Abstract
Cardiac transplantation is an effective treatment for heart failure refractive to therapy. Although immunosuppressive therapeutics have increased first year survival rates, chronic rejection remains a significant barrier to long-term graft survival. Chronic rejection manifests as patchy interstitial fibrosis, vascular occlusion and progressive loss of graft function. Recent evidence from experimental and patient studies suggests that the development of cardiomyocyte hypertrophy is another hallmark of chronic cardiac allograft rejection. This pathologic hypertrophy is tightly linked to the immune cytokine IL-6, which promotes facets of chronic rejection in concert with TGF-β and IL-17. These factors potentiate downstream mediators, such as CTGF, which promote the fibrosis associated with the disease. In this article, we summarize contemporary findings that have revealed several elements involved in the induction and progression of chronic rejection of cardiac allografts. Further efforts to elucidate the interplay between these factors may direct the development of targeted therapies for this disease.
Keywords: chronic allograft vasculopathy, chronic rejection, CTGF, fibrosis, hypertrophy, IL-6, IL-17, TGF-β
The advent of effective immunotherapeutic agents allowed cardiac transplantation to become a realistic treatment modality for end-stage cardiac diseases refractive to other therapies. Modern immunosuppressant regimens effectively limit acute rejection and prolong short-term survival of solid organ grafts. Indeed, the 1-year survival rate is approaching 90% for heart transplant recipients [1]. However, comprehensive evaluation of the success of clinical transplantation must take long-term survival, with its less favorable prospects, into account. For well over a decade, chronic rejection has been recognized as the most significant barrier to long-term graft survival [2–8] and has been defined by hallmarks of occlusive vasculopathy, interstitial fibrosis and progressive loss of graft function.
Cardiac allograft vasculopathy
In cardiac grafts undergoing chronic rejection, vessels undergo occlusive remodeling characterized by neointima development (Figure 1). This process is referred to by many names, although herein we will use cardiac allograft vasculopathy (CAV). CAV in humans was first reported in 1970 [9]. It has been suggested that some level of intimal thickening can be observed in 75% of cardiac transplants within the first year post-transplant [10,11], which may then develop CAV, estimated to occur in up to 80% of cardiac transplants within the first 5 years post-transplant [12,13]. Currently, no effective therapeutic exists for CAV other than retransplantation. Hence, the current approach to CAV is prevention. While treatment regimens utilizing cyclosporine A, azathioprine, steroids and antilymphocyte antibodies have not successfully prevented the incidence of the disease [8], recent findings suggest that newer immunosuppressive treatments may decrease the incidence and severity of CAV [8,14,15]. However, these effects may be attributable to more robust immunosuppression, as incidence of acute rejection has been decreased by these agents in some circumstances as well [16,17]. Although numerous factors may contribute to the development of CAV, it appears that graft/host incompatibility plays a central role, as CAV affects graft vessels up to, but not beyond, the suture line [8]. This supports the widely held notion that graft-derived factors, probably donor alloantigens, are critical for the disease [3,4,6–8,18].
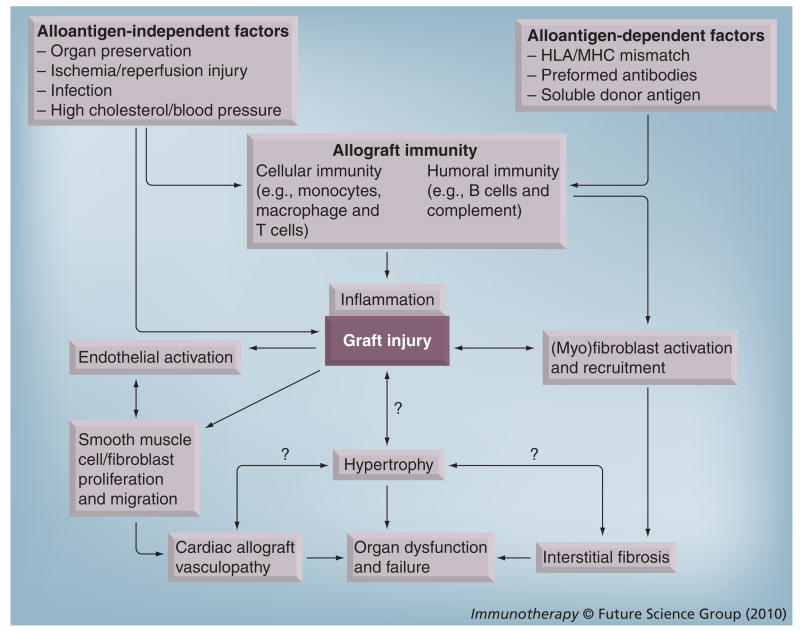
Figure 1. Multiple processes contribute to the development of chronic rejection.
Both alloantigen-dependent and -independent factors promote immune responses against allografts and activation of graft endothelium. Complex interplay of these factors results in graft infiltration/inflammation and injury. Injury prompts reparative processes characterized by fibroblast activation and recruitment, as well as proliferation and migration of smooth muscle-like cells in the vasculature. These processes lead to the development of chronic rejection hallmarks of chronic allograft vasculopathy and interstitial fibrosis. Graft hypertrophy, characterized by increased cardiomyocyte size and thickening of the left ventricle wall, accompanies these pathologies, although the interrelationship between these processes is not yet understood. Ultimately, these graft pathologies lead to organ dysfunction and failure.
Graft fibrosis
Another hallmark of chronic rejection is the development of patchy interstitial fibrosis (Figure 1). Fibrosis of cardiac grafts is characterized by the accumulation of thick web-like fibers of extracellular matrix (ECM) surrounding cardiomyocytes, readily visible in stained myocardial biopsy sections [19]. It has been suggested that graft fibrosis occurs largely in response to CAV [8], however the relationship between these elements of graft pathology is uncertain. Although its origins are not fully understood, fibrosis can have detrimental effects on organ function and survival. Ultimately, enhanced ECM accumulation observed in tissue fibrosis is the result of competition between programs that promote ECM degradation and those that promote ECM synthesis [20,21]. The balance between these two programs is regulated by numerous factors in the context of cardiac allograft fibrosis. Indeed, initiating factors for fibrotic responses can include both alloantigen-dependent (cellular and humoral immune attack) and alloantigen-independent factors (health of graft, ischemia reperfusion injury and infections), but both provide the common physiologic effect of tissue damage (Figure 1). Tissue damage can then prompt the production of cytokines, chemokines and growth factors [22]. Some of these mediators promote infiltration by immune cells, fibroblasts [23] and progenitor cells [24,25]. Infiltrating cells can then elaborate production of cytokines and growth factors that drive proliferative responses in inflammatory cells, fibroblasts and epithelial cells. Cytokines and growth factors subsequently promote cellular differentiation [26].
