Abstract
Proteasome inhibitor PS-341 (also known as Bortezomib) and histone deacetylase (HDAC) inhibitors have emerged as novel therapeutic agents for a variety of malignancies. In this study, we examined whether PS-341 and the HDAC inhibitor trichostatin A (TSA) induced apoptosis in head and neck squamous cell carcinoma (HNSCC), a common and lethal malignancy. We found that, while TSA treatment alone did not induce apoptosis in HNSCC cells, it significantly enhanced PS-341-induced apoptosis in HNSCC cells in vitro. Consistently, TSA significantly improved PS-341-mediated inhibition of HNSCC tumor growth in nude mice. Mechanistically, we found that TSA increased PS-341-induced Noxa expression and caspase activation in HNSCC cells. The knock-down of Noxa significantly reduced apoptosis induced by co-treatment of PS-341 and TSA. Taken together, our results provide new insight into the mechanisms of synergistic antitumor activity of PS-341 and HDAC inhibitor regimen, offering a new therapeutic strategy for HNSCC patients.
Keywords: PS-341, Proteasome inhibitor, HDAC inhibitor, HNSCC, Noxa, Apoptosis
Introduction
HNSCC ranks amongst the 10 most common cancers worldwide with more than 780,000 new cases diagnosed each year [1]. Despite the latest innovations in both basic science and clinical research, the overall survival rate for HNSCC remains low, and it is reported that 25% of HNSCC patients develop a second cancer within 5 years of diagnosis (2–3). Thus, improvement on conventional therapy is urgently needed to effectively treat HNSCC. Recently, the proteasome inhibitor PS-341, also known as Bortezomib or Velcade, has been proposed as an alternative approach for conventional cisplatin-based chemotherapy to overcome cisplatin resistance in head and neck malignancies (4). PS-341 is a dipeptidyl boronic acid derivative that specifically inhibits the function of the 26S proteasome (5). This ability distinguishes PS-341 from other proteasome inhibitors such as MG-132, which also inhibit thiol proteases like cathepsin B and calpains as well as the 26S proteasome (5, 6). PS-341 potently induces apoptosis in a broad range of human cancer cell lines, including myeloma, prostate and breast cancers, and head and neck squamous cell carcinoma (4–5, 7). Activation of the anti-apoptotic transcription factor nuclear factor kappa B (NF-κB) is dependent on the 26S proteasome. The inhibition of NF-κB by PS-341 has been found to induce apoptosis in several human cancer cells and is considered to be an important target of the PS-341 anti-tumor effect (4–5). Very recently, we and others have demonstrated that PS-341 activates the pro-apoptotic endoplasmic reticulum (ER) stress in numerous human cancer cells in addition to the inhibition of the pro-survival NF-κB signaling pathway (4–8). We found that the PS-341 induced in endoplasmic reticulum (ER) stress and subsequently activated a coordinated cellular response called unfolded protein response (UPR) in HNSCC cells (7). PS-341 induced activation of the ER transmembrane stress sensing kinase PERK and subsequent attenuation of general protein synthesis in HNSCC cells. PS-341 robustly increased protein levels of the pro-apoptotic transcription factors ATF4 and CHOP [4,8]. Mechanistically, PS-341 induced the expression of the pro-apoptotic Bcl-2 homology domain 3 only (BH3-only) protein Noxa through ATF4 in HNSCC cells. The knock-down of Noxa significantly reduced PS-341-mediated apoptosis in HNSCC cells, suggesting that induction of new gene transcription is required for PS-341-mediated apoptosis (4). However, although PS-341 was able to induce apoptosis in HNSCC cell lines, clinical studies showed that the cytotoxicity of PS-341 remains limited when used as a single agent (9).
Recently, histone deacetylase (HDAC) inhibitors such as TSA have emerged as novel therapeutic agents for human cancers (10–16). TSA, a classic potent inhibitor of HDAC, is a hydroxamic acid-derived compound from the metabolic product of streptomycete [10]. There are at least 18 HDACs, which are subdivided into 4 classes: Class I (HDAC 1, 2, 3, and 8), Class IIa (HDAC 4, 5, 7, and 9), Class IIb (HDAC 6 and 10), Class III (SIRT 1, 2, 3, 4, 5, 6, and 7) and Class IV (HDAC 11) (10–11). TSA is known to inhibit zinc-dependent deacetylases, including Class I, II, and IV HDACs (10). It has been shown that HDACs are highly over-expressed in a variety of human cancers, suppressing transcription of tumor suppressor genes such as p21WAF/CIP1 through chromatin structure modulation mediated by deacetylating lysine-4 residues of histone-H3 (12–13). HDAC inhibitors can reverse this process by blocking HDAC activity and promoting acetylation of histone-H3 to reactivate transcription of these dormant tumor suppressor genes, thereby inducing cytotoxicity in cancer cells (10–11, 16). Interestingly, there are several studies showing that HDAC inhibitors induce synergistic cytotoxicity in myeloma and pancreatic cancer cells when used simultaneously with PS-341 (17–24). Use of HDAC inhibitors, including TSA, have demonstrated that inhibition of HDAC activity abrogates the formation of a cytoprotective structure termed “aggresome”, which promotes the degradation of the ubiquitin conjugated proteins in many cancer cells upon PS-341 treatment (22). Studies proposed that the simultaneous treatment of a HDAC inhibitor such as TSA with proteasome inhibitor PS-341 restores or even enhances the diminished cytotoxicity of PS-341 through prevention of the protective response towards the accumulation of misfolded proteins upon PS-341 treatment in many cancer cells (22–24). However, the possible effects and therapeutic mechanism of this combination in HNSCC remains undefined. In this report, we examined whether TSA enhanced PS-341-mediated apoptosis in HNSCC cells. Our results revealed that the co-treatment of PS-341 and TSA in HNSCC cells enhanced apoptosis by increasing Noxa expression. The significantly enhanced anti-tumor activity that results from a combination of PS-341 and TSA offers promise as a novel treatment for HNSCC patients.
