Abstract
The role of costimulation has previously been confined to the very early stages of the CD8+ T cell response. In this study we demonstrate the requirement for CD27 costimulation during the later phase but not programming of the primary CD8+ T cell response to Influenza virus and reveal a novel mechanism of action for CD27 costimulation. CD27 signals, during the later phase of the primary CD8+ T cell response, prevent apoptosis of antigen-specific CD8+ T cells. Blocking CD27L (CD70) on days 6 and 8 post-infection reduces the number of NP(366–374)-specific CD8+ T cells, increases their sensitivity to CD95/Fas-mediated apoptosis, and upregulates FasL on CD4+ T cells. This reduction of NP(366–374)-specific CD8+ T cells requires the presence of CD4+ T cells and Fas signaling. Lack of CD27 signals also decreases the quality of memory CD8+ T cell responses. Memory CD8+ T cells, which express surface CD27 similar to naïve cells, however, do not require CD27 costimulation during a secondary response. Thus, CD27 acts indirectly to regulate primary antigen-specific CD8+ T cell responses by preventing apoptosis of CD8+ T cells during the later phase of the primary response and is required for optimal quality of memory cells, but is not required during normally primed secondary CD8+ T cell responses.
Keywords: T Cells, Cytotoxic, Costimulation, Apoptosis, Lung, Viral Infection
INTRODUCTION
Production and maintenance of virus-specific CD8+ T-cells is critical to the adaptive immune response against viruses. TCR, cytokine and costimulatory signals are all required for the development of an effective anti-viral CD8+ T cell response. CD8+ T cells are known to express different cell surface antigens such as costimulatory molecules, adhesion molecules and cytokine receptors as they progress from naïve to effector and memory cells (1–3). These antigens are used to define different cell subsets, but presumably function by interacting with cytokines or costimulatory molecules required during differentiation. TCR stimulation is needed as the first signal for T cell activation; however, additional costimulation or cytokine signals are needed for a full response to develop. Early antigenic (4, 5) signals as well as those from cytokines such as IL-2 (6) have been shown to program CD8+ T cell responses. Although costimulation is classically thought to occur only during APC-CD8 interactions in the draining lymph node during the early stages of an immune response, neither the role of costimulation in programming nor its requirement during the later phase of a primary virus-specific CD8+ T cell response is known.
The number of known costimulatory molecules has increased drastically since the characterization of CD28 as a costimulator of T cells nearly 20 years ago (7). Many TNFR family members such as CD30 (8, 9), OX40 (10), and 4-1BB (11, 12) have also been shown to be involved in T cell costimulation. The TNFR family member CD27 is known to be present on naïve CD8+ T lymphocytes (13, 14) as well as NK cells (15) and certain B cell subsets (16, 17). CD27 expression is enhanced by TCR cross-linking or stimulation of CD3 on naïve cells (2, 18), but down-regulated after ligation with CD27L (CD70) (19), or PMA stimulation (20). Proliferation of T cells stimulated with PHA or anti-CD3 antibody is enhanced by adding anti-CD27 antibodies (20) or CD27L (19, 21). CD27 down-regulation results from decreased transcription (19) and proteolytic cleavage of membrane bound CD27 and gives rise to soluble CD27, which can be used as an indication of T cell activation (22). CD27L is transiently expressed on activated T and B cells upon antigen receptor engagement as well as some dendritic cells (23, 24). Activated T cells up-regulate CD27L and can directly stimulate naive T cells through CD27 which causes down-regulation of CD27 and upregulation of the CD25 (25). CD27 is also expressed on and is a marker of memory CD8+ T cells. However, TCR stimulation of memory cells does not induce CD27 upregulation on these cells and a role for CD27 signaling has not been established during a recall response (26).
More recently it has been shown that mice lacking CD27 are deficient in responding to TCR stimulation and infection with Influenza A virus (27). Infected CD27−/− mice produced lower CD4+ and CD8+ T cell numbers in the lung as compared to infected wild-type animals. These decreases translated to reduced CD4+ and CD8+ T cell numbers during a secondary challenge. However, effector function of CD8+ T cells, IFN-γ production and cytotoxicity were not affected by lack of CD27. CD27L transgenic mice, on the other hand, deplete their naïve T cell pool by inducing CD44hiCD62L− effector-memory cells (28, 29). This suggests that the CD27/CD27L interaction leads to the production of effector memory cells, which is important for the secondary immune response. CD8+ T cells lacking CD27 costimulation, as well as 4-1BB and OX40, during the primary infection, have also been show to have a decreased capacity to expand to secondary infection (30).
In the current study we investigated the mechanism of CD27 action on CD8+ T-cell immune responses to Influenza A infection. By treating infected mice with a monoclonal antibody (mAb) to CD27L we can examine differences in CD8+ T-cell expansion, survival, and effector function during specific phases of an active viral immune response attributed to CD27 signaling. Our studies show that CD27/CD27L interactions play a definitive role in the primary CD8+ T cell response. Blocking CD27L decreases the number of antigen-specific CD8+ T cells at the peak of the response (day10), but has no effect during the programming of the antiviral CD8+ T cell response (up to day 7 post infection). Furthermore, treatment with anti-CD27L mAb starting on day 6 post infection, significantly decreases the number of CD8+ T cells responding to the immunodominant NP(366–374) epitope of Influenza Type A virus infected animals. This decrease was due to enhanced apoptosis and requires CD4+ T cells, as blocking CD27L does not affect CD8+ T cell responses in Class II deficient animals. This apoptosis was CD95/Fas but not TNFα or TRAIL dependent. CD27-CD27L interactions do not participate in the expansion of virus-specific memory CD8+ T cells during a secondary response, as blocking CD27L does not modify the recall response of animals primed in the absence of treatment. Memory formation is, however, disrupted if CD27L is blocked during the primary response. Although the quantity of memory generated is not affected, quality is, as cells exhibit a qualitative deficit in their ability to expand in response to a secondary infection. CD27 costimulation therefore has an indirect yet crucial effect on survival during the late phase of the primary CD8+ T cell response and is required for the quality of memory cells generated, but is not required during secondary CD8+ T cell responses.
MATERIALS AND METHODS
Mice and Infections
C57Bl/6J, Fas deficient, and TNFR1 deficient mice (The Jackson Laboratory, Bar Harbor, ME) and MHC class II deficient mice (Taconic Farms, Germantown, NY) were kept in an AAALAC certified barrier facility at the Drexel University College of Medicine Queen Lane Campus animal facility. TRAIL deficient animals were a generous gift from Amgen. Animal work was carried out according to approved IACUC protocols. Mice were primed or infected at 7–9 weeks of age. Mice were completely anesthetized with 2-2-2-tribromoethanol (250mg/kg i.p., Acros, Geel, Belgium) before intranasal (i.n.) inoculations. Mice were infected by i.n. administration of 256 hemagglutination units of A/HKX31 (HKX31, H3N2) Influenza virus in a total volume of 20μl. The primary response was examined by harvesting cells from lung, mediastinal lymph nodes, spleen, and blood on various days post infection. For secondary responses mice were primed i.p. with 1280 hemagglutination units of Influenza A/PR8/34 (PR8, H1N1) virus in a total volume of 100μl followed by the same i.n. infection as in the primary response 30 or 60 days post priming. Cells were recovered on day 5 or 7 post infection from the same tissues as in primary infections for analysis of the secondary response. Infected mice were treated i.p. with 100μg anti-CD27L monoclonal antibody (clone M681, rat IgG1, Amgen Seattle, WA) or rat IgG1 isotype control (no azide, low endotoxin, eBioscience) on days 0, 2, 4, 6, and 8 post-infection for treatment during the primary response, on days 6 and 8 during the primary response, and days 0, 2, and 4 post infection for treatment during secondary immune response.