One specific type of cellular differentiation that has been recently reported to contribute to cardiac fibrosis is endothelial to mesenchymal transformation [27,28]. However, an essential contribution of such developmental reprogramming in organ fibrosis is controversial, as the protein used to define mesenchymal fibroblasts in these studies has been reported to be expressed by numerous cell types in other systems [29]. A role for endothelial to mesenchymal transformation in cardiac fibrosis may seem more plausible in light of the more established epithelial to mesencymal transformation hypothesis of developmental reprogramming in kidney fibrosis. However, recent observations maintaining that epithelial to mesenchymal transformation readily occurs in vitro have challenged whether the process occurs in vivo [30,31].
Regardless of their etiology, the cells that differentiate to myofibroblasts are probably the most important cell type with respect to accumulation of excessive ECM [32,33]. Myofibroblasts are characterized morphologically [34] and by the expression of fetal isoform α-smooth muscle actin, extradomain-A-containing fibronectin and type I collagen [35–38]. In addition to myofibroblasts, it has been reported that cardiac myocytes can contribute to the production of collagen type I [39,40]. However, it is generally thought that the cell that regulates homeostasis of the ECM within the heart is the fibroblast [41], an abundant cell type that facilitates structural and functional connections in healthy cardiac tissue [42–44].
Cardiac fibrosis can occur in a reactive fashion to stimuli such as cytokines or hypertension, as well as a reparative fashion in response to cell death [45–50]. In both cases, fibrosis has significant implications for graft function, as it provides increased tensile strength but also stiffness to the myocardial wall. Furthermore, the sheathing of individual cardiomyocytes with ECM alters cell-to-cell contacts, which can result in disruption of the electophysiology of cardiac myocytes [49] and probably decrease the energy supply for cardiomyocytes. If this occurs while the workload for graft contractility is increased [50], the intense stress of such conditions triggers cell death and further reparative fibrosis. Hence, breaking this positive feedback loop of cardiac remodeling should be a priority for chronic rejection therapeutics.
Graft dysfunction & pathologic remodeling
Progressive loss of graft function in chronic rejection might occur in response to CAV-mediated impedance of vascular flow and tissue stiffening associated with fibrosis. Recent findings from the use of noninvasive echocardiographic imaging in patients and a murine model of chronic rejection have revealed an association between the development of cardiac hypertrophy and chronic rejection [51,52]. In murine studies, the heterotopic cardiac allograft model alters the hemodynamic load of cardiac grafts. Hence, even in the presence of appropriate controls, judicious interpretation must be exercised with regard to the physiologic implications of cardiac hypertrophy in these studies. However, the concommitance of hypertrophy with the development of fibrosis suggests a pathologic phenomenon [51]. This is consistent with previous in vitro demonstrations that hypertrophic stimuli induce cardiac myocytes to produce CTGF [53] and TGF-β [54], factors known to promote fibrosis of cardiac allografts [55,56]. In patient studies, hypertrophy was associated with graft vasculopathy, and it has been suggested that hypertrophy could provide a noninvasive surrogate marker for patient survival [52,57]. Hence, cardiac hypertrophy is concomitant with fibrosis and vascular pathology. Although causal relationships between these factors remain to be uncovered, echocardiographic analysis may provide a surrogate marker that is valuable for monitoring and diagnosing chronic rejection.
Etiology of chronic rejection
Although chronic rejection is highly prevalent, the etiology of the disease is less clear. Chronic rejection has been used to describe late graft loss from antigraft immunity [58]. However, both immune and nonimmune parameters indicate risk of chronic rejection [18], and contributions by alloantigen-dependent and -independent factors are well established [3,6,8,59]. The multifaceted nature of factors contributing to chronic rejection (Figure 1) may partially explain why correlative associations abound while causative relationships have been more elusive.
TGF-β: a key agent in chronic rejection
Perhaps no factor has been associated with chronic rejection more frequently than TGF-β – a cytokine whose effects are linked to both graft acceptance and the development of chronic rejection. TGF-β overexpression is linked with chronic rejection [56,60] and may negatively impact graft survival through chemotactic and profibrotic effects [61]. TGF-β plays an important role in fibrosis of various causes in multiple organs [62] and is reported to induce the differentiation of cardiac myofibroblasts [63]. Indeed, fibroblasts themselves can make TGF-β [64] and myofibroblasts are rescued from apoptosis by TGF-β [65].
However, in addition to its deleterious fibrotic effects on the graft, TGF-β mediates immunosuppressive and antiproliferative functions [61,66] that may be indispensable for graft and host survival [67]. The importance of TGF-β in establishing graft tolerance has been recently demonstrated by the premature rejection of allografts in CD4+ cell-depleted recipients with abrogated T-cell TGF-β signaling [68]. This may be explained in part by the critical role TGF-β plays in the induction and function of regulatory T cells, which are believed to contribute to graft acceptance [69–71]. Somewhat paradoxically, regulatory T cells that prevent acute allograft rejection could be a significant source of TGF-β that drives chronic rejection. Hence, while TGF-β promotes chronic rejection, TGF-β signaling in T lymphocytes is required for some mechanisms of graft tolerance. Thus, global disruption of TGF-β-dependent mechanisms of allograft acceptance may prevent manifestations of chronic rejection, but do so at the cost of accelerated immune-mediated rejection.
One approach to the contradicting roles of TGF-β has been through the local neutralization of TGF-β using decorin. Decorin is able to bind active TGF-β and sequester it in the ECM, which inhibits the interaction of TGF-β with its receptor [72,73]. It should be noted that decorin can inhibit TGF-β-mediated upregulation of α-smooth muscle actin and decrease TGF-β-induced collagen gel contraction in vitro [74]. This indicates that decorin is able to suppress myofibroblast differentiation, a process thought to be critical in the fibrotic lesions of chronic rejection [75]. Previous studies utilizing decorin gene transfer have shown promise in limiting the development of fibrotic lesions in both the kidney and lung [76,77]. Recent observations by our group revealed that ectopic decorin expression within the graft was able to ameliorate fibrosis in a murine model of chronic cardiac allograft rejection [78]. These findings cumulatively raise the possibility that targeting TGF-β in a local milieu might selectively block its deleterious functions while sparing the graft-protective functions. While decorin gene transfer appears hopeful as a therapeutic, it remains to be seen if localized TGF-β suppression will disrupt protective activities of TGF-β within the graft. Because of this potential limitation, complimentary approaches have aimed to identify facilitators and downstream mediators of deleterious TGF-β effects in chronic rejection. If identified, these factors may prompt the development of therapeutics that are able to negate the fibrosis-inducing activity of TGF-β while sparing its anti-inflammatory and antiproliferative effects.
