 11. The thermal cycling conditions were: five cycles 95°C for 30 seconds, 58°C for 30 seconds, 72°C for 1 minute; 30 cycles 95°C for 30 seconds,55°C for 30 seconds, 72°C for 1 minute; and a final extension of 5 minutes at 72°C. A fraction of the PCR products, including a negative control containing all reaction components except the genomic DNA, was run on a 1% agarose gel to ensure that the specific 221 base pair (bp) band was generated. Per the methods of Desan et al. 16, the PCR product was digested with Bsp1286I (New England Biolabs, Beverly, MA), which generates 95- and 126-bp bands in individuals homozygous for the C allele, 95-, 126- and 221-bp bands in heterozygous individuals, and a 221-bp band in individuals homozygous for the T allele. The restriction fragments were resolved by electrophoresis on a 3% agarose gel.
Analytic methods
Unconditional logistic regression was used to calculate odds ratios for the relationship between shift work and endometriosis and associated 95% confidence intervals (CIs) using STATA 8.0 (College Station, TX). All models were adjusted for the matching variables age and reference year as well as relevant confounders. Potential confounding factors, chosen as potentially related to both exposure and outcome but not on a plausible causal pathway, were race/ethnicity, household income, education, marital status, gravidity, parity and use of alcohol, cigarettes and oral contraceptives. Each potential confounder, treated categorically, was evaluated individually in a model containing the relevant shift and the match variables to determine which, if any, changed the estimate of the association between shift work and endometriosis by 10% or more. Nulliparity was treated as a binary variable, and gravidity as a four-level ordered categorical variable. Those confounders were then included in a multivariate model to determine a final, conservatively adjusted estimate of association.
For logistic regressions, those who had worked only days or never worked outside the home served as the reference group. Analyses of lifetime occupational shifts worked compared the reference group to those who had ever worked any non-day shifts; those who had ever worked evenings at any job; those who had worked more than 50% of shifts at any job during evenings; those who had ever worked nights at any job; and those who had worked more than 50% of shifts at any job during nights. Those who changed sleep times on their days off were compared to those who had not, by shift worked, and compared to the reference group. Duration analyses compared the reference group to those who had worked each of the shifts examined for 2 or fewer years, between 2 and 5 years, and more than 5 years. Duration of shift worked was also examined by case status using Cuzick’s nonparametric test for trend across ordered groups 17.
Logistic regression was also used to perform analyses of the risk of endometriosis associated with hClock T3111C polymorphism, using the T/T genotype as the reference group. Analyses treating T/C and C/C as separate groups and grouping them together as “any C” were conducted. The matching variables, age and reference year, were included in all models. Because there was no reason to believe that gene distribution was related to variables measured in the study, confounding was not further evaluated in these analyses. To determine whether the association between shift work and endometriosis varied by polymorphism status, we tested for interaction between polymorphism status and the types of shift work examined in the main analyses using logistic regression. Specifically, interaction was defined by a significant (α <=0.05) Wald test of the cross product term.
Because a previous study found a positive association between endometriosis and working night shifts was absent among nulliparous women 18, we performed subanalyses of this association in paras and nulliparas separately.
Results
Demographic characteristics are shown in Table 1. Cases and controls were similarly distributed in terms of age, race/ethnicity, marital status, education, income, and use of oral contraceptives. Cases had fewer pregnancies and fewer births than controls. Cases were more likely than controls to use alcohol and tobacco at the reference date.
Table 1.
Sociodemiographic and health characteristics of case and control participants.
