Abstract
Congestive heart failure (CHF) is associated with neurohumoral activation. Only very few studies have examined the progression of autonomic dysfunction in CHF in humans and scanty data are available in animal models of CHF. This study was performed to assess the changes in cardiac autonomic modulation during the progression of CHF in a rat model, using an innovative analysis of heart rate variability. Progression of cardiovascular autonomic dysfunction was assessed in a rat model of CHF induced by coronary artery ligation. Spectral and symbolic analyses were performed on heart period (approximated with pulse interval, PI) and systolic arterial pressure (SAP) signals, acquired ~2 and ~4 weeks after the surgical procedure. As CHF developed, symbolic analysis revealed a decrease of rhythmical physiological sympathetic modulation, as indicated by the reduction of the percentage of stable patterns. In addition, symbolic analysis revealed that runs of short-long-short and/or long-short-long PI values and high-low-high and/or low-high-low SAP values were more and more frequent as CHF progressed. On the contrary, spectral analysis of PI and SAP series was not able to detect any impairment of autonomic regulation. Indeed, low frequency and high frequency powers derived from both PI and SAP series were not significantly changed. These data indicate that the autonomic cardiovascular modulation is altered during the progression of CHF and that symbolic analysis seems to be more suitable than spectral analysis to describe alterations of heart period dynamics and of cardiovascular regulation in this animal model of CHF.
Keywords: nonlinear dynamics, autonomic nervous system, congestive heart failure
1. Introduction
Congestive heart failure (CHF) is a pathological condition characterized by altered neurohumoral regulation. The renin-angiotensin-aldosterone system (Dzau et al., 1981; Francis, 1998; Kjaer and Hesse, 2001; Middlekauff and Mark, 1998), the immune system (Deswal et al., 2001; Gullestad and Aukrust, 2001; Petersen and Felker, 2006), and the sympathetic nervous system (Cohn and Yellin, 1984; Leimbach et al., 1986; Malliani and Montano, 2002; Malliani and Pagani, 1983; Watson et al., 2006; Zucker, 2006) are all activated. Sympathetic activation in animals with CHF has been demonstrated by several different methods, including measurements of plasma catecholamines (Anker, 1998; Francis, 1988), noradrenaline spillover (Esler and Kaye, 2000; Hasking et al., 1986) and direct recordings of renal and muscle sympathetic activity (Ferguson et al., 1992; Grassi et al., 1995). However, the imbalance of autonomic regulation favoring sympathetic activation may be not limited to an increased firing rate. For example, in humans changes in the rhythmical properties of autonomic outflow, reflected in modifications of the oscillatory components of heart rate variability (HRV), have been described (van de Borne et al., 1997; Guzzetti et al., 1995; Guzzetti et al., 2001). In patients with CHF there was an increase of the amplitude of low frequency (LF) oscillations in the early stages of the disease and a reduction of the LF component at the end stage (Guzzetti et al., 1995). In severe CHF patients the absence of the LF oscillations of HRV has been ascribed to the loss of rhythmic properties of the sympathetic discharge (Van de Borne et al., 1997) triggered by an increase of sympathetic afferent activity and/or an impairment of baroreflex function that might modify the sympathetic central pattern generator. A reduction of the LF component in CHF patients is a strong predictor of mortality, independent of the absolute level of sympathetic firing (La Rovere et al., 2003).
However, spectral analysis results in CHF condition are limited by several factors. Indeed, the reliability of spectral estimates is quite low in conditions characterized by a very low variability, unstable rhythms the frequency of which might escape outside the assigned frequency bands and non linear components generating harmonics that are not independent (Malliani et al., 1991). This is mostly the case in an animal model of CHF such as the myocardial ischemic rats. Recently, a new non linear method for the investigation of HRV based on symbolic analysis has been validated and found to provide more information on cardiac autonomic modulation than the linear spectral methods in normal as well as in cardiac patients (Guzzetti et al., 2005; Porta et al., 2007a; Porta et al., 2007b).
The aim of the present study was to test whether symbolic analysis can be considered a more powerful tool than spectral analysis in detecting the changes in autonomic modulation of cardiovascular function that occur as CHF develops in rats following an acute myocardial infarction induced by coronary artery ligation, that is one of the most used animal CHF models.
2. Materials and methods
2.1 The CHF animal model
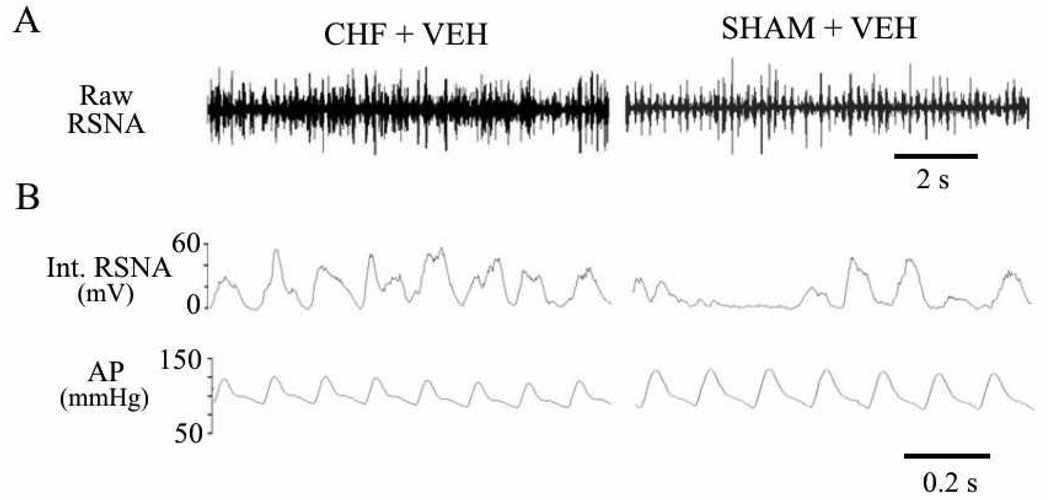
Coronary artery ligation induces CHF in rats that mimics the CHF syndrome in humans. The typical manifestations of CHF in this model have been reported elsewhere (Francis et al., 2001a; Kang et al., 2006). In brief, over the 4–6 weeks following coronary artery ligation renal sympathetic nerve activity (RSNA) increases and baroreflex regulation of RSNA and HR is impaired. Volume regulation is also impaired, as indicated by reduced sodium and water excretion and increased left ventricular end-diastolic pressure and heart/body weight and lung/body weight ratios. Consumption of a sodium rich drinking solution, if offered, is increased. Arterial pressure and pulse pressure are reduced but heart rate is not affected. Echocardiography reveals a reduction in left ventricular ejection fraction immediately after coronary ligation, with no significant further changes over the ensuing 4 weeks. Left ventricular volume increases progressively, as does left ventricular volume/mass ratio Representative analog tracings from a CHF and a sham-operated (SHAM) control rat are shown in Figure 1.