Materials and Methods
Cell culture and reagents
HNSCC cell lines, UMSCC1, UMSCC9 and UMSCC23, were obtained from Dr. Thomas Carey at The University of Michigan and FaDu was purchased from ATCC. These cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum and penicillin-streptomycin (Invitrogen). Cells were maintained at 37 °C with 5% carbon dioxide. PS-341 was purchased from L.L.C. Laboratories. PS-341 was dissolved in diemthyl sulfoxide as a 10 mM stock solution, aliquoted, stored at −70 °C, and subsequently diluted with 1X phosphate buffered saline pH 7.4 (Invitrogen) before use. Trichostatin A was purchased from Sigma-Aldrich. TSA was dissolved in DMSO as a 2.5 mM stock solution, aliquoted, stored at −20 °C, and subsequently diluted with cell culture medium before use.
Trypan Blue Assay and DNA Ladder
For cytotoxicity assay, UMSCC1, UMSCC9, UMSCC23, and FaDu cells were seeded on 12-well culture plates at 2 × 105 cells per well. Cells were then treated with PS-341 and/or TSA and incubated at 37 °C with 5% CO2 for 24 hrs. After 24 hrs of exposure to these chemotherapeutic agents, viability of the cells was determined using Trypan blue assay. The assays were performed in triplicate samples, and the results are representative of three independent experiments.
For the DNA ladder assay, cells were seeded onto 6 cm tissue culture dish at 7 × 105 cells per dish then treated with PS-341 and/or TSA at 37 °C with 5% CO2 for 24 hrs. Both of the detached and attached cells were scraped, harvested,, and lysed using DNA lysis buffer (10 mM Tris-HCl pH 7.4, 10 mM NsCl, 10 mM EDTA pH 8.0, and 10% SDS) with 100 µg/ml proteinase K for 2 hrs at 50°C. Genomic DNA was extracted with phenol-chloroform-isoamyl alcohol (Roche) for at least twice according to manufacturer’s protocol. DNA was precipitated with 3 M sodium acetate and ethyl alcohol. Precipitated DNA was washed with 70% ethanol and resuspended in TE with RNAase pH 8.0, and 5 µg of each DNA sample was resolved on a 1.5% agarose gel to visualize fragments.
Western Blot and Northern Blot Analysis
Cells (2 × 106) were plated in 10-cm tissue culture dishes a day before PS-341, TSA, and PS-341 plus TSA treatment. Whole-cell extracts were prepared with whole cell lysate buffer (Sigma-Aldrich) with protease inhibitors. 50 µg of lysates were separated by 8–15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinyldiene difluoride (PVDF) membrane with a Bio-Rad (Hercules, CA) semidry transfer apparatus. The membranes were blotted with 5% milk at room temperature for 1 hour and subsequently probed with primary antibodies at 4 °C overnight. Primary antibodies were acquired from the following sources: ATF-4, GADD-34 and histone H3 from Santa Cruz (Santa Cruz, CA); Caspase 3, Caspase 7, and Caspase 9 from Cell Signaling (Beverly, MA); acetyl-histone 3 from BD Biosciences (BD Biosciences, CA); and Noxa from Abcam, Inc. (Cambridge, MA). Secondary antibodies mouse anti-rabbit-IgG-HPR (Promega) and goat anti-mouse-IgG-HRP (Bio-Rad) were used to detect the primary antibodies. The signals were detected using ECL reagents (Pierce, Rockford, IL, USA). For Northern blot, cells were treated with PS-341, TSA or PS-341 plus TSA and total RNA was harvested using Trizol (Invitrogen) according to the manufacturer’s instructions. Five micrograms of total RNA was resolved on 1.5% agarose formaldehyde gels and transferred to Zeta-Probe GT Genomic Tested Blotting (nitrocellulose) membrane (Biorad) overnight. The membranes were hybridized with 32P-labelled Noxa cDNA probes and exposed to autoradiographic film as described previously [4].
siRNA Transfection
Noxa siRNA SMART pool (D-005275-05) and luciferase control siRNA were purchased from Dharmacon. UMSCC1 or UMSCC23 cells (3.5 × 105) were plated in 6cm tissue culture dishes a day before transfection. Noxa siRNA or luciferase siRNA (100 µM) was transfected into cells overnight with Oligofectamine diluted in Opti-Mem (Invitrogen) according to the manufacturer’s protocol. Forty-eight hours following transfection cells were treated with PS-341 or PS-341 plus TSA for 16 hr.
In Vivo Tumor Growth Model
All animal practices in this study were in accordance with the institutional animal welfare guidelines of the University of California Los Angeles. 8 week old nude mice were obtained from Jackson Laboratory and injected subcutaneously with UMSCC1 cells (2 × 106) into the right and left flank (day 0). One week after tumor cell inoculation, a group of mice (n = 5) were intraperitoneally injected with PS-341 (200µg/kg), TSA (1mg/kg), PS-341 plus TSA or vehicle control for 3 weeks. Tumor sizes were measured daily and calculated using the formula: (length × width2)/2.