| Cases | Controls | |||
|---|---|---|---|---|
| Number | Percent | Number | Percent | |
| Total | 235 | 100 | 545 | 100 |
| Age (years) | ||||
| <20 | 6 | 2.5 | 8 | 1.5 |
| 20-24 | 14 | 6.0 | 27 | 5.0 |
| 25-29 | 21 | 8.9 | 40 | 7.3 |
| 30-34 | 30 | 12.8 | 65 | 11.9 |
| 35-39 | 48 | 20.4 | 131 | 24.0 |
| 40-44 | 67 | 28.5 | 150 | 27.5 |
| 45-49 | 49 | 20.8 | 124 | 22.7 |
| Race/Ethnicity | ||||
| Non-Hispanic (NH) Caucasian | 195 | 83.0 | 463 | 84.9 |
| NH African-American | 7 | 3.0 | 25 | 4.6 |
| NH Asian/Pacific Islander | 12 | 5.1 | 30 | 5.5 |
| NH Native American | 2 | 0.9 | 2 | 0.4 |
| NH Other | 6 | 2.5 | 12 | 2.2 |
| Hispanic Asian/Pacific Islander | 4 | 1.7 | 1 | 0.2 |
| Hispanic Non-Asian/Pacific Islander | 9 | 3.8 | 12 | 2.2 |
| Marital status | ||||
| Married | 139 | 59.1 | 344 | 63.1 |
| Single, never married | 27 | 11.5 | 79 | 14.5 |
| Divorced/Separated/Widowed | 35 | 14.9 | 69 | 12.7 |
| Living as married | 34 | 14.5 | 53 | 9.7 |
| Highest educational level completed | ||||
| < 12 years | 8 | 3.4 | 8 | 1.5 |
| 12 years | 40 | 17.0 | 99 | 18.2 |
| Some college | 73 | 31.1 | 198 | 36.3 |
| College graduate | 72 | 30.6 | 130 | 23.9 |
| Post graduate | 42 | 17.9 | 110 | 20.2 |
| Family Income | ||||
| <$25,000 | 34 | 14.5 | 73 | 13.4 |
| $25,000-<$35,000 | 31 | 13.2 | 67 | 12.3 |
| $35,000-<$50,000 | 52 | 22.1 | 113 | 20.7 |
| $50,000-<$70,000 | 57 | 24.3 | 121 | 22.2 |
| $70,000-<$90,000 | 23 | 9.8 | 93 | 17.1 |
| ≥$90,000 | 28 | 11.9 | 67 | 12.3 |
| Refused/unknown | 10 | 4.3 | 11 | 2.0 |
| Parity | ||||
| 0 births | 113 | 48.1 | 149 | 27.3 |
| 1 births | 38 | 16.2 | 119 | 21.8 |
| 2 births | 34 | 27.2 | 171 | 31.4 |
| 3 births or more | 20 | 8.5 | 106 | 19.5 |
| Gravidity | ||||
| 0 pregnancies | 87 | 37.0 | 108 | 19.8 |
| 1 pregnancies | 35 | 14.9 | 98 | 18.0 |
| 2 pregnancies | 59 | 29.5 | 141 | 25.9 |
| 3 births or more | 54 | 23.0 | 198 | 36.3 |
| Tobacco use | ||||
| Never | 133 | 56.6 | 328 | 60.2 |
| Former | 54 | 23.0 | 132 | 24.2 |
| Current | 48 | 20.4 | 85 | 15.6 |
| Alcohol use | ||||
| Never | 70 | 29.8 | 184 | 33.8 |
| Former | 43 | 18.3 | 126 | 23.1 |
| Current | 122 | 51.9 | 235 | 43.1 |
| Oral contraceptive use | ||||
| Never | 25 | 10.6 | 85 | 15.6 |
| Former | 182 | 77.5 | 394 | 72.3 |
| Current | 28 | 11.9 | 66 | 12.1 |
Six participants, all cases, had never worked a paid job for six months or longer since the age of 18. Among those who did work outside the home, cases worked a lifetime mean of 17.9 years, with a standard deviation of 7.6 years, and controls worked a lifetime mean of 18.0 years, with a standard deviation of 7.6 years.
Compared with working only the day shift in all reported jobs, or having reported no jobs, ever working a job with any amount of evening or night shift work was modestly but not significantly associated with case status (odds ratio (OR), adjusted for age and reference year: 1.32, 95% confidence interval (CI) 0.94, 1.87.) No single variable made a difference of more than 10% in the point estimate, nor did using all the variables in a single model (OR 1.30, 95% CI 0.89, 1.88.) Results of the separate multivariate analyses of evening and night shift work, and of sleep changes during days off, are presented in Table 2. Confounders changing any model estimates more than 10% were race/ethnicity and education, which both altered only the smallest groups (those who worked night shifts more than 50% of the shifts worked in a given job.) Thus, all the adjusted models in Table 2 include, in addition to the matching variables age and reference year, both of these variables.
Table 2.