CD27L binding inhibition assay
M681 anti-CD27L antibody’s neutralizing activity was demonstrated using a CD27 binding competition assay. CD27L expressing 2PK-3 murine lymphoma cells (ATCC TIB-203) were incubated with 8 μg/ml mCD27-hIgG1 Fc (Amgen) in the presence or absence of rat anti-mouse CD27L monoclonal antibodies M681 or M682 (Amgen). A hIgG1-Fc construct of irrelevant specificity (huHB15Fc, Amgen) was used as a negative control to determine non-specific binding. After incubating for 30 minutes on ice the cells were washed and mCD27-hIgG1 Fc binding was detected using a biotinylated goat anti-human IgG1 secondary antibody (Jackson ImmunoResearch Laboratories) followed by streptavidin-PE (BD Biosciences). Fluorescence was measured on a FACSCalibur instrument (BD Biosciences) and analyzed using FCS Express software (De Novo Software).
Isolation of Pulmonary Lymphocytes
Pulmonary lymphocytes were isolated from individual mice by removing each lobe and mincing into smaller pieces. The tissue was then digested for 2hrs at 37°C with 3.0 mg/ml collagenase D, and 0.15μg/ml DNase I (Roche, Indianapolis, IN) in RPMI 1640 (Mediatech, Herdon, VA) containing 5% heat inactivated FBS (Gibco, Carlsbad, CA), 2mM L-glutamine, 100 I.U./ml penicillin, 100μg/ml streptomycin (Mediatech),. The digested tissue was then run through a 40μm cell strainer (Falcon, Bedford, MA) and washed in the same media as above. Total mononuclear cells were separated over a density gradient of Lympholyte-M (Cedarlane Laboratories, Hornby, Ontario, Canada) centrifuged at 1300g for 20 minutes at room temperature. Media gradient interface was harvested and washed before viable cells were counted in ethidium bromide/acridine orange under UV light.
Flow Cytometry
Influenza Virus NP specific CD8+ T-cells were detected using MHC Class I tetramers. Tetramers were prepared by linking four biotinylated monomeric H-2Db Class I MHC molecules refolded in the presence of equimolar amounts of β2-microglobulin and excess peptide representing the immuno-dominant nuclear protein (NP) epitope (366–374, ASNENMETM, NP366) to allophycocyanin (APC) labeled streptavidin (Molecular Probes). Cells were co-labeled with PE-Cy5 (eBioscience) or Alexa-flour 405 (Cal-Tag) anti-mouse CD8a and PE anti-mouse Fas or FasL (Pharmingen). PE-conjugated anti-CD27 (clone LG.7F9, eBioscience) was used for CD27 expression stains. Active caspase-3 was detected using CaspaTag™ CASPASE-3, 7 in-situ assay kit (Chemicon). Cells were incubated for 1hr at 37°C with fluorescein labeled DEVD peptide prior to surface staining. Intracellular staining for Ki-67 was performed after surface staining using the FITC labeled antibody set (Pharmingen). Cells were then permeabilized, fixed and stained using the Cytofix/Cytoperm Kit (Pharmingen). Cells were stained on ice for 30min. in 20μl of FACS Wash buffer containg HBSS (Mediatech), 3% Heat inactivated Horse Serum (Gibco), 0.02% Sodium Azide (Fisher, Pittsburgh, PA). Cells were then rinsed twice in wash buffer and fixed in 1% paraformaldehyde (Fisher). Data was collected on a FACSCalibur using Cell Quest software (Becton Dickinson) or FACS Aria using FACS Diva software . Analysis was performed using FlowJo software (Tree Star).
Cytotoxicity Assays
EL4 target cells, 0.5 × 106 cells/ml, were loaded with 10 μg NP(366–374) peptide for 16 hours at 37°C with 5% CO2. Cells were then washed in RPMI 5% and resuspended in a 100 μCi 51Cr solution (Sodium Chromate 1μCi/μL, Perkin Elmer) per 2 × 106 cells. Cells were incubated for 75 min at 37°C with 5% CO2 and resuspended every 15 min with gentle tapping. After 75 min the cells were washed twice in RPMI 5%, then resuspended in RPMI 10% and diluted to 1 × 105 cells/ml. Target cells were then added to wells of 96-well V- bottom plates (Costar), 104 cells/100μl per well, containing different ratios of effector cells. Plates were spun for 30 sec at 500g and incubated for 6 h at 37°C with 5% CO2. After 6 h plates were spun down at 500g for 5 min and 30 μl of supernatant were transferred to 96-well Lumaplates (Packard Instruments). Plates were allowed to dry overnight then read with a Top Count-NXT Microplate Luminescence-Scintillation Counter (Packard Instruments).
Fas Stimulations
Pulmonary mononuclear cells collected at day 7 post infection from anti-CD27L antibody treated or untreated mice were cultured 1.0 × 106cells/well in 24 well plates coated with anti-CD95 10μg/ml (clone Jo2 BD Pharmingen) at 37°C for 6h. Cells were then stained for activated Caspase 3 as described above along with the LIVE/DEADR Fixable Violet Dead Cell Stain Kit (Invitrogen). Cells were also stained with Alexa-flour 405 anti-mouse CD8a (Cal-Tag) and NP(366–374) tetramers to define antigen-specific populations. Fas-specific apoptosis was calculated as ((%Caspase 3+ in Fas-coated wells – %Caspase 3+ uncoated wells) / (100% – %Caspase 3+ uncoated wells)) × 100%.
Intracellular IFN-γ Staining
Freshly isolated pulmonary mononuclear cells were aliquoted into sterile round bottom polystyrene tubes 1.0 × 106cells/tube. Cells were pelleted and resuspended in 200μl of RPMI 1640 supplemented with 10% heat inactivated FBS (Gibco), 2mM L-glutamine, 100 I.U./ml penicillin, 100μg/ml streptomycin (Mediatech), that contained 10 μg/ml of NP(366–374) peptide. Cells were then incubated at 37°C with 5% CO2 for 12 hours adding brefeldin A for the last six hours. After the incubation, cells were washed once with wash buffer and surface stained for CD8 as described above. Cells were then stained with APC anti-mouse IFN-γ using the Cytofix/Cytoperm Kit (Pharmingen).
Memory cell transfer experiments
CD8+ T cells from spleens of mice infected and treated or not treated with anti-CD27L 60 days prior were isolated and purified using the Spin-Sep Murine cell enrichment kit (Stemcell Technologies). 2.5×104 NP(366–374)-specific memory CD8+ cells were transferred into naïve host by tail vein injection. Mice were then infected the following day as described above.
RESULTS
CD27 expression and M681 blocking anti-mouse CD27L monoclonal antibody
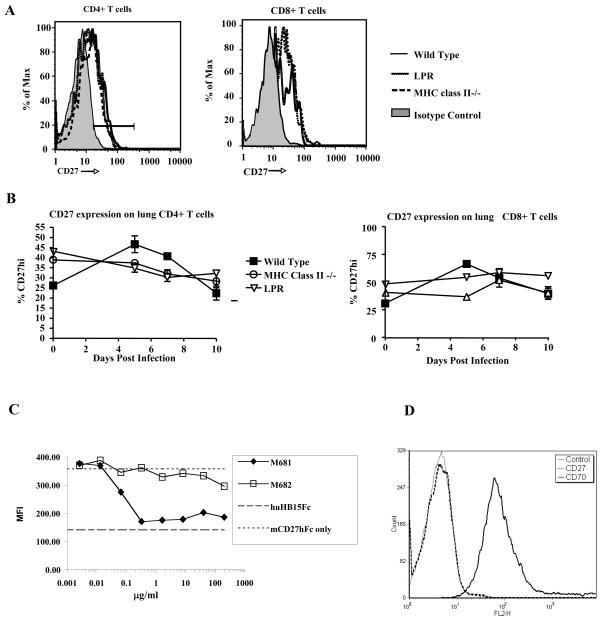
CD27 expression throughout the course of influenza virus infection was determined in several mouse strains (Fig. 1A,B). Little to no variation was seen in the levels or percent of CD4+ or CD8+ T cells expressing CD27 in the lung while responding to influenza virus. M681 an IgG1 anti-murine CD27L monoclonal antibody was shown to inhibit soluble murine CD27 binding to CD27L (Fig. 1C). When M681 antibody was added to an assay that measured the binding of soluble mouse CD27-Fc fusion protein to CD27L-expressing 2PK-3 murine lymphoma cells, M681 inhibited binding by >90% (Fig. 1C). The non-neutralizing murine CD27L-specific monoclonal antibody M682 failed to block CD27-Fc fusion protein binding to 2PK-3 cells and served as a negative control (Fig. 1C). Non-specific binding of fusion proteins was determined with a hIgG1-Fc construct of irrelevant specificity (huHB15Fc fusion protein). NP(366–374)-specific CD8+ T cells or any other lung cell population were not deleted in mice by day 7 post treatment (Fig. 2B,C, data not shown). Thus the M681 monoclonal antibody is a neutralizing anti-CD27L antibody and does not deplete cells in vivo.
