Abstract
Aim
To describe and discuss the merits of various direct and indirect methods applied in vitro (mainly on blood or milk) or in vivo (allergic test) for the diagnosis of brucellosis in animals.
Methods
The recent literature on brucellosis diagnostic tests was reviewed. These diagnostic tests are applied with different goals, such as national screening, confirmatory diagnosis, certification, and international trade. The validation of such diagnostic tests is still an issue, particularly in wildlife. The choice of the testing strategy depends on the prevailing brucellosis epidemiological situation and the goal of testing.
Results
Measuring the kinetics of antibody production after Brucella spp. infection is essential for analyzing serological results correctly and may help to predict abortion. Indirect ELISAs help to discriminate 1) between false positive serological reactions and true brucellosis and 2) between vaccination and infection. Biotyping of Brucella spp. provides valuable epidemiological information that allows tracing an infection back to the sources in instances where several biotypes of a given Brucella species are circulating. Polymerase chain reaction and new molecular methods are likely to be used as routine typing and fingerprinting methods in the coming years.
Conclusion
The diagnosis of brucellosis in livestock and wildlife is complex and serological results need to be carefully analyzed. The B. abortus S19 and B. melitensis Rev. 1 vaccines are the cornerstones of control programs in cattle and small ruminants, respectively. There is no vaccine available for pigs or for wildlife. In the absence of a human brucellosis vaccine, prevention of human brucellosis depends on the control of the disease in animals.
Brucellae are Gram-negative, facultative intracellular bacteria that can infect many species of animals and man. Ten species are recognized within the genus Brucella. There are 6 “classical” species: Brucella abortus, Brucella melitensis, Brucella suis, Brucella ovis, Brucella canis, and Brucella neotomae (1,2). This classification is based mainly on differences in pathogenicity and host preference (3). Distinction between species and between biovars of a given species is currently performed using differential tests based on phenotypic characterization of lipopolysaccharide (LPS) antigens, phage typing, dye sensitivity, requirement for CO2, H2S production, and metabolic properties (1,2).
The main pathogenic species worldwide are B. abortus, responsible for bovine brucellosis; B. melitensis, the main etiologic agent of ovine and caprine brucellosis; and B. suis, responsible for swine brucellosis. These 3 Brucella species cause abortion (“abortion storm” in naive heifers), and when brucellosis is detected in a herd, flock, region, or country, international veterinary regulations impose restrictions on animal movements and trade, which result in huge economic losses. These are the reasons why programs to control or eradicate brucellosis in cattle, small ruminants, and pigs have been implemented worldwide (4).
B. ovis and B. canis are responsible for ram epididymitis and canine brucellosis, respectively. In the case of B. neotomae, only strains isolated from desert wood rat (Neotoma lepida) in North America have been reported. Recently 4 new Brucella species have been described: Brucella pinnipedialis and Brucella ceti, isolated predominantly from seals and cetaceans, respectively (5); Brucella microti, isolated from common voles (Microtus arvalis) (6), soil (7), and foxes (Vulpes vulpes) (8); and Brucella inopinata, isolated from a breast implant (9).
There is a general host restriction pattern among the different Brucella species, meaning that different Brucella species infect different preferred hosts. Even within the B. suis species, different biovars preferentially infect different animal host species (1-3). Indeed, B. suis biovars 1 and 3 infect suidae, biovar 2 infects suidae and hare (Lepus europeanus), biovar 4 infects reindeer (Rangifer tarandus tarandus) and caribou (Rangifer tarandus granti), and biovar 5 has been isolated from rodents in Russia.
All Brucella species may also infect wildlife species. Classical Brucella species have been isolated from a great variety of wildlife species such as bison, elk, feral swine, wild boar, fox, hare, African buffalo, reindeer, and caribou (10). In order to implement appropriate control measures to address wildlife brucellosis, it is very important to distinguish between a spill-over of infection contracted from domestic animals and a sustainable infection (10). In the latter case, the concern of the livestock industry is to prevent the re-introduction of the infection in livestock (spill-back), particularly in regions or states that are “officially brucellosis-free.” If the status of “officially brucellosis-free” is lost, domestic animals must be tested prior to being traded, which imposes huge costs. This is exemplified by recent episodes of cattle being infected with B. abortus transmitted by elk in the Greater Yellowstone Area in the USA (11) and of outdoor reared pigs infected with B. suis biovar 2 transmitted by wild boar in France (12).