Relationships between endometriosis and lifetime occupational shifts worked, and between endometriosis and sleep time change on non-working nights by shift.
| Cases | Controls | OR1 (95% CI) | OR2 (95% CI) | |
|---|---|---|---|---|
| Days only/no job | 61 | 168 | 1.0 (reference) | 1.0 (reference) |
| Any evening shifts | 160 (72.4) | 357 (68.0) | 1.25 (0.88, 1.77) | 1.27 (0.90, 1.81) |
| Did not change sleep time | 25 (11.3) | 74 (14.1) | 0.94 (0.55, 1.61) | 1.00 (0.57, 1.73) |
| Changed sleep time | 135 (61.1) | 283 (53.9) | 1.33 (0.93, 1.91) | 1.34 (0.93, 1.93) |
| More than 50% evening shifts | 97 (61.4) | 193 (53.5) | 1.41 (0.96, 2.07) | 1.45 (0.98, 2.15) |
| Did not change sleep time | 15 (9.5) | 37 (10.3) | 1.14 (0.58, 2.22) | 1.20 (0.60, 2.37) |
| Changed sleep time | 82 (51.9) | 156 (43.2) | 1.47 (0.99, 2.20) | 1.51 (1.00, 2.28) |
| Any night shifts | 65 (51.6) | 133 (44.2) | 1.40 (0.92, 2.14) | 1.48 (0.96, 2.29) |
| Did not change sleep time | 43 (34.1) | 91 (30.2) | 1.36 (0.85, 2.17) | 1.45 (0.89, 2.35) |
| Changed sleep time | 22 (17.5) | 42 (13.9) | 1.50 (0.82, 2.73) | 1.55 (0.84, 2.85) |
| More than 50% night shifts | 21 (25.6) | 33 (16.4) | 1.94 (1.02, 3.68) | 1.98 (1.01, 3.85) |
| Did not change sleep time | 13 (15.9) | 24 (11.9) | 1.63 (0.77, 3.47) | 1.75 (0.80, 3.83) |
| Changed sleep time | 8 (9.8) | 9 (4.5) | 2.81 (1.00, 7.84) | 2.54 (0.88, 7.35) |
OR: odds ratio; CI: confidence interval.
Adjusted for age and reference year.
Adjusted for age, reference year, race/ethnicity, and education
Endometriosis risk was moderately but not significantly increased among women who ever worked a job with any amount of evening shift work, who ever worked night shift work, or who worked a job with more than 50% of hours during evening shift (ever any evenings OR 1.27, 95% CI 0.90, 1.81; ever any nights OR 1.48, 95% CI 0.96, 2.29; more than 50% evenings OR 1.45, 95% CI 0.98, 2.15). Working more than 50% of job hours during night shift was associated with a doubling of endometriosis risk (OR 1.98, 95% CI 1.01, 3.85). In general, changing sleep patterns on days off further increased the elevations in endometriosis risk seen with shift work, with highest risk among women working more than 50% of hours during night shifts and changing sleep patterns (OR 2.54, 95% CI 0.88, 7.35.) This association reached statistical significance among those who ever worked a job with more than 50% of hours during evening shifts and changed sleep patterns (OR 1.51, 95% CI 1.00, 2.28.)
As shown in Table 3, duration had differing effects on endometriosis risk associated with evening and night shift work. For night shift work, endometriosis risk elevations were higher with increasing duration of work (test for trend: any night shift work χ2 = 2.69, p = 0.10; >50% night shift work χ2 = 3.74, p = 0.05). Women who worked more than 50% of job hours during night shifts for more than five years had the highest risk of endometriosis (OR 5.32, 95% CI 1.21, 23.48.)
Table 3.
Relationships between endometriosis and duration of shifts worked.