Figure 1. CD27 expression and M681 anti-CD27L blocking monoclonal antibody blocking CD27-CD27L interactions.
(A) Expression of CD27 on CD4+ T cells (left panel) or CD8+ T cells (right panel), in the lungs of C57Bl/6, LPR and MHC class II deficient mice, on 7 days post-infection.
(B) Kinetics of CD27 expression on the surface of lung CD4+ (left panel) or CD8+ (right panel) T cells during the primary immune response to influenza virus, in these mouse strains.
(C) M681 antibody inhibits the binding of soluble mouse mCD27-Fc fusion protein to 2PK-3 cells. CD27L positive mouse 2PK-3 cells incubated with mCD27-hIgG1 Fc and the indicated concentrations of M681 or M682 rat anti-mouse CD27L monoclonal antibodies. As controls the cells were incubated with mCD27-hIgG1 alone (positive control) or a hIgG1-Fc construct of irrelevant specificity (huHB15Fc) (negative control). The non-neutralizing anti CD27L antibody M682 is also shown. Data are presented as mean channel of fluorescence of live-gated cells and are representative of three independent experiments.
(D) Mouse 2PK-3 cells expressing CD27L (mCD27L) were stained with either M681 rat anti-mouse CD27L monoclonal antibody or with an antibody to CD27, to show specificity of the M681 antibody binding.
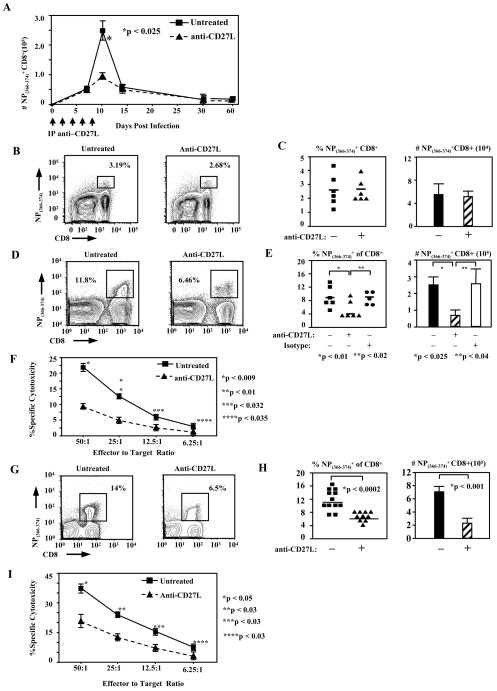
Figure 2. CD27L signals are required during the later phase of the NP(366–374)-specific CD8+ T cell responses.
(A) Absolute numbers of pulmonary CD8+ T cells specific for the immunodominant NP(366–374) epitope of influenza virus, found in the lungs of anti-CD27L treated or untreated animals, at different time points after infection. Quantitation of virus-specific CD8+ T cells at several time points post infection was done by flow-cytometry, using H2-Db tetramers complexed with the NP(366–374) peptide. Data represents average number (± SEM) of tetramer-staining CD8+ T cells recovered from the lung of infected animals.
(B) Mice were infected with influenza virus and then treated or not with anti-CD27L antibody (days 0, 2, 4, and 6). Representative FACS plots are shown, indicating the percentage of NP(366–374) tetramer staining cells within the CD8+ T cell population in the lungs of infected mice, 7 days after i.n. infection with influenza virus.
(C) Pooled data shown, from two independent experiments indicating the percentages (left panel) or total numbers (right panel) of lung NP(366–374) specific CD8+ T cells tetramers in mice infected 7 days earlier with influenza virus, that were treated or not with anti-CD27L antibody. Each symbol represents a single animal and the horizontal line marks the average for each group. Bars represent average numbers (± SEM) of NP(366–374)- specific CD8+ T cells in the lung of animals in each treatment group; n=6 mice.
(D) Day 10 NP(366–374) specific CD8+ T cells in mice treated with anti-CD27L antibody every other day throughout the primary infection, starting on day 0 up to day 10. Representative FACS plots are shown, indicating percentages of pulmonary CD8+ T cells staining positive for NP(366–374)-specific tetramers, at day 10 after infection, in mice that were treated or not with anti-CD27L antibody.
(E) Pooled data from 2 independent experiments showing percentages (left panel) and absolute numbers (right panel) of influenza-specific CD8+ T cells recovered from the lung of anti-CD27L treated, isotype control treated or untreated animals 10 days post influenza virus infection. Each symbol represents a single animal and horizontal lines mark the average for each treatment group. Bars represent average (± SEM) number of NP(366–374)-specific CD8+ T cells in the lung of animals in each treatment group; n = 6 mice.
(F) Specific cytotoxicity of lung CD8+ T cells from untreated or anti-CD27L antibody treated (days 0, 2, 4, 6 and 8) mice, against NP(366–374)-peptide loaded targets. Each symbol is the mean (± SEM) of the values obtained from three animals, at the indicated effector to target ratio.
(G) Mice were infected with influenza virus and then treated or not with anti-CD27L antibody only on days 6 and 8 post-infection. Representative FACS plots are shown, indicating percentages of lung NP(366–374)-specific CD8+ T cells, on day 10 post influenza infection, in untreated animals or animals treated with anti-CD27L mAb on days 6 and 8 post-infection. (H) Pooled data from 4 independent experiments showing percentages (left panel) and absolute numbers (right panel) of day 10 lung influenza-specific CD8+ T cells in animals treated only on days 6 and 8 post influenza infection. Each symbol represents a single animal and horizontal lines mark the average for each treatment group. Bars represent average (± SEM) number of NP(366–374)-specific CD8+ T cells in the lung of animals in each treatment group.
(I) Specific cytotoxicity of lung CD8+ T cells from untreated or anti-CD27L antibody treated (only days 6 and 8) mice, against NP(366–374)-peptide loaded targets. Each symbol is the mean (± SEM) of the values obtained from three animals, at the indicated effector to target ratio.
