Abstract
Previous efforts to differentiate human embryonic stem cells (hESCs) into endothelial cells have not achieved sustained expansion and stability of vascular cells. To define vasculogenic developmental pathways and enhance differentiation, we used an endothelial cell–specific VE-cadherin promoter driving green fluorescent protein (GFP) (hVPr-GFP) to screen for factors that promote vascular commitment. In phase 1 of our method, inhibition of transforming growth factor (TGF)β at day 7 of differentiation increases hVPr-GFP+ cells by tenfold. In phase 2, TGFβ inhibition maintains the proliferation and vascular identity of purified endothelial cells, resulting in a net 36-fold expansion of endothelial cells in homogenous monolayers, which exhibited a transcriptional profile of Id1highVEGFR2highVE-cadherin+ ephrinB2+. Using an Id1-YFP hESC reporter line, we showed that TGFβ inhibition sustains Id1 expression in hESC-derived endothelial cells and that Id1 is required for increased proliferation and preservation of endothelial cell commitment. Our approach provides a serum-free method for differentiation and long-term maintenance of hESC-derived endothelial cells at a scale relevant to clinical application.
Human embryonic stem cells (hESCs), which self-renew indefinitely1, offer a plentiful source of endothelial cells for therapeutic revascularization. However, few studies have identified specific developmental stimuli sufficient to support the specification and maintenance of large numbers of functional and vascular-committed endothelial cells from hESCs2–7. Although small numbers of hESC-derived endothelial cells have been generated in short-term cultures, these cells have not been subjected to sustained expansion, angiogenic profiling or interrogated as to the stability of vascular fate. As a result, molecular pathways that maintain vascular identity and long-term expansion of hESC-derived endothelial cells remain unknown.
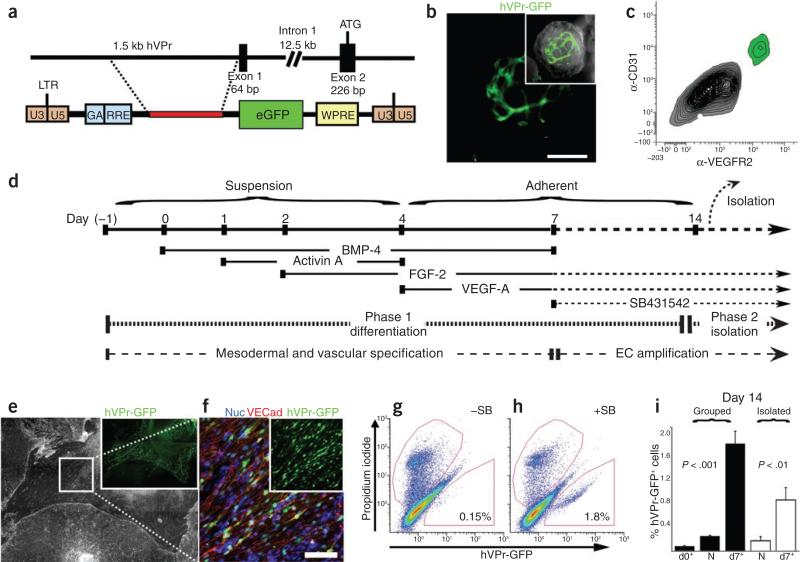
To detect the emergence of endothelial cells from differentiating hESCs in real time, we generated a cell line for endothelial cell–specific lineage tracing. We cloned a 1.5-kilobase fragment from a bacterial artificial chromosome (BAC) containing the genomic locus of the human endothelial cell–specific gene VE-cadherin (CDH5). The promoter sequence of this gene, encompassing a region upstream of exon 1, was inserted into a lentiviral vector upstream of GFP (hVPr-GFP; Fig. 1a). Human endothelial cells transduced with this vector showed robust expression of GFP, in contrast to transduced human mesenchymal and fibroblastic cells, which did not express GFP (Supplementary Fig. 1a–c). Endothelial-specific expression of the reporter was also evident in transduced, spontaneously differentiating hESCs (RUES1 line; Fig. 1b and Supplementary Fig. 1d–j): hVPr-GFP+ cells were organized into capillary-like structures expressing endothelial cell markers, including VE-cadherin, CD31 and CD34 (Supplementary Figs. 1d–g and 2a,b), and were negative for alpha smooth muscle actin (α-SMA) and CD45, a marker of hematopoietic cells (Supplementary Figs. 1h–j and 2c).
Figure 1.