CTGF is a downstream mediator of fibrosis in chronic rejection
CTGF is induced by TGF-β in multiple cell types [79], including cardiac myocytes and fibroblasts [80], and has been frequently implicated in cardiac disease [81]. Recent investigations have highlighted an association of CTGF with the development of vascular injury. Adventitial application of recombinant CTGF stimulated neointimal hyperplasia associated with vascular injury, which phenotypically recapitulates smad3 (a TGF-β signaling mediator) gene transfer [82]. Furthermore, it has been reported that the C-terminal domain of CTGF can induce cardiomyocyte hypertrophy [83]. CTGF drives connective tissue development and scar tissue formation [84,85]. CTGF is also upregulated in fibrotic disorders, including chronic rejection of cardiac and kidney grafts [56,81,86,87]. CTGF can mediate a number of profibrotic effects attributed to TGF-β such as enhanced accumulation of ECM and fibroblast proliferation [81]. Because CTGF can be induced by TGF-β and the two share similar profibrotic and remodeling effects, CTGF has been a target for limiting the downstream fibrotic effects of TGF-β, while hopefully sparing its immune-modulatory functions [5,56,88]. Indeed, recent findings by our group in a murine model of chronic cardiac allograft rejection indicate that CTGF neutralization can limit the development of interstitial fibrosis [55], suggesting that CTGF-targeting therapeutics might be effective at limiting the development of interstitial fibrosis in patients.
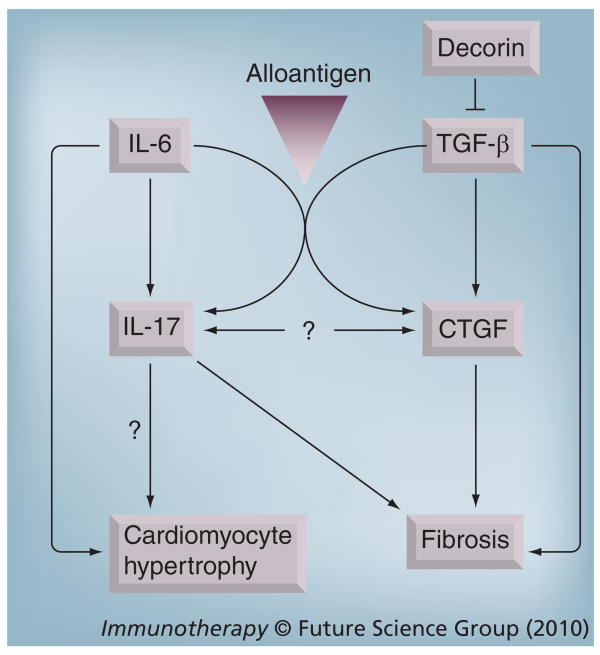
It should be noted that although transduction of cardiac allografts with TGF-β induces CTGF and chronic rejection, transduction of syngeneic grafts with TGF-β is insufficient to induce CTGF or chronic rejection [56]. Thus, the in vivo induction of CTGF by TGF-β depends on factors associated with the transplant setting (Figure 2). An obvious demarcation between allogeneic and syngeneic grafts is the development of alloimmune responses. In turn, alloimmune responses can be associated with the production of some factors known to crosstalk with TGF-β signaling [89]. One such factor is IL-6 (Figure 2), which has been reported to modulate TGF-β-mediated effects in multiple cell types [90–92].
Figure 2. Proposed interactions of TGF-β, IL-6, IL-17 and CTGF in the evolution of cardiac allograft hypertrophy and fibrosis.
In the context of alloantigen, TGF-β and IL-6 induce CTGF and IL-17. The relationship between IL-17 and CTGF remains to be established, although both of these factors, along with TGF-β, promote graft fibrosis. IL-6 induces cardiomyocyte hypertrophy, which might involve induction of IL-17.
IL-6 in chronic rejection
IL-6 is a pleiotropic cytokine with effects on cardiac biology [93] and broad effects upon immunologic responses [94–97]. Among its immune-stimulating functions, IL-6 enhances immune cell differentiation and lineage commitment while promoting inflammation through leukocyte recruitment and survival [98–105]. Recent observations have invoked a renaissance of interest in IL-6 as a modifier of immune responses and usurper of immune tolerance [106–108]. As is often the case, these new observations have reframed our understanding of historic data and brought new questions to the forefront. This is especially true regarding the associations of IL-6 with transplant pathology, as an association of IL-6 with TGF-β in rejecting cardiac grafts was observed more than a decade ago [109]. If not fully realized at the time, it is clearer now that IL-6 can instigate numerous phenomena associated with transplant rejection.
Several recent investigations have implicated a role for IL-6 in acute rejection. Studies placing IL-6-/- donor grafts in wild-type recipients and wild-type grafts in IL-6-/- recipients suggested that graft-origin IL-6 may be more essential than recipient-origin IL-6 in promoting acute rejection [110]. As the authors suggest, IL-6 may serve as an immune ‘danger’ signal in this context, consistent with recent demonstration of the ability of IL-6 to mediate disruption of established allograft tolerance by Toll-like receptor signals [111]. Whether similar functions of IL-6 are operating in the etiology of chronic rejection remains to be explored; however, a critical role for IL-6 in the onset and progression of chronic rejection has now been reported [51]. In cardiac allografts transiently depleted of CD4+ cells, which normally undergo chronic rejection, neutralization of IL-6 prevented chronic rejection. Though enumeration of graft-reactive cytokine production by recipient splenocytes did not reveal significant differences [51], intragraft expression of IL-6 and IL-17 were decreased in response to anti-IL-6 monoclonal antibodies (mAbs) [55].
IL-6 effects in chronic rejection are likely to extend beyond the alloimmune response. As summarized above, cardiac hypertrophy is emerging as a new hallmark of chronic rejection in cardiac grafts, associated with the onset and progression of chronic cardiac allograft rejection in patients [57] as well as experimental systems [51]. Previous findings have revealed an association between elevated IL-6 levels and pathological cardiac hypertrophy [112–116], and increased myocardial IL-6 levels correlate with donor heart dysfunction [117]. Indeed, IL-6 upregulation appears to extend beyond the heart, as several reports have correlated elevated levels of IL-6 in serum with cardiac dysfunction [118–121]. In chronic rejection of murine cardiac allografts transiently depleted of CD4+ cells, IL-6 was required for hypertrophic remodeling, as IL-6-neutralizing mAb normalized contractile parameters and prevented left ventricular wall thickening associated with hypertrophy [51].