CD27 acts during the later phase of the primary CD8+ T cell response
Previous data have demonstrated that mice deficient in CD27 have decreased Influenza-specific CD8+ T cells at the peak of the primary response on day 10, but show little to no difference at day 6 post-infection (27). To distinguish between the specific requirement of CD8+ T cells for CD27 signaling during an immune response, either early during programming of T cells or later in the response, and to exclude potential confounding effects of the absence of CD27 during T cell development, we treated wild type C57Bl/6J Influenza type A virus infected mice with a blocking non-depleting monoclonal antibody directed against CD27L, starting on day 0 post infection and then every other day until day 8. Digested lung tissue from infected mice was analyzed for NP(366–374)-specific CD8+ T cells at various time points post infection (Fig. 2A). Neither relative percent of CD8+ T cells nor absolute numbers of NP(366–374)-specific cells differed in treated animals on day 7 post- infection as compared to untreated animals (Fig. 2B,C). In untreated animals, 2.26±0.52% (mean±SEM) of total CD8+ T cells were specific for NP(366–374) as compared with 2.38±0.32% (n=6) in anti-CD27L treated mice (Fig. 2B,C). Absolute numbers were likewise unaffected in lungs from untreated and anti-CD27L treated animals containing 6.39±1.51×104 and 5.82±0.70×104 NP(366–374)-specific CD8+ T cells respectively (Fig. 2C). However, on day 10, the peak of the immune response, pulmonary NP(366–374)-specific CD8+ T cells were greatly reduced where NP(366–374)-specific cells made up 8.40±1.38% of pulmonary CD8+ T cells in untreated animals and 3.80±1.18% (p<0.01) of pulmonary CD8+ T cells in anti-CD27L treated animals and 8.64±1.08% for isotype controls (p<0.02) (Fig. 2D,E). Absolute numbers of NP(366–374) CD8+ T cells in mice treated every other day up to day 8 were reduced to 0.87±0.33×105 from 2.57±0.55×105 and 2.64±0.78 x105 for anti-CD27L antibody, untreated and isotype control treated respectively (n=6, p<0.025, p<0.04) (Fig. 2E). We also tested the ability of pulmonary lymphocytes to kill NP(366–374)-peptide loaded targets. The percent maximum killing was decreased in anti-CD27L antibody treated animals by between 57% and 70% at each effector to target ratio (Fig. 2F). We therefore reasoned that CD27 signaling might be important during the later stages as opposed to early programming events of the primary immune response. Treatment with anti-CD27L antibody starting on day 6 post-infection decreased the percent of pulmonary CD8+ T cells specific for NP(366–374) from 10.8±0.9% in untreated animals to 5.93±0.33% (p<0.0002), as determined on day 10 post-infection (Fig. 2G, H). This was accompanied with a decrease in absolute NP(366–374)-specific CD8+ T cell numbers from 7.70±1.26×105 to 2.06±0.37×105 (n=12 untreated and n=10 anti-CD27L treated, p<0.001) with anti-CD27L treatment (Fig. 2H) and also by a 44% to 54% decrease in in vitro lung NP(366–374)-specific CD8+ T cell cytotoxicity in anti-CD27L antibody treated animals (Fig. 2I). The reduction of NP(366–374)-specific CD8+ T cells was similar to the nearly three fold reduction seen when anti-CD27L antibody was delivered throughout the entire course of infection. Thus the effect of CD27 signaling on the antiviral CD8+ T cells response mainly occurs at the later stages of the response. This is especially interesting, as costimulation is classically believed to play a role during the early priming stages of a developing immune response and suggests temporal differences between various costimulatory requirements.
CD27 signals prevent apoptosis of antigen-specific CD8+ T cells during the later phase of the virus- specific response
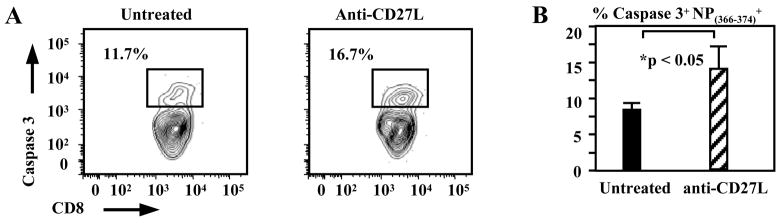
The above data demonstrated the critical interval for CD27 costimulation in the anti-viral CD8+ T cell response. We therefore explored the mechanism by which blocking CD27 is affecting the response during the later phase. Mechanisms by which CD27 may increase the number of pulmonary virus-specific CD8+ T cells are to promote proliferation or survival or aid in the migration of activated CD8+ T cells into infected tissues. Previous data has shown that although proliferation is decreased in CD27 deficient T cells, the number of cell divisions, however, is unchanged (27). We found that antigen-specific CD8+ T cells are not sequestered in other tissues such as the blood, mediastinal lymph node, or spleen after anti-CD27L antibody treatment as cell numbers in these tissues were decreased as compared to untreated controls (data not shown), therefore inhibition of migration does not seem to be a plausible mechanism. To examine proliferation of NP(366–374)-specific CD8+ T cells, lymphocytes isolated from the lungs of anti-CD27L antibody treated or untreated animals were stained for Ki-67 expression levels on days 7 through 10 post-infection after treatements on days 6 and 8. The percent of Ki-67 expressing NP(366–374) CD8+ T cells did not differ between treated and untreated animals (data not shown). Treatment with anti-CD27L antibody did however increase the number of NP(366–374)-specific CD8+ T cells on day 7 staining positive for activated caspase 3 to 14.68±3.89% from 8.7±1.52% (n=6, p<0.05) in untreated animals (Fig. 3A,B). Thus although CD27 costimulation is not required to enter cell cycle in vivo, CD27 signaling does play a role in the survival of antigen-specific CD8+ T cells.
Figure 3. CD27 signals inhibit caspase-mediated apoptosis in antigen-specific CD8+ T cells.
(A) NP(366–374)-specific CD8+ T cells stain positive for caspase 3, as a marker of apoptosis, after anti-CD27L monoclonal antibody treatment in the late phase of the primary CD8+ T cell response to influenza infection. FACS plots are shown, indicating percentages of NP(366–374)-specific CD8+ T cells staining positive for active caspase 3 on day 7 post virus infection, in the lung of animals treated on day 6 post infection with anti-CD27L antibody or left untreated.
(B) Pooled percentages of antigen-specific CD8+ T cells staining positive for active caspase 3 in untreated animals (solid bar) or animals treated with anti-CD27L antibody (cross-hatched bar) on day 6 post infection, shown. Bars mark the average percentage (± SEM) of 6 mice from each treatment group, on day 7 post infection.
CD8+ T cell apoptosis in the absence of CD27 signaling requires the presence of CD4+ T cells and Fas signaling
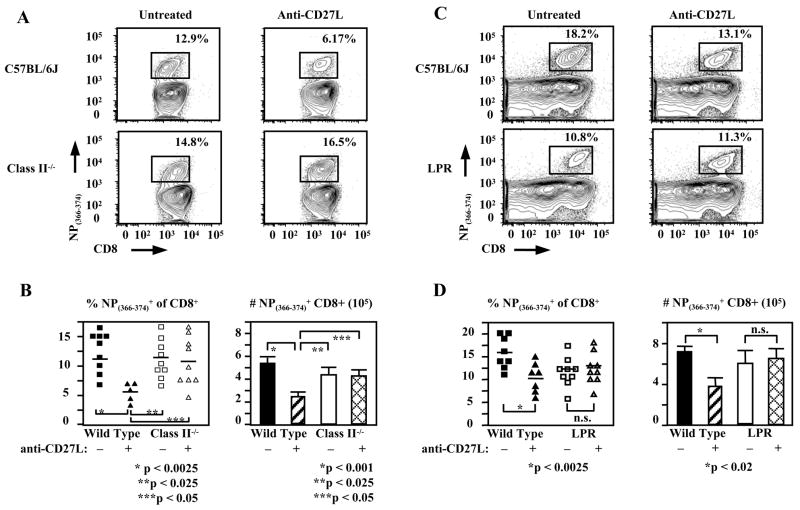
We also sought to determine whether CD27 was acting directly on CD8+ T cells or indirectly through another cell type. Since our antibody is directed against the ligand of CD27, treatment could be affecting CD4 or CD8 T cells, B cells or APCs. We therefore repeated our experiments using MHC class II deficient mice in order to examine whether activated CD4+ T cells play a role in this model. Interestingly, anti-CD27L treatment of MHC class II knockout animals had no effect on the influenza-specific CD8+ T cell response. Class II deficient animals had 11.7±1.93% of CD8+ T cells specific for NP(366–374) or 4.46±0.57×105 cells as compared to 10.9±1.46% or 4.33±0.56×105 antigen-specific CD8+ T cells with anti-CD27L treatment (n≥5 for all samples, p<0.0025) (Fig. 4A,B). This data suggests that inhibition of the antigen-specific CD8+ T cell response by CD27L blockade requires the presence of CD4+ T cells. This may be a direct or an indirect effect. It may be mediated by blocking CD27 signaling on CD4+ T cells that could be affecting the CD8+ T cell response either directly or indirectly through CD4+ T cell-APC interactions. On the other hand blocking CD27 signaling may be occurring on another cell type, which interacts with CD4+ T cells to downmodulate the CD8+ T cell response.
Figure 4. The decreased CD8+ T cell response when blocking CD27L is CD4+ T cell and Fas dependent.