Brucellosis is an established zoonosis: infections have been attributed to at least 5 of the 6 classical Brucella species in terrestrial mammals. Studies from around the world indicate that elimination of the animal brucellosis reservoir has resulted in a substantial decline in the incidence of human disease (13). Currently, laboratory workers are among those most frequently infected (14). Marine mammal strains of Brucella have been reported to cause the infection of a laboratory worker in the UK (15), as well as naturally-acquired infections in Peru (16) and New Zealand (17).
Brucella species and biovars, preferential hosts, and pathogenicity for humans are depicted in Table 1.
Table 1.
Brucella species and biovars, preferential hosts and pathogenicity for humans
| Species | Biovars | Colony morphology | Preferential host(s) | Pathogenicity in humans* |
|---|---|---|---|---|
|
B. melitensis |
1-3 |
smooth |
sheep, goat |
high |
|
B. abortus |
1-6, 9 |
smooth |
cattle |
high |
|
B. suis |
1, 3 |
smooth |
pig |
high |
| 2 |
smooth |
wild boar, hare |
low |
|
| 4 |
smooth |
reindeer, caribou |
high |
|
| 5 |
smooth |
rodent |
no |
|
|
B. neotomae |
- |
smooth |
desert rat |
moderate |
|
B. ovis |
- |
rough |
ram |
no |
|
B. canis |
- |
rough |
dog |
moderate |
|
B. pinnipedialis |
- |
smooth |
cetacean |
?† |
|
B. ceti |
- |
smooth |
seal |
? |
|
B. microti |
- |
smooth |
soil, vole, fox |
? |
| B. inopinata | - | smooth | human | ? |
The most important clinical sign of brucellosis is abortion at the first gestation. Usually, infected females will abort only once, although they may remain infected their entire life. The clinical diagnosis of brucellosis in animals on the basis of abortion is, however, equivocal since many pathogens can induce abortion. Laboratory testing is therefore essential. The aim of this article is to review the recent literature on laboratory testing techniques designed to diagnose brucellosis.
Overview of diagnostic methods
This section reviews the different methods used to diagnose brucellosis in livestock and wildlife. Diagnostic tests can be applied with different goals: confirmatory diagnosis, screening or prevalence studies, certification, and, in countries where brucellosis is eradicated, surveillance in order to avoid the reintroduction of brucellosis through importation of infected animals or animal products. The validation of such diagnostic tests is still an issue, particularly in wildlife.
Diagnostic methods include direct tests, involving microbiological analysis or DNA detection by polymerase chain reaction (PCR)-based methods and indirect tests, which are applied either in vitro (mainly to milk or blood) or in vivo (allergic test). The choice of a particular testing strategy depends on the prevailing epidemiological situation of brucellosis in susceptible animals (livestock and wildlife) in a country or a region. Isolation of Brucella spp. or detection of Brucella spp. DNA by PCR is the only method that allows certainty of diagnosis. Biotyping provides valuable epidemiological information that allows tracing of infections back to their sources in countries where several biotypes are co-circulating. However, when one particular biovar is overwhelmingly predominant, classical typing techniques are of no use because they do not allow the differentiation of isolates belonging to the same biovar of a given biotype species. In this context, new fingerprinting methods such as multiple locus variable (number of tandem repeats) analysis (MLVA), which measures the number of tandem repeats at a given locus and multi-locus sequence analysis (MLSA) can differentiate isolates within a given biovar. These methods are gaining wider acceptance and will in the coming years almost certainly be used as routine typing and fingerprinting methods for molecular epidemiological purposes (19-21).
Direct diagnosis
Staining. Stamp staining is still often used, even though this technique is not specific: other abortive agents such as Chlamydophila abortus (formerly Chlamydia psittaci) or Coxiella burnetii are also stained red. It provides valuable information for the analysis of aborted material (1). Brucella spp. is a coccobacillus measuring 0.6-1.5 µm long and 0.5-0.7 µm wide. They generally occur singly and are observed in clusters of two or more. Brucella spp. is a Gram-negative bacterium that can resist weak acidic treatment and therefore appears red after Stamp staining.
