Abstract
The P2X7 receptor (P2X7R)4 is highly expressed on the macrophage cell surface and activation of infected cells by extracellular ATP has been shown to kill intracellular bacteria and parasites. Furthermore, single nucleotide polymorphisms (SNPs) that decrease receptor function reduce the ability of human macrophages to kill Mycobacterium tuberculosis and are associated with extrapulmonary tuberculosis. In this paper we show that macrophages from people with the 1513C (rs3751143) loss-of-function P2X7R SNP are less effective in killing intracellular Toxoplasma gondii after exposure to ATP compared with macrophages from people with the 1513A wild-type allele. Supporting a P2X7R-specific effect on T. gondii, macrophages from P2X7R knock-out mice (P2X7R−/−) are unable to kill T. gondii as effectively as macrophages from wild-type mice. We show that P2X7R-mediated T. gondii killing occurs in parallel with host cell apoptosis and is independent of NO production.
Introduction
The purinergic P2X7 receptor (P2X7R) functions as a pro-inflammatory receptor in cells of the monocyte/macrophage lineage. P2X7R cell surface membrane expression is up-regulated by interferon-γ and the receptor is activated by extracellular ATP released from a variety of cellular sources including platelets and damaged cells (1). Activation of monocyte and macrophage P2X7R has been shown to kill intracellular Mycobacterium (2–11), Chlamydia (12–14), and Leishmania species (15). The P2X7R gene (P2RX7) is highly polymorphic and a number of non-synonymous single nucleotide polymorphisms (SNPs) have been described that alter receptor function. The majority of SNPs, including 946G>A (rs28360457, Arg-307 to Gln), 1096C>G (rs2230911, Thr-357 to Ser), 1405A>G (rs2230912, Arg-460 to Gln), 1513A>C (rs3751143, Glu-496 to Ala) and 1729T>A (rs1653624, Ile-568 to Asn) confer a loss-of-function phenotype. Significantly, the loss-of-function 1513A>C SNP reduces the in vitro ability of human macrophages to control Mycobacterium tuberculosis (10). Furthermore, inheritance of the 1513A>C SNP has been associated with susceptibility to extrapulmonary tuberculosis in humans (11, 16).
Similar to Mycobacterium, Chlamydia, and Leishmania spp., the apicomplexan parasite, Toxoplasma gondii, is able to infect and survive in cells of the monocyte/macrophage lineage. Toxoplasma gondii is an obligate intracellular protozoa that infects approximately one-third of humans worldwide (17). Human infection occurs after ingestion of either tissue cysts in raw/undercooked meat or oocysts from infected cat faeces and can be acquired congenitally following primary maternal infection. Although severe disease can occur in the immunocompetent human host, infection is usually asymptomatic or a mild illness characterised by malaise, lymphadenopathy, fever and headache (17). Considerable morbidity and mortality in immunosuppressed individuals, in particular toxoplasmic encephalitis, is caused by reactivation of chronic infection and severe fetal abnormalities can occur in association with primary maternal infection (17).
Herein we describe three immunocompetent people with toxoplasmosis who prompted further investigation of P2X7R as a factor influencing host-response to T. gondii infection. We show, in studies using human macrophages and P2X7R knockout (P2X7R−/−) mice, that P2X7R activation by extracellular ATP kills T. gondii parasites in infected cells. P2X7R-mediated T. gondii death occurs in parallel with host-cell apoptosis and is independent of NO production.
Materials and Methods
P2X7R function and genotyping in toxoplasmosis subjects
Subjects from the Nepean Hospital, Penrith, New South Wales, Australia provided informed consent for study of their peripheral blood mononuclear cells. The experimental protocol was approved by the Sydney West Area Health Service and the University of Sydney Human Ethics Committees and the UTS Human Research Ethics Committee, with approval code: UTS HREC 2004-077A. Peripheral blood was collected, mononuclear cells were separated and macrophages generated and cultured as described previously (9). Ethidium influx after activation of cells with 1 or 3mM ATP was measured by flow cytometry (7) and P2RX7 genotyping was performed as described previously (9). Peripheral blood samples provided the DNA obtained from participants in the National Collaborative Chicago-based Congenital Toxoplasmosis Study (NCCCTS) (18). Genotyping of these samples was performed using Taqman™ technology (19) for P2RX7 single nucleotide polymorphisms at rs28360457, rs1718119, rs2230911, rs2230912, rs3751143, rs1653624 and rs1621388 (NCBI Entrez SNP, http://www.ncbi.nlm.nih.gov/sites/entrez). Ethical approval for the NCCCTS was obtained from the Institutional Review Boards of the University of Chicago and Michael Reese Hospital and Medical Center, and oversight was provided by an Internal Data Safety Monitoring Committee, the Data Safety Monitoring Board, and the NIH.
Murine macrophage culture
The immortalised mouse macrophage-like cell line RAW 264.7, was cultured as described previously (20). All animal research was approved and conducted in accordance with the University of Technology, Sydney Animal Care and Ethics Committee, with approval code UTS ACEC 2008-30. Murine bone marrow was isolated from BALB/c (Animal Resource Centre, Murdoch, Western Australia), C57BL/6J (Animal Resource Centre, Murdoch, Western Australia) and mice on a C57BL/6J background with the P2RX7 gene deleted (P2X7R−/− mice, originally obtained from Pfizer, Ann Arbor, MI, USA, and subsequently bred at the Ernst Facility, University of Technology, Sydney) and bone marrow macrophages were cultured as described previously (21).
Toxoplasma gondii viability assays
Three assays were used to assess viability of T. gondii in vitro, ensuring a robust assessment of effect of activation of cells by ATP (1 or 3mM) on survival and replication of tachyzoites.
YFP-YFP RH T. gondii (a gift from Dr Boris Striepen, the University of Georgia, Athens, GA, USA) replication in cultured monocyte-macrophages was monitored as described previously (22) at the University of Chicago Cellular Screening Centre using an F3 robot and Acumen eX3 microplate cytometer. Hourly measurements of fluorescent parasites were conducted for a total period of 24 hours, with three initial measurements taken prior to the addition of ATP to half of the cell samples for each person immediately prior to the fourth measurement.
