Abstract
Coriander (Coriandrum sativum L.) has been cultivated for a long time in different parts of Iran. The chemical profiles of different accessions were analyzed by means of GC-MS. The essential oil content of the dried seeds was varied from 0.1 to 0.36 %. Thirty-Four different compounds were identified in essential oil of all accessions. Linalool (40.9−79.9%), neryl acetate (2.3−14.2%), γ-terpinene (0.1−13.6%) and α-pinene (1.2−7.1%) were identified as main components in the oil of Coriander accessions. Almost all studied accessions contain more that 60% linalool showing high quality of Coriander seeds produced in Iran and suitability of the accessions for use as initial genetic materials for breeding of homogenous and talent Coriander cultivars.
Keywords: Coriandrum sativum L. Essential oil, linalool, accession, diversity
1. Introduction
Coriander (Coriandrum sativum L.), commonly called Geshniz in Persian, is an annual herb in the family Apiaceae. Coriander is native to south-western parts of Asia to North Africa. It is a soft, hairless plant growing to 50 cm tall. The leaves are variable in shape, broadly lobed at the base of the plant and slender and feathery higher on the flowering stems. The flowers are borne in small umbels, white or very pale pink. The fruit is a globular dry schizocarp which commonly called seed (Omidbaigi, 1997).
The fresh and dried leaves (Coriandri herba) of Coriander are commonly used as vegetable, spice and also in cooking as an ingredient in many foods. The dried fruits or seeds (Coriandri fructus) are also used as a condiment in pickle spices, seasonings, curry powders, sausages, cakes, pastries, biscuits, buns. The aromatic seeds of Coriander are used as carminative, expectorant, stimulant and stomachic and appetite stimulant. It is widely used in treating indigestion, flatulence, diarrhea and colic and other stomach disorders in Iranian folk medicine (Zargari, 1991). The dried seed is used for the preparation of steam-distilled essential oil and solvent-extracted oleoresin for the aroma and flavor industries (Agri-facts, 1998). Coriander seed oil is included among the 20 major essential oils in the world market (Lawrence, 1992) and its commercial value depends on its physical properties, chemical composition and aroma (Smallfield, Van Klink, Perry, Dodds 2001) Linalool is the main volatile compound in seeds, typically constituting more than 50% of the total essential oil (Ramadan, Mörsel, 2003).
Variation in essential oil compositions can occurs as a result of differing soil conditions, altitude, climatic conditions, seasonal factors and other environmental features, leading in some cases to the evolution of different chemical variants or chemotypes (Heywood, 2002). As it is chemical constituents of essential oils that are the basis of their exploitation, particular attention has to be paid to the variation in the constituents. Variation in oil content and compositions and other characteristics of Coriander accessions from different parts of the world has been studied in extensive analysis (Diederichsen, 1996; Diederichsen & Hammer, 2003; López, Widrlechner, Simon, Rai, Boylston, Isbell, Bailey, Gardner & Wilson, 2008). Iran is one of the commercial Coriander producers (Agri-facts, 1998). Although, C. sativum has been cultivated for a long time in different parts of Iran, there is no information available on chemical characters of different accessions. So far local landraces have been used for cultivation and no attempt has been done for breeding of this plant in Iran. The aim of present study was to investigate the chemical profile of different Iranian Coriander landraces.
2. Experimental
2.1. Plant materials and oil extraction
The seeds of different accessions of Coriander were prepared from vegetable gardens and commercial fields in different parts of Iran during 2007. Essential oil of dried seeds of each accession was extracted by hydro-distillation of 30 g grinded seeds in three replications using a Celevenger apparatus.
2.2. GC and GC-MS analyses
GC-FID analysis of the oil was conducted using a Thermoquest-Finnigan instrument equipped with a DB-5 fused silica column (60 m × 0.25 mm i.d., film thickness 0.25 μm). Nitrogen was used as the carrier gas at the constant flow of 1.1 ml/min. The split ratio was 1/50. The oven temperature was raised from 60 °C to 250 °C at a rate of 5 °C/min. The injector and detector (FID) temperatures were kept at 250 °C and 280 °C, respectively.