(A) Representative FACS plots indicating day 10 pulmonary NP(366–374)-specific CD8+ T cells from influenza infected wild type and MHC class II deficient mice, untreated or treated with anti-CD27 on days 6 and 8 post infection shown.
(B) Squares (untreated) or triangles (anti-CD27L treated) represent a single animal and horizontal line marks the average for each treatment group. Bar graphs showing mean (± SEM) of pooled absolute numbers of lung virus-specific CD8+ T cells on day 10 post infection. (C) Representative FACS plots are shown, indicating percentages of day 10 lung NP(366–374)-specific CD8+ T cells in LPR and wild type mice treated during the late phase (day 6 and 8) of the primary response to influenza infection. (D) Pooled data from three independent experiments showing the effect of anti-CD27L treatment on percentages and absolute numbers of CD8+ T cells specific for NP(366–374) on day 10 post infection, in wild-type and LPR mice.
We next sought to determine the mechanism of apoptosis when CD27L is blocked. Ligation of TRAIL or FasL by their receptors on CD8+ T cells are major mediators of apoptosis and contraction in CD8+ T cell responses (31–33). To determine if CD27 is working by modulating TRAIL, TNFα, or Fas-mediated apoptosis we examined the effects of anti-CD27L mAb treatment in TRAIL, TNFR1, or Fas deficient animals. Although TRAIL plays an important role in controlling CD8+ T cell responses (31), the effect of blocking CD27 signaling is not abrogated in TRAIL deficient animals. Neither percentages nor absolute numbers of NP(366–374)- specific CD8+ T cells differ between TRAIL deficient and wild type animals treated with anti-CD27L antibody (data not shown). No effect was seen in TNFR1 knockout mice either, however the inhibitory effect of blocking CD27L was abrogated in Fas deficient animals. Relative cell numbers of NP(366–374)-specific CD8+ T cells were slightly decreased in Fas deficient animals, but were not further decreased by anti-CD27L antibody treatment (Fig. 4C,D). Absolute numbers of antigen-specific CD8+ T cells did not decrease in Fas deficient animals, 6.06±1.40×105 as compared to 7.29±0.40×105 in wild type C57Bl/6J mice while anti-CD27L antibody treatment of Fas deficient mice had no effect as cell numbers were 6.37±1.37×105 as compared to a decrease to 3.90±0.75×105 in wild type animals treated with anti-CD27L (n≥7 in all groups) (Fig. 4D). Thus inhibition of CD8+ T cell responses by CD27L blockade requires intact Fas signaling, leading to increased apoptosis of antigen-specific CD8+ T cells.
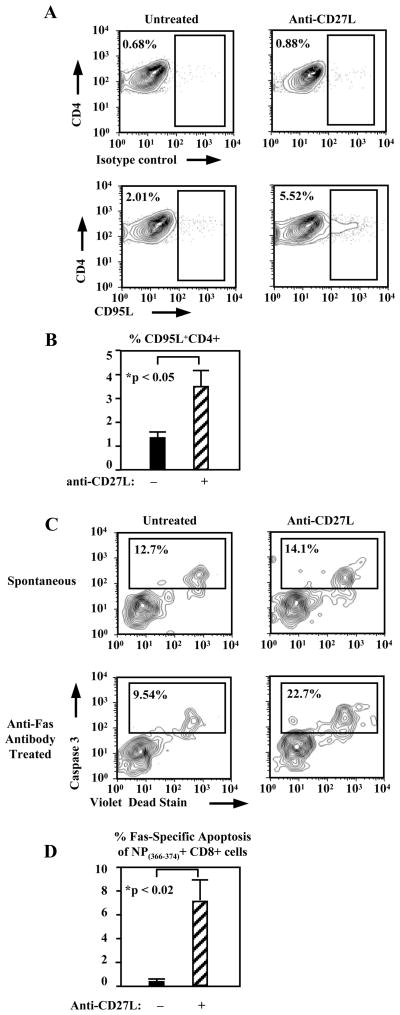
Given that both CD4+ T cells and Fas signaling were required for apoptosis of virus-specific CD8+ T cells, we examined whether FasL is upregulated on CD4+ T cells when CD27L is blocked. Indeed this did prove to be the case where 1.31±0.22% of pulmonary CD4+ T cells expressed FasL in untreated animals on day 7 post infection which increased to 3.58±1.17% (n=6, p<0.05) with anti-CD27L antibody treatment (Fig. 5A,B). The sensitivity of antigen-specific CD8+ T cells to Fas mediated apoptosis was also increased in the absence of CD27 costimulation during in vitro Fas stimulation. Fas-specific apoptosis was not increased over spontaneous apoptosis in NP(366–374)-specific CD8+ T cells from untreated mice however anti-CD27L antibody treated mice had clearly increased Fas-specific apoptosis to 7.02±2.09% (n=3, p<0.02) (Fig. 5C,D). Thus blocking CD27L not only increases FasL expression on CD4+ T cells but also increases antigen-specific CD8+ T cells sensitivity to Fas-mediated apoptosis. Although the above do not definitively prove that CD4+ T cells are mediating this apoptosis, our data strongly suggest this.
Figure 5. Lack of CD27 signals increases FasL expression on pulmonary CD4+ T cells and Fas sensitivity of NP(366–374)-specific CD8+ T cells.
(A) Representative FACS plots showing percentages of lung CD4+ T cells staining for FasL (bottom) or isotype control antibody (top), on day 7 after virus infection, in mice treated with anti-CD27L antibody or left untreated.
(B) Pooled percentages from 2 independent experiments showing the mean percentage (± SEM) of CD4+ T cells expressing high FasL levels (n=6). (C) Activated caspase 3 staining of day 7 NP(366–374)-specific CD8+ T cells from lung of untreated or anti-CD27L antibody treated mice. (D) Mean percentage (± SEM) of NP(366–374)-specific CD8+ T cells staining positive for activated caspase 3, in lungs of mice treated or not with anti-CD27L antibody.