In addition, a flow cytometry-based viability assay for intracellular T. gondii tachyzoites was established that allowed rapid assessment of viability of thousands of intracellular T. gondii tachyzoites. Cells were infected with T. gondii tachyzoites, incubated overnight and extracellular tachyzoites removed. Intracellular tachyzoites were released by mechanically lysing host cells and parasite viability assessed by flow cytometry. Prior to experiments, anti-p30 T. gondii monoclonal antibody was first conjugated to Alexa Fluor 647 using the Alexa Fluor 647 protein labelling kit according to the manufacturer’s instructions. Antibody was combined with Alexa Fluor 647 dye, incubated at room temperature and separated from unbound dye by size exclusion chromatography. Tachyzoite viability was assessed using flow cytometry by staining the cell suspension with anti-p30 T. gondii-Alexa Fluor 647 conjugated monoclonal antibody diluted 1:100 and Sytox Green diluted 1:10000. Previously prepared 97 mL tachyzoite suspensions were combined with 1mL anti-p30 T. gondii-Alexa Fluor 647 conjugated monoclonal antibody and 2mL Sytox Green working solution (diluted 1:200) and incubated at room temperature for 30 minutes protected from light, mixing the suspension gently after 15 minutes. Following incubation, 500 mL PBS plus 1% BSA and 0.05% NaN3 was added and the suspension transferred to a FACS tube for analysis on a BD FACSCalibur flow cytometer. Tachyzoites were identified by log increases in anti-p30 T. gondii-Alexa Fluor 647 conjugated monoclonal antibody fluorescence, measured on the FL-4 detector. Tachyzoite viability was analysed based on Sytox Green uptake, measured on the FL-1 detector. Following the acquisition of 5000 gated tachyzoite events, listmode data files were removed from the FACSCalibur Macintosh computer using a portable USB storage device and transferred to a PC for analysis with the free flow cytometry analysis software, WinMDI, version 2.9. Tachyzoites were gated for viability analysis, with the resulting bimodal populations corresponding to viable (Sytox Green non-fluorescent) and non-viable (Sytox Green fluorescent) tachyzoites. Percent viable T. gondii tachyzoites were plotted using GraphPad Prism, version 5.00.
For the RAW 264.7 line, cells were seeded into Labtek II 8 well chamber slides (Nunc) at a density of 5×104/well and left to grow overnight. The following day, 1×105 freshly lysed T. gondii tachyzoites were added to appropriate wells and left to invade for 2 hours at 37°C in 5% CO2. Uninvaded parasites were removed and then the infected cells were treated with ATP, pH 7.4, for 1 hour at 37°C in 5% CO2. The media was carefully removed and the chamber discarded. Determination of viability was undertaken using acridine orange and ethidium bromide as described previously (23–25). Cells and parasites were viewed on an Olympus BX51 fluorescent microscope with excitation filter 470/20nm. A minimum of 300 cells or intracellular parasites were counted per sample and the assay was repeated in duplicate on at least three separate occasions. Images were taken using an Olympus DP70 digital camera at 1000× magnification.
In vivo parasite burden and NO assay
Eight-week-old male mice were infected by intraperitoneal injection of 500 tachyzoites of T. gondii RH or ME49 strain. Splenic parasite burdens were determined for individual mice using a modification of the method described previously (26). Briefly, spleens were removed and placed into RPMI containing 5% fetal calf serum. Spleens were weighed and single cell suspensions were made by passing spleens through a 70µm sieve. Cells were pelleted at 1500 g then resuspended in 4 ml RPMI containing 5% fetal calf serum. One hundred microlitres was added to the first well of a 96 well plate and splenocytes were serially diluted 1/2 across the plate. Plates were incubated at 37°C in 5% CO2 for 8 days before wells were examined for the presence of parasites. Parasite burden was determined as the last well in which a single parasite was visible and the number of parasites per gram was calculated thus: (mean of reciprocal titers from each duplicate/weight of homogenized spleen) × 400, where 400 is the reciprocal fraction of the homogenized spleen inoculated into the first sample well. Serum was collected from the same mice and assayed for NO using the Griess assay, as described previously (27).
Annexin-V-FITC and propidium iodide apoptosis
RAW 264.7 cells were seeded into 6-well plates containing drop-in Teflon cups (Savillex, USA) at a density of 1×106 cells per well. Toxoplasma gondii tachyzoites (1×106) were infected into each well overnight, prior to the addition of ATP to activate P2X7 receptors. Apoptosis was assessed after addition of ATP using the Annexin-V-FITC apoptosis detection kit (Calbiochem/Merck, Germany), according to the manufacturer’s instructions. Annexin-V-FITC and propidium iodide fluorescence were assessed using a FACSCalibur flow cytometer (Becton Dickinson, USA).
Results
Description of three immunocompetent subjects with toxoplasmosis and reduced or absent P2X7R function
Subject 1 (S1), a 14-year-old male, presented to the Nepean Hospital, Penrith, Australia, with a 2-year history of fatigue, lethargy and generalized painless lymphadenopathy. There was no resolution of symptoms after multiple courses of antibiotic therapy. Toxoplasma gondii serology was positive for IgM and negative for IgG. A biopsy of an enlarged left axillary lymph node showed lymphadenitis consistent with toxoplasmosis. Symptoms resolved after treatment with sulfadiazine and pyrimethamine and convalescent serology was positive for Toxoplasma IgG and negative for IgM. Subject 2 (S2), a 20-year-old female, presented with an enlarged submandibular lymph node 5 weeks following a dental extraction. Excision lymph node biopsy and histology was consistent with T. gondii lymphadenitis and T. gondii serology was positive for IgM and IgG. Repeat serology 2 years later was positive for IgG and negative for IgM. Subject 3 (S3), a 24-year-old pregnant female, had a routine fetal ultrasound scan at 18 weeks gestation, which showed borderline fetal cerebral ventriculomegaly. A repeat scan at 22 weeks gestation showed prominent cerebral ventriculomegaly. A diagnostic amniocentesis was performed and PCR for the T. gondii-gene was positive.
In all three subjects there was no evidence of any major underlying immunodeficiency: HIV serology was negative; absolute lymphocyte numbers and lymphocyte subsets were normal; absolute neutrophil counts were normal; and serum immunoglobulin levels were normal (Fig. 1A). However, all three people displayed impaired macrophage P2X7R function measured by ATP-induced ethidium bromide uptake that was either reduced to approximately half (S1) or completely absent (S2 and S3) compared to control people (Fig. 1B). Moreover, P2RX7 genotyping showed S1 was heterozygous for 1513A>C, S2 was heterozygous for both 946G>A and 1096C>G and S3 was heterozygous for both 946G>A and 1513A>C, loss-of-function SNPs (Fig. 1C).
Figure 1. Studied acute toxoplasmosis subjects have normal immune function but have low P2X7R function due to P2X7R loss-of-function SNPs.
(A) Hb = haemoglobin (Hb; g/L), WCC = white cell count (WCC; ×109 cells/L), absolute lymphocyte counts (Abs lymph; × 109 cells/L), CD4+ lymphocyte counts (CD4; ×106 cells/L), CD8+ lymphocyte counts (CD8; ×106 cells/L), serum immunoglobulins (Serum Ig), HIV serology for human immunodeficiency virus (HIV) was negative for all subjects. (ND = not determined). (B) Monocyte-derived macrophages from S1–3 and from subjects wild-type for known loss-of-function P2X7R SNPs were treated with 1mM ATP, followed by quantification of ethidium bromide flux through P2X7R generated pores by time-resolved flow cytometry. P2X7R function of cultured monocyte derived macrophages from S1–3 was decreased in comparison to three normal control subjects. (C) Sequencing of the P2RX7 gene shows the presence of one or more non-synonymous SNPs in all three toxoplasmosis subjects. a loss-of-function SNP.