Results
PS-341-mediated apoptosis in HNSCC cells enhanced by TSA
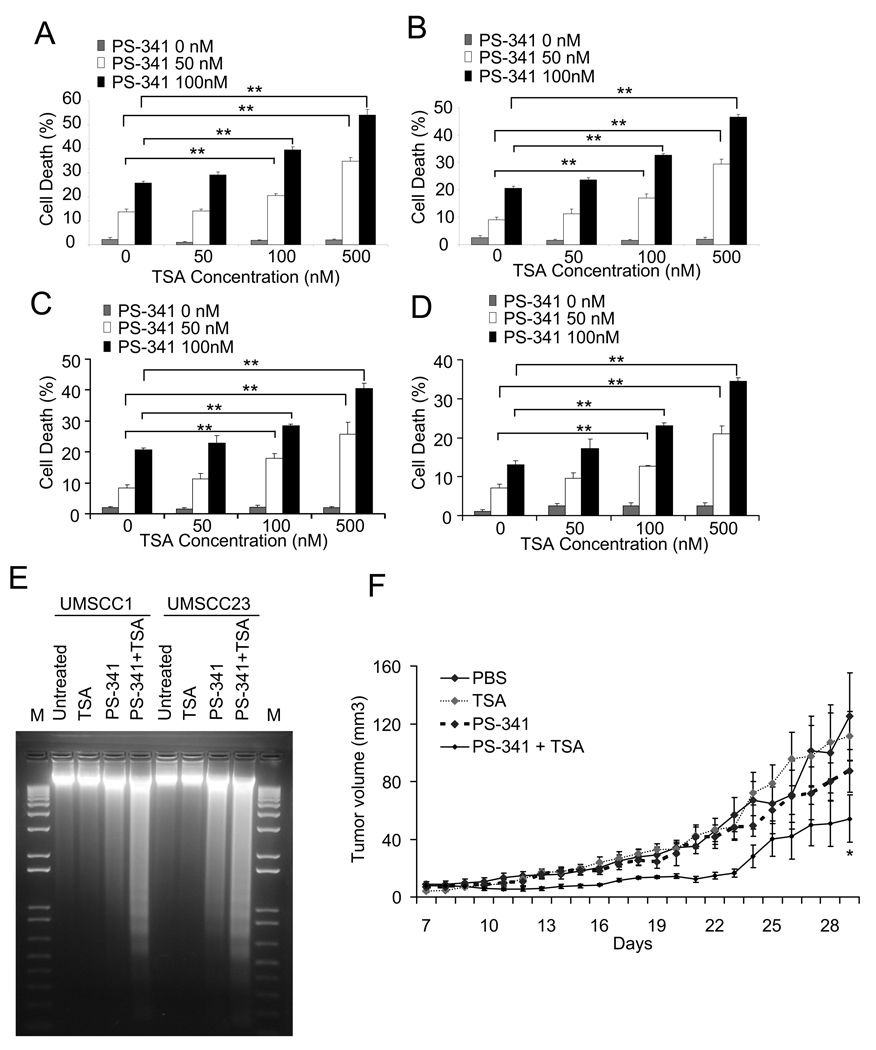
To examine the consequence of combined exposure to PS-341 and TSA, UMSCC1 cells were treated with PS-341, TSA, or in combination for 24 hr. TSA treatment alone did not affect cell death in HNSCC cells. While the low dose of PS-341 modestly induced cell death in UMSCC1 cells, the addition of TSA resulted in a markedly enhanced overall cell death in a dose-dependent manner (Fig. 1A). Also, TSA significantly enhanced cell death induced by the low dose of PS-341 in other HNSCC cell lines, including UMSCC23, UMSCC9 and FaDu cells (Fig. 1B, C and D). To confirm that TSA enhanced PS-341-induced apoptosis, we isolated genomic DNA and performed a DNA ladder assay on both UMSCC1 and UMSCC23 cells. As shown in Fig. 1E, the induction of genomic DNA fragmentation in UMSCC1 and UMSCC23 cells was significantly enhanced by the co-treatment with PS-341 and TSA (Fig. 1E). To explore whether TSA could enhance PS-mediated inhibition of tumor growth in vivo, UMSCC1 cells were inoculated into nude mice and treated with PS-341, TSA, or in combination for 3 weeks. As shown in Fig. 1F, UMSCC1 tumor growth in nude mice was significantly inhibited by co-treatment of PS-341 and TSA compared to PS-341 or TSA treatment alone.
Figure 1.
TSA enhances PS-341-mediated apoptosis in HNSCC cells. A, TSA enhanced the cell death induced by PS-341 in UMSCC1 cells. Cell viability was determined by the trypan blue exclusion assay. The assays were performed with triplicate samples, and the results are representative of three independent experiments (**P < 0.01). Error bars depict standard deviation. B, C and D, TSA enhanced PS-341-induced cell death in UMSCC23 (B), UMSCC9 (C), and FaDu (D). **P < 0.01. E, TSA enhanced PS-341-mediated apoptosis in HNSCC cells. UMSCC-1 and UMSCC-23 cells were treated with PS-341 alone, TSA alone, and PS-341 and TSA together for 24 h. After treatment, the detached and attached cells were pooled, and genomic DNA was extracted with phenol-chloroform-isoamyl alcohol and genomic DNAs were separated on a 1.5% agarose gel. M, 1 kb DNA ladder. F. TSA enhanced PS-341-mediated inhibition of HNSCC tumor growth in vivo. UMSCC1 cells were subcutaneously inoculated into nude mice for one week and then treated with vehicle control, TSA, PS-341, or PS-341 plus TSA for three weeks. Tumor size was daily measured for indicated days. *P < 0.05 (n = 5; PS-341 plus TSA vs PS-341 or TSA).