GC-MS analysis was carried out on a Thermoquest-Finnigan Trace GC-MS instrument equipped with the same column and temperature programming as mentioned for GC analysis. Transfer line temperature was 250 °C. Helium was used as the carrier gas at a flow rate of 1.1 ml/min with a split ratio equal to 1/50.The constituents of the essential oils were identified by calculation of their retention indices under temperature-programmed conditions for n-alkanes (C6 – C24) and the oil on a DB-5 column under the same conditions. Identification of individual compounds was made by comparison of their mass spectra with those of the internal reference mass spectra library (Wiley 7.0) or of authentic compounds and confirmed by comparison of their retention indices with those of authentic compounds or with those reported in the literature (Adams 2007). Semi-quantitative data was obtained from FID area percentages without the use of correction factors.
2.3. Data analysis
Essential oil data were first standardized, and then the average Euclidean distance was calculated for each variety-pair. The resulting distance matrix was used to construct an UPGMA (Unweighted Pair Method with Arithmetic Mean) cluster analysis (Mohammadi, Prasanna, 2003). The SPSS software was used to produce a distance matrix and a dendrogram. The SPSS software was also used to construct a Pearson correlation matrix between main components of the oils.
3. Results and discussion
The essential oil content of the dried fruits of different accessions was varied from 0.1−0.36 %. Maximum oil content was observed in accession no. 1 from Maraghe (0.36%), no. 7 from Zarand (0.35%) and no. 13 from Arak (0.35%). Thirty-Four different compounds were identified in essential oil of all accessions (Table 1). Linalool, γ-terpinene, neryl acetate, α-pinene, p-cymene, dodecanal, 2E-dodecanal were identified as main components in the oil of all accessions. Essential oil of 10 accessions including accessions no.1 collected from Maraghe (70.0%), no.3 from Khoramabad (73.0%), no.4 from Estahbanat (79.9%), no.9 from Tabriz a (77.6%), no.10 from Hamedan (70.6%), no.12 from Bajestan (74.2%), no.14 from Amol (74.1%), no.15 from Yasooj (79.9%), no.16 from Lahijan (74.6%) and no.18 from Yazd (74.9%), showed a linalool content more than 70%.
Table 1.
Chemical constituents of essential oils of different Iranian Coriandrum sativum accessions
| No | Component | RI | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | α-thujene | 925 | - | - | - | - | - | - | - | - | 0.1 | 0.3 | - | - | - | 0.1 | - | - | 0.3 | - | - |
| 2 | α-pinene | 939 | 3.3 | 1.4 | 1.2 | 2.8 | 1.4 | 5.4 | 5.4 | 7.1 | 5.9 | 5.1 | 6.1 | 5.8 | 4.3 | 6.6 | 2.8 | 1.4 | 5.1 | 4.8 | 2.8 |
| 3 | sabinene | 970 | 0.2 | - | - | 0.1 | 0.8 | 0.2 | 0.5 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | - | 0.1 | - | 0.2 | 0.1 | 0.2 | |
| 4 | β-pinene | 978 | 0.4 | - | - | 0.3 | 0.2 | 1.2 | 0.6 | 1.1 | 0.5 | 0.6 | 0.5 | 0.7 | 0.5 | 0.2 | 0.3 | 0.2 | 0.6 | 0.3 | 0.6 |