ATP-dependent macrophage killing of Toxoplasma gondii is reduced in people with decreased P2X7R function
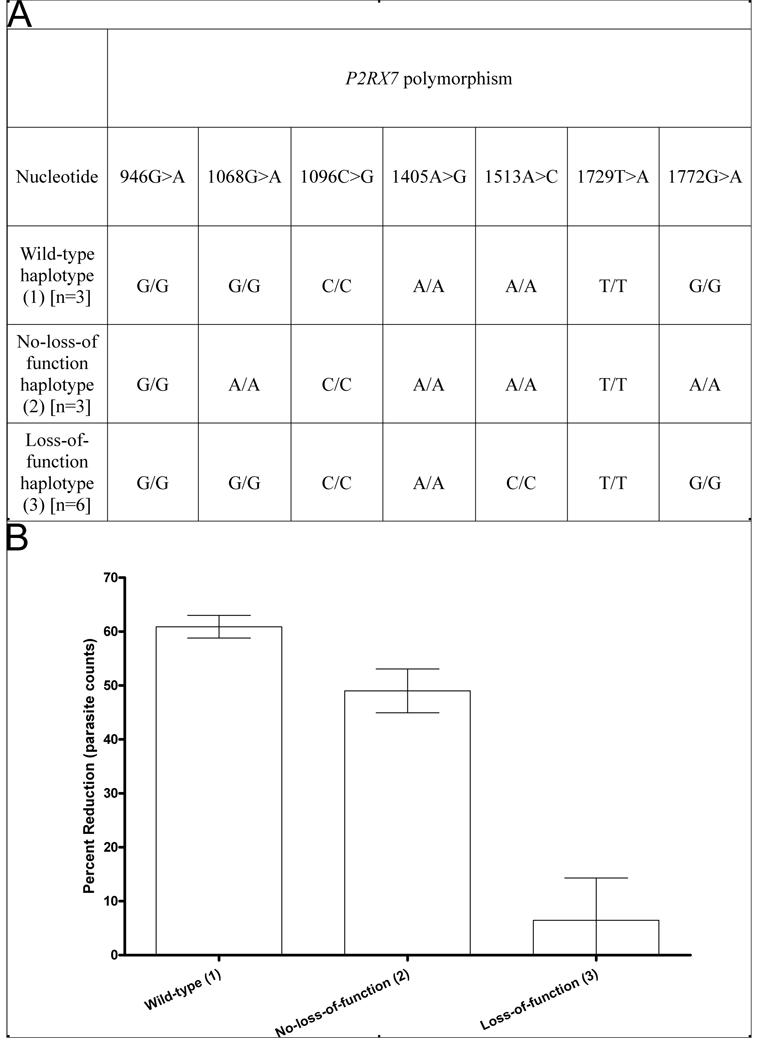
Genotyping of the NCCCTS cohort (Fig. 2A) identified people with the most common loss of function polymorphism in P2RX7, the 1513A>C polymorphism (28), as well as those with wild-type P2RX7 or polymorphisms in linkage disequilibrium (1068G>A and 1772G>A) that do not reduce the function of the receptor (28). A reduction in parasite burden was observed following ATP treatment of monocyte-derived macrophages from people with wild-type P2RX7 or with no loss of function polymorphisms (Fig. 2B). Conversely, ATP treatment of monocyte-derived macrophages cultured from people who are homozygous for the 1513A>C loss-of-function polymorphism had minimal effect on the number of intracellular T. gondii tachyzoites (Fig. 2B).
Figure 2. Activation of human monocyte-derived macrophages with ATP fails to affect the viability of Toxoplasma gondii in the absence of functional P2X7 receptors.
(A) P2RX7 haplotypes identified in NCCCTS samples after genotyping for seven single nucleotide polymorphisms; haplotype 1 is wild type at all seven SNP locations, haplotype 2 is homozygous for the non-loss-of-function SNPs at nucleotides 1068 and 1772, and haplotype 3 is homozygous for the loss of function single nucleotide polymorphism at nucleotide 1513. (B) Intracellular T. gondii (RH) tachyzoite numbers were assessed in ATP-treated (3mM) monocyte-derived macrophages from NCCCTS samples. The reduction in fluorescence of YFP-labelled T. gondii parasites measured by flow cytometry in ATP-treated cells was calculated at 24 hours as a percentage of the untreated control for each subject. Results are the mean ± SE, n=3–6. There is a significantly lower percentage of parasites following ATP treatment in cells expressing functional P2X7R than in cells with the P2X7R 1513A>C loss-of-function SNP (p = 0.0015 using a General Linear Model, 1-factor ANOVA with Tukey’s post-hoc test).