| Cases | Controls | OR1 (95% CI) | OR2 (95% CI) | |
|---|---|---|---|---|
| Days only/no job | 61 (26.0) | 168 (30.8) | 1.0 (reference) | 1.0 (reference) |
| Any evening shifts | ||||
| <=2 years | 85 (36.2) | 203 (37.3) | 1.15 (0.78, 1.69) | 1.16 (0.77, 1.75) |
| 2-5 years | 61 (26.0) | 105 (19.3) | 2.63 (1.06, 2.52) | 1.66 (1.04, 2.63) |
| >5 years | 28 (11.9) | 69 (12.7) | 1.19 (0.70, 2.03) | 1.39 (0.79, 2.44) |
| Days only/no job | 61 (38.6) | 168 (46.1) | 1.0 (reference) | 1.0 (reference) |
| More than 50% evening shifts | ||||
| <=2 years | 28 (17.7) | 64 (17.7) | 1.10 (0.64, 1.89) | 1.13 (0.64, 1.99) |
| 2-5 years | 43 (27.2) | 72 (19.9) | 1.71 (1.05, 2.76) | 1.76 (1.04, 2.95) |
| >5 years | 26 (16.5) | 57 (15.8) | 1.42 (0.81, 2.48) | 1.64 (0.91, 2.96) |
| Days only/no job | 61 (26.0) | 168 (30.8) | 1.0 (reference) | 1.0 (reference) |
| Any night shifts | ||||
| <=2 years | 157 (66.8) | 350 (64.2) | 1.25 (0.88, 1.77) | 1.29 (0.89, 1.86) |
| 2-5 years | 9 (3.8) | 20 (3.7) | 1.27 (0.55, 2.95) | 1.50 (0.62, 3.65) |
| >5 years | 8 (3.4) | 7 (1.3) | 3.39 (1.17, 9.81) | 3.79 (1.22, 11.69) |
| Days only/no job | 61 (74.4) | 168 (83.6) | 1.0 (reference) | 1.0 (reference) |
| More than 50% night shifts | ||||
| <=2 years | 8 (9.8) | 18 (9.0) | 1.31 (0.53, 3.24) | 0.89 (0.31, 2.56) |
| 2-5 years | 7 (8.5) | 10 (5.0) | 2.14 (0.76, 6.00) | 2.18 (0.71, 6.62) |
| >5 years | 6 (7.3) | 5 (2.5) | 4.01 (1.13, 14.19) | 5.32 (1.21, 23.48) |
OR: odds ratio; CI: confidence interval
Adjusted for age and reference year.
Adjusted for age, reference year, race/ethnicity, marital status, education, gravidity, nulliparity.
As shown in Table 4, women who reported ever working more than one type of shift in a four-week period appeared to have a modestly increased risk of endometriosis, but this increase was only slightly higher than that among women whose shift did not rotate, and neither reached statistical significance. The association did not vary depending on whether a job included evenings or nights in the shifts worked.
Table 4.
Relationships between endometriosis and working more than one type of shift in an average 4-week period.
| Cases | Controls | OR1 (95% CI) | OR2 (95% CI) | |
|---|---|---|---|---|
| Days only/no job | 61 (26.0) | 168 (30.8) | 1.0 (reference) | 1.0 (reference) |
| No shift rotation3 | 85 (36.2) | 194 (35.6) | 1.23 (0.83, 1.81) | 1.17 (0.78, 1.76) |
| Any shift rotation3 | 89 (37.9) | 183 (33.6) | 1.35 (0.92, 1.99) | 1.38 (0.92, 2.05) |
| Evening shift | ||||
| Without rotation | 74 (33.5) | 184 (35.1) | 1.12 (0.75, 1.68) | 1.17 (0.78, 1.76) |
| With rotation | 86 (38.9) | 173 (33.0) | 1.39 (0.94, 2.05) | 1.38 (0.92, 2.05) |
| Night shift | ||||
| Without rotation | 33 (26.2) | 71 (23.6) | 1.37 (0.82, 2.30) | 1.55 (0.91, 2.63) |
| With rotation | 32 (25.4) | 62 (20.6) | 1.43 (0.85, 2.41) | 1.37 (0.80, 2.34) |
Adjusted for age and reference year.
Adjusted for age, reference year, and nulliparity.
Includes three participants who remembered that they worked shifts other than days but not which shifts were worked. Two of these participants recalled working more than one type of shift in a four-week period, and one recalled not doing so. Inclusion did not change estimates. These participants are excluded from shift-specific analyses.
The T3111C polymorphism of hClock was not associated with disease (adjusted for age and reference year, T/T OR 1.00, reference category; T/C OR 0.87, 95% CI 0.62, 1.23; C/C OR 1.28 95% CI 0.65, 2.53; any C versus T/T OR 0.92, 95% CI 0.66, 1.28); there were no significant interactions between polymorphism and shift worked (Table 5.) Distribution of T3111C was in Hardy-Weinberg equilibrium among the controls (likelihood-ratio χ2: 0.698, p =0.403.)
Table 5.