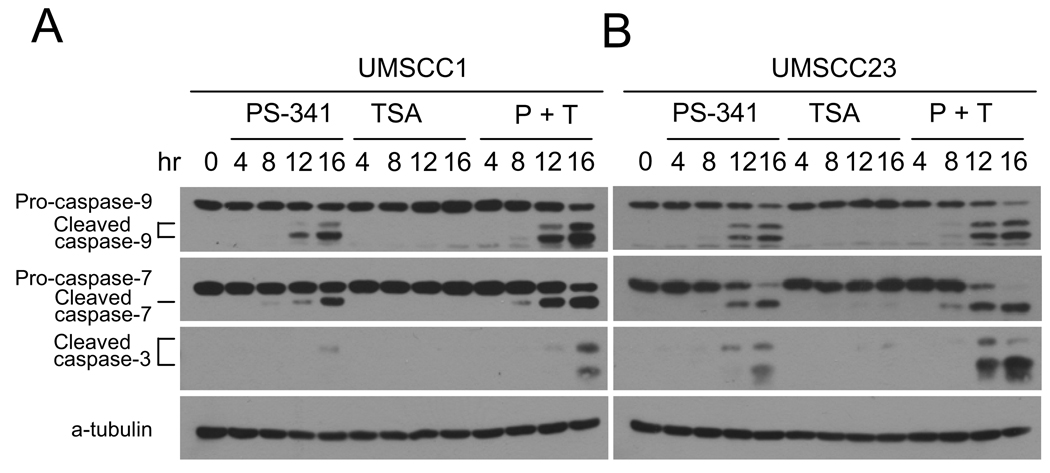
Previously, we found that PS-341 induced apoptosis in HNSCC cells involves activating intrinsic caspase cascades. Thus, we performed Western blot analysis to determine whether TSA promoted PS-341-induced caspase activation. As shown in Fig. 2A and B, TSA significantly enhanced the activation of the apical caspase-9 induced by PS-341 in both UMSCC1 and UMSCC23 cells. Consistently, the activation of executioner caspase-3 and -7 was also increased by the co-treatment of PS-341 and TSA when compared to PS-341 treatment alone. For both UMSCC1 and UMSCC23 cells, individual treatment with TSA did not trigger caspase activation. In conclusion, our results suggest that the co-treatment with PS-341 and TSA synergistically promotes apoptosis in HNSCC cells.
Figure 2.
TSA enhances caspase activation induced by PS-341 in HNSCC cells. A, TSA enhanced caspase activation induced by PS-341 in UMSCC1 cells. UMSCC-1 cells were treated with PS-341 alone, TSA alone, or PS-341 and TSA together for 0, 4, 8, 12, or 16 h. Whole cell extracts were prepared, and 50- µg aliquots of proteins were probed with anti-caspase-3, anti-caspase-7, and anti-caspase-9 antibodies. For the loading control, the membrane was stripped and re-probed with monoclonal antibodies against α-tubulin. B, TSA enhanced caspase activation induced by PS-341 in UMSCC23 cells. P+T, PS-341 + TSA.
TSA does not modulate PS-341-induced ER stress in HNSCC cells
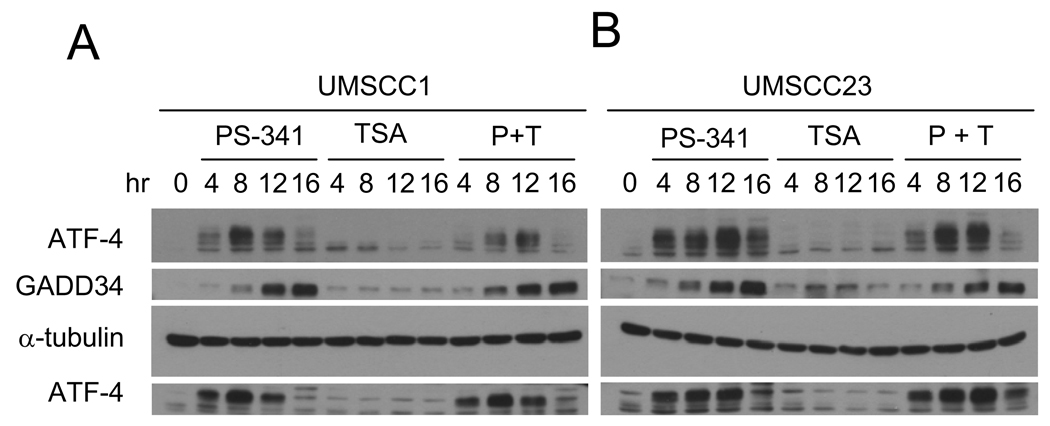
Previously, we demonstrated that PS-341 induced apoptosis depends on stimulation of ER-stress mediated by the accumulation of unfolded or misfolded proteins in the ER (4,7). Thus, we examined whether TSA modulated PS-341-induced ER stress responses by examining the expression of the ER stress markers ATF4 and GADD34. ATF4 is a transcription factor that is known to be induced upon ER-stress to regulate the expression of the genes involved in integrated stress response (25–27). ATF4 up-regulates expression of GADD34 and directly interacts with pro-apoptotic molecules such as CHOP/GADD153 to mediate ER stress induced apoptosis (28). As shown in Fig. 3, the individual treatment with TSA did not induce ATF4 and GADD34 expression in both UMSCC1 (Fig. 3A) and UMSCC23 cells (Fig. 3B) while PS-341 treatment alone strongly induced ATF4 and GADD34 expression. However, TSA did not enhance ATF4 and GADD34 expression induced by PS-341 (Fig. 3A and B). Taken together, these results suggest that it is unlikely that TSA enhanced PS-341-mediated apoptosis by modulating ER-stress in HNSCC cells.
Figure 3.
TSA does not modulate PS-341-induced ER stress in HNSCC cells. A and B, TSA did not affect the expression of ATF4 and GADD34 induced by PS-341 in HNSCC cells. Whole cell extracts were prepared, and 50- µg aliquots of proteins were examined with polyclonal antibodies against ATF-4 and GADD-34. For loading control, the membranes were stripped and re-probed with monoclonal antibodies against α-tubulin. P + T, PS-341 + TSA.