ATP-dependent macrophage killing of Toxoplasma gondii is reduced in P2X7R−/− mice
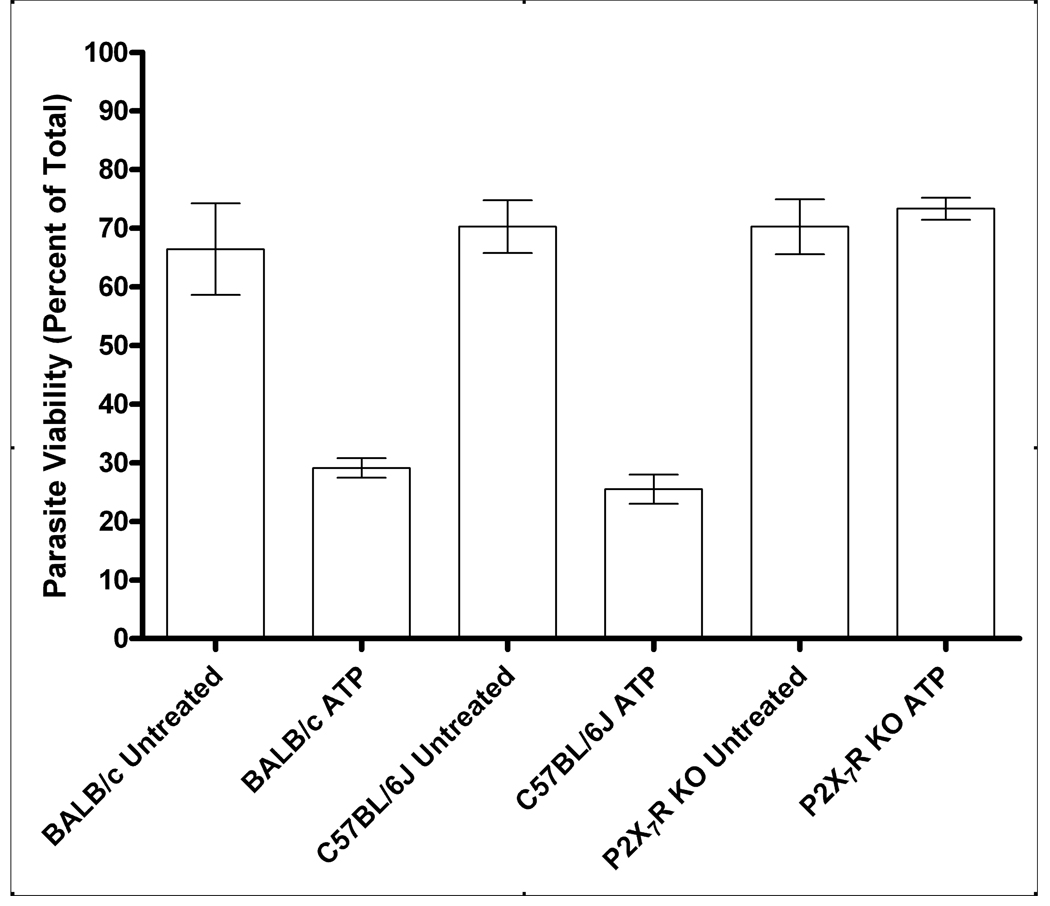
The number of samples and the quantity of cells from people in the NCCCTS cohort with polymorphisms in their P2X7R was relatively limited. Therefore, P2X7R−/− mice on a C57BL/6J background were also used to more definitively assess the ability of the P2X7R to mediate killing of T. gondii tachyzoites by macrophages. C57BL/6 mice possess a proline to leucine polymorphism at amino acid 451 in the C-terminal tail of the P2X7R (29). This polymorphism has variable effects on P2X7R function (29–34), so we included comparative analyses of BALB/c cells in our investigations since this strain of mouse is known to possess fully functional P2X7R (29–34) and is also known to be more resistant to T. gondii than C57BL/6J mice (35). P2X7R function of macrophages from BALB/c, C57BL/6J and P2X7R−/− mice was determined, confirming 100%, 50% and zero P2X7R-dependent pore opening, respectively (Supplemental Fig. 1). ATP treatment of macrophages from BALB/c and C57BL/6J mice resulted in a marked reduction in viability of T. gondii RH strain. In contrast, ATP treatment of P2X7R−/− murine macrophages produced no significant loss of T. gondii viability (Fig. 3). Very similar results were seen with the relatively avirulent, type 2 ME49 strain of T. gondii; exposure of BALB/c macrophages to ATP reduced parasite viability from 81 ± 4% to 49 ± 12%, in C57BL/6J macrophages, from 83 ± 2% to 49 ± 12% but, in P2X7R KO mice, parasite viability remained unchanged at 82 ± 4% (versus 83 ± 3% in non-activated cells; results are mean ± SE, n=3).
Figure 3. Deletion of the P2RX7 gene affects the ability of murine macrophages to control Toxoplasma gondii.
T. gondii RH strain tachyzoites (3×106) were added to 1×105 macrophages and parasite viability was assessed by flow cytometry 24 hours after addition of 3mM ATP. Parasite viability was significantly reduced in ATP-treated macrophages from BALB/c (p<0.0001) and C57BL/6J (p<0.0001) mice compared with untreated controls. Parasite viability was not significantly reduced after the addition of ATP to macrophages from P2X7R−/− mice (p>0.05). Results are the mean ± SE, n=4. Statistical analysis was performed using a General Linear Model 1-factor AVOVA with Tukey’s post-hoc test.
There were significant differences in the number of parasites recovered from the spleens of ME49-infected mice: on day 12 pi, for BALB/c mice, 1693 ± 238 parasites per gram spleen were recovered versus 2353 ± 500 for C57BL/6J mice and 2892 ± 298 for P2X7R−/− mice (results are means ± SE, n=6, and there were significantly more parasites recovered from the P2X7R−/− mice, p<0.05, Mann-Whitney non-parametric test).
P2X7R-mediated killing of Toxoplasma gondii is independent of NO production but is associated with host cell apoptosis
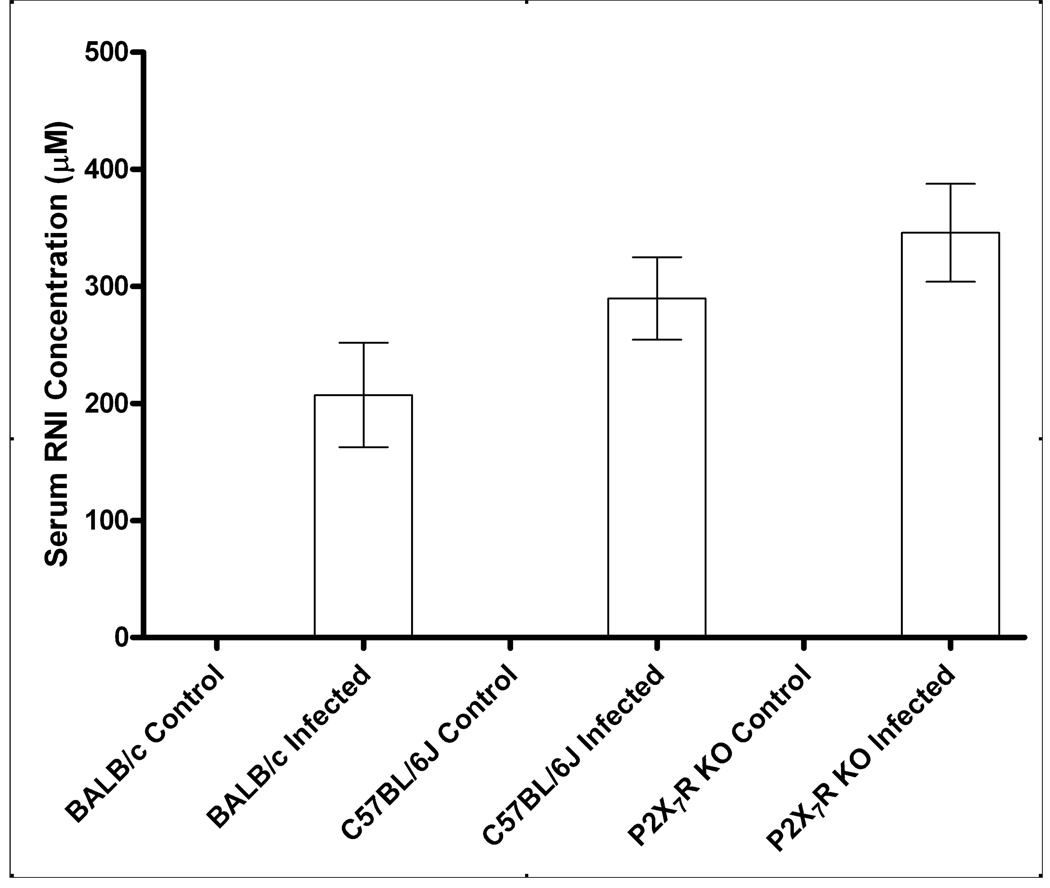
To test if killing of T. gondii after P2X7R activation is dependent on NO production, we compared serum NO levels from P2X7R−/− mice to BALB/c and C57BL/6J mice after infection by intraperitoneal injection of T. gondii RH strain tachyzoites. There was a trend towards higher serum NO levels in the P2X7R-deficient infected mice although none of the differences achieved statistical significance; thus, control of T. gondii RH strain via the P2X7R is independent of NO production (Fig. 4).