Culture. Bacterial isolation is always required for the biotyping of strains (see “Molecular methods,” below). For the definitive diagnosis of brucellosis, the choice of samples depends on the clinical signs observed. In the case of clinical brucellosis, valid samples include aborted fetuses (stomach, spleen, and lung), fetal membranes, vaginal secretions, colostrum, milk, sperm, and fluid collected from arthritis or hygroma. At slaughter, in order to confirm suspected cases of acute or chronic brucellosis, the preferred tissues are the genital and oropharyngeal lymph nodes, the spleen, and the mammary gland and associated lymph nodes. For the isolation of Brucella spp., the most commonly used medium is the Farrell medium, which contains antibiotics able to inhibit the growth of other bacteria present in clinical samples. Some Brucella species, like B. abortus wildtype (biovars 1-4), need CO2 for growth, while others, like B. abortus wildtype (biovars 5, 6, 9), B. abortus S19 vaccine strain, B. melitensis, and B. suis, do not (1). For liquid samples (milk or blood), sensitivity is increased by the use of a biphasic medium like the Castaneda medium, originally described for use with human blood cultures. Growth may appear after 2-3 days, but cultures are usually considered negative after 2-3 weeks of incubation (1). The identification of Brucella spp. is based on morphology, staining and metabolic profile (catalase, oxidase, and urease) (1,2).
Biotyping. Biotyping of Brucella spp. is performed using different tests, the most important being agglutination tests with antibodies against rough or smooth LPS, ie, against the A or M epitopes of 'O' chain polysaccharides (O-LPS); lysis by phages, dependence on CO2 for growth, measured usually in primary cultures; production of H2S; growth in the presence of basal fuchsine or thionine; and the crystal violet or acriflavine tests (1). In order to be carried out proficiently, these techniques must be carried out using standardized procedures by experienced personnel. Therefore, they are often performed only in reference laboratories.
Molecular methods. New techniques allowing identification and sometimes quick typing of Brucella have been developed and are in use in certain diagnostic laboratories (19-24).
Identification. Several PCR based methods have been developed. The best validated methods are based on the detection of specific sequences of Brucella spp., such as the 16S-23S genes, the IS711 insertion sequence or the bcsp31 gene encoding a 31-kDa protein (25,26). These techniques were originally developed on bacterial isolates and are now also used to detect Brucella spp. DNA in clinical samples. DNA extraction methods known to influence the sensitivity of PCR assays need to be evaluated (27). It is worth noting that most of these techniques have been validated on human samples, but some reports evaluate their application to clinical veterinary samples (28,29). Recently, one technique has been favorably evaluated for the diagnosis of B. suis biovar 2 in clinical samples originating from wild boar in Switzerland (30). It is difficult to provide sensitivity and specificity estimates for these techniques because the test protocols described in the respective articles are not the same. Nevertheless, as a general rule, brucellosis PCR techniques show a lower diagnostic sensitivity than culture methods, although their specificity is close to 100% (23,28,29). The best results have so far been obtained by combining culture and PCR detection on clinical samples (28-30).
Molecular typing. For typing of Brucella spp., the multiplex AMOS PCR, named for its applicability to “abortus, melitensis, ovis, suis” species, is often used. This PCR and PCR protocols derived from it allow discrimination between Brucella species and between vaccine and wild-type strains. They do not, however, allow discrimination among all the biovars of a given Brucella species (22-24). The multiplex “Bruce ladder” PCR is the first method designed to identify and differentiate all of the known Brucella species and the vaccine strains in the same test (31).
The lack of PCR-based methods to discriminate among biovars within a species stimulated the development of other molecular typing techniques for Brucella spp., such as restriction fragment length polymorphism analysis based on the number of IS711 insertion sequences. However, this has not proven to be a useful tool (32). PCR restriction fragment length polymorphism based on the restriction analysis of Brucella genes encoding outer membrane proteins is a simple tool to implement, but the degree of polymorphism is low and the robustness is weak (33). Several new fingerprinting techniques show promise for differentiating isolates within the same biovar of a given species: single-nucleotide polymorphisms, which detect single-nucleotide differences in the DNA sequence of members of a species; MLSA, which detects DNA sequence variations in a set of housekeeping genes and characterizes strains by their unique allelic profiles; and MLVA, which analyzes the variability of loci containing repeated sequences (19-21).