TSA promotes histone H3 acetylation in HNSCC cells
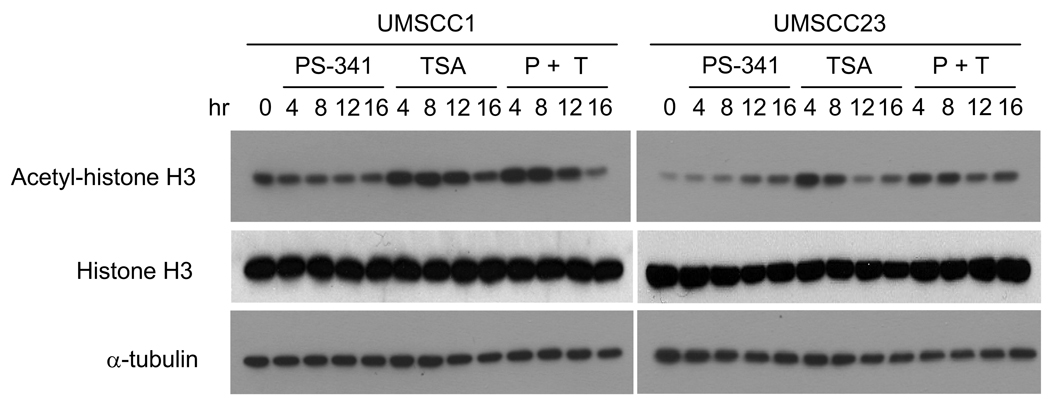
Since HDAC inhibitors such as TSA have been reported to induce cytotoxicity by inhibiting HDACs’ ability to deacetylate core histones in many cancer cells (10–12), we examined whether TSA treatment induced acetylation of histone H3 in HNSCC cells. Studies with HDAC inhibitors including TSA have been shown to transcriptionally re-activate these dormant tumor suppressor genes through epigenetic modification in many solid tumors (10–12, 14, 16). Consistently, Western blot analysis revealed that TSA alone enhanced acetylation of histone H3 in both UMSCC1 and UMSCC-23 cells in time-dependent manner; however, acetylation of H3 was not affected by PS-341 alone in both cell lines (Fig 4A and 4B). Of note, TSA and/or PS-341 treatment did not affect expression of histone H3. Thus, our data suggest that TSA might alter acetylation of H3 to synergistically enhance PS-341-induced apoptosis in HNSCC by modulating gene transcription.
Figure 4.
Promoting histone H3 acetylation in HNSCC cells by TSA. A and B, TSA induced hyperacetylation of histone H3 in HNSCC cells. UMSCC1 and UMSCC23 cells were treated with PS-341 alone (0.5 µM), TSA alone (300 nM), or PS-341 and TSA together for indicated times. 50- µg aliquots of proteins were probed with anti-acetyl-histone H3 antibodies (1:1000). For a loading control, the membrane were stripped and re-probed with monoclonal antibodies against α-tubulin.
PS-341-induced Noxa enhanced by TSA
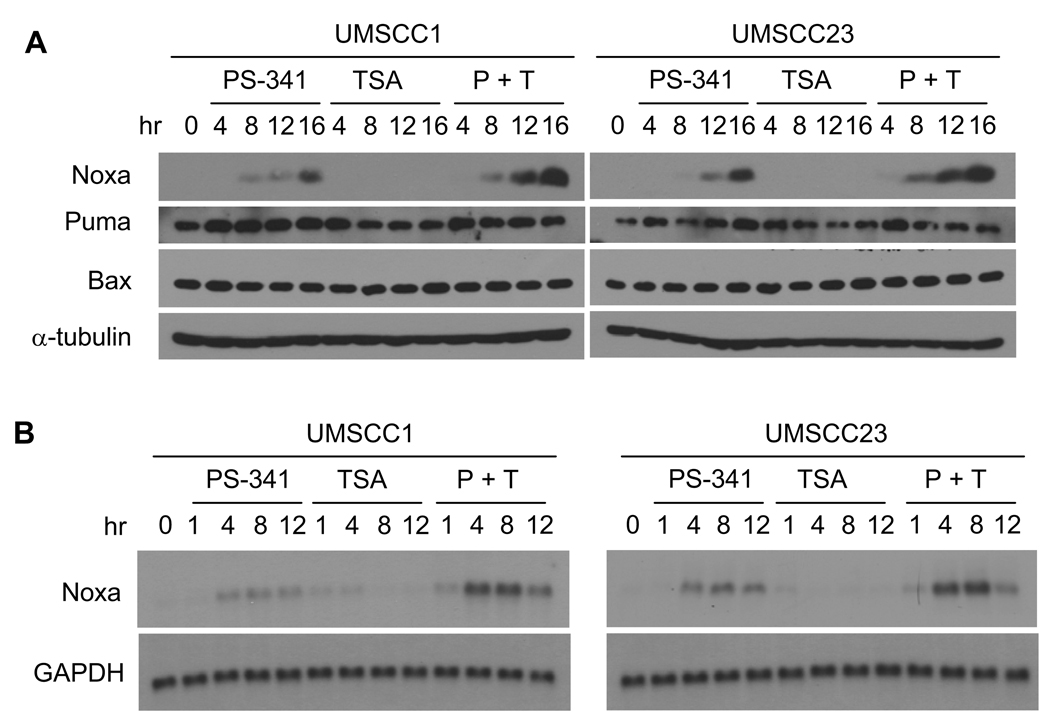
Recently, we and others have demonstrated that the cytotoxicity of PS-341 involves the regulation of the pro-apoptotic molecule Noxa expression in many solid tumors including HNSCC (4, 29). Since our data revealed that TSA modulated acetylation of histone H3, we examined whether TSA enhanced PS-341-induced Noxa expression in SCC cell lines. As shown in Fig. 5, Western blot analysis revealed that TSA strongly enhanced PS-341 induced Noxa expression in both UMSCC1 and UMSCC23 cells in a time-dependent manner while TSA treatment alone did not affect Noxa expression. In contrast, TSA did not modulate the expression of another BH3-only Bcl-2 family member, Puma, in UMSCC1 and UMSCC23 cells (Fig. 5A). Moreover, there was no notable change of Bax expression upon the co-treatment with PS-341 and TSA, thereby suggesting that Puma and Bax might not be critical pro-apoptotic proteins involved in enhanced apoptosis by PS-341 and TSA. Consistent with our Western blot analysis, TSA also enhanced mRNA expression of Noxa induced by PS-341 in both UMSCC1 and UMSCC23 cells in time dependent manner compared with PS-341 treatment alone (Fig. 5B).
Figure 5.