Figure 4. NO production in response to infection with Toxoplasma gondii is unaffected by deletion of the P2RX7 gene.
Mice were infected by intra-peritoneal injection with 500 RH T. gondii tachyzoites and euthanized 8 days post infection. Serum was collected and assayed for NO using the Griess Assay. Results are the mean ± SE; BALB/c control/infected, n=5/8; C57BL/6J control/infected, n=5/9; P2X7R−/− control/infected, n=5/5. There were no statistically significant differences in serum NO levels between strains for mice infected with T. gondii (using a General Linear Model 1-factor ANOVA with Tukey’s post-hoc test).
RAW 264.7 cells express functional P2X7R (Supplemental Fig. 2). Exposure to ATP induced rapid apoptosis of T. gondii-infected RAW 264.7 cells; more than 70% of cells were positive for Annexin-V by 1 hour (Fig. 5A). At 5 hours post-exposure to ATP, a significant percentage (>30%) of apoptotic cells had progressed towards lysis (Fig. 5A). Similar levels of ATP-induced apoptosis and necrosis were seen in uninfected RAW 264.7 cells (data not shown).
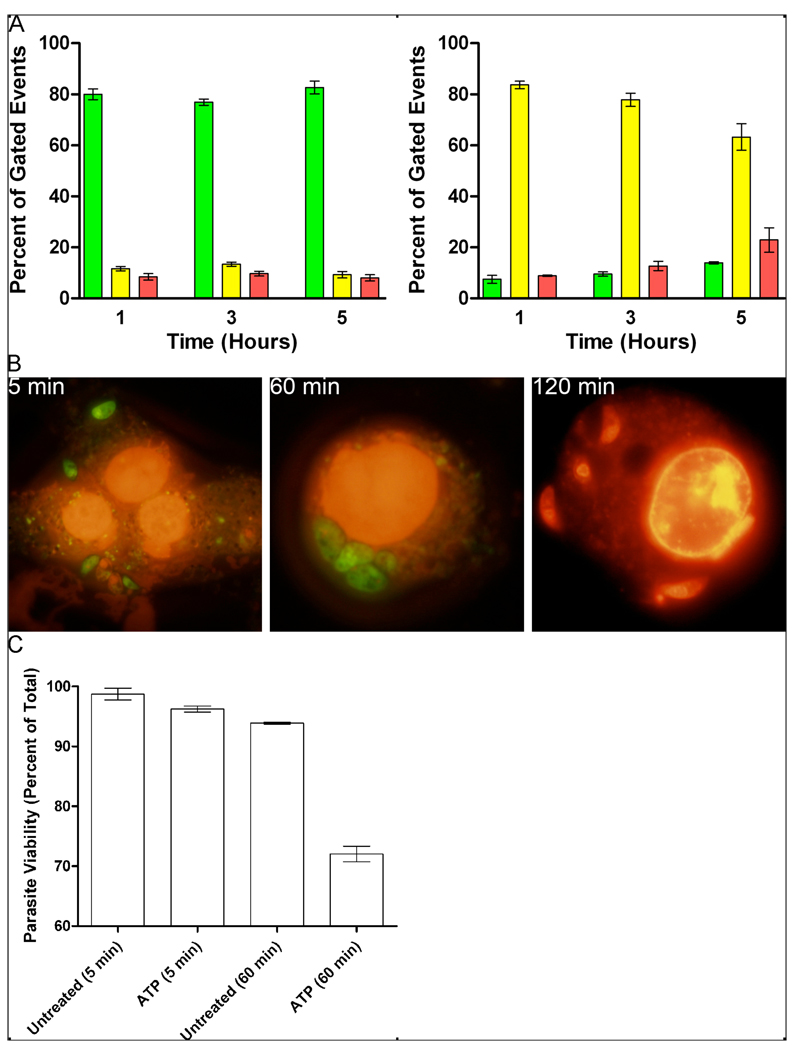
Figure 5. T. gondii killing occurs in parallel to host cell apoptosis in murine macrophages.
(A) Murine macrophages undergo rapid apoptosis after treatment with ATP. RAW 264.7 cells (1×106) were infected with RH T. gondii tachyzoites (3×106). After overnight incubation, RAW 264.7 cells were untreated (left panel) or treated for 1 hour with 1 mM ATP to activate P2X7R (right panel), apoptosis was quantified by flow cytometry after Annexin-V-FITC/propidium iodide staining 1, 3 and 5 hours after addition of 1mM ATP. Results are mean ± SE, n=3; green bars are viable cells, yellow are apoptotic cells and red are late apoptotic or necrotic cells. (B, C) 1×106 RAW 264.7 cells were infected with 3×106 RH T. gondii tachyzoites for 2 hours. Extracellular parasites were washed away and the monolayer was then treated with 1mM ATP for 5, 60 or 120 minutes. Parasite viability was determined after staining with acridine orange (viable, green fluorescence) and ethidium bromide (non-viable, red fluorescence). The number of non-viable tachyzoites was significantly increased at both 60 and 120 minutes after ATP treatment (p<0.05). Results are the mean ± SE, n=4. Statistical analysis was performed using a General Linear Model 2-factor AVOVA with Tukey’s post-hoc test, comparing the untreated control with experimental treatments at each time point. Treatment of extracellular parasites with ATP for 120 minutes did not affect their viability (data not shown).
In situ staining of RAW264.7 cells with acridine orange and ethidium bromide confirmed the Annexin V/propidium iodide flow cytometry results. Acridine orange is able to penetrate all cells and stains nucleic acids green, whereas ethidium bromide, which binds to nucleic acids and fluoresces orange, is only able to enter cells once the integrity of the cell membrane is compromised. Thus, live cells have normal nuclear staining with green chromatin apparent in organised nuclear structures, whereas non-viable cells exhibit orange staining of nuclear material and/or condensed or fragmented chromatin (23–25). Cells were viewed and counted on a fluorescence microscope and the viability of RAW 264.7 cells was assessed according to these criteria (Fig. 5C). Thus, 54% of RAW264.7 cells were non-viable after 1 hour and 86% non-viable within 2 hours of exposure to ATP, compared with 4% and 7%, respectively, for untreated cells. Furthermore, at 2 hours post-exposure to ATP, intensely stained, condensed, fragmented areas of chromatin were evident within the nuclei of host cells (Fig. 5C, panel 3). At later time points, microscopic examination revealed extensive cell lysis.