To summarize, classical typing methods are able to discriminate between biovars of Brucella but, in a near future, single-nucleotide polymorphisms, MLSA, and MLVA will be used as routine typing techniques to allow the discrimination of strains within a given biovar, allowing molecular epidemiological analysis.
Indirect diagnostic tests
These tests are derived from research done mainly on brucellosis diagnosis in cattle. To a large extent the characteristics of the different tests can be transposed to sheep and goat, except for the milk ring test, which is not an accepted test in these species because it generates too many false-positive results (4).
In pigs, infection by Yersinia enterocolitica serotype O:9 (YO9) is not uncommon in some areas, particularly in Europe. Since YO9 and Brucella share a polysaccharide ‘O’ chain, Brucella spp. antigens used in serological tests react equally well with the surface smooth LPS of YO9 and are therefore unable to distinguish between antibodies to these 2 pathogens. Thus, as determined by the World Organization for Animal Health (Office International des Epizooties, OIE), none of the conventional serological tests used for the diagnosis of porcine brucellosis is reliable for diagnosis in individual pigs (4).
Several studies of brucellosis serology have been performed in wildlife as well as in zoo collections, with the goal of assessing the presence or spread of Brucella spp. within different wild species and to classify species or individuals as exposed or non-exposed (4). Brucellosis serology is usually performed using the same antigens as in livestock serology because the immunodominant Brucella antigens, associated to the smooth LPS, are to a large extent shared by all naturally occurring biovars of B. abortus, B. melitensis, B. suis, B. neotomae, B. ceti, B. pinnipedialis, and B. microti. Most brucellosis serological tests have been directly transposed to wild species, without validation, from domestic livestock populations, where their use has quite often not been validated either.
In order to validate serological tests, results should be analyzed according to the true infectious status of an animal. The presence of anti-Brucella antibodies suggests exposure to Brucella spp., but it does not indicate which Brucella species induced production of those antibodies. Moreover, seropositivity does not necessarily mean that the animals have current or active infection at the time of sampling. In fact, studies of experimental and natural infections indicate that nearly all animal species vulnerable to Brucella infection can lose their antibody titers. This means that the actual prevalence of brucellosis may be higher than that indicated by antibody screening. Therefore, the “gold standard” in brucellosis remains the isolation of Brucella spp. If brucellosis is suspected in livestock or in wildlife because of positive serological results, attempts to isolate the organism are considered mandatory and should always be performed (10).
A sound description of the different tests for the diagnosis of brucellosis is available online on the OIE Web site (http://www.oie.int) in the form of the Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. In the following section, only the primary benefits and shortcomings of the tests will be addressed. Sensitivities and specificities of indirect tests, as documented in the literature, are depicted in Table 2.
Table 2.
Sensitivity and specificity of indirect tests for the diagnosis of cattle brucellosis as published in the literature*
| Tests | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Serological tests |
||
| SAT (SAW)/MAT |
81.5 |
98.9 |
| CFT |
90-91.8 |
99.7-99.9 |
| BAT |
87 |
97.8 |
| iELISA |
97.2 |
97.1 - 99.8 |
| cELISA |
95.2 |
99.7 |
| FPA |
96.6 |
99.1 |
| Milk tests |
||
| MRT |
88.5 |
77.4 |
| FPA |
76.9 |
100 |
| iELISA |
98.6 |
99.0 |
| Cellular tests |
||
| Skin test | 78-93 | 99.8 |
*Abbreviations: SAT – Slow agglutination test; SAW – Slow agglutination of Wright; MAT – Micro agglutination Test; CFT – Complement fixation test; BAT – Buffered Brucella antigen test; iELISA – indirect ELISA; cELISA – competitive ELISA; FPA – Fluorescence polarization assay; MRT – Milk ring test. The data in the table come from references 4,34-41.