Figure 1.
Representative recordings from a conscious rat with CHF 4 weeks after coronary ligation (left panels) and a sham-operated (SHAM) control rat (right panels). A, raw recordings of RSNA. B, fast time base displays of integrated RSNA (mV) and simultaneously recorded arterial pressure (AP, mmHg), illustrating loss of the normal modulation of the RSNA signal in the CHF rat.
Recorded data from two prior studies (Francis et al., 2002; Francis et al., 2001b) were sampled for spectral and symbolic analysis. The data were acquired from adult male Sprague–Dawley rats that underwent coronary artery ligation to induce CHF, or a sham operation, with echocardiographic confirmation of the extent of ischemic injury. Some of the rats had undergone placement of an intracerebroventricular cannula two weeks earlier. The only treatment these rats received was intracerebroventricular or intraperitoneal infusion of vehicle. At approximately two (Francis et al., 2002) or four (Francis et al., 2001b) weeks after coronary ligation, the rats were re-anesthetized to implant renal nerve recording electrodes and arterial and venous cannulas. RSNA and blood pressure were recorded in the conscious, freely mobile state 4 hours after recovery from pentobarbital (50 mg/kg) anesthesia. The surgical preparations and recording techniques are described in detail in previous publications (Francis et al., 2001b; Francis et al., 2001a). All experimental procedures were approved by University of Iowa Institutional Animal Care and Use Committee. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
The data reported here were obtained from CHF rats studied approximately 2 weeks (CHF-2wk, n=7) or 4 weeks (CHF-4wk, n=7) after coronary ligation, and from SHAM rats studied at 4 weeks (n=7). In this model, left ventricular ejection fraction at 2–3 weeks (Francis et al., 2001b) and at 6 weeks (Francis et al., 2001b) has been demonstrated to be significantly reduced compared to SHAM animals, while no differences were observed between these two groups of CHF animals.
2.2 Spectral and symbolic analysis of cardiovascular variability
In order to increase temporal resolution of pulse interval (PI), the blood pressure signal was sampled at 12000 Hz. PI was derived from the interdiastolic interval with a resolution of 0.8 ms; systolic arterial pressure (SAP) was calculated as well. Spectral and symbolic analysis were carried out over short PI and SAP series of 200 ± 50 beats manually selected from the entire recordings using the first stationary segment detected. In addition, the same analysis was performed over longer data sequences of 500 beats, iterated with maximum overlapping (499 beats) without any selection by the operator, being a completely automatic approach. Spectral and symbolic parameters were extracted for each frame and the distribution of the parameters over the entire recording was derived. We selected the median of the distribution as the most representative parameter value in a specified experimental condition (Porta et al., 2007a).
Spectral analysis is a method capable of providing a quantitative evaluation of the sympathovagal interaction modulating cardiovascular functions (Malliani et al., 1991; Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996). This methodology, using an autoregressive approach, performs a frequency domain analysis of HRV. The best model order was automatically selected using the Akaike figure of merit in the range from 8 to 14. The power spectrum of short term HRV contains three components; a very low (VLF, below 0.2 Hz), a low (LF, 0.2–0.8 Hz) and a high (HF, 0.8–2 Hz) frequency component. The LF and HF components are expressed in absolute (msec2) and normalized (nu) units (Dias da Silva et al., 2002). Normalization consists in dividing the power of a spectral component by the total power minus the VLF component and multiplying by 100. On HRV series, the LF power expressed in normalized units is a marker of sympathetic modulation and HF power both in absolute and normalized units is a marker of vagal modulation.
Spontaneous baroreflex sensitivity can be determined based on the power spectrum of the SAP and PI series. The baroreflex sensitivity is derived as the square root of the ratio of PI to SAP power in the LF band and indicated as αLF (Pagani et al., 1988). This index was considered to reliably estimate whether the coherence value in the LF band was significant. We tested the significance of the coherence in LF band according to the method proposed by Porta et al. (Porta et al., 2002). This method is based on the generation of a set of isospectral uncoupled surrogates and on the calculation of a threshold for the uncoupling (i.e. coherence values smaller than the threshold indicated that oscillations of SAP and PI series at that specific frequency were not linearly correlated).
Symbolic analysis is a non-linear method based on the conversion of the series into a sequence of symbols. The full dynamic of the series (the min-max range) is spread over six bins, each of which is identified by a number (symbol) from 0 to 5. Original values inside each bin are substituted by the symbol defining the specific bin, thus obtaining a symbolic series. The symbolic series is converted into a series of patterns of three symbols. Four different families of patterns can be identified (Porta et al., 2001; Porta et al., 2007b): 0V (patterns with no variation, all symbols are equal), 1V (patterns with one variation, two consecutive symbols are equal and the remaining one is different), 2LV (patterns with two like variations, the second and the third symbol change with respect to the previous one and the changes have the same sign) and 2UV (patterns with two unlike variations, the second and the third symbol change with respect to the previous one and the changes have opposite sign) (Porta et al., 2001). This method has been recently applied to evaluate cardiac autonomic control from HRV (Guzzetti et al., 2005). It has been demonstrated that the percentage (%) of occurrence of the 0V pattern - i.e., 0V% - is a marker of sympathetic modulation of HR, while 2UV% is a marker of vagal modulation (Porta et al., 2007b).