TSA enhances PS-341-induced Noxa expression. A, TSA enhanced PS-341-induced Noxa expression as determined by Western blot analysis. Cells were treated with PS-341 alone, TSA alone, or PS-341 and TSA together for indicated time periods. 50-µg aliquots of cell lysates were probed with anti-Noxa, anti-Puma, or anti-Bax antibodies. α-Tubulin was utilized as an internal control. B, TSA enhanced Noxa mRNA expression induced by PS-341. Total RNA was isolated and determined by Real-time PCR.
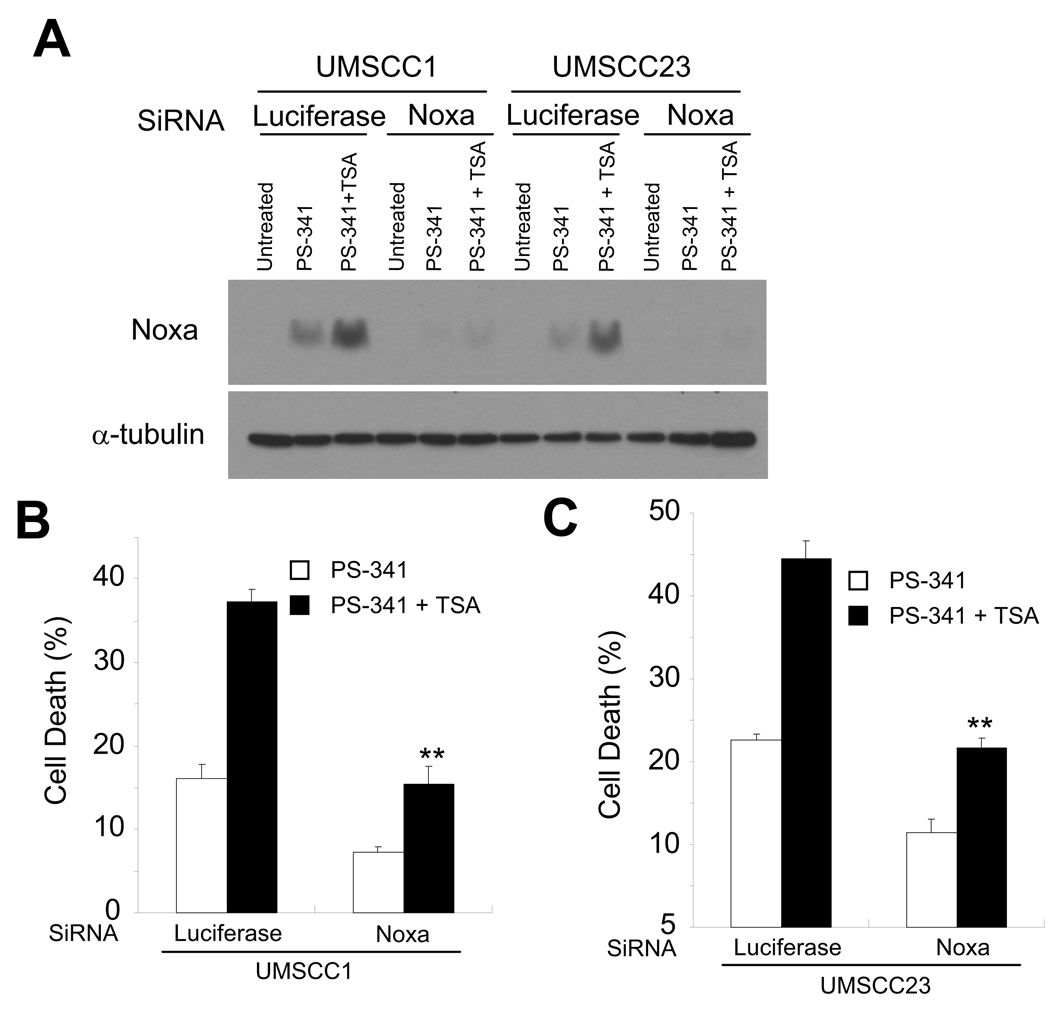
To further determine whether elevated expression of Noxa is the downstream factor responsible for increased apoptosis, we utilized small interference RNA (siRNA) to knock-down Noxa expression in UMSCC1 and UMSCC23 cells. Western blot analysis showed that Noxa siRNA completely inhibited Noxa expression in UMSCC1 and UMSCC23 cells induced by co-treatment of TSA and PS-341 compared to cells transfected with luciferase siRNA (Fig. 6A). The co-treatment of PS-341 and TSA resulted in 38% of apoptosis in control UMSCC1 cells and 15% in Noxa knocked down cells, showing a significant increase in cell viability upon depletion of Noxa expression (Fig 6B). Similarly, PS-341 and TSA co-treatment induced 44% of apoptosis in control UMSCC23 cells but only 22% in Noxa knocked down cells, indicating that Noxa is a primary pro-apoptotic molecule involved in apoptosis induced by PS-341 alone as well as co-treatment with TSA and PS-341 in both UMSCC1 and UMSCC23 cells. In conclusion, our results suggest that the enhanced apoptosis induced by co-treatment of PS-341 and TSA is mediated by increasing Noxa expression.
Figure 6.
Noxa plays a critical role in TSA- and PS-341-induced apoptosis in HNSCC cells. A, Knock-down of Noxa in UMSCC1 and UMSCC23 cells. SCC cells were transfected with Noxa siRNA or control luciferase siRNA using fugene. Cell lysates were probed with anti-Noxa antibodies. α-Tubulin was utilized as an internal control. B, Knock-down of Noxa inhibited PS-341 and TSA-induced apoptosis. Cells were transfected with siRNA as described in A. 48 h after transfection, cells were treated with PS-341 alone or PS-341 and TSA together for 16 h. Cell viability was determined by Trypan blue exclusion assay. The results are average values from three independent experiments (** P < 0.01).