Endometriosis risk and hCLOCK T3111C polymorphism.
| Cases | Controls | All participants OR1 (95% CI) | |
|---|---|---|---|
| T/T | 127 (58.3) | 257 (56.4) | 1.0 (reference) |
| T/C | 76 (34.9) | 175 (38.4) | 0.84 (0.59, 1.29) |
| C/C | 15 (6.9) | 24 (5.3) | 1.16 (0.58, 2.31) |
| T/T | 127 (58.3) | 257 (56.4) | 1.0 (reference) |
| any C | 91 (41.7) | 199 (43.6) | 0.88 (0.63, 1.22) |
| T/T days only/no job | 34 (16.6) | 69 (15.6) | 1.0 (reference) |
| T/T any evening shifts | 85 (41.6) | 179 (40.6) | 0.98 (0.60, 1.60) |
| T/C days only/no job | 20 (9.8) | 61 (13.8) | 0.66 (0.34, 1.26) |
| T/C any evening shifts | 51 (25.0) | 108 (24.4) | 0.96 (0.57, 1.64) |
| C/C days only/no job | 3 (1.5) | 7 (1.6) | 0.93 (0.22, 3.87) |
| C/C any evening shifts | 11 (5.4) | 17 (3.9) | 1.34 (0.56, 3.18) |
| T/T days only/no job | 34 (28.8) | 69 (27.6) | 1.0 (reference) |
| T/T any night shifts | 32 (27.1) | 61 (24.4) | 1.10 (0.60, 1.99) |
| T/C days only/no job | 20 (16.9) | 61 (24.4) | 0.64 (0.34, 1.26) |
| T/C any night shifts | 25 (21.2) | 51 (20.4) | 1.01 (0.54, 1.90) |
| C/C days only/ no job | 3 (2.5) | 7 (2.8) | 0.85 (0.20, 3.58) |
| C/C any night shifts | 4 (3.4) | 1 (0.4) | 8.43 (0.90, 78.7) |
| T/T days only/no jobs | 34 (16.7) | 69 (15.6) | 1.0 (reference) |
| T/T any evening shifts | 85 (41.7) | 179 (40.6) | 0.98 (0.60, 1.60) |
| Any C days only/no jobs | 23 (11.3) | 68 (15.4) | 0.68 (0.27, 1.28) |
| Any C any evening shifts | 62 (30.4) | 125 (28.3) | 1.01 (0.61, 1.69) |
| T/T days only/no jobs | 34 (28.8) | 69 (27.6) | 1.0 (reference) |
| T/T any night shifts | 32 (27.1) | 61 (24.4) | 1.10 (0.60, 1.99) |
| Any C days only/no jobs | 23 (19.5) | 68 (27.2) | 0.67 (0.36, 1.27) |
| Any C any night shifts | 29 (24.6) | 52 (20.8) | 1.15 (0.62, 2.13) |
OR: odds ratio; CI: confidence interval
Main effects models adjusted for age, reference year, and race/ethnicity. Interaction models adjusted for age and reference year.
Among the controls, neither gravidity nor nulliparity was related to ever working evening or night shift. An analysis by parity showed results consistent with the main analyses after controlling for age and reference year (nulliparous women: any shift OR 1.21, 95% CI 0.71, 2.07; any evening shift OR 1.09, 95% CI 0.63, 1.88; any night shift OR 1.57, 95% CI 0.78, 3.15; parous women: any shift OR 1.49, 95% CI 0.93, 2.39; any evening shift OR 1.45, 95% CI 0.90, 2.33; any night shift OR 1.43, 95% CI 0.81, 2.52) (data not shown.)
Discussion
In this population-based case control study, night shift work was associated with a 50% increase in risk of endometriosis, and working more than 50% of shifts on a job at night was associated with a nearly twofold risk increase. Evening shift work was not significantly related to risk of endometriosis, but showed a similar pattern of elevated risk with more than 50% evening shifts. Changing sleep patterns on days off was associated with further increases in risk among those who worked more than 50% of evening or night shift. We found no relationship between hClock T3111C polymorphism and endometriosis, nor any effect of the polymorphism on the relationship of shift work to endometriosis.