| 5 | myrcene | 983 | 0.2 | - | - | 0.2 | 0.1 | 0.8 | 0.3 | 0.5 | 0.4 | 0.3 | 0.3 | 0.3 | 0.4 | 0.6 | 0.2 | 0.1 | 0.3 | 0.2 | 0.3 |
| 6 | undecane | 1000 | - | - | - | - | - | - | - | - | - | 0.1 | - | - | - | - | - | - | 0.1 | - | - |
| 7 | α-terpinene | 1010 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 | - | - | - | - | - |
| 8 | p-cymene | 1018 | 2 | 1.1 | 0.8 | 1.1 | 1.3 | 3.2 | 3.6 | 2.4 | 1.2 | 1.3 | 1.6 | 2.6 | 1.2 | 1.4 | 1.1 | 1.3 | 1.3 | 1.1 | 3.6 |
| 9 | limonene | 1026 | 0.3 | - | - | 0.1 | 0.3 | 1.0 | 0.4 | 0.5 | 0.2 | 0.4 | 0.2 | 0.2 | 0.2 | 0.3 | 0.1 | 0.3 | 0.4 | 0.1 | 0.4 |
| 10 | γ-terpinene | 1052 | 9.3 | 6.7 | 6.7 | 0.1 | 8.1 | 11.9 | 10.7 | 9.6 | 7.6 | 9.1 | 13.6 | 5.8 | 9.7 | 9.6 | 8.12 | 8.1 | 7.1 | 9.12 | 8.7 |
| 11 | linalool | 1085 | 70.1 | 62.8 | 73 | 79.9 | 61.7 | 40.9 | 64.2 | 64.6 | 77.6 | 70.6 | 69.1 | 74.2 | 68.3 | 74.1 | 79.9 | 69.7 | 74.6 | 74.9 | 69.2 |
| 12 | camphor | 1123 | 0.2 | - | 0.2 | 0.1 | - | - | 0.3 | 0.1 | 0.3 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | - | 0.3 | 0.1 | - | |
| 13 | citronella | 1127 | 0.3 | 0.4 | - | 0.2 | 0.2 | - | 0.2 | 0.3 | 0.2 | 0.3 | 0.1 | 0.1 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| 14 | borneol | 1150 | - | - | - | 0.1 | - | - | - | - | 0.1 | 0.1 | 0.1 | 0.1 | - | - | 0.1 | - | 0.1 | 0.1 | - |
| 15 | terpinen-4-ole | 1164 | 0.1 | - | - | - | - | 0.3 | - | 0.2 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | - | - | - | 0.1 | - | - |
| 16 | decanal | 1180 | 0.4 | 0.2 | 1 | 0.1 | 0.2 | 1.0 | 0.2 | 0.4 | 0.3 | 0.3 | 0.1 | 0.1 | 0.6 | 0.3 | 0.1 | 0.2 | 0.3 | 0.1 | - |
| 17 | n-dodecane | 1200 | - | - | 0.2 | - | 0.4 | - | 0.3 | - | - | - | - | - | - | - | - | 0.4 | - | 0.3 | |
| 18 | nerol | 1212 | 0.8 | 0.7 | 0.8 | 0.5 | - | 1.8 | 0.3 | 0.6 | 0.2 | 0.3 | - | 0.4 | 0.6 | - | 0.5 | - | 0.3 | 0.5 | 0.3 |
| 19 | methyl | 1245 | - | - | - | 0.1 | - | - | - | - | - | - | - | - | - | - | 0.1 | - | - | 0.1 | - |
| 20 | 2E-decanal | 1260 | 0.2 | 0.6 | - | - | - | - | - | 0.2 | 0.2 | 0.2 | - | 0.1 | 0.5 | 0.2 | - | - | 0.2 | - | - |
| 21 | decyl alcohol | 1260 | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 22 | carvacrol | 1265 | 0.3 | 0.5 | 0.2 | 0.1 | - | 0.8 | 0.7 | - | - | - | - | - | - | - | 0.1 | - | - | 0.1 | 0.7 |
| 23 | thymol | 1276 | 0.2 | 0.3 | 0.4 | - | - | - | 0.8 | - | - | - | - | - | - | - | - | - | - | - | 0.8 |
| 24 | Undecanal | 1290 | - | 0.2 | 0.3 | - | - | - | - | - | 0.5 | - | - | 0.1 | 0.1 | - | - | - | - | - | - |
| 25 | citronellyl | 1337 | - | - | - | - | 0.6 | - | - | - | 0.03 | 0.1 | - | - | 0.1 | - | - | 0.6 | 0.1 | - | - |
| 26 | neryl acetate | 1342 | 6.9 | 10.8 | 7.5 | 5.3 | 14.2 | 14.7 | 8.4 | 4.9 | 2.6 | 7.2 | 2.8 | 6.5 | 6 | 4.1 | 2.3 | 10.2 | 5.9 | 4.3 | 8.4 |
| 27 | dodecanal | 1382 | 0.2 | 0.6 | 0.4 | 0.1 | 0.9 | 1.0 | 0.3 | - | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 | 1.0 | 0.1 | 0.9 | 0.2 | 0.1 | 0.3 |