Discussion
The proteasome inhibitor PS-341 has been offered as an alternative to conventional cancer therapy for various solid tumors (6, 29). In vitro and in vivo studies conducted by our laboratory and others have shown that PS-341 also has a promising antitumor activity in HNSCC cells (4,5). However, a higher concentration of PS-341 is required to induce apoptosis in solid tumors including HNSCC when compared with myeloma (4). Since we previously showed that PS-341-induced apoptosis requires the induction of the pro-apoptotic genes, in this study, we investigated whether a classic HDAC inhibitor TSA enhanced PS-341-induced apoptosis by epigenetic modification of histones. We revealed that a marked increase in cytotoxicity of PS-341 plus TSA treatment compared to PS-341 alone was associated with notable enhancement of DNA fragmentation caused by enhanced apoptosis in SCC cell lines. We demonstrated that TSA strongly enhanced PS-341-induced activation of caspase-9, -3 and -7. Our results suggest the synergy between PS-341 and TSA in HNSCC is accomplished by enhancing the intrinsic apoptotic pathway.
Previously, we have showed that the inhibition of the 26S proteasome by PS-341 results in endoplasmic reticulum (ER) stress which subsequently stimulates a coordinated cellular response called the unfolded protein response (UPR) to induce apoptosis in HNSCC (4–5, 7). Thus, we investigated whether TSA modulated PS-341-induced ER stress by examining the expression level of two ER-stress markers, ATF4 and its downstream factor GADD34 in HNSCC cells. The co-treatment of PS-341 and TSA induced a similar level of ATF4 and GADD34 when compared to PS-341 treatment alone, suggesting that TSA does not modulate PS-341-induced ER stress in HNSCC cells. Studies on the effect of HDAC inhibitors in many cancer cells suggested that the cytotoxicity of HDAC inhibitors is induced by epigenetic modulation on histone cores such as histone H3 (30–34). HDAC inhibitors including TSA induce cytotoxicity in many solid tumors by increasing acetylation of core histones to change the chromatin structure in order to transcriptionally re-activate dormant tumor suppressor genes (30–31). Therefore, we explored whether TSA increases the acetylation of H3 in HNSCC. Our data displayed that hyperacetylation of histone H3 was observed in UMSCC1 and UMSCC23 cells that were treated with TSA alone and co-treated with PS-341 and TSA. Individual treatment with PS-341 in both UMSCC1 and UMSCC23 cells showed no effect on acetylation of histone H3, suggesting that TSA may enhance PS-341-induced apoptosis by promoting gene expression. Previously, we found that PS-341 induced apoptosis through induction of Noxa (4). Intriguingly, the co-treatment with PS-341 and TSA in both UMSCC1 and UMSCC23 cells notably up-regulated Noxa compared to cells that were treated with PS-341 alone. While it is likely that TSA promoted Noxa expression through epigenetic modification on histone H3, currently, it is not clearly the precise mechanism by which PS-341-induced Noxa is enhanced by TSA. Mechanistic association of hyper-acetylation at histone H3 with Noxa expression upon PS-341 and TSA co-treatment needs to be further elucidated. Moreover, it remains a possibility that TSA may also affect other molecules or signaling pathways to promote PS-341-mediated apoptosis in HNSCC cells.
The cytotoxicity of PS-341 depends on ER-stress mediated by the accumulation of misfolded or unfolded proteins; nevertheless, cancer cells can attenuate the antitumor activity of PS-341 by preventing this accumulation of ubiquitin-conjugated proteins through the formation of a cytoprotective structure known as an aggresome (22–24). Aggresome formation promotes the degradation of the ubiquitin-conjugated proteins upon PS-341 treatment (10–11), increasing the survival rate of variety of malignancies including multiple myeloma (22) and ovarian cancer (24). Thus, it will be interesting to examine whether TSA enhances PS-341-induced apoptosis by inhibiting aggresome formation in HNSCC cells. In conclusion, our findings implicate that inhibition of the proteasome and HDACs with PS-341 and TSA, respectively, synergistically induces apoptosis in HNSCC cells by enhancing Noxa expression and promoting caspase activation. Our results provide an additional rationale for the usage of a combination of both agents in patients with HNSCC.
Acknowledgments
This work was supported by NIDCR grants DE13848 and DE15964.
Footnotes
No potential conflicts of interest were disclosed.