In situ staining with acridine orange and ethidium bromide also showed that loss of viability of intracellular tachyzoites of T. gondii occurred in parallel to host cell apoptosis. Thus, 5 minutes post-exposure to ATP, almost all tachyzoites were stained green with acridine orange (Fig. 5B, panel 1) and, after 1 hour, the parasites were still predominantly (73%) acridine orange-positive and ethidium bromide-negative (Fig. 5B, panel 2; Fig. 5C). However, by 2 hours post-exposure to ATP, 43% of the parasites were stained orange with ethidium bromide (Fig. 5B, panel 3; Fig. 5C). Meanwhile, tachyzoites exposed to ATP in cell-free media remained 100% viable, even after 2 hours (data not shown). This demonstrates that a direct toxic effect of exogenous ATP on T. gondii is unlikely; rather, ATP acts via effects on the host cell. By 24 hours post-exposure to 1mM ATP, flow cytometric assessment of parasite viability showed that only 25 ± 5% (mean ± SE, n=4) of T. gondii tachyzoites were viable, a result comparable with the effect of 3mM sodium nitroprusside (28 ± 5% viable, mean ± SE, n=4), a known toxin for T. gondii tachyzoites (36). This viability was statistically significantly different (p<0.001, General Linear Model 2-facot ANOVA with Tukey’s post-hoc test) from controls at the same time-point where 70 ± 8% (mean ± SE, n=4) of tachyzoites in control cultures were viable, a level not statistically different from the number of viable parasites after 2, 8 or 16 hours of normal in vitro culture (data not shown). The effects on intracellular T. gondii of exposure to ATP could be totally reversed by pre-treatment of RAW264.7 cells with oxidized ATP (data not shown), a potent antagonist of the murine P2X7R (37), further aiding in confirmation of the specificity of the effect.
Discussion
P2X7R genotyping and relative P2X7R activity of three acute toxoplasmosis patients from Nepean Hospital, Australia (Fig. 1) is consistent with previous studies showing 50% reduction in monocyte P2X7R function in 1513A>C heterozygotes and complete loss-of-function for compound heterozygotes (8, 9, 11). To follow up the surprising association between P2X7R function and acute symptoms in these patients, and to study the effect of polymorphisms at P2RX7 on function in more detail, we accessed cryopreserved cells from NCCCTS individuals of known genotypes/haplotypes (20). We demonstrated that ATP-dependent killing of tachyzoites of T. gondii in vitro was virtually non-existent in people with the 1513A>C polymorphism but readily observable in macrophages from people with wild-type receptors or in cells from people with polymorphisms that do not cause loss of receptor function (Fig. 2).
The number of cells available from NCCCTS subjects was limited and did not allow us to perform additional, confirmatory studies with antagonists of the P2X7R. Therefore, we compared parasite killing using macrophages from P2X7R−/− mice to BALB/c mice and C57BL/6J mice to confirm that reduced parasite killing is P2X7R specific; cells from the knockout mice were totally unable to affect the viability of T. gondii upon activation with ATP (Fig. 3). These data confirm that ATP-dependent macrophage killing of T. gondii is via P2X7R and does not involve other P2X or P2Y receptors, which are also activated by extracellular ATP (37). Parasite burden in vivo in BALB/c, C57BL/6J and P2X7R−/− mice appears to at least partially confirm that the P2X7R plays a role in controlling T. gondii. Thus, there were differences in the number of parasites recovered from the spleens of ME49-infected mice, in proportions consistent with their relative P2X7R function.
It perhaps needs to be noted that ATP-induced killing of T. gondii was comparable in BALB/c and C57/BL/6J mice even though the C57/BL6 strain is known to possess a proline to leucine polymorphism at amino acid 451 that reduces ATP-dependent pore formation by 50% (Supplemental Fig. 1). This could be because of the higher concentration of ATP used in the parasite-killing assay versus the pore-opening assay (3mM versus 1mM). However, it is also important to recognise that this particular polymorphism has quite variable effects on P2X7R function depending on the cell type and activity being examined. Thus, for example, it reduces ATP-dependent pore formation (29), impairs cell death in thymocytes (30), inhibits ATP-induced IL-2 production by splenocytes (31) and affects intercellular calcium waves in astrocytes (32). In contrast, this polymorphism has no affect on phospholipase D activation (30), no affect on expression of the P2X7R or sensitivity to ATP-induced cell death in splenic CD4+CD25+ T cells (33), and no affect on P2X7R-mediated calcium influx in bone marrow derived macrophages or splenocytes (34).
Activation of the P2X7R initiates a cascade of intracellular events including activation of NFκB (38), phospholipase D (39) and metalloproteases (40), release of reactive oxygen and nitrogen intermediates (41), and stimulation of caspases, leading to apoptosis (42). Loss of function polymorphisms in the P2X7R are also recognised (43, 44) to have a negative effect on the activation of the “inflammasome”, a complex of cytosolic proteins that regulates caspase-1 activation and, therefore, the processing of IL-1β and IL-18 from inactive to active forms. It is known that killing of mycobacteria and Leishmania amazonensis via the P2X7R is independent of NO (3, 6, 15); rather it is associated with apoptosis of host cells (2, 3, 7, 8, 10, 11, 15), as is P2X7R-mediated killing of Chlamydia (12). We confirmed that P2X7R-mediated killing of T. gondii is also not associated with NO levels (Fig. 4). To test if T. gondii killing was associated with apoptosis, we studied in vitro parasite death in RAW 264.7 cells after induction of apoptosis with ATP. Both flow cytometry and in situ staining with acridine orange and ethidium bromide enabled us to document that loss of viability of intracellular tachyzoites of T. gondii occurred in parallel to host cell apoptosis (Fig. 5).