A prior study of Norwegian women found that those who ever worked the night shift were 1.8 times as likely to report endometriosis as other women 18. This small study did not adjust for potential confounders, but our current findings are consistent with theirs. Moen and Schei dismissed their finding, noting, “Among only nulliparous women shift work was not related to endometriosis (OR 0.79, 95% CI 0.10, 6.5). In general, women without children are probably more prone to irregular working terms.” 18, p. 561 This generalization is uncited and, although perhaps true for Norwegian women, does not hold true in the United States. In a multivariate analysis of 1991 U.S. Bureau of Labor Statistics (BLS) data, Presser found that women were more likely to work shifts other than days on their principal job if they had preschool children, and less likely if they had school-age children, than women without children or with children 14 or older 19. As parity increases, so does the number of older children in a household; thus, the presence of only preschool children can be taken as representing lower parity and lower maternal age. We adjusted for nulliparity and gravidity, without substantive changes to the relationships in endometriosis and shift, and analysis limited to nulliparous women revealed relationships very similar to those in the whole group. To our knowledge, no other studies have evaluated shift work in endometriosis.
In the present study, although changing sleep patterns on days off appeared to increase risk of endometriosis among shift workers, rotating shifts did not. Our study measured rotation in very broad terms, and was not adequately powered to look at the pattern of shift rotation (speed and direction.) Generally speaking, the shorter the rotation, the more severe the health effects, presumably because of loss of synchrony between external and internal circadian cues. Changing sleep patterns on days off was more common in our population than shift rotation, and may have captured loss of synchrony or maladaption to shift work that could not be measured with our shift rotation item.
An explanation advanced for the relationship between breast cancer and night shift work may relate to the present findings. Melatonin is a potential agent of oncostasis 20,21, and is suppressed by light at night 22. Melatonin may inhibit estradiol secretion 23, or melatonin and estradiol may be mutually inhibitory 24. If melatonin inhibits cancer, it might also inhibit the inappropriate growth of ectopic endometriotic tissue.
A second potential explanation for the role of shift work in endometriosis is that working at night might cause sustained stress, in all such workers or in a subset whose vulnerability is defined by as-yet undetermined factors (such as job strain, adaptability to shift work, or family difficulties.) Although sustained stress suppresses gonadotrophin release25, it may also exacerbate inflammatory disorders despite the anti-inflammatory effects of cortisol. Endometriosis has a significant chronic inflammatory component 26
Family 27, 28, twin 29, 30 and genealogical database 31 studies suggest that endometriosis has a genetic basis, with one study showing the most parsimonious explanatory model has additive effects of individual-level environmental and genetic mechanisms 29. At the time of our study design, the only well-characterized sequence variant of any of the human circadian protein genes common enough in general populations to be studied in our case-control population was the T3111C polymorphism (rs 1801260) in the 3’ untranslated region of the hClock gene (4q12-GDB:9785615), which codes for a helix-loop-helix-PAS subunit of a transcriptional activator of the Period and Cryptochrome genes. We did not find evidence of an association between risk of endometriosis and this polymorphism, nor that this polymorphism modified the association between shift work and endometriosis, but it is possible that circadian genetics may still play a role in this relationship Additional work to evaluate the role of this gene in adaptation to shift work and diurnal preference may be of interest, as may explorations of variants of other components of the human circadian oscillator.
Strengths of this study include nearly complete case ascertainment in a defined population, such that the full spectrum of diagnosed disease was represented. The control population, drawn randomly from GH rolls, has a race, income, and educational profile very similar to that of western Washington 32. Because cases and controls were GH members, there was no difference in the ability to seek care and hence no disparity of socioeconomic status introduced by access to care. This strength was attenuated by the loss of participants from the originally eligible pool through refusal to participate in the parent study, refusal to be re-contacted for subsequent studies, loss to follow-up, and refusal to participate in the present study. None of these steps individually lost many subjects, but the overall loss was considerable, though comparable to similar ancillary studies. Although controls reporting prior diagnoses of endometriosis were excluded from the study, the possibility exists that some controls had undiagnosed asymptomatic or mildly symptomatic endometriosis, potentially attenuating odds ratios.
One limitation is that cases were slightly more likely to agree to be re-contacted than controls. The final response rate was lower among cases, probably because of the more involved follow-up interview. To defray the possibility that shift workers would be less likely to participate, we attempted contact from 8 AM to 9 PM, and scheduled interviews at participant convenience. Given the relationship to shift work we found, if shift workers with endometriosis had been less likely to participate because of the more involved interview, the risk observed would have been an underestimate. We found strong, consistent relationships, and have no reason to believe that this was an important influence on the results.
Another limitation is that because we do not know the mechanism by which shift work may affect endometriosis, we could not determine the etiologically relevant time point at which to evaluate shift work. If exposure leads to disease only in a specific timeframe, then our estimates of risk based on ever/never working shift work underestimate the association between shift work and disease.