Sequential TGFβ activation followed by inhibition during phase 1 differentiation promotes a tenfold expansion of hVPr-GFP+ heSC-derived cells. (a) A 1.5-kb fragment of the putative human Ve-cadherin promoter (hVPr) region was isolated from a BAC clone and placed upstream of GFP in a lentiviral expression vector (hVPr-GFP). (b) Spontaneously differentiating embryoid bodies exhibited expression of hVPr-GFP in tubular structures. Inset, merge of GFP and brightfield views. (c) Flow cytometric analysis showed hVPr-GFP+ cells were positive for the vascular markers CD31 and VeGFR2. (d) Schematic diagram showing the sequence in which BMP4, activinA, FGF-2, VeGF-A and SB431542 were added and removed from differentiation cultures. eC, endothelial cell. (e) Adherent hVPr-GFP cultures stimulated with SB431542 (10 μM) resulted in differentiation of heSCs into monolayers of hVPr-GFP+ adherent cells. Inset, hVPr-GFP+ cells alone. (f) Human VPr-GFP+ cells were immuno-positive for Ve-cadherin. Blue, nuclear counterstain. Inset, hVPr-GFP+ cells alone. (g,h) The proportion of hVPr-GFP+ cells was measured by flow cytometry at day 14 after culture in the absence (–SB; g) and presence (+SB; h) of SB431542. (i) Measurement of hVPr-GFP+ cells at day 14 when embryoid bodies were cultured either in groups or as isolated embryoid bodies and SB431542 was added at day 0, day 7 or not at all (N). error bars represent s.d. of experimental values performed in triplicate. Scale bars, 100 μm.
Using the hVPr-GFP hESC reporter line, we tracked the chronology and geometry of vasculogenic differentiation in differentiating embyroid bodies by time-lapse confocal microscopy. Beginning at day 5, we observed the specification and emergence of hVPr-GFP+ cells (Supplementary Video 1 and Supplementary Fig. 3), and by day 8, hVPr-GFP+ cells co-expressing vascular endothelial growth factor receptor (VEGFR)2 and CD31 (Fig. 1c) formed motile vessel-like structures (Supplementary Video 2). These data validated the ability of the hVPr-GFP reporter construct to specifically identify and track hESC-derived nascent endothelial cells.
We used the reporter line to develop a chemically defined, serum-free method for enhancing vascular differentiation. In phase 1, heterogenous embryoid body cultures of hVPr-GFP hESCs were sequentially stimulated with bone morphogenetic protein (BMP)4, activinA, fibroblast growth factor (FGF)-2 and VEGF-A8–10 (Fig. 1d). Although these growth conditions promoted formation of hVPr-GFP+ structures (Supplementary Fig. 4 and Supplementary Videos 3 and 4), the yield of dissociated hVPr-GFP+ endothelial cells obtained by fluorescence-activated cell sorting (FACS) was low, and these few isolated endothelial cells could not be expanded without the majority of cells assuming a non-endothelial cell phenotype (data not shown). We therefore screened for bioactive small molecules that would improve vascular differentiation. Screening of >20 molecules associated with early developmental signaling pathways (Supplementary Table 1) showed that the TGFβ-inhibitory molecule SB431542 (ref. 11) reproducibly increased the yield of hVPr-GFP+ cells. Adding SB431542 to differentiation cultures at day 7 resulted in the formation of hVPr-GFP+ VE-cadherin+ monolayers (Fig. 1e,f), which, upon dissociation, yielded tenfold more hVPr-GFP+ endothelial cells than cultures stimulated by cytokines alone (Fig. 1g–i). No hVPr-GFP+ cells were generated if SB431542 was added at the onset of differentiation (day 0), suggesting that vascular commitment depends on active TGFβ/activin/nodal signaling before day 7.
Kinetic analysis of differentiation suggested a shift from a pluripotent phenotype (Oct3/4+; Fig. 2a) to a vascular phenotype (CD31+; Fig. 2b,c) through a mesodermal intermediate (brachyury+; Fig. 2a). Addition of SB431542 to differentiating hESC cultures at day 7 accelerated the reduction of Oct3/4 and brachyury and increased the number of hVPr-GFP+CD31+ cells beginning at about day 9, while reducing expression of α-SMA (Fig. 2b,c). After isolation from heterogenous cultures by FACS, endothelial cells grown in the absence of TGFβ inhibition retained high expression of CD31 but also expressed α-SMA (Supplementary Video 5), indicating that these endothelial cell–like cells had not assumed a terminally committed vascular fate.
Figure 2.