Serological tests. This section refers to serological tests to detect “smooth” Brucella spp. infections. These tests do not detect infections caused by B. ovis and B. canis. For these infections, “rough” Brucella antigens must be used (4).
Slow Agglutination Test or Slow Agglutination of Wright (SAT or SAW). The principle of this test is to detect agglutinin antibodies mainly of the IgM isotype directed against Brucella spp. At an optimum concentration of antigen and antibodies, large antigen-antibody complexes form and precipitate at the bottom of the test tube. This reaction is slow because, in contrast to the rapid agglutination tests, it requires an overnight incubation at 37°C. This technique can also be practiced in micromethod (microagglutination test) in a reaction volume of 100 µL, without a change in performance. Reading the result is facilitated by the addition of a dye that stains the cells. The relative lack of specificity and sensitivity of this test has often been presented as a major drawback (Table 2). Nevertheless, this is a standardized and extremely robust test that has shown good results and has proven efficacious in several countries now declared officially free of brucellosis (34). The specificity of the test is increased by treating the serum with a chelating agent such as EDTA, which reduces cross-reactions due to IgM (1). Although this test is no longer recommended by the OIE for bovine brucellosis diagnosis (4), it is still widely used in human brucellosis diagnosis (1).
Buffered Brucella antigen tests. The Rose Bengal (RB) and buffered plate agglutination (BA) tests are the well-known buffered Brucella antigen tests. These tests are rapid agglutination tests lasting 4 minutes done on a glass plate with the help of an acidic-buffered antigen (pH 3.65 ± 0.05). These tests have been introduced in many countries as the standard screening test because it is very simple and thought to be more sensitive than the SAT (35). The OIE considers these tests “prescribed tests for trade” (4).
Complement fixation test. The Complement Fixation Test (CFT) allows the detection of anti-Brucella antibodies that are able to activate complement. Cattle immunoglobulins (Ig) that can activate bovine complement are the IgG and the IgM. According to some literature this test is not highly sensitive but shows an excellent specificity (34,36). Because the test is difficult to standardize, it is progressively being replaced by ELISAs (4). This test is a “prescribed test for trade” by the OIE (4).
ELISAs. ELISAs are divided into two categories, the indirect ELISA (iELISAs) and the competitive ELISA (cELISAs). Most iELISAs use purified smooth LPS as antigen but a good deal of variation exists in the anti-bovine Ig conjugate used (37). Most iELISAs detect mainly IgGs or IgG sub-classes. Their main quality is their high sensitivity but they are also more vulnerable to non-specific reactions, notably those due to YO9 infection. These cross-reactions seen in iELISAs motivated the development of cELISAs. The O-chain of the smooth LPS of Brucella contains specific epitopes that are not shared with the LPS of YO9. Therefore, by using monoclonal antibodies directed against specific epitopes of the Brucella LPS, the development of more specific cELISAs has been possible. These tests are more specific, but less sensitive, than iELISAs (38,42). The OIE considers these tests “prescribed tests for trade” (4).
Fluorescence Polarization Assay. The fluorescence polarization assay (FPA) is based on a physical principle: how quickly a molecule spins in a liquid medium correlates with its mass. Molecules of small size spin faster and depolarize a polarized light beam more, while bigger molecules spin more slowly and, consequently, depolarize light less. FPA measures the degree of depolarization in milli-polarization units (mP). During the test, serum samples are incubated with a specific antigen of B. abortus labeled with fluorescein isothiocyanate. In the presence of antibodies against Brucella spp., large fluorescent complexes are formed. In negative samples, the antigen remains uncomplexed. These smaller molecules spin more quickly and therefore cause greater depolarisation of the light than do the samples positive for Brucella spp..
This test can be easily automated and is very quick, since after mixing the labeled antigen and serum the reading is almost instantaneous. The test sensitivity seems slightly lower than that of iELISAs (36). The specificity varies between 98.8 and 99.0% (35). This test is already used in brucellosis control and certification programs in North America and in Europe. The OIE considers this test a “prescribed test for trade” (4).
Milk tests. These tests are prescribed by the OIE as tests to use in control and eradication programs but not for trading purposes (4).