IL-6 neutralization was also associated with minimal development of interstitial fibrosis, which might be due to its roles in immune function and hypertrophic remodeling [51]. Although IL-6 might promote fibrosis in chronic rejection indirectly through inflammation and cardiomyocyte hypertrophy, several lines of evidence indicate that IL-6 may itself have profibrotic effects (Figure 2). IL-6 upregulated collagen transcripts in cardiac fibroblast and cardiac myocyte co-culture experiments [122], while IL-6-neutralizing mAbs decreased cardiac fibroblast proliferation [123]. Related activities of IL-6 include enhanced fibroblast survival [124] and augmentation of TGF-β signaling through altered turnover and compartmentalization of its receptor [92]. In summary, recent findings implicate IL-6 to be an important player in alloimmunity, hypertrophy and fibrosis associated with chronic cardiac allograft rejection. Because IL-6 is involved in so many facets of the disease, it may present a unique target for future studies and perhaps therapeutics.
IL-17
The presence of IL-6 shunts T-cell responses to TGF-β toward the Th17 lineage, while TGF-β in the absence of IL-6 promotes the generation of Foxp3+ regulatory cells [90]. As both IL-6 and TGF-β are tightly associated with chronic rejection, the role of IL-17 in these effects must be considered (Figure 2). Several previous investigations have associated Th17 responses with chronic remodeling pathologies of the lung. First, Th17 responses against collagen type V have been correlated with the severity of obstructive modeling in human lung transplant patients with bronchiolitis obliterans syndrome [125]. More recently, Th17 cellular immune responses have been demonstrated to facilitate lung fibrosis in response to transferred MHC class I immunity [126]. Furthermore, Th17 responses were implicated in the development of inflammation and fibrosis in a murine model of hypersensitivity pneumonitis [127]. Two recent investigations have highlighted a role for IL-17 in chronic rejection of cardiac grafts. In a model of CAV, graft recipients lacking the Th1 transcription factor Tbet had exacerbated vasculopathy associated with enhanced infiltration by IL-17-producing CD4+ cells [128]. More recently, it has been described that IL-17-/- mice are protected from fibrosis in a model of chronic cardiac allograft rejection following transient depletion of CD4+ cells [68].
Beyond its roles in the control of immune responses described above, evidence suggests that IL-17 may more directly elicit fibrotic responses. IL-17 has been reported to promote collagen production by primary mouse cardiac fibroblasts [129], and these effects may involve modulation of the balance of matrix metalloproteinases and tissue inhibitors of metalloproteinases [130,131]. A profibrotic role for IL-17 is further supported by the ability of IL-17 neutralization to ameliorate the degree of fibrosis resulting from drug-induced heart failure 130]. Our group has recently reported an association of elevated intragraft IL-17 expression with chronic rejection and fibrosis in response to TGF-β gene transfer [55]. Furthermore, cardiac allografts in IL-17-deficient recipients transiently depleted of CD4+ cells develop significantly less fibrosis than wild-type controls [68]. It should be noted that Th17 responses can elaborate the production of IL-6 [132,133], which can directly or indirectly promote ECM accumulation in chronic rejection (Figure 2).
In summary, recent investigations have highlighted a complex interplay between the quadrumvirate of TGF-β, IL-6, IL-17 and CTGF in the pathology of chronic cardiac allograft rejection. Further studies are necessary to better understand both the interrelationships between these cytokines and the associations of each with chronic rejection of cardiac grafts in patients. It should be noted that while individual associations of TGF-β, IL-6, IL-17 and CTGF with chronic rejection of multiple organs have been established, the cytokine interactions discussed here for cardiac fibrosis may or may not be operative in other organs. Indeed, the evolution of hypertrophy and associated responses are likely to be unique to chronic remodeling of cardiac grafts. Hence, advancements in our understanding of these factors and their interrelationships in chronic rejection may facilitate the development of future therapeutics that breach the impasse of chronic rejection and make the transplantation of solid organs an even more effective therapy.
Future perspective
Current immunotherapeutics for transplant rejection have extreme side effects and have generally been ineffective against the development of chronic rejection. By contrast to broad immunosuppressive agents, the ‘holy grail’ of donor-specific tolerance in allograft recipients remains elusive, although our understanding of the mechanisms underlying immune tolerance is rapidly expanding. Until that goal is reached, targeted therapeutics against the pathologic manifestations of chronic rejection should be considered. Immunotherapeutic agents specifically targeting the factors described herein may provide alternate or supplementary treatments that are more efficacious in ameliorating the hallmark pathologies of chronic rejection. While it is ultimately uncertain whether therapeutics effective for treating the onset and development of chronic cardiac allograft rejection in experimental models will be efficacious clinically, it is clear that these factors have provided targets for future investigations.
Executive summary.
Hypertrophy should be considered as a new hallmark of chronic cardiac allograft rejection
Cardiomyocyte hypertrophy is an indicator of the onset and progression of chronic rejection, evidenced by echocardiographic evaluation of ventricular wall thickening in patients and animal models.
Echocardiographic analysis of cardiac hypertrophy may provide a noninvasive assay that helps predict the onset and progression of chronic rejection.
CTGF is a downstream mediator of fibrosis in chronic cardiac allograft rejection
Targeting CTGF may provide a fibrosis-specific therapeutic.
CTGF neutralization may ameliorate the fibrosis-associated chronic transplant rejection, and may do so without inflicting additional immune disruption to highly immunosuppressed patients.
IL-6 is a critical mediator of chronic rejection
IL-6 neutralization prevented the onset of hypertrophy and fibrosis in an experimental model of chronic rejection.
In transplant settings, IL-6 can disrupt established tolerance and promote the induction of Th17 responses.
IL-6 potentiates TGF-β-mediated induction of CTGF in experimental models of chronic cardiac allograft rejection.
Growing evidence suggests an involvement of IL-17 in the development of chronic rejection pathology, particularly fibrosis
IL-17 has been associated with the development of multiple fibrotic remodeling pathologies of the lung.
IL-17 upregulation has been observed in murine models of chronic cardiac allograft rejection. Furthermore, cardiac allografts in IL-17-/- recipients display reduced development of interstitial fibrosis.