| 28 | n-tetradecane | 1402 | 0.2 | 1.7 | 0.7 | - | 1.2 | 1.2 | 1.1 | - | - | - | - | - | - | - | - | 1.2 | - | - | 1.1 |
| 29 | 2E-dodecanal | 1408 | 3.7 | 8.1 | 3.7 | 2.2 | 3.5 | 7.7 | 0.8 | 2.2 | 0.6 | 2.1 | - | 0.1 | 4.3 | - | 1.7 | 3.5 | 1.1 | 1.7 | 0.8 |
| 30 | β-cayophyllene | 1427 | - | - | - | - | 0.2 | - | - | 1.7 | 0.03 | 0.1 | - | - | 0.1 | - | - | 0.2 | 0.1 | - | - |
| 31 | tetrahydro ionol | 1539 | 0.3 | 0.3 | 0.2 | 0.2 | - | - | 0.2 | 0.1 | 0.3 | 0.1 | - | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 | |||
| 32 | n-hexadecane | 1601 | 0.3 | 1.3 | 0.8 | 1.5 | 1 | 3.8 | 1 | 2.1 | - | - | - | - | - | - | 1.5 | 1 | 1.5 | 1 | |
| 33 | tetradecanal | 1615 | - | 0.6 | - | - | - | 0.3 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 34 | benzyl benzoate | 1730 | - | - | - | - | - | - | - | - | 0.1 | - | 4.5 | 0.5 | - | 0.3 | - | - | - | - | - |
| Total | 99.6 | 99.1 | 99.5 | 99.62 | 99.5 | 97.8 | 97.9 | 98.2 | 99.6 | 99.7 | 99.4 | 98.96 | 95.7 | 98.9 | 95 | 95.5 | 99.9 | 98.3 | 98.9 | ||
| Oil content | 0.36 | 0.32 | 0.28 | 0.31 | 0.27 | 0.25 | 0.15 | 0.35 | 0.25 | 0.1 | 0.1 | 0.3 | 0.22 | 0.35 | 0.3 | 0.15 | 0.25 | 0.2 | 0.15 |
Note: RI: observed retention indices
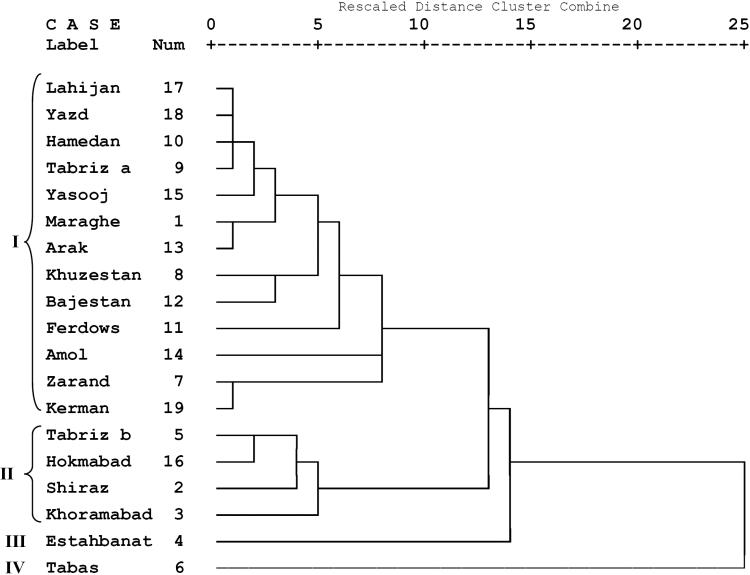
Linalool content in the oil of 8 accessions including no.2 from Shiraz (62.8%), no.5 from Tabriz b (61.7%), no.11 from Ferdows (69.1%), no.7 from Zarand (64.2%), no.8 from Khuzestan (64.6%), no.13 from Arak (68.3%), no.19 from Kerman (69.2%) and no.16 from Hokmabad (69.7%) were more than 60% of the oil. Among all, accession no.6 collected from Tabas showed minimum linalool content (40.9%). Cluster analysis divided all accessions into four major groups (Fig. 1). First group included 13 accessions that linalool (64.2−79.9%), γ-terpinene (5.8−13.6%), neryl acetate (2.3−8.4%), α-pinene (2.8−7.1%) and p-cymene (1.1−3.6%) are major components of the oils. Second group comprised four accessions from Shiraz, Khoramabad, Hokmabad and Tabriz b and linalool (61.7−73 %), neryl acetate (7.5−14.2 %), γ-terpinene (6.7−8.1 %), 2E-dodecanal (3.5−8.1 %), α-pinene (1.2−1.4 %), p-cymene (0.8−1.3%), n-tetradecane (0.7−1.7%), n-hexadecane (0.8−1.3%) and dodecanal (0.4−0.9%) identified as main constituents of the oils of this group. Third group included an individual accession from Estahbanat. Linalool (79.9%), neryl acetate (5.3%), α-pinene (2.8 %), 2E-dodecanal (2.2%), n-hexadecane (1.5%) and p-cymene (1.1%) were major components of the oil. Forth group also included an individual accession from Tabas. Linalool (40.9%) γ-terpinene (11.9%) neryl acetate (9.7%), 2E-dodecanal (8.7%), α-pinene (6.4%), p-cymene (4.2%), nerol (1.8%), β-pinene (1.2%), pentadecane (1.1%), limonene (1.0%), decanal (1.0%), dodecanal (1.0%), n-hexadecane (3.8%) and n-tetradecane (1.2%) represented as main constituents of this accession.
Figure 1.