Individual response to shift work is variable, and may depend on whether the individual chooses to work the shift 33. Even when an individual values the ability to work nonstandard hours, working nonstandard shifts disrupts social rhythms and presents challenges to sleep. We were not, unfortunately, able to consider the reasons for shift work in the present study, as lifetime recall of such variables is likely to be undependable, and cannot be validated.
Of the 5.3 million U.S. women who work shifts other than a regular daytime schedule, 1.1 million work a night shift 34. This suggests that many women may be at substantive risk for endometriosis, if the results of this exploratory study are reproducible in other contexts. Moreover, the current findings add to the growing body of evidence suggesting that some aspect of shift work is disruptive to estrogen-influenced body functions.
Supplementary Material
Table 6.
(Electronic appendix.) Endometriosis risk and hCLOCK T3111C polymorphism among NonHispanic Caucasians.
| Cases | Controls | Odds Ratio1 (95% CI) | |
|---|---|---|---|
| T3111C T/T | 104 (57.1) | 217 (55.5) | 1.0 (reference) |
| T3111C T/C | 66 (36.3) | 155 (39.6) | 0.88 (0.61, 1.28) |
| T3111C C/C | 12 (6.6) | 19 (4.9) | 1.38 (0.64, 2.97) |
| T3111C T/T | 104 (57.1) | 217 (67.6) | 1.0 (reference) |
| T3111C any C | 78 (30.9) | 174 (44.5) | 0.93 (0.65, 1.33) |
Adjusted for age and reference year.
Acknowledgments
Thanks to the participants of the study; Dean Nancy Fugate Woods for comments on the manuscript; Dana Mirick, Claudia Salinas, and Britton Trabert for technical discussions; Elizabeth Hosto, Berta Nicol-Blades, Dana Mirick, and David Doody for technical assistance; and Georgia Green for administrative support.
Support: NIH grants R01 HD33792, F31 NR009164, P30 CA015704; Maternal and Child Health Dissertation Award from the Maternal and Child Health Leadership Training Program at the University of Washington School of Public Health and Community Medicine; Woodrow Wilson-Johnson&Johnson Dissertation Grant in Women’s Health.
References
- 1.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstetrics and Gynecologic Clinics of North America. 1997;24(2):235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 2.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. Journal of Steroid Biochemistry and Molecular Biology. 2003;83:149–155. doi: 10.1016/s0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 3.Bao AM, Liu RY, van Someren EJW, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. European Journal of Endocrinology. 2003;148:227–232. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- 4.Carandente F, Angeli A, Crosignani P, et al. Circatrigintan rectal temperature and endocrine rhythms of clinically healthy, menstrually cycling women. Advances in Chronobiology. 1987;227B:533–548. [PubMed] [Google Scholar]
- 5.Chung F-F, Yao C-CC, Wan G-H. The associations between menstrual function and life style/working conditions among nurses in Taiwan. Journal of Occupational Health. 2005;47:149–156. doi: 10.1539/joh.47.149. [DOI] [PubMed] [Google Scholar]
- 6.Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health Care for Women International. 2002;23:703–714. doi: 10.1080/07399330290107449. [DOI] [PubMed] [Google Scholar]
- 7.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. Journal of the National Cancer Institute. 2001;93(20):1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 8.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. Journal of the National Cancer Institute. 2001;93(20):1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Kawachi I, Colditz GA, Stampfer MJ, et al. Prospective study of shift work and coronary heart disease in women. Circulation. 1995;92(11):178–182. doi: 10.1161/01.cir.92.11.3178. [DOI] [PubMed] [Google Scholar]
- 11.Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 12.Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology. 2001;25(S5):S102–108. doi: 10.1016/S0893-133X(01)00340-2. [DOI] [PubMed] [Google Scholar]
- 13.Johansson C, Willeit M, Smedh C, et al. Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology. 2003 Apr;28(4):734–739. doi: 10.1038/sj.npp.1300121. [DOI] [PubMed] [Google Scholar]