Acknowledgments
Adam J Booth was supported by NIH T32 HL00749 (Galen B Toews) and R01 HL085083 (Eric S White). D Keith Bishop was supported by NIH grants R01 HL070613 and AI061469.
Footnotes
Financial & competing interests disclosure: Neither author has competing financial interests or involvements relevant to the materials discussed in this manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics – 2010 update. A report from the American Heart Association. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Keck BM, Bennett LE, Rosendale J, Daily OP, Novick RJ, Hosenpud JD. Worldwide thoracic organ transplantation: a report from the UNOS/ISHLT International Registry for Thoracic Organ Transplantation. Clin Transpl. 1999:35–49. [PubMed] [Google Scholar]
- 3.Waaga AM, Gasser M, Laskowski I, Tilney NL. Mechanisms of chronic rejection. Curr Opin Immunol. 2000;12(5):517–521. doi: 10.1016/s0952-7915(00)00132-1. [DOI] [PubMed] [Google Scholar]
- 4.Mehra MR. Contemporary concepts in prevention and treatment of cardiac allograft vasculopathy. Am J Transplant. 2006;6(6):1248–1256. doi: 10.1111/j.1600-6143.2006.01314.x. [DOI] [PubMed] [Google Scholar]
- 5.Mannon RB. Therapeutic targets in the treatment of allograft fibrosis. Am J Transplant. 2006;6(5 Pt 1):867–875. doi: 10.1111/j.1600-6143.2006.01261.x. [DOI] [PubMed] [Google Scholar]
- 6.Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23(5 Suppl):S187–S193. doi: 10.1016/j.healun.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Weiss MJ, Madsen JC, Rosengard BR, Allan JS. Mechanisms of chronic rejection in cardiothoracic transplantation. Front Biosci. 2008;13:2980–2988. doi: 10.2741/2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornick P, Rose M. Chronic rejection in the heart. Methods Mol Biol. 2006;333:131–144. doi: 10.1385/1-59745-049-9:131. [DOI] [PubMed] [Google Scholar]
- 9.Bieber CP, Stinson EB, Shumway NE, Payne R, Kosek J. Cardiac transplantation in man. VII. Cardiac allograft pathology. Circulation. 1970;41(5):753–772. doi: 10.1161/01.cir.41.5.753. [DOI] [PubMed] [Google Scholar]
- 10.Yeung AC, Davis SF, Hauptman PJ, et al. Incidence and progression of transplant coronary artery disease over 1 year: results of a multicenter trial with use of intravascular ultrasound. Multicenter Intravascular Ultrasound Transplant Study Group. J Heart Lung Transplant. 1995;14(6 Pt 2):S215–S220. [PubMed] [Google Scholar]
- 11.Weis M, von Scheidt W. Cardiac allograft vasculopathy: a review. Circulation. 1997;96(6):2069–2077. doi: 10.1161/01.cir.96.6.2069. [DOI] [PubMed] [Google Scholar]
- 12.Wahlers T, Mugge A, Oppelt P, et al. Coronary vasculopathy following cardiac transplantation and cyclosporine immunosuppression: preventive treatment with angiopeptin, a somatostatin analog. Transplant Proc. 1994;26(5):2741–2742. [PubMed] [Google Scholar]
- 13.Ewel CH, Foegh ML. Chronic graft rejection: accelerated transplant arteriosclerosis. Immunol Rev. 1993;134:21–31. doi: 10.1111/j.1600-065x.1993.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Tanaka K, Chhabra A, Oeser B, Kobashigawa JA, Tobis JM. Vascular remodeling 1 year after cardiac transplantation. J Heart Lung Transplant. 2007;26(1):56–62. doi: 10.1016/j.healun.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Raichlin E, Bae JH, Khalpey Z, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116(23):2726–2733. doi: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 16.Eisen H. Long-term cardiovascular risk in transplantation – insights from the use of everolimus in heart transplantation. Nephrol Dial Transplant. 2006;21(Suppl 3):III9–III13. doi: 10.1093/ndt/gfl295. [DOI] [PubMed] [Google Scholar]
- 17.Segovia J, Gomez-Bueno M, Alonso-Pulpon L. Treatment of allograft vasculopathy in heart transplantation. Expert Opin Pharmacother. 2006;7(17):2369–2383. doi: 10.1517/14656566.7.17.2369. [DOI] [PubMed] [Google Scholar]
- 18.Tullius SG, Tilney NL. Both alloantigen-dependent and -independent factors influence chronic allograft rejection. Transplantation. 1995;59(3):313–318. [PubMed] [Google Scholar]
- 19.Torre-Amione G, Wallace CK, Young JB, et al. The effect of etanercept on cardiac transplant recipients: a study of TNFα antagonism and cardiac allograft hypertrophy. Transplantation. 2007;84(4):480–483. doi: 10.1097/01.tp.0000276990.78659.77. [DOI] [PubMed] [Google Scholar]
- 20.Weber KT, Sun Y, Tyagi SC, Cleutjens JP. Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol. 1994;26(3):279–292. doi: 10.1006/jmcc.1994.1036. [DOI] [PubMed] [Google Scholar]
- 21.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5(3):334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacs EJ, DiPietro LA. Fibrogenic cytokines and connective tissue production. FASEB J. 1994;8(11):854–861. doi: 10.1096/fasebj.8.11.7520879. [DOI] [PubMed] [Google Scholar]
- 23.Wu GD, Tuan TL, Bowdish ME, et al. Evidence for recipient derived fibroblast recruitment and activation during the development of chronic cardiac allograft rejection. Transplantation. 2003;76(3):609–614. doi: 10.1097/01.TP.0000066362.37931.6D. [DOI] [PubMed] [Google Scholar]
- 24.Wu GD, Bowdish ME, Jin YS, et al. Contribution of mesenchymal progenitor cells to tissue repair in rat cardiac allografts undergoing chronic rejection. J Heart Lung Transplant. 2005;24(12):2160–2169. doi: 10.1016/j.healun.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Wu GD, Nolta JA, Jin YS, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75(5):679–685. doi: 10.1097/01.TP.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 26.Sartore S, Chiavegato A, Faggin E, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 27.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13(8):952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 28.Goumans MJ, van Zonneveld AJ, ten Dijke P. Transforming growth factor β-induced endothelial-to-mesenchymal transition: a switch to cardiac fibrosis? Trends Cardiovasc Med. 2008;18(8):293–298. doi: 10.1016/j.tcm.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Mazzucchelli L. Protein S100A4: too long overlooked by pathologists? Am J Pathol. 2002;160(1):7–13. doi: 10.1016/S0002-9440(10)64342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook HT. The origin of renal fibroblasts and progression of kidney disease. Am J Pathol. 