CD27 signaling in the primary response affects the quality of resulting memory CD8+ T cells, but is not required during secondary responses
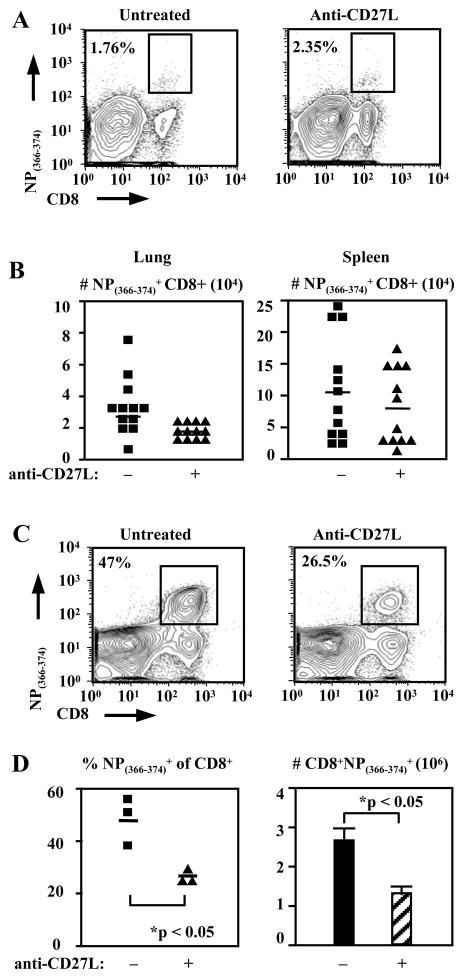
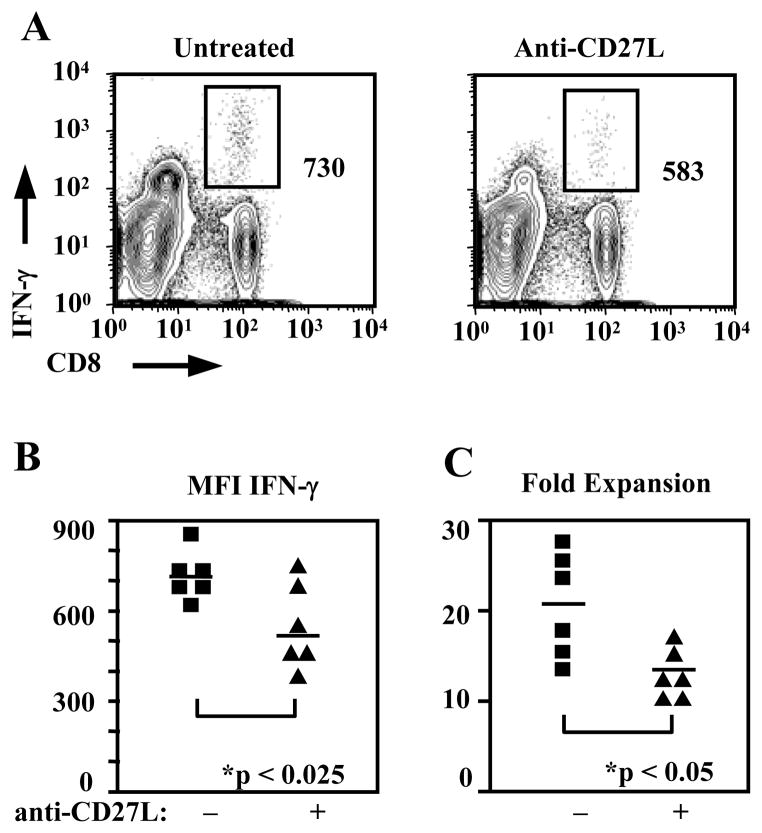
In order to assess the effect of CD27 on the formation of CD8+ T cell memory, animals were treated during the primary response to Influenza virus infection and examined after memory populations were allowed to form. Interestingly we saw little difference in the size of the antigen-specific memory CD8+ T cell populations. NP(366–374)-specific CD8+ T cells in the lung were 2.91±0.6% of CD8+ T cells in untreated animals as compared with 2.36±0.47% in treated animals (Fig. 6A). The absolute numbers were 2.69±0.63×104 antigen-specific memory CD8+ T cells in untreated animals and 1.61±0.20×104 in treated animals (n=12 animals per group) (Fig. 6B). Numbers were also not different in the spleens of anti-CD27L treated animals containing 0.80±0.16 x105 NP(366–374)-specific CD8+ T cells as compared with 1.09±0.23 x105 antigen-specific cells in untreated animals (n=12) (Fig. 6B). However, after reinfection these animals failed to produce similar antigen-specific CD8+ T cell populations in the lung at day 7, the peak of the secondary response. Anti-CD27L treatment during the primary decreased both virus-specific CD8+ T cell numbers and frequencies during the secondary response (Fig. 6C,D). Frequencies of NP(366–374)-specific CD8+ T cells in the lung were reduced to 28.2±1.90% in anti-CD27L antibody treated mice from 47.1±5.51% (n=3, p<0.05) in untreated mice (Fig. 6C,D). Total NP(366–374)-specific cells were likewise decreased to 1.28±0.19×106 with anti-CD27L mAb treatment from 2.62±0.41×106 (p<0.05) in animals with no treatment (Fig. 6D). To examine whether this reduced expansion during a secondary response was due to a qualitative defect of memory cells from anti-CD27L treated animals we performed adoptive transfers. Transfer of equal numbers (2.5×104) of NP(366–374)-specific CD8+ T memory cells from anti-CD27L treated or untreated animals into naïve hosts followed by rechallenge, results in decreased expansion of antigen-specific memory CD8+ T cells from 17.08±2.84 fold in untreated to 12.60±0.97 fold in anti-CD27L treated animals (n=6, p<0.05) (Fig. 7C). Furthermore, memory CD8+ T cells from anti-CD27L monoclonal antibody treated animals restimulated in vitro with NP(366–374) peptide produced significantly less IFN-gamma 567±52.2 MFI as compared to untreated animals 733±32.6 (n=6, p<0.025) (Fig. 7A,B). These results demonstrate that anti-CD27L treatment during a primary response decreases the subsequent influenza-specific CD8+ T-cell secondary response without changing memory population size. Therefore CD27 signaling is indispensable for optimal quality of antigen-specific CD8+ T cell memory generated following a primary immune response.
Figure 6. NP(366–374)-specific memory CD8+ T cell populations do not differ quantitatively but are qualitatively impaired in mice treated with anti-CD27L antibody.
(A) FACS plots indicating percentages of day 60 lung NP(366–374)-specific CD8+T cells in mice untreated (left) or treated (right) with anti-CD27L antibody.
(B) Pooled data from 3 experiments showing absolute numbers of NP(366–374)-specific CD8+ T cells isolated from lung and spleen 60 days post infection. Each symbol represents one animal and the horizontal line marks the mean for each treatment group; n = 12 mice.
(C) Animals were either untreated or treated during the primary immune response with anti-CD27L antibody and rechallenged with virus 60 days later. FACS plots indicating percentages of NP(366–374)-specific CD8+T cells in the lungs of mice on day 7 of the secondary immune response to Influenza virus infection
(D) Percentages and absolute numbers of NP(366–374)-specific CD8+ T cells in the lungs of mice at day 7 of the secondary immune response. The horizontal line marks the mean for each treatment group. Bar graphs represent the mean (± SEM) absolute number of NP(366–374)-specific CD8+ T cells recovered from lungs on day 7 post reinfection.
Figure 7. Memory cells respond less efficiently to secondary challenge after blocking CD27L during the primary response.
(A). Splenocytes isolated from mice infected with influenza virus 60 days earlier, and treated or not with anti- CD27L antibody during the primary immune response, were in vitro stimulated with NP(366–374) peptide for 5 h in the presence of Golgi plug. IFNγ intracellular staining of virus-specific memory CD8+ T cells in mice treated or not with anti-CD27L antibody, is shown.
(B) MFI values shown from six individual animals in each treatment group, after in vitro stimulation for 12 hours with NP(366–374) peptide.
(C) Treatment with anti-CD27L antibody during the primary immune response affects the expansion of memory virus-specific CD8+ T cells. Twenty-five thousand day 60 memory NP(366–374)-specific CD8+ T cells from animals that were either untreated or treated with anti-CD27L antibody during the primary response were transferred into naïve C57Bl/6 recipients. Recipients were then challenged with influenza A virus and the immune response examined 7 days later. The ratios of recovered to transferred NP(366–374)-specific CD8+ T cells from two independent experiments, are shown.
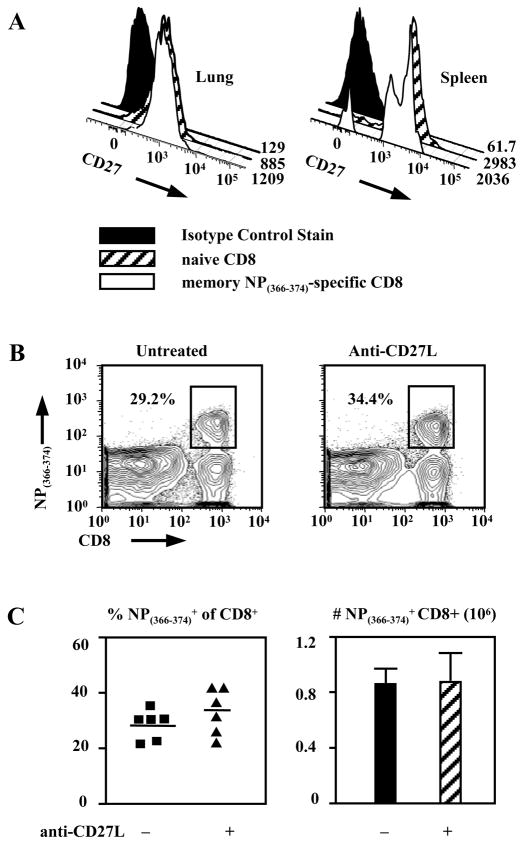
Finally, treatment with anti-CD27L antibodies allows us to examine the role of CD27 signaling during a secondary or recall response after an unaffected primary response. In these experiments mice were primed with influenza virus and then challenged in the presence or absence of anti-CD27L treatment. CD27 is present on antigen-specific memory CD8+ T cells (26), which we confirmed for these experiments (Fig. 8A). However blocking CD27 signaling does not diminish the NP(366–374)-specific recall response. CD8+ T cells specific for NP(366–374) made up 34.0±4.75% of total CD8+ T cells in anti-CD27L antibody treated animals and 29.8±2.52% in untreated (Fig. 8B,C). Total numbers of pulmonary NP(366–374)-specific CD8+ T-cells were 8.07±1.53×105 and 8.63±1.87×105 in treated and untreated mice respectively (Fig. 8C). Therefore, although CD27 is present on virus-specific memory CD8+ T cells it is not required for the expansion of properly formed memory cells.