Dendrogram showing the phenetic relationships among 19 Coriandrum sativum accessions based on Euclidean distances from essential oil data matrix.
The Pearson's coefficient correlations (Table 2) showed that there is significant negative correlation between linalool content and other major components such as γ-terpinene, neryl acetate, α-pinene, p-cymene, 2E-dodecanal and dodecanal.
Table 2.
Simple correlation between main constituents of different accessions of Coriandrum sativum.
| Compound | α-pinene | p-cymene | γ-terpinene | linalool | neryl acetate | dodecanal |
|---|---|---|---|---|---|---|
| p-cymene | 0.292 | |||||
| γ-terpinene | 0.408 | 0.354 | ||||
| linalool | −0.037 | −0.511* | −0.506* | |||
| neryl acetate | −0.607** | 0.187 | −0.067 | −0.546* | ||
| dodecanal | −0.278 | 0.029 | 0.217 | −0.566* | 0.594** | |
| 2E-dodecanal | −0.465* | −0.081 | 0.001 | −0.657** | 0.552* | 0.461* |
Notes:
Correlation is significant at the 0.01 level
Correlation is significant at the 0.05 level.
It has been shown that essential oil content and composition of C. sativum can be influenced by cultivation practices, ontogenetic and genetic factors (Hornok, 1976; Msaada, Ben Taarit, Chahed, Kchouk & Marzouk, 2007; Telci, Toncer & Sahbaz, 2006). The essential oil content of coriander fruits varies from very low (0.03%) to a maximum report of 2.7% (Bandara, Wildschut, Russel, Ost, Simo, Weber, 2000; Purseglove, Brown, Green, Robbins, 1981). Dobos and Novak reported a range of variation of oil content between 0.2 and 1.3% among 36 different Coriander accessions from Austria (Dobos & Novak, 2005). The reported variation of oil content is considerably higher than those of Iranian accessions obtained in present study. Msaada, et al. reported that essential oil yield and compositions of Coriander fruit increase during maturation process. Geranyl acetate (46.27%) and linalool (10.96%) were the main compounds of immature fruits while essential oils of mature fruits consist mainly on linalool (87.54%) and cis-dihydrocarvone (2.36%) (Msaada, Ben Taarit, Chahed, Kchouk & Marzouk, 2007). The ecological variation of significant effect on seed yields, oil content and composition of different Coriander varieties were reported. Linalool content was 63.5−71.0% and 42.1−52.7% in var. microcarpum and var. vulgar, respectivly (Telci, Toncer & Sahbaz, 2006). Raal et al. analyzed the oil of Coriander seeds from different geographical origins of Europe. The major constituent of the oils were linalool (58.0−80.3%), γ-terpinene (0.3−11.2%), α-pinene (0.2−10.9%), p-cymene (0.1−8.1%), camphor (3.0−5.1%) and geranyl acetate (0.2−5.4%) (Raal, Arak & Orav 2004). The main constituents of the essential oil of Coriander growing in 6 different zones of Argentina were linalool (68.9−83.7%), γ-terpinene (2.2−5.1%), camphor (3.2−4.8%), α-pinene (1−6.5%), geraniol (1.4−3.2%) and geranyl acetate (0.8−3.8%). The contents of cis- and trans-linalool oxide (0.1−0.4%) were low (Bandoni, Mizrahi & Juarez, 1998).