TGFβ inhibition after endothelial cell isolation during phase 2 increases yield and preserves vascular identity of purified endothelial cells. (a–c) Human VPr-GFP heSCs were sequentially stimulated with cytokines (–SB) and SB431542 (+SB) (Fig. 1d and Online Methods) and cultures were assessed for the prevalence of pluripotency (Oct3/4) and mesodermal transcripts (brachyury) (a), CD31 and α-SMA transcripts (b) and endothelial cell markers hVPr-GFP and CD31 (c) at multiple time points during differentiation. The secondary axis in b shows values for cells shown in solid bars. (d) Isolated endothelial cells that were cultured in the absence of SB431542 were stained for both Ve-cadherin and α-SMA and showed rare cells that were positive for both markers (arrowhead in the inset). Inset, α-SMA alone. (e–i) Human VPr-GFP+ cells were isolated from differentiation cultures at day 14 by FACS and further cultured in the absence (e) or presence (f) of SB431542. (g) Flow cytometric assessment of CD31 was performed after 5 d of isolated culture (total cells are shown in white and CD31+ cells are shown in black in the bar graph). (h) After isolation and 5 d of culture in the presence or absence of SB431542, the incidence of α-SMA+ cells was measured. (i) After 5 d of culture following isolation, unstimulated cultures showed reduced incidence of cells positive for phospho-histoneH3 (PHH3+), relative to SB431542-stimulated cultures. The mean incidences of α-SMA and phospho-histoneH3 positive cells were obtained by counting positively stained cells in multiple parallel wells. (j) The yield of endothelial cells (eCs) from heSCs is schematized relative to a 50,000 heSC input at day 0. The relative difference in endothelial cell (eCs) number is indicated at day 14 (upon isolation from differentiation cultures), and day 20 (after expansion in isolated conditions). The ratio of input heSCs to committed heSC-derived endothelial cells after 20 d is also shown. Relative transcript abundance was measured by QPCR and normalized to the housekeeping gene β-actin (ACTB). error bars in (a–c and g–i) represent s.d. of experimental values performed in triplicate. Scale bars, 100 μm.
Expression of α-SMA in hESC-derived endothelial cells suggested a degree of plasticity that is not present in terminally differentiated endothelial cells (human umbilical vein endothelial cells; HUVEC, Fig. 2b). Indeed, extended culture (>10 d after FACS isolation) of hESC-derived endothelial cells in the absence of TGFβ inhibition yielded a substantial number of cells co-expressing VE-cadherin and α-SMA (Fig. 2d). One explanation for the increased percentage of endothelial cells in SB431542-stimulated cultures is maintenance of the vascular-committed state after specification. To test the capacity of TGFβ inhibition to promote expansion of pure populations of hESC-derived endothelial cells, we dissociated day 14 differentiation cultures, isolated hVPr-GFP+ cells by FACS and expanded them for an additional 5 d with or without SB431542 (Phase 2, Fig. 2e–i). SB431542-treated cultures yielded more cells in the 5-d period, and a higher percentage of the population retained an α-SMA−CD31+VE-cadherin+ phenotype (Fig. 2e–h). In addition to preserving the vascular phenotype, SB431542 also increased cell proliferation, as indicated by a higher percentage of phospho-histoneH3+ (PHH3) mitotic endothelial cells (Fig. 2i and Supplementary Videos 6 and 7).
In aggregate, TGFβ inhibition in phase 1 and 2 resulted in a 36-fold expansion in the total number of vascular-committed hESC-derived endothelial cells, with 7.4 CD31+ VE-cadherin+ endothelial cells generated from every one hESC input over 20 d, compared with 0.2 endothelial cells per input hESC derived from control culture conditions (Fig. 2j). Similar levels of expansion of hESC-derived endothelial cells were achieved with four additional hESC lines and one induced pluripotent stem cell line using the same protocol except that either SB431542 or soluble TGFβRII receptor decoys was used interchangeably to inhibit activation of the activin/nodal branch of TGFβ superfamily signaling (Supplementary Fig. 5a–e). These results demonstrate that the effect of TGFβ inhibition shown for the RUES1 line is applicable to other pluripotent cell lines.
To define the vasculogenic transcriptional signature of hESC-derived endothelial cells at different time points during phases 1 and 2, we carried out Affymetrix microarray analyses of several hESC-derived populations and mature cell types (Fig. 3a). The yield of freshly isolated phase 1 endothelial cells in the absence of TGFβ inhibition was insufficient for microarray analyses, underscoring the value of our approach for generating sufficient expanding (phase 1) and vascular-committed (phase 2) endothelial cells for molecular profiling.
Figure 3.