Figure 8. CD27 signaling is not required for secondary expansion of memory CD8+ T cells.
(A) Expression of CD27 on day 60 NP(366–374)-specific memory CD8+ T cells is equivalent to that of naïve CD8+ T cells. FACS histograms and MFI values shown.
(B) Representative FACS plots indicating percentages of NP-specific CD8+ T cells in the lungs of mice on day 7 post rechallenge; mice were treated on days 0, 2, and 4 post rechallenge with anti-CD27L antibody (right panel) of left untreated (left panel).
(C). Percentages of lung NP(366–374)-specific CD8+ T cells (left panel) and mean (± SEM.) absolute numbers of NP(366–374)-specific CD8+ T cells (right panel) recovered from the lung on day 5 of the secondary immune response, in mice that were treated or not with anti-CD27L antibody. The horizontal line marks the mean of each treatment group. Data pooled from two independent experiments.
DISCUSSION
In this paper we examine the effects of CD27 in both the primary and secondary anti-influenza CD8+ T-cell immune response. By blocking the murine CD27L in-vivo using an anti-CD27L antibody, we demonstrate that CD27-CD27L interactions are essential for developing a robust primary anti-viral CD8+ T cell immune response. This confirms in our model what has been previously demonstrated in CD27 deficient animals (27). Although CD27−/− mice have reduced antiviral CD8+ T cell responses, CD8+ T-cells appear to gain effector function at the same frequency as wild-type mice. This data, however, must be viewed with caution since antagonistic anti-CD27 mAb treatment of mice inhibits the CD3-mediated expansion and differentiation of the thymocytes CD4-8-25+ precursor population (34). Consequently, the quality of thymic development may be perturbed in CD27−/− animals although thymic phenotype is normal, and therefore, CD8+ T cells from CD27−/−animals may not be representative of normal CD8+ T cells. Our experiments, using a blocking anti-CD27L antibody circumvents this important drawback. We demonstrate that CD27 is expressed on lung CD4+ and CD8+ T cells during influenza virus infection and that blocking CD27 signaling during the primary response decreases the frequencies and absolute numbers of NP(366–374)-specific CD8+ T cells found in the lung, but blocking as late as day 6 post infection can still mediate this effect. This suggests that CD8+ T cells require CD27 signaling to survive and maintain effector cells subsequent to initial APC encounter in the draining lymph node during the programming of the response. This raises the question of what cells are responsible for providing this costimulation during the later stages of the immune response and if costimulation is occurring at the site of infection as well as the draining node. Inducible lymphoid tissue such as iBALT may lend an answer to this question (35), however if costimulation is occurring outside germinal centers this would be of significant interest.
DCs are critical in the expansion of effector and the formation of memory CD8+ T cells (36). Expression of CD27L on DCs may represent the initial source of CD27 stimulation, but direct interaction between CD27L on DCs and CD27 on CD8+ T cells has not been demonstrated in vivo. We should note that staining of lung DC and T cells for CD27L expression using the FR-70 monoclonal antibody did not differ from isotype control stains (data not shown), however in vitro generated and activated bone-marrow derived DC could be readily stained as previously reported (29).
Furthermore, we have demonstrated that CD27 signaling affects CD8+ T cells in vivo during a primary response and that requires the presence of CD4+ T cells. Recent studies have also shown the need for CD4+ T cell help in the formation of memory, but not CD8+ T cell memory expansion (37, 38). This has been proposed to be either direct through CD40-CD40L signaling or indirect through CD4+ mediated APC activation (39–41), but may also be mediated through CD27 signaling in light of our results. CD27 however seems to be more important for CD8+ T cell expansion than for CD4+ T cell expansion (27), further obscuring this molecule’s function. Our data show that blocking CD27 signaling indirectly affects CD8+ T cells through CD4+ T cells that play a role in deleting CD8+ T cells when CD27 signaling is absent. Although lack of CD27 signals lead to increased FasL expression on CD4+ T cells and increased apoptosis of CD8+ T cells, and we believe this suggests a direct effect of CD27 signals on CD4+ T cells, we have not formally proven this. It is possible that we are blocking CD27L on CD4+ T cells but that the apoptosis of CD8+ T cells is mediated by a third cell type. On the other hand, it is possible that we are inhibiting CD27 signaling on another cell type such as an APC which in turn instructs the CD4+ T cell or another cell to induce apoptosis of CD8+ T cells. Thus although we have ruled out a direct action of CD27 signals on CD8+ T cells, how CD27 signals prevent Fas mediated killing of CD8+ T cells and the precise interplay of cells is still unclear.
CD27 may also affect the ability of CD4+ T cells to produce cytokines that support CD8+ T cell survival. Although TRAIL is an important mediator of CD8+ T cell responses (31), neither TRAIL nor TNFR1 mediate the effects of blocking CD27 signaling. The effect of blocking CD27L is negated in the absence of Fas. Even though both Fas and FasL can have weak costimulatory effects on normal T lymphocytes (42, 43), models lacking these molecules have significant lymphoproliferative abnormalities (44, 45), pointing to the more important role of Fas as a mediator of contraction in T cell responses. This lymphoproliferative disorder is unlikely to affect our studies as we used 6–10 week old lpr/lpr mice on a C57Bl/6 background. Lpr/lpr mice on a C57Bl/6 background show a much slower development of lymphadenopathy and autoimmunity compared to MRL-lpr/lpr mice (46). Indeed our 10 week old Lpr/lpr mice on a C57Bl/6 background showed no lymphadenopathy and splenomegaly, and this is in agreement with others (46). IL-2 and IL-7 (47) can upregulate Fas and significantly upregulate FasL predisposing T cells to activation-induced cell death (AICD) (48, 49). Blocking CD27L did increase the expression of FasL on CD4+ T cells. Blocking CD27 signaling leads to increased sensitivity of CD8+ T cells to Fas apoptosis, which may be initiated by FasL expressing activated CD4+ T cells (32) or DCs (33) in the infected lung, leading to the increased apoptosis we have demonstrated during the later phase of the CD8+ T cell response. Decreased FLIP expression upon IL-2 stimulation also leaves T cells more sensitive to Fas mediated apoptosis (48). IL-2 produced by CD4+ T cells may therefore lead to the increased AICD in the absence of CD27 signaling. IL-2 has been shown to hasten contraction in vivo when administered during the expansion phase of the CD8+ T cell response (50). CD27 signaling has been shown to prevent the terminal effector differentiation attributed to IL-2 stimulation (51) and thus may inhibit this induced contraction in vivo. Here we show a definitive relationship between CD27 signaling, CD4+ T cells, and Fas mediated AICD of virus-specific CD8+ T cells that suggests that CD27 signaling suppresses IL-2 production by CD4+ T cells at the later phase of the immune response leading to a decrease in AICD of virus-specific CD8+ T cells. This previously unsuspected role of CD27 signaling is novel and reveals the complexity of roles costimulation molecules play at different stages of CD8+ T cell responses. We have also demonstrated that CD27 costimulation does not have a role in programming CD8+ T cell responses, but may disrupt that program if not present during the later stages of the response.