The vulnerability of T. gondii to ATP-induced, P2X7R-mediated killing shares much in common with similar phenomena observed with Mycobacteria (2–11), Chlamydia (12–14) and Leishmania (15) species, not least the association with apoptosis and independence from NO generation. However, there are likely to be differences too; P2X7R-dependent killing of Mycobacteria and Chlamydia species is actually dependent on phospholipase D, which is associated with both apoptosis and phagosome-lysosome fusion (4–6, 13). This scenario seems less likely for T. gondii as the parasite inhibits phagosome-lysosome fusion after active penetration of the host cell and modification of phagosomal membrane proteins (45–47). A role for caspase-1 and subsequent release of mature IL-1β, which can cause apoptosis in surrounding cells (37), can also be ruled out because RAW264.7 cells lack the key adaptor protein, ASC (apoptosis-associated speck-like protein containing a C-terminal caspase-activating recruiting domain), to form the inflammasome (48). Whilst the limitations of the correlative link between host cell apoptosis and parasite killing must be acknowledged, we believe that, on balance, this effect is likely to be P2X7R-specific and does not imply that just any pro-apoptotic agent will cause killing of this parasite. This is because of the extremely well demonstrated ability of T. gondii to inhibit apoptosis induced by a remarkable spectrum of extrinsic and intrinsic pro-apoptotic stimuli in a variety of host cell types (49–55). It is also worth noting that apoptosis induced by CD-95 ligation or by H202 or necrosis induced by complement does not directly influence the survival of mycobacteria within macrophages (3). Thus, whilst we have shown that host cell apoptosis is a potential effector mechanism of parasite killing via P2X7R activation, the precise mechanism underpinning this remains to be definitively determined.
Our observations suggest that inheritance of SNPs that reduce P2X7R function might cause a defective response to T. gondii infection, higher risk of reactivation in immunocompromised people and more severe congenital toxoplasmosis. Genotyping P2RX7 in these patient groups and identifying those at risk would allow for more intensive monitoring for reactivation of infection and, if required, suppressive treatment using anti-Toxoplasma therapy.
Supplementary Material
Footnotes
This work was supported by an Australian Research Council Discovery Project grant (DP0666515) to NCS and JSW, and an Australian Research Council/National Health and Medical Research Council Research Network for Parasitology Researcher Exchange and Travel Award to MPL. MPL was supported by postgraduate scholarships from the Institute for the Biotechnology of Infectious Diseases and the Faculty of Science, the University of Technology, Sydney.
Abbreviations used in this paper: NCCCTS, National Collaborative Chicago-based Congenital Toxoplasmosis Study; P2X7R, P2X7 receptor; P2X7R−/−, P2X7 receptor knockout; SNP, single nucleotide polymorphism
References
- 1.Di Virgilio FD, Falzoni S, Mutini C, Sanz JM, Chiozzi P. Purinergic P2X7 receptor: a pivotal role in inflammation and immunomodulation. Drug Dev. Res. 1998;45:207–213. [Google Scholar]
- 2.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular Bacillus Calmette-Guerin. J. Exp. Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 4.Kusner DJ, Adams J. ATP-induced killing of virulent Mycobacterium tuberculosis within human macrophages requires phospholipase D. J. Immunol. 2000;164:379–388. doi: 10.4049/jimmunol.164.1.379. [DOI] [PubMed] [Google Scholar]
- 5.Stober CB, Lammas DA, Li CM, Kumararatne DK, Lightman SL, McArdle CA. ATP-mediated killing of Mycobacterium bovis Bacille Calmette-Guerin within human macrophages is calcium dependent and associated with the acidification of mycobacteria-containing phagosomes. J. Immunol. 2001;166:6276–6286. doi: 10.4049/jimmunol.166.10.6276. [DOI] [PubMed] [Google Scholar]
- 6.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria by macrophages is a P2X7-dependent process inducing bacterial death by phagosome-lysosome fusion. J. Immunol. 2001;167:3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 7.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley SJ. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J. Immunol. 2003;171:5442–5446. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- 8.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, Britton WJ. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J. Infect. Dis. 2005;192:149–155. doi: 10.1086/430622. [DOI] [PubMed] [Google Scholar]
- 9.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung L-P, Skarratt KK, Saunders BM, Tan KS, Gu BJ, Fuller SJ, Britton WJ, Petrou S, Wiley JS. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J. Biol. Chem. 2006;281:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 10.Placido R, Auricchio G, Falzoni S, Battistini L, Colizzi V, Brunetti E, Di Virgilio F, Mancino G. P2X7 purinergic receptors and extracellular ATP mediate apoptosis of human monocytes/macrophages infected with Mycobacterium tuberculosis reducing the intracellular bacterial viability. Cell. Immunol. 2006;244:10–18. doi: 10.1016/j.cellimm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Goldberg H, Marks GB, Wiley JS, Britton WJ. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am. J. Res. Crit. Care Med. 2007;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 12.Coutinho-Silva R, Perfettini J-L, Perrsechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am. J. Physiol. Cell Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 13.Coutinho-Silva R, Stahl L, Raymond M-N, Jungas T, Verbecke P, Burnstock G, Darville T, Ojcius DM. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 14.Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW, Jr, Ojcius DM. Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J. Immunol. 2007;179:3707–3714. doi: 10.4049/jimmunol.179.6.3707. [DOI] [PubMed] [Google Scholar]
- 15.Chavez SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, Coutinho-Silva R, Rossi-Bergmann B. Modulation of P2X7 purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009;11:842–849. doi: 10.1016/j.micinf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Nino-Moreno P, Portales-Perez D, Hernandez-Castro B, Portales-Cervantes L, Flores-Meraz V, Baranda L, Gomez-Gomez A, Acuna-Alonzo V, Granados J, Gonzalez-Amaro R. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo subjects with pulmonary tuberculosis. Clin. Exp. Immunol. 2007;148:469–477. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod R, Kieffer F, Sautter M, Hosten T, Pelloux H. Why prevent, diagnose and treat congenital toxoplasmosis? Mem. Inst. Oswaldo Cruz. 2009;104:320–344. doi: 10.1590/s0074-02762009000200029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLeod R, Boyer K, Karrison T, Swisher C, Roizen N, Jalbrzikowski J, Remington J, Heydemann P, Noble AG, Mets M, Holfels E, Withers S, Latkany P, Meier P. Outcome of treatment for congenital toxoplasmosis, 1981–2004: the National Collaborative Chicago-based, Congenital Toxoplasmosis Study. Clin. Infect. Dis. 2006;42:1383–1394. doi: 10.1086/501360. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson SE, de Roubaix L-A, Cortina-Borja M, Tan HK, Mui EJ, Cordell HJ, Kirisitis MJ, Miller EN, Peacock CS, Hargrave ACa, Coyne JJ, Boyer K, Bessieres M-H, Buffolano W, Ferret N, Franck J, Keiffer F, Meier P, Nowakowska DE, Paul M, Peyron F, Stray-Pedersen B, Prusa A-R, Thulliez P, Wallon M, Petersen E, McLeod R, Gilbert RE, Blackwell JM. Genetic and epigenetic factors at COL2A1 and ABCA4 influence clinical outcome in congenital toxoplasmosis. PLoS ONE. 2008;3:e2285. doi: 10.1371/journal.pone.0002285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobbin CA, Smith NC, Johnson AM. Heat shock protein 70 is a potential virulence factor in murine Toxoplasma infection via modulation of host NF-κB and nitric oxide. J. Immunol. 2002;169:958–965. doi: 10.4049/jimmunol.169.2.958. [DOI] [PubMed] [Google Scholar]