Molecular profiling of heSC-derived endothelial cells reveals a signature defined by high Id1 expression. Human VPr-GFP embryoid bodies and highly purified hVPr-GFP+ cells were compared to mature vascular cells by microarray analysis. (a) RNA was extracted for microarray analysis from human VPr-GFP embryoid bodies cultured in the presence of recombinant cytokines alone until day 14; isolated endothelial cells (99.8% pure) from hVPr-GFP embryoid bodies cultured in the presence of recombinant cytokines and the TGFβ inhibitor SB431542 until day 14; isolated endothelial cells (>95% pure) from hVPr-GFP embryoid bodies cultured in the presence of recombinant cytokines and the TGFβ inhibitor SB431542 until day 14, followed by 10 d additional culture in the presence of cytokines and SB431542; HUVeCs; human umbilical vein smooth muscle cells; and CD34+ umbilical cord blood cells. expressed factors are displayed in an ordered array, as labeled, with highly expressed factors shown in red, minimally expressed factors shown in blue and factors for which transcripts are below a significant expression level shown in gray. (b–f) Following the endothelial cell differentiation protocol (Fig. 1d), Id1-YFP heSC-derived cells were sorted by FACS, separating the CD31+ population into Id1-YFPhigh-expressing cells (b (green) and c) and Id1-YFPlow-expressing cells (b (red) and d). Insets, brightfield views on the day after isolation of Id1-YFPhigh (c) and Id1-YFPlow (d) cells. (e) After 3 d culture in the presence of SB431542, both populations were transferred to conditions with and without SB431542 for an additional 4 d (+SB and –SB, respectively). (f) Total cells and mean fluorescence intensity (MFI) measurements of Id1:YFP (black) and CD31+ (white) were measured for: CD31+Id1low (I) and CD31+Id1high (II) populations upon isolation; and for four populations following culture conditions (as shown in e, III–VI). Scale bars, 100 μM.
Phase 1 hESC-derived endothelial cells showed increased levels of factors typical of arterial-like endothelial cells (VEGFR2, VEGFR1, Id1, CD31, CD34, VE-cadherin, vWF, thrombomodulin, ephrinB2 and E-selectin) but not of lymphatic endothelial cells (Prox1 and podoplanin). Markers of vascular progenitor cells, including CD133 and Id1 (refs. 12–17), were also highly expressed in phase 1 endothelial cells and downregulated upon in vitro culture. Transcription factors expressed primarily in committed endothelial cells, including HoxA9 (ref. 18), were not expressed in phase 1 endothelial cells. Accordingly, we defined a comprehensive vasculogenic expression profile of the hESC-derived endothelial cell population as VE-cadherin +VEGFR2highId1highthrombomodulinhighephrinB2+CD133+HoxA9−, whereas mature endothelial cells were identified by a VE-cadherin+VEG FR2lowId1lowephrinB2+CD133−HoxA9+ phenotype.
Id1 was one of numerous transcription factors upregulated in phase 1 endothelial cells. Because it has been shown to modulate differentiation and maintenance of vascular cell fate19, we focused on Id1 as a potential mediator of the pro-angiogenic effect of TGFβ-inhibition observed in our study. To track Id1 expression in live hESC differentiation cultures, we used a stable BAC transgenic hESC line20 containing yellow fluorescent protein driven by the Id1 promoter (Id1-YFP) (Fig. 3b–f) (Nam, H.S. and Benezra, R., unpublished data). Differentiated endothelial cells were isolated at day 14 from Id1-YFP cultures (Fig. 1d), sub-fractionating the CD31+ population into Id1-YFP high-expressing (Fig. 3c) and low-expressing (Fig. 3d) cells, and these populations were serially expanded for 7 d with or without the TGFβ inhibitor (Fig. 3e,f). Flow cytometric analysis of these cells revealed a direct relationship between upregulation of Id1 expression and TGFβ inhibition. Notably, although SB431542 increased the percentage of the CD31+ population, the mean fluorescence intensity of CD31 on these cells was lower than that of unstimulated cells. These data suggested that TGFβ inhibition increased expansion of hESC-derived endothelial cells by maintaining high levels of Id1 expression and preserving an immature proliferative phenotype.
To determine the requirement for Id1 in mediating endothelial cell commitment, we transduced hVPr-GFP+ cells with lentiviral short hairpin (sh)RNA targeted against the Id1 transcript (Fig. 4a,b). In the presence of SB431542, knockdown of Id1 reduced the numbers of both VEGFR2+ vascular progenitors and hVPr-GFP+ cells at day 14. When the Id1 shRNA construct was introduced after isolation of the hVPr-GFP+ fraction (Fig. 4c), it elicited a marked decrease in CD31+ endothelial cells after 5 d of SB431542 treatment (Fig. 4d). These results identified TGFβ inhibition–mediated Id1 upregulation as a primary effector in promoting endothelial cell expansion and maintaining long-term vascular identity.