References
- 1.Lemaire F, Millon R, Wasylyk B, et al. Differential expression profiling of head and neck squamous cell carcinoma (HNSCC) Br J Cancer. 2003;89:1940–1949. doi: 10.1038/sj.bjc.6601373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 3.Pisani P, Bray F, Ferlay J, Parkin DM. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Fribley A, Evenchik B, Wang CY, et al. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281:31440–31447. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 5.Fribley A, Zeng Q, Wang CY. Proteasome inhibitor PS-341 induces apoptosis through induction of endoplasmic reticulum stress-reactive oxygen species in head and neck squamous cell carcinoma cells. Mol Cell Biol. 2004;24 doi: 10.1128/MCB.24.22.9695-9704.2004. 9695-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming JA, Lightcap ES, Sadis S, Thoroddsen V, Bulawa CE, Blackman RK. Complementary whole-genome technologies reveal the cellular response to proteasome inhibition by PS-341. Proc Natl Acad Sci USA. 2002;99:1461–1466. doi: 10.1073/pnas.032516399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fribley A, Wang CY. Proteasome inhibitor induces apoptosis through induction of endoplasmic reticulum stress. Can Biol Ther. 2006;5:745–748. doi: 10.4161/cbt.5.7.2971. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Mora-Jensen H, Ye Y, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only proteain NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106:2200–2205. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen C, Saigal K, Nottingham L, Arun P, Chen Z, Waes CV. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative Nuclear Factor-κB subunits in head and neck cancer. Clin Cancer Res. 2008;14:4175–4185. doi: 10.1158/1078-0432.CCR-07-4470. [DOI] [PubMed] [Google Scholar]
- 10.Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Marks PA, Xu WS. Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem. 2009;107:600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bi G, Jiang G. the molecular mechanism of HDAC inhibitors in anticancer effects. Cell Mol Immunol. 2006;3:285–290. [PubMed] [Google Scholar]
- 13.Prystowsky MB, Adomako A, Belbin TJ, et al. The histone deacetylase inhibitor LBH589 inhibits expression of mitotic genes causing G2/M arrest and cell death in head and neck squamous cell carcinoma cell lines. J Pathol. 2009;218:467–477. doi: 10.1002/path.2554. [DOI] [PubMed] [Google Scholar]
- 14.Long J, Zhao J, Yan Z, Liu Z, Wang N. Antitumor effects of a novel sulfur-containing hydroxamate histone deacetylase inhibitor H40. Int J Cancer. 2009;124:1235–1244. doi: 10.1002/ijc.24074. [DOI] [PubMed] [Google Scholar]
- 15.Karagiannis TC, El-Osta A. Modulation of cellular radiation responses by histone deacetylase inhibitors. Oncogene. 2006;25:3885–3893. doi: 10.1038/sj.onc.1209417. [DOI] [PubMed] [Google Scholar]
- 16.Mottet D, Castronovo V. Histone deacetylases: target enzymes for cancer therapy. Clin Exp Metastasis. 2008;25:183–189. doi: 10.1007/s10585-007-9131-5. [DOI] [PubMed] [Google Scholar]
- 17.Bai J, Demirjian A, Sui J, Marasco W, Callery MP. Histone deacetylase inhibitor trichostatin A and proteasome inhibitor PS-341 synergistically induce apoptosis in pancreatic cancer cells. Biochem Biophys Res. 2006;348:1245–1253. doi: 10.1016/j.bbrc.2006.07.185. [DOI] [PubMed] [Google Scholar]
- 18.Zhang QL, Wang L, Zhao WL, et al. The proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/lymphoma cells apoptosis. Leukemia. 2009;23:1507–1514. doi: 10.1038/leu.2009.41. [DOI] [PubMed] [Google Scholar]
- 19.Lin Z, Bazzaro M, Wang MC, Chan KC, Peng S, Roden R. Combination of proteasome and HDAC inhibitors for uterine cervical cancer treatment. Clin Cancer Res. 2009;15:570–577. doi: 10.1158/1078-0432.CCR-08-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan J, Friedman J, Nottingham L, Chen Z, Ara G, Waes CV. Nuclear factor-κB p65 small interfering RNA or proteasome inhibitor bortezomib sensitizes head and neck squamous cell carcinomas to classic histone deacetylase inhibitors and novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2007;6:37–50. doi: 10.1158/1535-7163.MCT-05-0285. [DOI] [PubMed] [Google Scholar]
- 21.Heider U, Rademacher J, Sezer O, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in cutaneous T cell lymphoma. Eur J Hematol. 2009;82:440–449. doi: 10.1111/j.1600-0609.2009.01239.x. [DOI] [PubMed] [Google Scholar]
- 22.Hideshima T, Bradner JE, Anderson KC. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci USA. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catley L, Weisberg E, Anderson KC, et al. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–3449. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bazzaro M, Lin Z, Roden R, et al. Ubiquitin proteasome system stress underlies synergistic killing of ovarian cancer cells by Bortezomib and a novel HDAC6 inhibitor. Clin Cancer Res. 2008;14:7340–7347. doi: 10.1158/1078-0432.CCR-08-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ameri K, Harris AL. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Niknejad N, Morley M, Dimitroulakos J. Activation of the integrated stress response regulates lovastatin-induced apoptosis. J Biol Chem. 2007;282:29748–29756. doi: 10.1074/jbc.M705859200. [DOI] [PubMed] [Google Scholar]
- 27.Milani M, Rzymski T, Harris AL, et al. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009;69:4415–4423. doi: 10.1158/0008-5472.CAN-08-2839. [DOI] [PubMed] [Google Scholar]
- 28.Kojima E, Takeuchi A, Isobe K, et al. The function of GADD34 is a recovery from a shutoff of protein systhesis induced by ER stress-elucidation by GADD34-deficient mice. FASEB J. 2003;17:1573–1575. doi: 10.1096/fj.02-1184fje. [DOI] [PubMed] [Google Scholar]
- 29.Ri M, Iida S, Ueda R, et al. Bortezomib-induced apoptosis in mature T-cell lymphoma cells partially depends on upregulation of Noxa and functional repression of Mcl-1. Cancer Sci. 2009;100:341–348. doi: 10.1111/j.1349-7006.2008.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richon VM, O’Brien JP. Histone deacetylase inhibitors: a new class of potential therapeutic agents for cancer treatment. Clin Cancer Res. 2002;8:662–664. [PubMed] [Google Scholar]
- 31.Inoue S, Riley J, Gant TW, Dyer MJS, Cohen GM. Apoptosis induced by histone deacetylase inhibors in leukemic cells is mediated by Bim and Noxa. Leukemia. 2007;21:1773–1782. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- 32.Kanao K, Mikami S, Mizuno R, Shinojima T, Murai M, Oya M. Decreased acetylation of histone H3 in renal cell carcinoma: a potential target of histone deacetylase inhibitors. J Urol. 2008;180:1131–1136. doi: 10.1016/j.juro.2008.04.136. [DOI] [PubMed] [Google Scholar]
- 33.Zhang B, West EJ, Lyeth BG, et al. HDAC inhibitor increases histone H3 acetylation and reduces microglia inflammatory response following traumatic brain injury in rats. Brain Res. 2008;1226:181–191. doi: 10.1016/j.brainres.2008.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller CP, Rudra S, Keating MJ, Wierda WG, Palladino M, Chandra J. Caspase-8 dependent histone acetylation by a novel proteasome inhibitor, NPI-0052: a mechanism for synergy in leukemia cells. Blood. 2009;113:4289–4299. doi: 10.1182/blood-2008-08-174797. [DOI] [PMC free article] [PubMed] [Google Scholar]