2.3 Statistical analysis
A one way ANOVA (Bonferroni’s test) was used to test the significance between the three different groups (SHAM, 2 weeks and 4 weeks CHF groups). If the normality test was not fulfilled, a Kruskal-Wallis one-way ANOVA on ranks was utilized (Dunn’s test). A value of p< 0.05 was considered statistically significant. Data are presented as mean ± SD.
3. Results
3.1 Spectral analysis
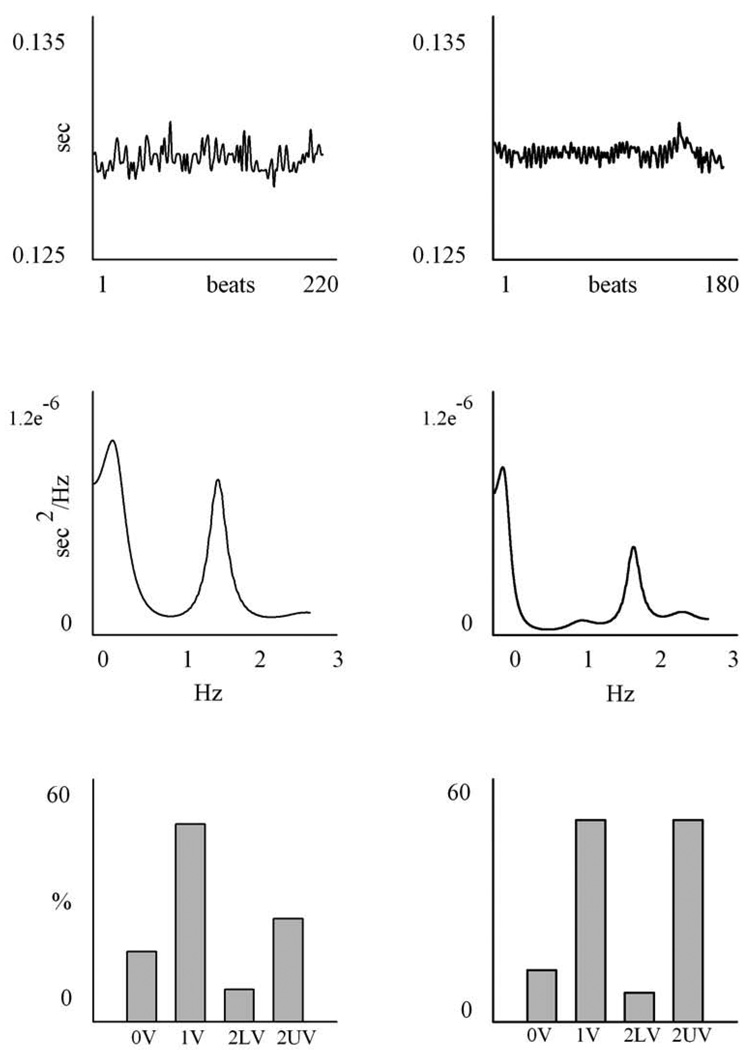
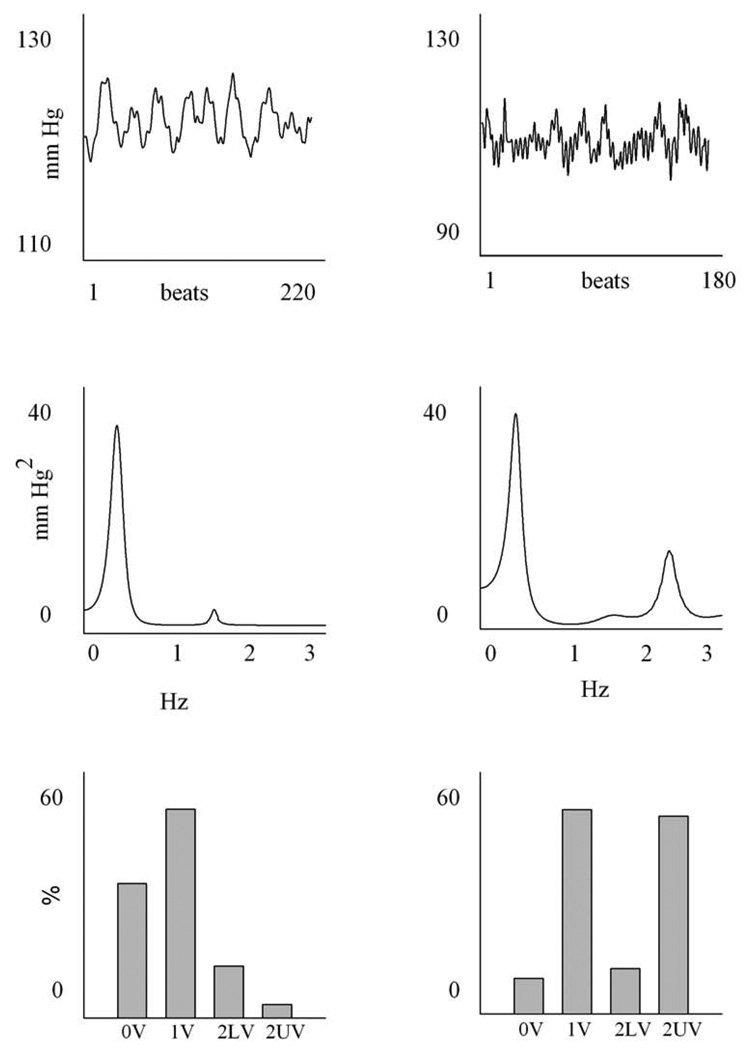
An example of spectral analysis of PI and SAP variability in one SHAM (left) and in one CHF-4wk (right) animal is reported in Figs. 2 and 3. All values are summarized in Table 1.