Figure 4.
TGFβ inhibition upregulates Id1 expression and is necessary for the increased yield of functional endothelial cells capable of in vivo neo-angiogenesis. (a,b) Human VPr-GFP heSCs that were stably transduced with control (a) or Id1-specific (b) shRNAs were differentiated according to the protocol shown in Figure 1d and assessed at day 14 for the prevalence of VeGFR2+ (blue) and hVPr-GFP+ (green) cells. The insets show plots of side scatter on the y axis and hVPr-GFP on the x axis. (c) Control and Id1-specific shRNAs were added to HUVeC or freshly isolated (at day 14) hVPr-GFP+ cells, and the relative Id1 transcript levels were measured after 3 d. *, P < 0.05. error bars, s.d. of experimental values performed in triplicate. (d) Control and Id1-specific shRNAs were added to freshly isolated hVPr-GFP+ cells, which were cultured in the absence or presence of SB431542. After 5 d, the total cell number and proportion of CD31+ cells was measured by flow cytometry. error bars, s.d. of experimental values performed in triplicate. Scr, scrambled control shRNA. (e–g) Human VPr-GFP+ cells were isolated by FACS at day 14 and expanded in monolayer culture (e) for 8 d while retaining expression of both the endogenous Ve-cadherin (f) and the hVPr-GFP transgene (g). Panel e shows a mosaic view of one well of a 24-well dish. A magnified view of the boxes in e and f are shown in f and g, respectively. (h,i) expanded cells were injected in Matrigel plugs into immunodeficient mice and excised after 10 d following intravital labeling of functional vasculature with lectin (GIB4, blue). h, View of hVPr-GFP+ cells alone; i, view of hVPr-GFP+ cells merged with GIB4+ cells. Scale bars, 100 μM.
To demonstrate that our cultured endothelial cells could form functional vessels, we grew purified hVPr-GFP+ cells from day 14 differentiation cultures for an additional 8 d in the presence of SB431542. These endothelial cells showed high proliferative potential (up to ten cell divisions) and generated homogenous hVPr-GFP+VE-cadherin+ monolayers (Fig. 4e–g) with retention of hVPr-GFP fluorescence at the single-cell level (arrowheads in Fig. 4g). These cells were subcutaneously injected in Matrigel plugs into nonobese (NOD)/severe combined immunodeficient (SCID) mice and 10 d later extracted after intravenous injection of lectin into live animals. In Matrigel plugs, hVPr-GFP+ cells co-localized with lectin+ cells, forming chimeric vessels along with host cells (Fig. 4h–i and Supplementary Videos 8 and 9). These data indicated that the endothelial cells generated by our methods could function in vivo.
A prerequisite to therapeutic vascularization using hESC-derived cells is generation of abundant durable endothelial cells that upon expansion maintain their angiogenic profile without differentiating into nonendothelial cell types. Here, we show that differentiation of hESCs into a large number of stable and proliferative endothelial cells can be achieved by early-stage TGFβ-mediated mesoderm induction followed by TGFβ inhibition beginning at day 7 (phase 1) and after isolation at day 14 (phase 2). Using this approach, we achieved a 36-fold net expansion of committed endothelial cells. The increased yield allowed transcriptional analysis, which revealed a molecular signature that sheds light on the regulatory influences that govern embryonic vasculogenesis. Indeed, genes encoding factors associated with vascular progenitor identity (Id1high, VEGFR2high, CD133)12–17,19 as well as vascular markers (PECAM, VE-cadherin, ephrinB2) were highly expressed in hESC-derived endothelial cells and, among these factors, Id1 was found to act downstream of TGFβ inhibition to increase endothelial cell yield by promoting proliferation and preserving vascular commitment. These studies establish TGFβ modulation of Id1 expression as a determinant of hESC-derived endothelial cell identity and set the stage for large-scale generation of authentic long-lasting human endothelial cells for therapeutic vascularization.
Our use of vascular-specific hVPr-GFP and Id1-YFP hESC reporter lines in small-molecule screens allowed the discovery of the TGFβ inhibitor SB431542 as a key stimulus for human endothelial cell differentiation and proliferation in serum-free conditions. In murine ESCs, TGFβ and serum factors promote smooth muscle cell differentiation, whereas inhibition of this pathway promotes formation of CD31+ cells21. Our data show that stage-specific TGFβ inhibition, beginning on day 7 at a point following TGFβ-mediated mesoderm induction, increases the mitotic index and maintenance of hESC-derived endothelial cells by upregulation of Id1 expression. Differentiation of hVPr-GFP hESCs with TGFβ inhibition generated endothelial cells at yields tenfold greater than those of cells differentiated with angiogenic factors alone, and after purification, TGFβ inhibition supported endothelial cell expansion for up to ten cell divisions while retaining the angiogenic surface phenotype. The ability of TGFβ inhibition to increase endothelial cell yield in both differentiating (phase 1) and purified (phase 2) cultures resulted in a 36-fold increase in the absolute number of hESC-derived endothelial cells, with 95% of the population maintaining endothelial cell identity. As such, we have established a means of generating a homogenous population of stable endothelial cells in ratios that greatly exceed hESC input and are relevant to therapeutic vasculoplasty.