Milk Ring Test. The test consists of mixing colored Brucella whole-cell antigen with fresh bulk/tank milk. In the presence of anti-Brucella antibodies, antigen-antibody complexes form and migrate to the cream layer, forming a purple ring on the surface. In the absence of antigen-antibody complexes, the cream remains colorless. This test is not considered sensitive but this lack of sensitivity is compensated by the fact that the test can be repeated, usually monthly, due to its very low cost. This test is prescribed by the OIE for use only with cow milk (4).
ELISAs and Fluorescence Polarization Assay. These two tests, discussed above in the context of serum samples, can also be applied to milk samples to detect infected animals. These tests are less sensitive when applied to milk than to serum samples. Indeed, before they can be used on tank milk, which may come from hundreds of cows, their sensitivity must first be checked on pools of samples (39,40). This lower sensitivity in the case of tank milk can often be compensated by increasing the testing frequency. These tests are prescribed by the OIE for testing the milk of cattle and small ruminants (4).
Skin test. The skin test is an allergic test that detects the specific cellular immune response induced by Brucella spp. infection. The injection of brucellergene, a protein extract of a rough strain of Brucella spp., is followed by a local inflammatory response in a sensitized animal. This delayed-type hypersensitivity reaction is measured by the increase in skin thickness at the site of inoculation. This test is highly efficient in discriminating between true brucellosis cases and false positive serological reactions. The skin test is highly specific but its weak sensitivity makes it a good test for herds but not for individual certification. It cannot discriminate between infection and vaccination (41). This test is prescribed as an alternative test by the OIE (4).
Strategic use of serological tests
This section highlights the strategic use of certain serological tests in order to discriminate 1) between false positive serological reactions and true brucellosis and 2) between vaccination and infection.
Brucellosis is an infectious disease but animals are not always contagious. Indeed, excretion of Brucella spp. only occurs at certain times, mainly when abortion occurs. During an abortion, billions of Brucella spp. are excreted and this is a major source of infection for congeners and for professionals in contact with aborted materials. In order to avoid contamination from aborted material, it is important (1) to isolate pregnant heifers in their sixth month of pregnancy, given that brucellosis induces abortion usually in late pregnancy; and 2) to predict abortion and eliminate animals likely to abort, before they become a source of infection. Vaccination does not provide complete protection from exposure to Brucella spp. (4). This raises the key question: when a pregnant animal is infected, regardless of whether or not it has been vaccinated, is it possible to predict whether it will abort? Key factors that determine the answer to this question are the kinetics of antibody production and the type of antibodies produced.
Kinetics of the immune responses in cattle
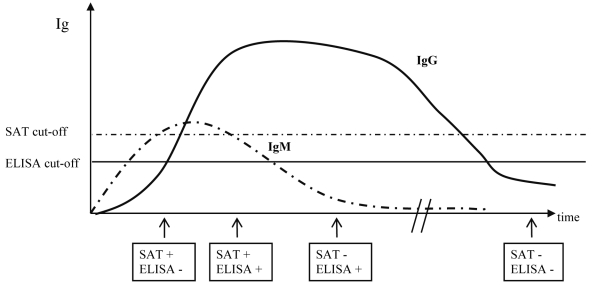
Indirect diagnostic tests are based on the detection of immune responses induced by infection. These tests show different sensitivities and specificities depending on numerous variables, such as the infection dose and route, the presence of so-called “cross-reactive bacteria” antigenically similar to Brucella spp., the kinetics of the induced immune response, and previous vaccination (37,43). Serology is the method of choice for screening in any sound control or eradication program. Strong humoral immune responses are induced after exposure. Humoral IgG responses persist after the peak of the response (3-4 weeks post-infection) and remain detectable over long periods of time (up to several years); in contrast, the IgM response is rapidly induced 2-3 weeks after exposure and may disappear after a few months (37,43,44). The cell-mediated immune response (induced 3-4 weeks after exposure), as measured by the brucellosis skin test, is long-lasting and can be detected for several years (41). Thus, given the kinetics of the immune responses induced after infection, the time when different tests are performed after exposure has a major impact on the results, as depicted in Figure 1.
Figure 1.