2010;176(1):22–24. doi: 10.2353/ajpath.2010.090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 176(1):85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki J, Isobe M, Aikawa M, et al. Nonmuscle and smooth muscle myosin heavy chain expression in rejected cardiac allografts. A study in rat and monkey models. Circulation. 1996;94(5):1118–1124. doi: 10.1161/01.cir.94.5.1118. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: TGF-β signaling through Smad3-dependent and -independent pathways. Am J Transplant. 2006;6(9):2080–2088. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 34.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J Cell Mol Med. 2008;12(1):22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subramanian SV, Kelm RJ, Polikandriotis JA, Orosz CG, Strauch AR. Reprogramming of vascular smooth muscle α-actin gene expression as an early indicator of dysfunctional remodeling following heart transplant. Cardiovasc Res. 2002;54(3):539–548. doi: 10.1016/s0008-6363(02)00270-5. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian SV, Orosz CG, Strauch AR. Vascular smooth muscle α-actin expression as an indicator of parenchymal cell reprogramming in cardiac allografts. Transplantation. 1998;65(12):1652–1656. doi: 10.1097/00007890-199806270-00020. [DOI] [PubMed] [Google Scholar]
- 37.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol. 1998;142(3):873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muro AF, Moretti FA, Moore BB, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177(6):638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madani S, De Girolamo S, Munoz DM, Li RK, Sweeney G. Direct effects of leptin on size and extracellular matrix components of human pediatric ventricular myocytes. Cardiovasc Res. 2006;69(3):716–725. doi: 10.1016/j.cardiores.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Parkes JG, Liu Y, Sirna JB, Templeton DM. Changes in gene expression with iron loading and chelation in cardiac myocytes and non-myocytic fibroblasts. J Mol Cell Cardiol. 2000;32(2):233–246. doi: 10.1006/jmcc.1999.1068. [DOI] [PubMed] [Google Scholar]
- 41.Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- 42.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65(1):40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 43.Eghbali M. Cardiac fibroblasts: function, regulation of gene expression, and phenotypic modulation. Basic Res Cardiol. 1992;87(Suppl 2):183–189. doi: 10.1007/978-3-642-72477-0_16. [DOI] [PubMed] [Google Scholar]
- 44.Camelliti P, Green CR, Kohl P. Structural and functional coupling of cardiac myocytes and fibroblasts. Adv Cardiol. 2006;42:132–149. doi: 10.1159/000092566. [DOI] [PubMed] [Google Scholar]
- 45.Weber KT, Janicki JS, Shroff SG, Pick R, Chen RM, Bashey RI. Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res. 1988;62(4):757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- 46.Weber KT, Jalil JE, Janicki JS, Pick R. Myocardial collagen remodeling in pressure overload hypertrophy. A case for interstitial heart disease. Am J Hypertens. 1989;2(12 Pt 1):931–940. doi: 10.1093/ajh/2.12.931. [DOI] [PubMed] [Google Scholar]
- 47.Weber KT, Brilla CG. Factors associated with reactive and reparative fibrosis of the myocardium. Basic Res Cardiol. 1992;87(Suppl 1):291–301. doi: 10.1007/978-3-642-72474-9_25. [DOI] [PubMed] [Google Scholar]
- 48.Silver MA, Pick R, Brilla CG, Jalil JE, Janicki JS, Weber KT. Reactive and reparative fibrillar collagen remodelling in the hypertrophied rat left ventricle: two experimental models of myocardial fibrosis. Cardiovasc Res. 1990;24(9):741–747. doi: 10.1093/cvr/24.9.741. [DOI] [PubMed] [Google Scholar]
- 49.Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27(3):341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 50.Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41(3):532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 51.Diaz JA, Booth AJ, Lu G, Wood SC, Pinsky DJ, Bishop DK. Critical role for IL-6 in hypertrophy and fibrosis in chronic cardiac allograft rejection. Am J Transplant. 2009;9(8):1773–1783. doi: 10.1111/j.1600-6143.2009.02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Describes an association of pathologic hypertrophy and fibrosis with IL-6 in a mouse model of chronic cardiac rejection.
- 52.Torre-Amione G. Cardiac allograft hypertrophy: a new target for therapy, a surrogate marker for survival? Am J Transplant. 2009;9(1):7–8. doi: 10.1111/j.1600-6143.2008.02503.x. [DOI] [PubMed] [Google Scholar]
- 53.Matsui Y, Sadoshima J. Rapid upregulation of CTGF in cardiac myocytes by hypertrophic stimuli: implication for cardiac fibrosis and hypertrophy. J Mol Cell Cardiol. 2004;37(2):477–481. doi: 10.1016/j.yjmcc.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi N, Calderone A, Izzo NJ, Jr, Maki TM, Marsh JD, Colucci WS. Hypertrophic stimuli induce transforming growth factor-β 1 expression in rat ventricular myocytes. J Clin Invest. 1994;94(4):1470–1476. doi: 10.1172/JCI117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Booth AJ, Csencsits-Smith K, Wood SC, Lu G, Lipson KE, Bishop DK. Connective tissue growth factor promotes fibrosis downstream of TGF-β and IL-6 in chronic cardiac allograft rejection. Am J Transplant. 2010;10(2):220–230. doi: 10.1111/j.1600-6143.2009.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Investigates the interrelationships between IL-6, TGF-β and CTGF in two experimental models of chronic cardiac allograft rejection.
- 56.Csencsits K, Wood SC, Lu G, et al. Transforming growth factor β-induced connective tissue growth factor and chronic allograft rejection. Am J Transplant. 2006;6(5 Pt 1):959–966. doi: 10.1111/j.1600-6143.2006.01292.x. [DOI] [PubMed] [Google Scholar]
- 57.Raichlin E, Villarraga HR, Chandrasekaran K, et al. Cardiac allograft remodeling after heart transplantation is associated with increased graft vasculopathy and mortality. Am J Transplant. 2009;9(1):132–139. doi: 10.1111/j.1600-6143.2008.02474.x. [DOI] [PubMed] [Google Scholar]; ▪▪ Implicates the development of left ventricle hypertrophy as an indicator for development of graft vasculopathy and that left ventricle hypertrophy is associated with decreased survival of heart transplant recipients.