- 21.Phinney DG, Kopen G, Isaacson RL, Prockop DJ. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, and differentiation. J. Cell. Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 22.Gubbels M-J, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob. Agents Chemother. 2003;47:309–316. doi: 10.1128/AAC.47.1.309-316.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duke RC, Witter RZ, Nash PB, Young JD, Ojcius DM. Cytolysis mediated by ionophores and pore-forming agents: role of intracellular calcium in apoptosis. FASEB J. 1994;8:237–246. doi: 10.1096/fasebj.8.2.8119494. [DOI] [PubMed] [Google Scholar]
- 24.Borel E, Mayencon M, Kaiser K, Picot S, Peyron F. Fluorogenic detection of viable Toxoplasma gondii. Parasite. 1998;5:371–373. doi: 10.1051/parasite/1998054371. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi M, Kowalewski-Grochowska K, Bahar MA, Kilani RT, Winkler-Lowen B, Guilbert LJ. Infection of placental trophoblasts by Toxoplasma gondii. J. Infect. Dis. 2003;188:608–616. doi: 10.1086/377132. [DOI] [PubMed] [Google Scholar]
- 26.Buffet PA, Sulahian A, Garin YJF, Nassar N, Derouin F. Culture microtitration: a sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995;39:2167–2168. doi: 10.1128/aac.39.9.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller CMD, Akratos C, Johnson AM, Smith NC. The production of a 70 kDa heat shock protein by Toxoplasma gondii RH strain in immunocompromised mice. Int. J. Parsitol. 2000;30:1467–1473. doi: 10.1016/s0020-7519(00)00118-1. [DOI] [PubMed] [Google Scholar]
- 28.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signaling. 2009;5:257–262. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adriouch S, Dox C, Welge V, Seman M, Koch-Noble F, Haag F. A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J. Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 30.Le Stunff H, Auger R, Kanellopoulos J, Raymond M-N. The Pro-451 to Leu polymorphism within the C-terminal tail of the P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J. Biol. Chem. 2004;279:16918–16926. doi: 10.1074/jbc.M313064200. [DOI] [PubMed] [Google Scholar]
- 31.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suadicani SO, Iglesias R, Spray DC, Scemes E. Point mutation in the mouse P2X7 receptor affects intercellular calcium waves in astrocytes. ASN NEURO. 2009;1(1) doi: 10.1042/AN20090001. art:e00005.doi:10.1042/AN20090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asward F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell. Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong S, Schwarz N, Brass A, Seman M, Haag F, Koch-Nolt F, Schilling WP, Dubyak GR. Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J. Immunol. 2009;183:578–592. doi: 10.4049/jimmunol.0900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown CR, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J. Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 36.Peng B-W, Lin J, Lin J-Y, Jiang M-S, Zhang T. Exogenous nitric oxide induces apoptosis in Toxoplasma gondii tachyzoites via a calcium signal transduction pathway. Parasitology. 2003;126:541–550. [PubMed] [Google Scholar]
- 37.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 38.Ferrari D, Wesselborg S, Bauer MK, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappBp65. J. Cell. Biol. 1997;29:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gargett CE, Cornish EJ, Wiley JS. Phospholipase D activation by P2Z purinoreceptor agonists in human lymphocytes is dependent on divalent cation influx. Biochem. J. 1996;313:529–535. doi: 10.1042/bj3130529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107:4946–4952. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 41.Hewinson J, Moore SF, Glover C, Watts AG, MacKenzie AB. A key role for redox signaling in rapid P2X7 receptor-induced IL-1β processing in human monocytes. J. Immunol. 2008;180:8410–8420. doi: 10.4049/jimmunol.180.12.8410. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari D, Los M, Bauer MK, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptosis and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 43.Sluyter R, Dalitz JG, Wiley JS. P2X7 receptor polymorphism impairs extracellular adenosine 5-triphosphate-induced interleukin-18 release from human monocytes. Genes Immun. 2004;5:588–591. doi: 10.1038/sj.gene.6364127. [DOI] [PubMed] [Google Scholar]
- 44.Sluyter R, Shemon AN, Wiley JS. Glu-496 Ala polymorphism in the P2X7 receptor impairs ATP-induced IL-1β release from human monocytes. J. Immunol. 2004;172:3399–3405. doi: 10.4049/jimmunol.172.6.3399. [DOI] [PubMed] [Google Scholar]
- 45.Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 46.Sibley LD, Krahenbuhl JL, Adams GM, Weidner E. Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J. Cell. Biol. 1986;103:867–874. doi: 10.1083/jcb.103.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morisaki JH, Heuser JE, Sibley LD. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J. Cell. Sci. 1995;108:2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- 48.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1β in mouse macrophages. J. Immunol. 2008;180:7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 49.Nash PB, Purner MB, Leon RP, Clarke P, Duke RC, Curiel TJ. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J. Immunol. 1998;160:1824–1830. [PubMed] [Google Scholar]
- 50.Goebel S, Gross U, Luder CG. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J. Cell Science. 2001;114:3495–3505. doi: 10.1242/jcs.114.19.3495. [DOI] [PubMed] [Google Scholar]
- 51.Payne TM, Molestina RE, Sinai AP. Inhibition of caspase activation and a requirement for NF-kappaB function in the Toxoplasma gondii-mediated blockade of host apoptosis. J. Cell Science. 2003;116:4345–4358. doi: 10.1242/jcs.00756. [DOI] [PubMed] [Google Scholar]
- 52.Keller P, Schaumburg F, Fischer SF, Hacker G, Gross U, Luder CG. Direct inhibition of cytochrome c-induced caspase activation in vitro by Toxoplasma gondii reveals novel mechanisms of interference with host cell apoptosis. FEMS Microbial. Lett. 2006;258:312–319. doi: 10.1111/j.1574-6968.2006.00241.x. [DOI] [PubMed] [Google Scholar]
- 53.Vutova P, Wirth M, Hippe D, Gross U, Schulze-Osthoff K, Schmitz I, Luder CG. Toxoplasma gondii inhibits Fas/CD95-triggered cell death by inducing aberrant processing and degradation of caspase 8. Cell Microbiol. 2007;9:1556–1570. doi: 10.1111/j.1462-5822.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 54.Hippe D, Lytocvchenko O, Schmitz I, Luder CG. Fas/CD95-mediated apoptosis of type II cells is blocked by Toxoplasma gondii primarily via interference with the mitochondrial amplification loop. Infect. Immun. 2008;76:2905–2912. doi: 10.1128/IAI.01546-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luder CG, Stanway RR, Chaussepied M, Langsley G, Heussler VT. Intracellular survival of apicomplexan parasutes and host cell modification. Int. J. Parasitol. 2009;39:163–173. doi: 10.1016/j.ijpara.2008.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.