Linalool (77.48 %), γ-terpinene (4.64 %), α-pinene (3.97 %), limonene (1.28 %), geraniol (0.64 %) and 2-decenal (0.16 %) have been reported as main oil components of C. sativum from Brazil (De Figueiredo, Nakagawa &Marques, 2004). The composition of the essential oils of C. sativum from Bulgaria was analyzed (Stoyanova, Konakchiev & Berov 2002). The main components of the essential oil were linalool (63.3%), α-pinene (6.1%) and p-cymene (5.0%) (Dimri, Khan & Narayana, 1976).
Variation in seed oil content and composition of Coriander due to genetic or environmental factors has been reported in several other studies (Kalra, Patra, Singh, Singh, Mengi, Naqvi & Kumar, 1999; Singh, Patra, Kalra, Singh, Kumar, Singh& Singh, 2002). This study revealed some variations in oil content and composition of Coriander seed in Iran which can be because of the influence of agricultural practices, environmental and genetic factors. Linalool as an alifatic terpene was major component of all accessions of Coriander collected from different parts of Iran. With an exception in case of accession no. 6 from Tabas, all other studied accessions contain more that 60% linalool showing high quality of Coriander seeds produced in Iran which can be used in food, pharmaceutical and other related industries. Beside, the results revealed the suitability of the accessions for use as initial genetic materials for breeding of homogenous and talent Coriander cultivars.
Acknowledgments
Shahid Beheshti University Research Council is acknowledged for financial support (Research project No: 600/4630)
References
- Adams RP. Identification of essential oils components by gas chromatography/quadrupole mass spectroscopy. Illinois: Allured Publishing Corporation; 2007. [Google Scholar]
- Agri-facts Coriander. Practical information for Alberta's agriculture industry. 1998 Accessed online at: http://agric.gov.ab.ca/agdex/100/147_20-2.html#top.
- Bandara M, Wildschut C, Russel E, Ost L, Simo T, Weber J. Alberta, Agriculture, Food, and Rural Development. Crop Diversification Centres Annual Report. Alberta; Canada: 2000. Special crops program (Brooks). Accessed online at http://www.agric.gov.ab.ca/ministry/pid/cdc/00/sc_brooks.html. [Google Scholar]
- Bandoni AL, Mizrahi I, Juarez MA. Composition and quality of the essential oil of coriander (Coriandrum sativum L.) from Argentina. Journal of Essential Oil Research. 1998;10:581–584. [Google Scholar]
- De Figueiredo RO, Nakagawa J, Marques M. Composition of coriander essential oil from Brazil. Future for Medicinal and Aromatic Plants. 2004;629:135–137. [Google Scholar]
- Diederichsen A, Hammer K. The infraspecific taxa of coriander (Coriandrum sativum L.). Genetic Resources and Crop Evolution. 2003;50:33–63. [Google Scholar]
- Diederichsen A. Promoting the conservation and use of underutilized and neglected crops 3. Int. Plant Genet. Res. Inst. (IPGRI); Rome, Italy: 1996. Coriander. [Google Scholar]
- Dimri BP, Khan MNA, Narayana MR. Some promising selections of Bulgarian Coriander (Coriandrum sativum L.) for seed and essential oil with a note on cultivation and distillation of oil. Indian Perfumer. 1976;20:13–21. [Google Scholar]
- Dobos G, Novak J. Comparison of the composition of the essential oil of some winter-annually cultivated coriander accessions (Coriandrum sativum L.). Zeitschrift fur Arznei- & Gewurzpflanzen. 2005;10:144–145. [Google Scholar]