Figure 2.
An example of a time series, power spectrum and symbolic analysis of PI. The column on the left is a SHAM animal, the one on the right is a 4 weeks CHF rat. The upper panel shows the PI series; the units on the x axis are beats, while those on the y axis are seconds. The middle panels show the power spectrum of the time series: the frequency, expressed in Hz, is reported on the x-axis, while the power spectral density of PI series, expressed in sec2/Hz is reported on the y-axis. The lower panel represents the symbolic pattern distribution of the time series: all the patterns are divided into the four families of patterns, i.e. 0V, 1V, 2LV, 2UV and the percentages of occurrence of the four families are shown.
Figure 3.
An example of a time series, power spectrum and symbolic analysis of SAP. The column on the left is a SHAM animal, the one on the right is a 4 weeks CHF rat. The upper panel shows the SAP series; the units on the x axis are beats, while those on the y axis are mmHg. The middle panels show the power spectrum of the time series: the frequency, expressed in Hz, is reported on the x-axis, while the power spectral density of SAP series, expressed in mmHg2/Hz is reported on the y-axis. The lower panel represents the symbolic pattern distribution of the time series: all the patterns are divided into the four families of patterns, i.e. 0V, 1V, 2LV, 2UV and the percentages of occurrence of the four families are shown.
Table 1.
Spectral parameters of pulse interval (PI), systolic arterial pressure (SAP) and baroreflex sensitivity calculated from time series using autoregressive spectral analysis. All values were expressed as mean ± SD.
| SHAM | 2 WEEKS | 4 WEEKS | |
|---|---|---|---|
| (n=7) | (n=7) | (n=7) | |
| Pulse Interval | |||
| Variance (ms2) | 2 ± 1.4 | 1.96 ± 1 | 1.44 ± 0.8 |
| LF Hz | 0.42 ± 0.15 | 0.5 ± 0.2 | 0.48 ± 0.2 |
| LF (ms2) | 0.36 ± 0.3 | 0.31 ± 0.2 | 0.17 ± 0.12 |
| LF (nu) | 22.6 ± 17.1 | 16.4 ± 11.1 | 12.7 ± 7.7 |
| HF Hz | 1.4 ± 0.2 | 1.65 ± 0.4 | 1.62 ± 0.5 |
| HF (ms2) | 0.59 ± 0.4 | 0.81 ± 0.55 | 0.66 ± 0.41 |
| HF (nu) | 44.5± 13.6 | 39.9 ± 15.5 | 45.1 ± 21.6 |
| Systolic Pressure | |||
| Variance (mmHg2) | 14.2 ± 13.1 | 16.2 ± 7.8 | 9.1 ± 5.2 |
| LF Hz | 0.43 ± 0.04 | 0.34 ± 0.1 | 0.4 ± 0.06 |
| LF (mmHg2) | 10.1 ± 9.0 | 5.6 ± 4.5 | 3.9 ± 2.7 |
| HF Hz | 1.35 ± 0.2 | 1.62 ± 0.4 | 1.75 ± 0.3 |
| HF (mmHg2) | 2.0 ± 2.6 | 5.7 ± 3.6 | 2.9 ± 3.2 |
| Baroreflex | |||
| αLF (ms/mmHg) | 0.19 ± 0.11 | 0.25 ± 0.15 | 0.23 ± 0.11 |
LF: low frequency; HF: high frequency; nu: normalized units.
In the PI series, the total variance as well the LF and HF components both in absolute and normalized units were all similar in the three groups.
As to SAP variability, the total variance was not significantly modified in the three groups, as well as the power and central frequency of LF and HF components. Also baroreflex sensitivity was similar in the three groups, being αLF not different between the SHAM and the CHF groups. The coherence analysis showed that PI and SAP series were significantly linked in correspondence of the central frequency of the LF component in a large percentage of animals (i.e. 7 out of 7, 5 out of 7 and 5 out of 7 at LF in SHAM, CHF-2wk and CHF-4wk groups respectively).
Results of the automatic spectral analysis over overlapped sequences are reported in Table 2. This analysis confirmed the analysis carried out over manually-selected sequences: indeed, automatic analysis did not reveal any significant change among the groups both on PI and SAP variabilities.
Table 2.
Spectral parameters of pulse interval (PI), systolic arterial pressure (SAP) and baroreflex sensitivity calculated from longer (500 beats), overlapped time series using autoregressive spectral analysis.
| SHAM | CHF-2wk | CHF-4wk | |
|---|---|---|---|
| (n=7) | (n=7) | (n=7) | |
| Pulse Interval | |||
| Variance (ms2) | 3.8 ± 1.94 | 2.8 ± 0.66 | 4.5 ± 4.8 |
| LF Hz | 0.62 ± 0.08 | 0.65 ± 0.11 | 0.69 ± 0.07 |
| LF (ms2) | 0.16 ± 0.28 | 0.23 ± 0.26 | 0.15 ± 0.21 |
| LF (nu) | 6.03± 7.7 | 12.7±15.2 | 6.5 ± 6.0 |
| HF Hz | 1.4 ± 0.14 | 1.47 ± 0.3 | 1.56 ± 0.17 |
| HF (ms2) | 0.55 ± 0.4 | 0.65 ± 0.68 | 1.1 ± 1.8 |
| HF (nu) | 42.4± 21.07 | 40.7 ± 19.6 | 29.9 ± 18.6 |
| Systolic Pressure | |||
| Variance (mmHg2) | 15.3 ± 12.5 | 17.7±10.5 | 9.9 ± 6.2 |
| LF Hz | 0.43 ± 0.05 | 0.38 ± 0.11 | 0.3 ± 0.19 |
| LF (mmHg2) | 9.7 ± 9.0 | 3.5 ± 2.7 | 2.4 ± 1.9 |
| HF Hz | 1.5 ± 0.2 | 1.5 ± 0.4 | 1.6 ± 0.3 |
| HF (mmHg2) | 1.9 ± 2.8 | 4.7 ± 4 | 1.4 ± 1.5 |
| Baroreflex | |||
| αLF (ms/mmHg) | 0.1±0.17 | 0.2±0.19 | 0.15±0.13 |
All values were expressed as mean ± SD. LF: low frequency; HF: high frequency; nu: normalized units.