In summary, we have further defined the role of CD27 in the context of primary and secondary anti-viral CD8+ T cell responses and identified novel mechanisms behind this. The mechanisms of CD27 action as well as the temporal manner in which it functions in vivo were previously undefined. We have demonstrated that continued stimulation through CD27 during later stages of the primary effector CD8+ T cell response is required for mounting an optimal antiviral response. In the absence of CD27 signaling increased AICD dampens the primary response. CD27 signaling during the primary response is also required for the optimal quality but not quantity of memory CD8+ T cells. CD27 signaling however is not required during the secondary response. Our findings demonstrate that disruption of CD27 costimulatory signals even after the initial priming event can significantly affect the course of the immune response and CD8+ T cell programming underscoring the importance of determining the temporal aspect of costimulation delivery to CD8+ T cell responses.
Abbreviations
- Kg
kilogram
- i.n
intranasal
- mM
milimolar
- RPMI 1640
cell culture medium RPMI 1640
- iBALT
inflammatory bronchus-associated lymphoid tissue
- MFI
mean fluorescence intensity
Footnotes
This work was supported by NIH R01 AI66215 awarded to P.D.K.
References
- 1.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 2.Hintzen RQ, de Jong R, Lens SM, Brouwer M, Baars P, van Lier RA. Regulation of CD27 expression on subsets of mature T-lymphocytes. J Immunol. 1993;151:2426–2435. [PubMed] [Google Scholar]
- 3.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 5.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–894. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CA, Gruss HJ, Davis T, Anderson D, Farrah T, Baker E, Sutherland GR, Brannan CI, Copeland NG, Jenkins NA, et al. CD30 antigen, a marker for Hodgkin’s lymphoma, is a receptor whose ligand defines an emerging family of cytokines with homology to TNF. Cell. 1993;73:1349–1360. doi: 10.1016/0092-8674(93)90361-s. [DOI] [PubMed] [Google Scholar]
- 9.Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, Jerzy R, Dower SK, Cosman D, Goodwin RG. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990;248:1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- 10.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nature Immunology. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 11.Halstead ES, Mueller YM, Altman JD, Katsikis PD. In vivo stimulation of CD137 broadens primary antiviral CD8(+) T cell responses. Nat Immunol. 2002;3:536–541. doi: 10.1038/ni798. [DOI] [PubMed] [Google Scholar]
- 12.Pollok KE, Kim YJ, Zhou Z, Hurtado J, Kim KK, Pickard RT, Kwon BS. Inducible T cell antigen 4–1BB. Analysis of expression and function. J Immunol. 1993;150:771–781. [PubMed] [Google Scholar]
- 13.Hintzen RQ, de Jong R, Lens SM, van Lier RA. CD27: marker and mediator of T-cell activation? Immunol Today. 1994;15:307–311. doi: 10.1016/0167-5699(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 14.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugita K, Robertson MJ, Torimoto Y, Ritz J, Schlossman SF, Morimoto C. Participation of the CD27 antigen in the regulation of IL-2-activated human natural killer cells. J Immunol. 1992;149:1199–1203. [PubMed] [Google Scholar]
- 16.Maurer D, Fischer GF, Fae I, Majdic O, Stuhlmeier K, Von Jeney N, Holter W, Knapp W. IgM and IgG but not cytokine secretion is restricted to the CD27+ B lymphocyte subset. J Immunol. 1992;148:3700–3705. [PubMed] [Google Scholar]
- 17.Maurer D, Holter W, Majdic O, Fischer GF, Knapp W. CD27 expression by a distinct subpopulation of human B lymphocytes. Eur J Immunol. 1990;20:2679–2684. doi: 10.1002/eji.1830201223. [DOI] [PubMed] [Google Scholar]
- 18.Kobata T, Agematsu K, Kameoka J, Schlossman SF, Morimoto C. CD27 is a signal-transducing molecule involved in CD45RA+ naive T cell costimulation. J Immunol. 1994;153:5422–5432. [PubMed] [Google Scholar]
- 19.Hintzen R, Lens S, Beckmann M, Goodwin R, Lynch D, van Lier R. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol. 1994;152:1762–1773. [PubMed] [Google Scholar]
- 20.van Lier R, Borst J, Vroom T, Klein H, Van Mourik P, Zeijlemaker W, Melief C. Tissue distribution and biochemical and functional properties of Tp55 (CD27), a novel T cell differentiation antigen. J Immunol. 1987;139:1589–1596. [PubMed] [Google Scholar]
- 21.Goodwin RG, Alderson MR, Smith CA, Armitage RJ, VandenBos T, Jerzy R, Tough TW, Schoenborn MA, Davis-Smith T, Hennen K, et al. Molecular and biological characterization of a ligand for CD27 defines a new family of cytokines with homology to tumor necrosis factor. Cell. 1993;73:447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 22.Hintzen RQ, de Jong R, Hack CE, Chamuleau M, de Vries EF, ten Berge IJ, Borst J, van Lier RA. A soluble form of the human T cell differentiation antigen CD27 is released after triggering of the TCR/CD3 complex. J Immunol. 1991;147:29–35. [PubMed] [Google Scholar]
- 23.Akiba H, Miyahira Y, Atsuta M, Takeda K, Nohara C, Futagawa T, Matsuda H, Aoki T, Yagita H, Okumura K. Critical Contribution of OX40 Ligand to T Helper Cell Type 2 Differentiation in Experimental Leishmaniasis. J Exp Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesselaar K, Xiao Y, Arens R, van Schijndel GMW, Schuurhuis DH, Mebius RE, Borst J, van Lier RAW. Expression of the Murine CD27 Ligand CD70 In Vitro and In Vivo. J Immunol. 2003;170:33–40. doi: 10.4049/jimmunol.170.1.33. [DOI] [PubMed] [Google Scholar]
- 25.Agematsu K, Kobata T, Sugita K, Hirose T, Schlossman SF, Morimoto C. Direct cellular communications between CD45R0 and CD45RA T cell subsets via CD27/CD70. J Immunol. 1995;154:3627–3635. [PubMed] [Google Scholar]
- 26.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 28.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 29.Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 30.Hendriks J, Xiao Y, Rossen JWA, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During Viral Infection of the Respiratory Tract, CD27, 4–1BB, and OX40 Collectively Determine Formation of CD8+ Memory T Cells and Their Capacity for Secondary Expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 31.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 32.Tateyama M, Oyaizu N, McCloskey TW, Than S, Pahwa S. CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood. 2000;96:195–202. [PubMed] [Google Scholar]
- 33.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Gravestein LA, van Ewijk W, Ossendorp F, Borst J. CD27 cooperates with the pre-T cell receptor in the regulation of murine T cell development. J Exp Med. 1996;184:675–685. doi: 10.1084/jem.184.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, Randall TD. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10:927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 36.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 38.Sun JC, Bevan MJ. Defective CD8 T Cell Memory Following Acute Infection Without CD4 T Cell Help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 40.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 41.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 42.Sun M, Ames KT, Suzuki I, Fink PJ. The cytoplasmic domain of Fas ligand costimulates TCR signals. J Immunol. 2006;177:1481–1491. doi: 10.4049/jimmunol.177.3.1481. [DOI] [PubMed] [Google Scholar]
- 43.Alderson MR, Armitage RJ, Maraskovsky E, Tough TW, Roux E, Schooley K, Ramsdell F, Lynch DH. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–318. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 45.Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159:1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morse HC, 3rd, Roths JB, Davidson WF, Langdon WY, Fredrickson TN, Hartley JW. Abnormalities induced by the mutant gene, lpr. Patterns of disease and expression of murine leukemia viruses in SJL/J mice homozygous and heterozygous for lpr. J Exp Med. 1985;161:602–616. doi: 10.1084/jem.161.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jaleco S, Swainson L, Dardalhon V, Burjanadze M, Kinet S, Taylor N. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J Immunol. 2003;171:61–68. doi: 10.4049/jimmunol.171.1.61. [DOI] [PubMed] [Google Scholar]
- 48.Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- 49.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 50.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 51.Carr JM, Carrasco MJ, Thaventhiran JED, Bambrough PJ, Kraman M, Edwards AD, Al-Shamkhani A, Fearon DT. CD27 mediates interleukin-2-independent clonal expansion of the CD8+ T cell without effector differentiation. PNAS. 2006;103:19454–19459. doi: 10.1073/pnas.0609706104. [DOI] [PMC free article] [PubMed] [Google Scholar]