- 14.Miller BH, Olson SL, Turek F, Levine JE, Horton TH, Takahashi JS. Circadian Clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Current Biology. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt VL, Weiss NS. Recommendations for the design of epidemiologic studies of endometriosis. Epidemiology. 2000;11(6):654–659. doi: 10.1097/00001648-200011000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Desan PH, Oren DA, Malison R, et al. Genetic polymorphism at the CLOCK gene locus and major depression. Am J Med Genet. 2000 Jun 12;96(3):418–421. doi: 10.1002/1096-8628(20000612)96:3<418::aid-ajmg34>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Cuzick J. A Wilcoxon-type test for trend. Statistics in Medicine. 1985;4:87–90. doi: 10.1002/sim.4780040112. [DOI] [PubMed] [Google Scholar]
- 18.Moen M, Schei B. Epidemiology of endometriosis in a Norwegian county. Acta Obstetrica et Gynecologica Scandinavica. 1997;76:559–562. doi: 10.3109/00016349709024584. [DOI] [PubMed] [Google Scholar]
- 19.Presser HB. Job, family and gender: Determinants of nonstandard work schedules among employed Americans in 1991. Demography. 1995;32(4):577–598. [PubMed] [Google Scholar]
- 20.Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005 March;16(2):254–258. doi: 10.1097/01.ede.0000152525.21924.54. 2005. [DOI] [PubMed] [Google Scholar]
- 21.Blask DE, Brainard GC, Dauchy RT, et al. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Research. 2005;65(23):11174–11184. doi: 10.1158/0008-5472.CAN-05-1945. [DOI] [PubMed] [Google Scholar]
- 22.Brainard GC, Hanifin JP, Greeson JM, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. Journal of Neuroscience. 2001;21(16):6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez-Barcelo EJ, Cos S, Mediavilla D, Martinez-Campa C, Gonzalez A, Alonso-Gonzalez C. Melatonin-estrogen interactions in breast cancer. Journal of Pineal Research. 2005;38(4):217–222. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 24.Kostoglou-Athanassiou I, Athanassiou P, Treacher DF, Wheeler MJ, Forsling ML. Neurohypophysial hormone and melatonin secretion over the natural and suppressed menstrual cycle in premenopausal women. Clinical Endocrinology. 1998;49:209–216. doi: 10.1046/j.1365-2265.1998.00504.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalantaridou SN, Makrigiannakis A, Zoumakis E, Chrousos GP. Stress and the female reproductive system. Journal of Reproductive Immunology. 2004;62:61–68. doi: 10.1016/j.jri.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Ness RB, Modugno F. Endometriosis as a model for inflammation-hormone interactions in ovarian and breast cancers. European Journal of Cancer. 2006;42:691–703. doi: 10.1016/j.ejca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Moen MH, Magnus P. The familial risk of endometriosis. Acta Obstetrica et Gynecologica Scandinavica. 1993;72:560–564. doi: 10.3109/00016349309058164. [DOI] [PubMed] [Google Scholar]
- 28.Lamb K, Hoffmann RG, Nichols TR. Family trait analysis: a case-control study of 43 women with endometriosis and their best friends. American Journal of Obstetrics and Gynecology. 1986;154(3):596–601. doi: 10.1016/0002-9378(86)90608-3. [DOI] [PubMed] [Google Scholar]
- 29.Treloar S, O’Connor D, O’Connor V, Martin N. Genetic influences on endometriosis in an Australian twin sample. Fertility and Sterility. 1999;71(4):701–710. doi: 10.1016/s0015-0282(98)00540-8. [DOI] [PubMed] [Google Scholar]
- 30.Hadfield R, Mardon H, Barlow D, Kennedy S. Endometriosis in monozygotic twins. Fertility and Sterility. 1997;68(5):941–942. doi: 10.1016/s0015-0282(97)00359-2. [DOI] [PubMed] [Google Scholar]
- 31.Stefansson H, Geirsson RT, Steinthorsdottir V, et al. Genetic factors contribute to the risk of developing endometriosis. Human Reproduction. 2002;17(3):555–559. doi: 10.1093/humrep/17.3.555. [DOI] [PubMed] [Google Scholar]
- 32.Saunders KW, Davis RL, Stergachis A. Group Health Cooperative. In: Strom BL, editor. Pharmacoepidemiology. 4. West Sussex, England: John Wiley & Sons, Ltd.; 2005. pp. 223–239. [Google Scholar]
- 33.Fenwick R, Tausig M. Scheduling stress: family and health outcomes of shift work and schedule control. American Behavioral Scientist. 2001;44(7):1179–1198. [Google Scholar]
- 34.Beers TM. Flexible schedules and shift work: replacing the ‘9 to 5’ workday? Monthly Labor Review. 2000 June;:33–40. 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