- 58.Libby P, Pober JS. Chronic rejection. Immunity. 2001;14(4):387–397. doi: 10.1016/s1074-7613(01)00119-4. [DOI] [PubMed] [Google Scholar]
- 59.Izutani H, Miyagawa S, Shirakura R, et al. Evidence that graft coronary arteriosclerosis begins in the early phase after transplantation and progresses without chronic immunoreaction. Histopathological analysis using a retransplantation model. Transplantation. 1995;60(10):1073–1079. doi: 10.1097/00007890-199511270-00002. [DOI] [PubMed] [Google Scholar]
- 60.Jain S, Furness PN, Nicholson ML. The role of transforming growth factor β in chronic renal allograft nephropathy. Transplantation. 2000;69(9):1759–1766. doi: 10.1097/00007890-200005150-00001. [DOI] [PubMed] [Google Scholar]
- 61.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 62.Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 63.Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-β1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97(9):900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 64.Noronha IL, Niemir Z, Stein H, Waldherr R. Cytokines and growth factors in renal disease. Nephrol Dial Transplant. 1995;10(6):775–786. [PubMed] [Google Scholar]
- 65.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor β(1) Am J Respir Cell Mol Biol. 1999;21(6):658–665. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 66.Brattain MG, Markowitz SD, Willson JK. The type II transforming growth factor-β receptor as a tumor-suppressor gene. Curr Opin Oncol. 1996;8(1):49–53. doi: 10.1097/00001622-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Massague J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 68.Faust SM, Lu G, Marini BL, et al. Role of T cell TGF-β signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol. 2009;183(11):7297–7306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪▪ Highlights the necessity of TGF-β in some mechanisms of allograft tolerance and suggests a role for IL-17 in chronic remodeling pathologies of chronic cardiac allograft rejection.
- 69.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 70.Yong Z, Chang L, Mei YX, Yi L. Role and mechanisms of CD4+CD25+ regulatory T cells in the induction and maintenance of transplantation tolerance. Transpl Immunol. 2007;17(2):120–129. doi: 10.1016/j.trim.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 71.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114(10):1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature. 1990;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- 73.Border WA, Noble NA, Yamamoto T, et al. Natural inhibitor of transforming growth factor-β protects against scarring in experimental kidney disease. Nature. 1992;360(6402):361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z, Garron TM, Li XJ, et al. Recombinant human decorin inhibits TGF-β1-induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35(4):527–537. doi: 10.1016/j.burns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 75.Pedagogos E, Hewitson TD, Walker RG, Nicholis KM, Becker GJ. Myofibroblast involvement in chronic transplant rejection. Transplantation. 1997;64(8):1192–1197. doi: 10.1097/00007890-199710270-00019. [DOI] [PubMed] [Google Scholar]
- 76.Isaka Y, Brees DK, Ikegaya K, et al. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med. 1996;2(4):418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- 77.Kolb M, Margetts PJ, Sime PJ, Gauldie J. Proteoglycans decorin and biglycan differentially modulate TGF-β-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1327–1334. doi: 10.1152/ajplung.2001.280.6.L1327. [DOI] [PubMed] [Google Scholar]
- 78.Faust SM, Lu G, Wood SC, Bishop DK. TGF-β neutralization within cardiac allografts by decorin gene transfer attenuates chronic rejection. J Immunol. 2009;183(11):7307–7313. doi: 10.4049/jimmunol.0902736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leask A, Abraham DJ. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81(6):355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- 80.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-β in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32(10):1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 81.Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 2009;195(3):321–338. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]; ▪ Extensive review of CTGF and its role in cardiac fibrosis.
- 82.Kundi R, Hollenbeck ST, Yamanouchi D, et al. Arterial gene transfer of the TGF-β signalling protein Smad3 induces adaptive remodelling following angioplasty: a role for CTGF. Cardiovasc Res. 2009;84(2):326–335. doi: 10.1093/cvr/cvp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayata N, Fujio Y, Yamamoto Y, et al. Connective tissue growth factor induces cardiac hypertrophy through Akt signaling. Biochem Biophys Res Commun. 2008;370(2):274–278. doi: 10.1016/j.bbrc.2008.03.100. [DOI] [PubMed] [Google Scholar]
- 84.de Winter P, Leoni P, Abraham D. Connective tissue growth factor: structure-function relationships of a mosaic, multifunctional protein. Growth Factors. 2008;26(2):80–91. doi: 10.1080/08977190802025602. [DOI] [PubMed] [Google Scholar]
- 85.Bonniaud P, Martin G, Margetts PJ, et al. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31(5):510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- 86.Cheng O, Thuillier R, Sampson E, et al. Connective tissue growth factor is a biomarker and mediator of kidney allograft fibrosis. Am J Transplant. 2006;6(10):2292–2306. doi: 10.1111/j.1600-6143.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 87.Yuan YC, Xia ZK, Mu JJ, Zhang QC, Yin BL. Increased connective tissue growth factor expression in a rat model of chronic heart allograft rejection. J Formos Med Assoc. 2009;108(3):240–246. doi: 10.1016/S0929-6646(09)60058-9. [DOI] [PubMed] [Google Scholar]
- 88.Csencsits K, Wood SC, Lu G, Bishop DK. Transforming growth factor-β1 gene transfer is associated with the development of regulatory cells. Am J Transplant. 2005;5(10):2378–2384. doi: 10.1111/j.1600-6143.2005.01042.x. [DOI] [PubMed] [Google Scholar]
- 89.Guo X, Wang XF. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009;19(1):71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 91.Chen RH, Chang MC, Su YH, Tsai YT, Kuo ML. Interleukin-6 inhibits transforming growth factor-β-induced apoptosis through the phosphatidylinositol 3-kinase/Akt and signal transducers and activators of transcription 3 pathways. J Biol Chem. 1999;274(33):23013–23019. doi: 10.1074/jbc.274.33.23013. [DOI] [PubMed] [Google Scholar]
- 92.Zhang XL, Topley N, Ito T, Phillips A. Interleukin-6 regulation of transforming growth factor (TGF)-β receptor compartmentalization and turnover enhances TGF-β1 signaling. J Biol Chem. 2005;280(13):12239–12245. doi: 10.1074/jbc.M413284200. [DOI] [PubMed] [Google Scholar]
- 93.Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O'Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. Am J Pathol. 2007;171(1):315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175(6):3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 95.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14(3):109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 96.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 97.Takatsuki F, Okano A, Suzuki C, et al. Human recombinant IL-6/B cell stimulatory factor 2 augments murine antigen-specific antibody responses in vitro and in vivo. J Immunol. 1988;141(9):3072–3077. [PubMed] [Google Scholar]