3.2 Symbolic analysis
An example of symbolic analysis of PI and SAP variability in one SHAM (left) and in one CHF-4wk (right) is reported in Fig. 2 and 3. Results are summarized in Table 3.
Table 3.
Symbolic parameters of pulse interval (PI) and systolic arterial pressure (SAP) calculated from time series using symbolic analysis.
| SHAM | 2 WEEKS | 4 WEEKS | |
|---|---|---|---|
| (n=7) | (n=7) | (n=7) | |
| Pulse Interval | |||
| 0V (%) | 15.4 ± 11.0 | 8.57 ± 3.7 | 7.8 ± 3.8* |
| 1V (%) | 45.4 ± 7.1 | 37.6 ± 7.8 | 29.3 ± 8.4* |
| 2LV (%) | 9.9 ± 3.9 | 13.8 ± 7 | 11.8 ± 4.2 |
| 2UV (%) | 29.2 ± 7.7 | 40.1 ± 14.8 | 51.1 ± 11.4* |
| Systolic Pressure | |||
| 0V (%) | 20.2 ± 6.1 | 11.1 ± 13.2 | 7.5 ± 10.1* |
| 1V (%) | 51.1 ± 2.3 | 32.5 ± 17.3* | 29.8 ± 15.3* |
| 2LV (%) | 15.4 ± 5.4 | 14.4 ± 10.5 | 7.7 ± 4.7 |
| 2UV (%) | 13.3 ± 6.9 | 41.1 ± 31.1 | 54.9 ± 23.1* |
All values were expressed as mean ± SD.
p<0.05 versus SHAM
As to PI variability, 0V% decreased in CHF, (15.4 ± 11.0 %, 8.57 ± 3.7 % and 7.8 ± 3.8 % in SHAM, CHF-2wk and CHF-4wk, respectively), being statistically significant in CHF-4wk compared to SHAM. The index 1V% decreased from SHAM to CHF-4wk, while 2LV% was similar in the three groups. The index 2UV% increased during the progression of CHF (29.2 ± 7.7 %, 40.1 ± 14.8 % and 51.1 ± 11.4 % in SHAM, CHF-2wk and CHF-4wk, respectively) and 2UV% in CHF-4wk was statistically significant compared to SHAM.
As to SAP variability, 0V% and 1V% both decreased significantly in the CHF groups, while 2LV% was similar among the three groups. The index 2UV% increased (13.3 ± 6.9 %, 41.1 ± 31.1% and 54.9 ± 23.1% in SHAM, CHF-2wk and CHF-4wk, respectively), being statistically significant in CHF-4wk compared to SHAM.
Results of automatic symbolic analysis of PI and SAP variability over overlapped sequences are reported in Table 4. This analysis carried out over the SAP series confirmed the analysis performed over manually-selected sequences. At variance with manual selection, the reduction of 0V% and the increase of 2UV% observed over PI series were not statistically significant.
Table 4.
Symbolic parameters of pulse interval (PI) and systolic arterial pressure (SAP) calculated from longer (500 beats), overlapped time series using symbolic analysis.
| SHAM | CHF-2wk | CHF-4wk | |
|---|---|---|---|
| (n=7) | (n=7) | (n=7) | |
| Pulse Interval | |||
| 0V (%) | 25.9 ± 13.9 | 20.6 ± 13.9 | 19.1 ± 5.6 |
| 1V (%) | 40.6 ± 6.2 | 37.7 ± 6.7 | 36.5 ± 5.8 |
| 2LV (%) | 6.0 ± 4.5 | 9.6 ± 9.6 | 5.9 ± 4.3 |
| 2UV (%) | 27.2 ± 10.8 | 31.7 ± 10.3 | 38.1 ± 8.5 |
| Systolic Pressure | |||
| 0V (%) | 28.8 ± 9.6 | 15.1 ± 11.4* | 11.5 ± 8.6* |
| 1V (%) | 49.6 ± 2.4 | 39.1 ± 14.1 | 31.7 ± 13.4* |
| 2LV (%) | 10.2 ± 7.2 | 9.8 ± 9.4 | 6.0 ± 5.2 |
| 2UV (%) | 11.1 ± 4.9 | 35.5 ± 26.7 | 50.7 ± 20.7* |
All values were expressed as mean ± SD.
p<0.05 versus SHAM
4. Discussion
The most important findings of this study are: 1) the confirmation that the progression of ischemia-induced CHF in rats is accompanied by significant changes in cardiovascular autonomic regulation, i.e. by increased sympathetic activity and loss of the sympathetic rhythmical modulation; 2) the demonstration that symbolic analysis is a more sensitive tool than spectral analysis to track the changes in cardiovascular autonomic modulation in rats; and 3) the predominance of rapid alternating patterns in the symbolic analysis of PI and SAP variabilities in rats with established CHF.