Outcome of slow agglutination test (SAT) and ELISA tests performed at different times post-infection. According to the time point post-infection at which sampling and testing occur, different serological results may be generated. Therefore, epidemiological information is extremely important for informing the interpretation of test results. Immunoglobulin (IgG) responses will be induced 1 or 2 weeks later than the IgM response but they will last for long periods of time, usually years. The intensity of the response is measured by serum antibody titers using slow agglutination test, which measures mainly IgM, and indirect ELISA (iELISA), which measures mainly IgG. Testing was performed 3 weeks, 4 weeks, 6-7 months, and more than a year post-infection. Adapted from references 41,43,44.
The kinetics of production and disappearance of the principal immunoglobulin isotypes during infection, and the activity of these immunoglobulins in the different serological tests, will usually permit the distinction between acute and chronic infections. For example, the immune responses against B. abortus in cattle are rapid IgM production 2-3 weeks after experimental infection, followed by IgG production 3-4 weeks after experimental infection (41,43,44). Therefore, the following principles apply:
1. The concomitant presence of IgM (detected in an agglutination test) and IgG (detected in iELISA) suggests acute brucellosis, while chronic brucellosis is characterized by the presence of IgG alone.
2. A positive response in an agglutination test, which detects mainly IgM, is not indicative of brucellosis if it is not confirmed by a positive IgG response by iELISA within one week.
The SAT, RB, and BAT are commonly used as screening tests for the diagnosis of bovine brucellosis. However, the OIE and the EU have recently decided not to recommend use of the SAT because they consider it inferior to the other standard tests (4,35). The CFT is used as a confirmatory test after a positive agglutination reaction. This test is gradually being replaced by iELISAs and, more recently, by the FPA (4). All these tests must be standardized and should be performed according to validated standard operating procedures in accredited laboratories.
Serology and vaccination
For over 60 years, the B. abortus S19 vaccine has been used in cattle and the B. melitensis Rev.1 vaccine has been used in sheep and goats to prevent abortion and infertility caused by natural infection with virulent strains of these Brucella species (45). These vaccines, combined with serologic surveillance tests, have been instrumental in the success of the brucellosis eradication program. Conventional serologic tests for brucellosis detect antibody against the LPS antigens induced by vaccination with S19 or Rev. 1 or exposure to virulent field strains. Therefore, no single serologic test can differentiate, beyond any reasonable doubt, animals vaccinated with S19 or Rev. 1 and animals infected with virulent Brucella spp. field strains. Nevertheless, strategic use of tests to detect different isotypes of immunoglobulins provides useful information in order to differentiate vaccination from infection. Indeed, more than 90% of heifers vaccinated with S19 were classified negative by classical serological tests (ie, SAT, RB, and CFT) at 16 weeks post-vaccination, while they were still classified positive by iELISAs (41). Moreover, under experimental conditions, the kinetics of antibody production differ between vaccination and infection such that iELISAs can be used to predict abortion in heifers and thus allow their elimination before congeners can be contaminated (41).
Recently, a rough mutant of B. abortus, strain RB51, has been proposed for use as a vaccine for cattle of all ages (46). Although RB51 expresses low levels of the O side chain, naive animals remain seronegative in surveillance tests following vaccination with RB51 (47). This is a major advantage in a control program based on vaccination combined with serological testing. Unfortunately, the efficacy of the RB51 vaccine in cattle is still questionable (45). RB51 has been shown to be non-protective in small ruminants (48). Currently, there is no vaccine available for humans, pigs, or wildlife (10,18).
Conclusion
The aim of an eradication program is not a “zero seropositivity” situation, but the absence of infection, given that seropositivity can occur after vaccination or YO9 infection (44). Criteria to declare a country or a region “officially free from brucellosis” have been laid down in international regulations such as in EU Directives and recommendations of the OIE. The epidemiology of brucellosis is complex and criteria other than test results are needed in order to guarantee the success of an eradication program (49). The S19 and Rev. 1 vaccines are still the cornerstones of control and eradication programs (4).