- 98.Hurst SM, Wilkinson TS, McLoughlin RM, et al. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705–714. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 99.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73(6):713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 100.Youker K, Smith CW, Anderson DC, et al. Neutrophil adherence to isolated adult cardiac myocytes. Induction by cardiac lymph collected during ischemia and reperfusion. J Clin Invest. 1992;89(2):602–609. doi: 10.1172/JCI115626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sporri B, Muller KM, Wiesmann U, Bickel M. Soluble IL-6 receptor induces calcium flux and selectively modulates chemokine expression in human dermal fibroblasts. Int Immunol. 1999;11(7):1053–1058. doi: 10.1093/intimm/11.7.1053. [DOI] [PubMed] [Google Scholar]
- 102.Weissenbach M, Clahsen T, Weber C, et al. Interleukin-6 is a direct mediator of T cell migration. Eur J Immunol. 2004;34(10):2895–2906. doi: 10.1002/eji.200425237. [DOI] [PubMed] [Google Scholar]
- 103.Fielding CA, McLoughlin RM, McLeod L, et al. IL-6 regulates neutrophil trafficking during acute inflammation via STAT3. J Immunol. 2008;181(3):2189–2195. doi: 10.4049/jimmunol.181.3.2189. [DOI] [PubMed] [Google Scholar]
- 104.McLoughlin RM, Jenkins BJ, Grail D, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA. 2005;102(27):9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Teague TK, Marrack P, Kappler JW, Vella AT. IL-6 rescues resting mouse T cells from apoptosis. J Immunol. 1997;158(12):5791–5796. [PubMed] [Google Scholar]
- 106.Yang XO, Nurieva R, Martinez GJ, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29(1):44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178(1):271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 108.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 109.Zhao XM, Frist WH, Yeoh TK, Miller GG. Expression of cytokine genes in human cardiac allografts: correlation of IL-6 and transforming growth factor-β (TGF-β) with histological rejection. Clin Exp Immunol. 1993;93(3):448–451. doi: 10.1111/j.1365-2249.1993.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liang Y, Christopher K, Finn PW, Colson YL, Perkins DL. Graft produced interleukin-6 functions as a danger signal and promotes rejection after transplantation. Transplantation. 2007;84(6):771–777. doi: 10.1097/01.tp.0000281384.24333.0b. [DOI] [PubMed] [Google Scholar]
- 111.Chen L, Ahmed E, Wang T, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182(10):6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Erten Y, Tulmac M, Derici U, et al. An association between inflammatory state and left ventricular hypertrophy in hemodialysis patients. Ren Fail. 2005;27(5):581–589. doi: 10.1080/08860220500200072. [DOI] [PubMed] [Google Scholar]
- 113.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. J Cell Physiol. 2005;202(3):891–899. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 114.Kurdi M, Randon J, Cerutti C, Bricca G. Increased expression of IL-6 and LIF in the hypertrophied left ventricle of TGR(mRen2)27 and SHR rats. Mol Cell Biochem. 2005;269(1–2):95–101. doi: 10.1007/s11010-005-3085-1. [DOI] [PubMed] [Google Scholar]
- 115.Sano M, Fukuda K, Kodama H, et al. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem. 2000;275(38):29717–29723. doi: 10.1074/jbc.M003128200. [DOI] [PubMed] [Google Scholar]
- 116.Briest W, Rassler B, Deten A, et al. Norepinephrine-induced interleukin-6 increase in rat hearts: differential signal transduction in myocytes and non-myocytes. Pflugers Arch. 2003;446(4):437–446. doi: 10.1007/s00424-003-1043-x. [DOI] [PubMed] [Google Scholar]
- 117.Birks EJ, Burton PB, Owen V, et al. Elevated tumor necrosis factor-α and interleukin-6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102(19 Suppl 3):III352–III358. doi: 10.1161/01.cir.102.suppl_3.iii-352. [DOI] [PubMed] [Google Scholar]
- 118.Hirota H, Izumi M, Hamaguchi T, et al. Circulating interleukin-6 family cytokines and their receptors in patients with congestive heart failure. Heart Vessels. 2004;19(5):237–241. doi: 10.1007/s00380-004-0770-z. [DOI] [PubMed] [Google Scholar]
- 119.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27(5):1201–1206. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 120.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31(2):391–398. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 121.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107(11):1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 122.Sarkar S, Vellaichamy E, Young D, Sen S. Influence of cytokines and growth factors in ANG II-mediated collagen upregulation by fibroblasts in rats: role of myocytes. Am J Physiol Heart Circ Physiol. 2004;287(1):H107–117. doi: 10.1152/ajpheart.00763.2003. [DOI] [PubMed] [Google Scholar]
- 123.Fredj S, Bescond J, Louault C, Delwail A, Lecron JC, Potreau D. Role of interleukin-6 in cardiomyocyte/cardiac fibroblast interactions during myocyte hypertrophy and fibroblast proliferation. J Cell Physiol. 2005;204(2):428–436. doi: 10.1002/jcp.20307. [DOI] [PubMed] [Google Scholar]
- 124.Liu X, Das AM, Seideman J, et al. The CC chemokine ligand 2 (CCL2) mediates fibroblast survival through IL-6. Am J Respir Cell Mol Biol. 2007;37(1):121–128. doi: 10.1165/rcmb.2005-0253OC. [DOI] [PubMed] [Google Scholar]
- 125.Burlingham WJ, Love RB, Jankowska-Gan E, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117(11):3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Describes a correlation between IL-17 responses and obliterative remodeling in lung transplant patients.
- 126.Fukami N, Ramachandran S, Saini D, et al. Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection. J Immunol. 2009;182(1):309–318. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simonian PL, Roark CL, Wehrmann F, et al. Th17-polarized immune response in a murine model of hypersensitivity pneumonitis and lung fibrosis. J Immunol. 2009;182(1):657–665. [PMC free article] [PubMed] [Google Scholar]
- 128.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205(13):3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B. Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2008;294(5):H2078–H2087. doi: 10.1152/ajpheart.01363.2007. [DOI] [PubMed] [Google Scholar]
- 130.Feng W, Li W, Liu W, Wang F, Li Y, Yan W. IL-17 induces myocardial fibrosis and enhances RANKL/OPG and MMP/TIMP signaling in isoproterenol-induced heart failure. Exp Mol Pathol. 2009;87(3):212–218. doi: 10.1016/j.yexmp.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 131.Cortez DM, Feldman MD, Mummidi S, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-β, NF-κB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293(6):H3356–H3365. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- 132.Chabaud M, Fossiez F, Taupin JL, Miossec P. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines. J Immunol. 1998;161(1):409–414. [PubMed] [Google Scholar]
- 133.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]