Two different methods, spectral and symbolic analysis, have been applied to the same experimental data in animals in order to test the hypothesis that the progression of ischemia-induced CHF is characterized by a progressive loss of cardiac autonomic modulation. While the first method is well-established to investigate cardiac autonomic modulation from short-term HRV (Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology, 1996), the second one has only recently been suggested (Guzzetti et al., 2005) and applied to healthy (Guzzetti et al., 2005; Porta et al., 2007b) and pathological subjects (Porta et al., 2007a; Maestri et al., 2007). Symbolic analysis is based on a non-linear transformation and on the conversion of the temporal series into a sequence of three-beat patterns. The percentages of two of the resulting families of patterns correlate with the state of cardiac autonomic modulation: indeed, 0V% increased and 2UV% decreased during experimental conditions characterized by an increased sympathetic modulation (e.g. head-up tilt), while the opposite was observed in experimental procedures increasing parasympathetic modulation (Guzzetti et al., 2005; Porta et al., 2007b).
The present study showed an increase in sympathetic nerve activity during the progression of CHF, consistent with previous reports (Floras, 2002). This increase in sympathetic activity was accompanied by a significant decrease in sympathetic modulation, as indicated by symbolic analysis of both the PI and the SAP series. Indeed, we found that 0V% in the PI series was significantly reduced 4 weeks after CHF. A reduction of 0V% has been reported in CHF subjects as well when compared to healthy subjects (Porta et al., 2007b). This impairment of the sympathetic rhythmical properties might be linked to a peripheral mechanism, such as the downregulation of β adrenoreceptors (Bristow et al., 1982; Lohse et al., 2003) or to the loss of central rhythmical modulation of sympathetic discharge to the periphery (Van de Borne et al., 1997). These results clearly indicate that symbolic indices are robust measures of autonomic modulation. Completely automatic analysis based on overlapped PI sequences confirms the negative trend of 0V% even though differences among experimental conditions were insignificant.
The decline of 1V% in PI variability observed in the CHF groups compared to SHAM has already been described in CHF (Porta et al., 2007a) and has been shown to be an independent predictor of major clinical and functional events (Maestri et al., 2007). This trend, although less clear, was present even when analysis was carried out automatically over overlapped sequences. However, the biological significant of this pattern is not yet clear.
This is the first study using symbolic analysis to investigate autonomic modulation of SAP variability. Results of symbolic analysis of SAP variability are consistent with those derived from HRV. Indeed, a significant reduction of 0V% in the CHF group was observed. Completely automatic analysis, based on overlapped sequences, confirmed the significant reduction of 0V% over SAP series.
We observed a significant increase of rapid alternating patterns in both PI and SAP series, as measured by the increase of 2UV% after 4 weeks of CHF. This trend was significant both when considering manual and automatic analyses of SAP series, while over PI series it was significant only when the sequences were manually selected. The 2UV pattern describes alternating sequences of short-long-short or long-short-long heart periods. In healthy subjects these very fast changes seem to be under vagal control. For example, in a recent work Porta et al (Porta et al., 2007b) reported that in healthy subjects the 2UV% progressively decreased with tilt angles, thus suggesting a progressive loss of vagal modulation. However, an increase in 2UV% has also been reported in CHF patients (Porta et al., 2007a). In this setting, in which it would be naïve to suggest that cardiac vagal modulation increased, other explanations must be considered. It has been suggested that the increase of 2UV% of the PI series (Porta et al., 2007a) in a pathological condition such as CHF might be related to mechanisms not strictly under autonomic nervous system control. With regard to that possibility, pulsus alternans is commonly observed in severe CHF (Kodama et al., 2004) and might alter PI through the activation of baroreflex. Conversely, rapid alternating sequences in the PI series could induce rapid alternating SAP values by altering the stroke volume (Kodama et al., 2004), thus resulting in a 2UV% increase over the SAP series. In any case, 2UV% was significantly enhanced even in the SAP series at 4 weeks. The present experimental protocol provides no clue regarding the temporal sequence of the interactions, thus limiting the possibility of addressing the causal relationship between the symbolic patterns in PI and SAP variability.
In contrast to symbolic analysis, spectral analysis did not reveal any significant change in sympathetic modulation during the progression of CHF. This finding was independent of the approach (i.e. manual or automatic). This conclusion is in agreement with that reported by Kruger et al (Kruger et al., 1997), that observed no difference in terms of spectral parameters at 2, 4 and 8 weeks after coronary ligation in rats. The LF power expressed in normalized units did show a slight tendency towards a decrease in the PI series, in agreement with previous results in humans and animals (Motte et al., 2005; Eaton et al., 1995; Ishise et al., 1998). The HF power of PI variability expressed in absolute units, an index of vagal modulation, was also unchanged at 2 weeks and 4 weeks after coronary ligation, thus confirming that spectral indexes failed to detect any changes in cardiac regulation in our experimental model. Similarly to PI, spectral analysis of SAP series did not reveal any difference in the sympathetic modulation when measured as the LF power expressed in absolute units in the three groups of animals. The low discriminative power of spectral analysis was also confirmed by the absence of change in baroreflex sensitivity when estimated using spectral-based baroreflex sensitivity indexes. It can be hypothesized that the loss of discriminative power of the spectral parameters could be related to the presence of rhythms with unstable frequencies that might escape outside the assigned and fixed limits of the frequency bands or to the presence of non linear components producing harmonics again escaping the band limits, thus being unaccounted by spectral analysis. Since symbolic analysis does not predefine the limits of the frequency bands, it seems to be more flexible in dealing with these issues.
A strength of this study was the application of spectral and symbolic analysis techniques to a body of data obtained from rats with echocardiographically defined CHF. The original analog recording studies in these animals (Francis et al., 2001b) demonstrated the expected increases in sympathetic nerve activity, along with impaired baroreflex regulation of sympathetic drive and heart rate, but they did not detect the more subtle changes in autonomic regulation of the cardiovascular system that could be found with symbolic analysis of cardiovascular variabilities. Symbolic analysis of PI and SAP variabilities might be a tool for noninvasive clinical assessment of the progression (or regression) of CHF.