Expression of Id1 has been shown to inhibit cell differentiation and growth arrest in multiple cell types22. The TGFβ signaling pathway, through the effectors Smad3 and ATF3, has been shown to repress Id1 promoter activity23. The link between TGFβ signaling, Id1 and preservation of proliferation and phenotypic identity of hESC-derived endothelial cells provides insight into the molecular mechanisms that regulate vascular ontogeny during human development. Indeed, these results point toward a biphasic role for TGFβ signaling during vasculogenesis, whereby early activation of this pathway is required for specification of mesodermal progenitors, and inhibition after vascular commitment functions to increase mitotic index and prevent the loss of endothelial identity. Our approach for vascular monitoring and differentiation may enable the identification of as-yet unrecognized vasculogenic and angiogenic modulators for preclinical studies aimed at the cell-based therapeutic revascularization of ischemic tissues.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturebiotechnology/.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Brivanlou for providing the RUES1 hESC line. D.J., M.S. and G.L. are Fiona and Stanley Druckenmiller Fellows of the New York Stem Cell Foundation. S.R. is supported by Howard Hughes Medical Institute; Ansary Stem Cell Institute; Anbinder and Newmans Own Foundation; National Heart, Lung, and Blood Institute R01 grants HL075234 and HL097797; Qatar National Priorities Research Program; and Empire State Stem Cell Board and New York State Department of Health, NYS C024180.
ONLINE METHODS
Human ESC culture
The experiments delineated in this report were performed primarily with the recently approved RUES1 hESC (generous gift from A. Brivanlou24), and corroborated using WMC2, WMC7, WMC8, generated at Weill Cornell Medical College (courtesy of Z. R./N.Z.) and H9 (Id1-YFP, courtesy of R.B./H.-s.N. and L.S./M.T.) and IPSc (courtesy L.S./G.L.). The permissions for use of these cell lines were obtained after comprehensive review by the Cornell-Rockefeller-Sloan Kettering Institute ESC research oversight committee. The funding for execution of these studies was secured from approved non-federal funding resources. Human ESC culture medium consisted of Advanced DMEM/F12 (Gibco) supplemented with 20% knockout serum replacement (Invitrogen), 1× non-essential amino acids (Gibco), 1× l-glutamine (Invitrogen), 1× penicillin/streptomycin (Invitrogen), 1× β-mercaptoethanol (Gibco) and 4 ng/ml FGF-2 (Invitrogen). Human ESCs were maintained on Matrigel using hESC medium conditioned by mouse embryonic fibroblasts (Chemicon).
Lentiviral vectors and transduction
Supernatants containing infectious particles were collected 40 and 68 h after transfection of HEK 293T with hVPr-GFP along with accessory vectors as previously described25. Viral supernatants were concentrated by ultracentrifugation and used to transduce undifferentiated RUES1 hESCs. After two passages, hESCs were disaggregated by accutase to form single cells, which were isolated and expanded to form multiple parallel cultures, each containing a relatively consistent level of viral incorporation. After expansion, these cultures were differentiated as adherent embryoid bodies and screened for the presence of GFP+ cells.
Id1-YFP hESC reporter line and lentiviral Id1 shRNA knockdown
A bacterial artificial chromosome (BAC) was modified to place YFP under control of the endogenous Id1 promoter locus. This construct was electroporated into the H9 hESC line, selected for BAC integration using antibiotic resistance and subcloned. Clones were assessed and selected based on expression of YFP in Id1 hESC derivatives after spontaneous differentiation. The Id1 and control shRNA lentiviral constructs were obtained from Open Biosystems and viral particles were assembled according to the manufacturer's recommendations (pLKO Lentiviral Packaging System).
Embryoid bodies
Human VPr-GFP hESCs were grown to confluence on Matrigel (BD Biosciences) and then incubated in 5 units/ml dispase (Gibco) until colonies were completely detached from the substrate. Human VPr-GFP embryoid bodies were washed and cultured in hESC medium on ultra-low attachment plates (Corning) and cultured in the conditions described, with replacement of cytokine-supplemented medium every 48 h. Embryoid bodies were fixed in 4% paraformaldehyde and frozen for cryosectioning and staining.