- Heywood VH. Biodiversity: Biomolecular Aspects of Biodiversity and Innovative Utilization. Şener B. Springer; 2002. The Conservation of Genetic and Chemical Diversity in Medicinal and Aromatic Plants. pp. 13–22. [Google Scholar]
- Hornok L. The effect of sowing date on the yield and essential oil content of coriander (Coriandrum sativum). Herba Hungarica. 1976;15:55–62. [Google Scholar]
- Kalra A, Patra NK, Singh HP, Singh HB, Mengi N, Naqvi AA, Kumar S. Evaluation of coriander (Coriandrum sativum) collection for essential oil. Indian Journal of Agricultural Sciences. 1999;69:657–659. [Google Scholar]
- Lawrence BM. A planning scheme to evaluate new aromatic plants for the flavor and fragrance industries.. In: Janick J, Simon JE, editors. New Crops: exploration, research, and commercialization. Proceedings of the Second National Symposium; John Wiley and Sons, Inc., New York. 1992. pp. 620–627. [Google Scholar]
- López PA, Widrlechner MP, Simon PW, Rai S, Boylston TD, Isbell TA, Bailey TB, Gardner CA, Wilson LA. Assessing phenotypic, biochemical, and molecular diversity in coriander ( Coriandrum sativum L.) germplasm. Genetic Resources and Crop Evolution. 2008;55:247–275. [Google Scholar]
- Mohammadi SA, Prasanna BM. Analysis of genetic diversity in crop plants-salient statistical tools and considerations. Crop Sciences. 2003;43:1235–1248. [Google Scholar]
- Msaada KHK, Ben Taarit M, Chahed T, Kchouk ME, Marzouk B. Changes on essential oil composition of coriander (Coriandrum sativum L.) fruits during three stages of maturity. Food Chemistry. 2007;102:1131–1134. [Google Scholar]
- Omidbaigi R. Approaches to Production and Processing of Medicinal Plants. Vol. 2. Tarrahan-e-Nashr; Tehran, Iran: 1997. pp. 1–424. [Google Scholar]
- Purseglove JW, Brown EG, Green CL, Robbins SRJ. Spices. Vol. 2. Longman; New York: 1981. pp. 736–788. [Google Scholar]
- Raal A, Arak E, Orav A. Chemical composition of coriander seed essential oil and their conformity with EP standards. Agraarteadus. 2004;15:234–239. [Google Scholar]
- Ramadan MF, Mörsel JT. Analysis of glycolipids from black cumin (Nigella sativa L.), coriander (Coriandrum sativum L.) and niger (Guizotia abyssinica Cass.) oilseeds. Food Chemistry. 2003;80:197–204. doi: 10.1021/jf0346713. [DOI] [PubMed] [Google Scholar]
- Singh HP, Patra NK, Kalra A, Singh HB, Kumar B, Singh SP, Singh AK. Genetic distance in coriander (Coriandrum sativum L.) for essential oil yield and yield traits. Journal of Spices and Aromatic Crops. 2002;11:101–105. [Google Scholar]
- Smallfield BM, Van Klink JW, Perry NB, Dodds G. Coriander spice oil: effects of fruit crushing and distillation time on yield and composition. Journal of Agriculture and Food Chemistry. 2001;49:118–123. doi: 10.1021/jf001024s. [DOI] [PubMed] [Google Scholar]
- Stoyanova A, Konakchiev A, Berov O. Investigation on the essential oil of coriander from Bulgaria. Herba Polonica. 2002;48:67–70. 2002. [Google Scholar]
- Telci I, Toncer OG, Sahbaz N. Yield, essential oil content and composition of Coriandrum sativum varieties (var. vulgare Alef and var. microcarpum DC.) grown in two different locations. Journal of Essential Oil Research. 2006;18:189–193. [Google Scholar]
- Zargari A. Medicinal plants. 5th ed. Vol. 2. Tehran University Publications; 1991. pp. 1–942. [Google Scholar]