A limitation of this study is that we do not have echocardiographic assessments of left ventricular function at the time points at which the recordings were obtained. As mentioned above, however, this model is associated with a well described and consistent progression of left ventricular dysfunction to left ventricular failure (Francis et al., 2001a; Kang et al., 2006). A second limitation is that the recordings used for spectral and symbolic analysis were made only four hours after the animals recovered from anesthesia. Thus the residual effects of surgical stress may have minimized differences between the study groups. However, the study was mostly suited to evaluate the performance of symbolic analysis in comparison with spectral one, and, notably, we observed that symbolic analysis was sensitive enough to detect differences in autonomic function even under these conditions.
In conclusion, symbolic analysis is capable of identifying changes in autonomic modulation in CHF rats that are not detected by spectral analysis. Therefore, symbolic analysis appears to be a sensitive tool for the evaluation of autonomic modulation in this ischemic model of CHF. While the decrease of 0V% can be related to the reduced sympathetic modulation that is well described in this condition, the pathophysiological significance of the decrease of 1V% and the increase of 2UV% remains unexplained and deserves further investigations.
Acknowledgments
Funding
This work was partly supported by a PUR 2007 University of Milan Grant and Italian Space Agency Research DCMC Grant to NM, and by NIH RO1 HL-063915 to RBF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker SD. Catecholamine levels and treatment in chronic heart failure. Eur Heart J. 1998;19:56–61. [PubMed] [Google Scholar]
- Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- Cohn NJ, Yellin AM. Learned precise cardiovascular control through graded central sympathetic modulation. J Hyperten. 1984;2:77–79. [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST) Circulation. 2001;103:2055–2059. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- Dias Da Silva VJ, Gnecchi-Ruscone T, Lavelli B, Bellina V, Manzella D, Porta A, Malliani A, Montano N. Opposite effects of iv amiodarone on cardiovascular vagal and sympathetic efferent activities in rats. Am J Physiol. 2002;283:543–548. doi: 10.1152/ajpregu.00608.2001. [DOI] [PubMed] [Google Scholar]
- Dzau VJ, Colucci WS, Hollenberg NK, Williams GH. Relation of the renin-angiotensin-aldosterone system to clinical state in congestive heart failure. Circulation. 1981;63:645–651. doi: 10.1161/01.cir.63.3.645. [DOI] [PubMed] [Google Scholar]
- Eaton GM, Cody RJ, Nunziata E, Binkley PF. Early left ventricular dysfunction elicits activation of sympathetic drive and attenuation of parasympathetic tone in the paced canine model of congestive heart failure. Circulation. 1995;92:555–561. doi: 10.1161/01.cir.92.3.555. [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D. Measurements of sympathetic nervous system activity in heart failure: the role of norepinephrine kinetics. Heart Fail Rev. 2000;5:17–25. doi: 10.1023/A:1009889922985. [DOI] [PubMed] [Google Scholar]
- Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol. 1992;69:523–531. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- Floras JS. The "unsympathetic" nervous system of heart failure. Circulation. 2002;105:1753–1755. doi: 10.1161/01.cir.0000013788.71817.16. [DOI] [PubMed] [Google Scholar]
- Francis GS. Extracardiac features of heart failure: catecholamines and hormonal changes. Cardiology. 1988;75:19–29. doi: 10.1159/000174442. [DOI] [PubMed] [Google Scholar]
- Francis GS. Plasma BNP concentration predicted the presence of heart failure. Evid Based Cardiovasc Med. 1998;2:55–56. doi: 10.1016/s1361-2611(98)80096-1. [DOI] [PubMed] [Google Scholar]
- Francis J, Wei SG, Weiss RM, Johnson AK, Felder RB. Central but not peripheral infusion of mineralocorticoid receptor antagonist decreases sympathetic drive early after coronary artery ligation in rats. FASEB J. 2002;16:A791. [Google Scholar]
- Francis J, Weiss R, Wei SG, Johnson AK, Felder RB. Progression of heart failure after myocardial infarction in the rat. Am J Physiol. 2001a;281:1734–1745. doi: 10.1152/ajpregu.2001.281.5.R1734. [DOI] [PubMed] [Google Scholar]
- Francis J, Weiss RM, Wei SG, Johnson AK, Beltz TG, Zimmerman K, Felder RB. Central mineralocorticoid receptor blockade improves volume regulation and reduces sympathetic drive in heart failure. Am J Physiol. 2001b;281:H2241–H2251. doi: 10.1152/ajpheart.2001.281.5.H2241. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation. 1995;92:3206–3211. doi: 10.1161/01.cir.92.11.3206. [DOI] [PubMed] [Google Scholar]
- Gullestad L, Aukrust P. The cytokine network in heart failure: pathogenetic importance and potential therapeutic targets. Heart failure monitoring. 2001;2:8–13. [PubMed] [Google Scholar]
- Guzzetti S, Borroni E, Garbelli PE, Ceriani E, Della Bella P, Montano N, Cogliati C, Somers VK, Malliani A, Porta A. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation. 2005;112:465–470. doi: 10.1161/CIRCULATIONAHA.104.518449. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Cogliati C, Turiel M, Crema C, Lombardi F, Malliani A. Sympathetic predominance followed by functional denervation in the progression of chronic heart failure. Eur Heart J. 1995;16:1100–1107. doi: 10.1093/oxfordjournals.eurheartj.a061053. [DOI] [PubMed] [Google Scholar]