Endothelial differentiation protocols
Embryoid bodies were generated and cultured in base hESC medium, supplemented with the cytokines as shown. Sequential administration of cytokines was implemented (Fig. 1d). Briefly, embryoid bodies were generated in hESC base medium without FGF-2. On the morning after generation of embryoid bodies (day 0), medium was supplemented with 20 ng/ml BMP4 (R&D Systems) (removed at day 7); on day 1, medium was supplemented with 10 ng/ml activinA (R&D Systems) (removed at day 4); on day 2, medium was supplemented with 8 ng/ml FGF-2 (Peprotech) (remained for the duration of culture); on day 4, embryoid bodies were transferred to adherent conditions on Matrigel-coated plates and medium was supplemented with 25 ng/ml VEGF-A (Peprotech) (remained for the duration of culture); on day 7, SB431542 (Tocris) was added at 10 μM concentration and remained for indicated duration. Cultures were dissociated using 0.5% Trypsin/EDTA (Gibco) or Accutase (eBioscience). Absolute yield as well as ratio of input hESCs to differentiated endothelial cells was calculated from the number of live cells recovered from differentiation cultures at days 0, 14 and 20. Purified endothelial cells could be frozen and thawed in 10% DMSO with >90% recovery.
Quantitative PCR
Total RNA was prepared from cultured cells using the RNeasy extraction kit (Qiagen) and reverse transcribed using Superscript II reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Relative quantitative PCR was performed on a 7500 Fast Real Time PCR System (Applied Biosystems) using either TaqMan PCR mix along with Id1 and β-actin primer pairs, or SYBR Green PCR mix (Applied Biosystems). Human-specific SYBR green primer pairs used were: PECAM – f, 5′-tctatgacctcgccctccacaaa–3′, r, 5′ gaacggtgtcttcaggttggtatttca-3′; Oct3/4 - f, 5′-aacctggagtttgtgccagggttt-3′, r, 5′-tgaacttcaccttccctccaacca-3′; Brachyury – f, 5′-cagtggcagtctcaggttaagaagga-3′, r, 5′-cgctactgcaggtgtgagcaa-3′; and a-SMA, f, 5′-aatactctgtctggatcggtggct-3′, r, 5′-acgagtcagagctttggctaggaa-3′. Cycle conditions were: one cycle at 50 °C for 2 min followed by 1 cycle at 95 °C for 10 min followed by 40 cycles at 95 °C for 15s and 60 °C for 1 min. Primers were checked for amplification in the linear range and primer dissociation and verified. Threshold cycles of primer probes were normalized to the housekeeping gene β-actin (ACTB) and translated to relative values.
Endothelial cell isolation and flow cytometry
Endothelial cells were isolated from differentiation cultures using Magnetic Activated Cell Sorting (MACS; Miltenyi Biotech) with an antibody against CD31 conjugated to magnetic microbeads. Alternatively, cells were isolated by virtue of the expression of GFP/YFP or a fluorophore conjugated antibody to human CD31 or VEGFR2 (BD) using a FACSAriaII (BD).
Microarray analysis
The Affymetrix Human Genome U133 2.0 array was used to analyze gene expression. In brief, using Qiagen RNeasy kits, total RNA was extracted from: Human VPr-GFP embryoid bodies that were cultured in the presence of recombinant cytokines alone until day 14; MACS-sorted endothelial cells isolated from hVPr-GFP embryoid bodies cultured in the presence of recombinant cytokines alone until day 14; MACS-sorted endothelial cells isolated from hVPr-GFP transduced embryoid bodies cultured in the presence of recombinant cytokines and the TGFβ inhibitor SB431542 until day 14; MACS-sorted endothelial cells isolated from hVPr-GFP embryoid bodies cultured in the presence of recombinant cytokines and the TGFβ inhibitor SB431542 until day 14, followed by 10 d additional culture in the presence of cytokines and SB431542; human umbilical vein endothelial cells; human umbilical vein smooth muscle cells; and CD34+ umbilical cord blood cells. The Superscript choice kit (Invitrogen) was used to make cDNA with a T7-(dT)24 primer incorporating a T7 RNA polymerase promoter. The biotin-labeled cRNA was made by in vitro transcription (Enzo Diagnostics). Fragmented cRNA was hybridized to the gene chips, washed, and stained with streptavidin phycoerythrin. The probe arrays were scanned with the Genechip System confocal scanner and Affymetrix Microarray suite 4.0 as used to analyze the data.
Matrigel plug
Human VPr-GFP embryoid bodies were differentiated for 14 d by our differentiation protocol followed by expansion in the presence of SB431542 for 10 d and injected subcutaneously into NOD/SCID mice in a suspension of Matrigel. After 2 weeks, Griffonia simplificolia IB4 lectin and/or Ulex europus agglutinin lectin were administered intra-vitally to Matrigel plug–bearing mice and plugs were harvested, fixed overnight in 4% paraformaldehyde and equilibrated in 30% sucrose before freezing and cryosectioning.
Immunofluorescence
Cryosections were immunocytochemically stained as previous described24. Briefly, samples were permeabilized in PBST and blocked in 5% donkey serum. Samples were incubated for 2 h in primary antibodies blocking solution, washed 3 times in PBS and incubated in CY3-conjugated secondary antibodies (Jackson Laboratories) for 1 h. After washing, some sections were counterstained for nucleic acids by TO-PRO3 (Invitrogen) before mounting and imaging by confocal microscopy. Primary antibodies included CD31 (DAKO), CD34 (DAKO), Phospho-HistoneH3, Smooth Muscle Actin (DAKO) and VE-cadherin (R&D). All imaging was performed using a Zeiss 510 META confocal microscope.
Live imaging and 3D rendering
Human VPr-GFP embryoid bodies were cultured in a TOKAI-HIT live cell-imaging chamber on a Zeiss 510 META confocal microscope. Laser intensity and interval were optimized to ensure viability of cells for the duration of the experiments. Three-dimensional reconstruction and rendering of optical z-stacks was performed using Improvision Volocity software.
Footnotes
Accession codes. GEO: GSE19735.
Note: Supplementary information is available on the Nature Biotechnology website.
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Yamahara K, et al. Augmentation of neovascularization in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLoS ONE. 2008;3:e1666. doi: 10.1371/journal.pone.0001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sone M, et al. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler. Thromb. Vasc. Biol. 2007;27:2127–2134. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- 4.Lu SJ, et al. Generation of functional hemangioblasts from human embryonic stem cells. Nat. Methods. 2007;4:501–509. doi: 10.1038/nmeth1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman O, et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells. 2009;27:1750–1759. doi: 10.1002/stem.100. [DOI] [PubMed] [Google Scholar]
- 6.Nourse MB, et al. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler. Thromb. Vasc. Biol. 2009;30:80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai H, et al. BMP4 regulates vascular progenitor development in human embryonic stem cells through a smad-dependent pathway. J. Cell Biochem. doi: 10.1002/jcb.22410. published online, doi:10.1002/jcb.22410 (30 November 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 9.Levenberg S, Zoldan J, Basevitch Y, Langer R. Endothelial potential of human embryonic stem cells. Blood. 2007;110:806–814. doi: 10.1182/blood-2006-08-019190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonicstem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 11.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Gehling UM, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 13.Kelly MA, Hirschi KK. Signaling hierarchy regulating human endothelial cell development. Arterioscler. Thromb. Vasc. Biol. 2009;29:718–724. doi: 10.1161/ATVBAHA.109.184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peichev M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 15.Rafii S, Lyden D. Cancer. A few to flip the angiogenic switch. Science. 2008;319:163–164. doi: 10.1126/science.1153615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 17.Lyden D, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 18.Rossig L, et al. Histone deacetylase activity is essential for the expression of HoxA9 and for endothelial commitment of progenitor cells. J. Exp. Med. 2005;201:1825–1835. doi: 10.1084/jem.20042097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410–418. doi: 10.1016/s0962-8924(03)00147-8. [DOI] [PubMed] [Google Scholar]
- 20.Placantonakis DG, et al. BAC transgenesis in human embryonic stem cells as a novel tool to define the human neural lineage. Stem Cells. 2009;27:521–532. doi: 10.1634/stemcells.2008-0884. [DOI] [PubMed] [Google Scholar]
- 21.Watabe T, et al. TGF-beta receptor kinase inhibitor enhances growth and integrity of embryonic stem cell-derived endothelial cells. J. Cell Biol. 2003;163:1303–1311. doi: 10.1083/jcb.200305147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jankovic V, et al. Id1 restrains myeloid commitment, maintaining the self-renewal capacity of hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:1260–1265. doi: 10.1073/pnas.0607894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang Y, Chen CR, Massague J. A self-enabling TGFbeta response coupled to stress signaling: Smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol. Cell. 2003;11:915–926. doi: 10.1016/s1097-2765(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 24.James D, Noggle SA, Swigut T, Brivanlou AH. Contribution of human embryonic stem cells to mouse blastocysts. Dev. Biol. 2006;295:90–102. doi: 10.1016/j.ydbio.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 25.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.