- Guzzetti S, Magatelli R, Borroni E, Mezzetti S. Heart rate variability in chronic heart failure. Auton Neurosci: Basic and Clinical. 2001;91:102–105. doi: 10.1016/S1566-0702(01)00274-0. [DOI] [PubMed] [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous system activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- Ishise H, Asanoi H, Ishizaka S, Joho S, Kameyama T, Umeno K, Inoue H. Time course of sympathovagal imbalance and left ventricular dysfunction in conscious dogs with heart failure. J Appl Physiol. 1998;84:1234–1241. doi: 10.1152/jappl.1998.84.4.1234. [DOI] [PubMed] [Google Scholar]
- Kang YM, Zhang ZH, Johnson RF, Yu Y, Beltz T, Johnson AK, Weiss RM, Felder RB. Novel effect of mineralocorticoid receptor antagonism to reduce proinflammatory cytokines and hypothalamic activation in rats with ischemia-induced heart failure. Circulation Research. 2006;99:758–766. doi: 10.1161/01.RES.0000244092.95152.86. [DOI] [PubMed] [Google Scholar]
- Kjaer A, Hesse B. Heart failure and neuroendocrine activation: diagnostic, prognostic and therapeutic perspectives. Clin Physiol. 2001;21:661–672. doi: 10.1046/j.1365-2281.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- Kodama M, Hirono S, Hanawa H, Yoshida T, Hayashi M, Tachikawa H, Kashimura T, Watanabe K, Aizawa Y. Linkage between mechanical and electrical alternans in patients with chronic heart failure. J Cardiovasc Electrophysiol. 2004;15:295–299. doi: 10.1046/j.1540-8167.2004.03016.x. [DOI] [PubMed] [Google Scholar]
- Kruger C, Kalenka A, Haunstetter A, Schweizer M, Maier G, Ruhle U, Ehmke H, Kubler W, Haass M. Baroreflex sensitivity and heart rate variability in conscious rats with myocardial infarction. Am J Physiol. 1997;273:2240–2247. doi: 10.1152/ajpheart.1997.273.5.H2240. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Pinna GD, Maestri R, Mortara A, Capomolla S, Febo O, Ferrari R, Franchini M, Gnemmi M, Opasich C, Riccardi PG, Traversi E, Cobelli F. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107:565–570. doi: 10.1161/01.cir.0000047275.25795.17. [DOI] [PubMed] [Google Scholar]
- Leimbach WN, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–919. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circulation Research. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- Maestri R, Pinna GD, Accardo A, Allegrini P, Balocchi R, D'addio G, Ferrario M, Menicucci D, Porta A, Sassi R, Signorini MG, La Rovere MT, Cerutti S. Nonlinear Indices of Heart Rate Variability in Chronic Heart Failure Patients: Redundancy and Comparative Clinical Value. J Cardiovasc Electrophysiol. 2007;18:425–433. doi: 10.1111/j.1540-8167.2007.00728.x. [DOI] [PubMed] [Google Scholar]
- Malliani A, Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension. 2002;39:63–68. doi: 10.1161/hy0102.099200. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M. The role of the sympathetic nervous system in congestive heart failure. Eur Heart J. 1983;4:49–54. doi: 10.1093/eurheartj/4.suppl_a.49. [DOI] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Mark AL. The treatment of heart failure: the role of neurohumoral activation. Intern Med. 1998;37:112–122. doi: 10.2169/internalmedicine.37.112. [DOI] [PubMed] [Google Scholar]
- Motte S, Mathieu M, Brimioulle S, Pensis A, Ray L, Ketelslegers JM, Montano N, Naeije R, Van De Borne P, Entee KM. Respiratory-related heart rate variability in progressive experimental heart failure. Am J Physiol. 2005;289:1729–1735. doi: 10.1152/ajpheart.01129.2004. [DOI] [PubMed] [Google Scholar]
- Pagani M, Somers VK, Furlan R, Dell'orto S, Conway J, Baselli G, Cerutti S, Sleight P, Malliani A. Changes in autonomic regulation induced by physical training in mild hypertension. Hypertension. 1988;12:600–610. doi: 10.1161/01.hyp.12.6.600. [DOI] [PubMed] [Google Scholar]
- Petersen JW, Felker GM. Inflammatory biomarkers in heart failure. Congest Heart Fail. 2006;12:324–328. doi: 10.1111/j.1527-5299.2006.05595.x. [DOI] [PubMed] [Google Scholar]
- Porta A, Faes L, Masé M, D'addio G, Pinna GD, Maestri R, Montano N, Furlan R, Guzzetti S, Nollo G, Malliani A. An integrated approach based on uniform quantization for the evaluation of complexity of short-term heart period variability: application to 24h Holter recordings in healthy and heart failure humans. Chaos. 2007a;17:015117. doi: 10.1063/1.2404630. [DOI] [PubMed] [Google Scholar]
- Porta A, Furlan R, Rimoldi O, Pagani M, Malliani A, Van De Borne P. Quantifying the strength of the linear causal coupling in closed loop interacting cardiovascular variability signals. Biol Cybern. 2002;86:241–251. doi: 10.1007/s00422-001-0292-z. [DOI] [PubMed] [Google Scholar]
- Porta A, Guzzetti S, Montano N, Furlan R, Malliani A, Cerutti S. Entropy, entropy rate and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans Biomed Eng. 2001;48:1282–1291. doi: 10.1109/10.959324. [DOI] [PubMed] [Google Scholar]
- Porta A, Tobaldini E, Guzzetti S, Furlan R, Montano N, Gnecchi-Ruscone T. Assessment of cardiac autonomic modulation during graded head-up tilt by symbolic analysis of heart rate variability. Am J Physiol. 2007b;293:H702–H708. doi: 10.1152/ajpheart.00006.2007. [DOI] [PubMed] [Google Scholar]
- Task force of the European Society of cardiology and the north American Society of pacing and electrophysiology. Standard of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Van De Borne P, Montano N, Pagani M, Oren R, Somers VK. Absence of low-frequency variability of sympathetic nerve activity in severe heart failure. Circulation. 1997;95:1449–1454. doi: 10.1161/01.cir.95.6.1449. [DOI] [PubMed] [Google Scholar]
- Watson AM, Hood SG, May CN. Mechanisms of sympathetic activation in heart failure. Clin Exp Pharmacol Physiol. 2006;33:1269–1274. doi: 10.1111/j.1440-1681.2006.04523.x. [DOI] [PubMed] [Google Scholar]
- Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]