In contrast, no vaccine is available against brucellosis in wildlife. It is striking to see that the ecology of infection remains so poorly understood. For example, scientists have speculated for decades whether brucellosis induces abortion in bison (50). Little is known about the pathology of B. suis biovar 2 in wild boars (51), and marine mammal brucellosis research is still in its infancy (18). Very little is known about brucellosis pathology in marine mammals (52) or their zoonotic potential (53). Emphasis should be put on multidisciplinary research addressing the ecology of infection, particularly on how to predict whether infection will persist in a population. Research should also analyze the factors of crucial importance for the maintenance of Brucella spp. in infected populations. Serology is the first tool in detecting subclinical infections. Better tests and testing strategies should be developed but the “gold standard” in brucellosis remains the isolation of Brucella spp. and is thus mandatory.
Brucellosis is not a sustainable disease in humans. The source of human infection resides always in domestic or wild animal reservoirs. Therefore, as a general rule, prevention of human zoonotic brucellosis depends predominantly on the control of the disease in animals (13,18).
Acknowledgments
We thank all our colleagues working in research and diagnostic laboratories, the veterinary inspectors, and the veterinary surgeons who over the years have contributed to the control and eradication of brucellosis in livestock and to the discovery of new Brucella species in wildlife.
References
- 1.Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the brucellosis laboratory. 1st edition. Paris: Institut National de la Recherche Agronomique; 1988. [Google Scholar]
- 2.Corbel MJ, Banai M. Genus I. Brucella Meyer and Shaw 1920, 173AL. In: Brenner DJ, Krieg NR, Staley JT, editors. Bergey’s manual of systematic bacteriology. vol. 2. New York: Springer; 2005. p. 370-86. [Google Scholar]
- 3.Moreno E, Cloeckaert A, Moriyon I. Brucella evolution and taxonomy. Vet Microbiol. 2002;90:209–27. doi: 10.1016/S0378-1135(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 4.Manual of standards for diagnostic tests and vaccines. Paris: Office International des Epizooties; 2009. [DOI] [PubMed] [Google Scholar]
- 5.Foster G, Osterman BS, Godfroid J, Jacques I, Cloeckaert A. Brucella ceti sp. nov. and Brucella pinnipedialis sp. nov. for Brucella strains with cetaceans and seals as their preferred hosts. Int J Syst Evol Microbiol. 2007;57:2688–93. doi: 10.1099/ijs.0.65269-0. [DOI] [PubMed] [Google Scholar]
- 6.Scholz HC, Hubalek Z, Sedlácek I, Vergnaud G, Tomaso H, Al Dahouk S, et al. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int J Syst Evol Microbiol. 2008;58:375–82. doi: 10.1099/ijs.0.65356-0. [DOI] [PubMed] [Google Scholar]
- 7.Scholz HC, Hubalek Z, Nesvadbova J, Tomaso H, Vergnaud G, Le Fleche P, et al. Isolation of Brucella microti from soil. Emerg Infect Dis. 2008;14:1316–7. doi: 10.3201/eid1408.080286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scholz HC, Hofer E, Vergnaud G, Le Fleche P, Whatmore AM, Al Dahouk S, et al. Isolation of Brucella microti from mandibular lymph nodes of red foxes, Vulpes vulpes, in lower Austria. Vector Borne Zoonotic Dis. 2009;9:153–6. doi: 10.1089/vbz.2008.0036. [DOI] [PubMed] [Google Scholar]
- 9.Scholz HC, Nockler K, Gollner C, Bahn P, Vergnaud G, Tomaso H, et al. Brucella inopinata sp. nov., isolated from a breast implant infection. Int J Syst Evol Microbiol. 2010;60:801–8. doi: 10.1099/ijs.0.011148-0. [DOI] [PubMed] [Google Scholar]
- 10.Godfroid J. Brucellosis in wildlife. Rev Sci Tech. 2002;21:277–86. doi: 10.20506/rst.21.2.1333. [DOI] [PubMed] [Google Scholar]
- 11.Beja-Pereira A, Bricker B, Chen S, Almendra C, White PJ, Luikart G. DNA genotyping suggests that recent brucellosis outbreaks in the Greater Yellowstone Area originated from elk. J Wildl Dis. 2009;45:1174–7. doi: 10.7589/0090-3558-45.4.1174. [DOI] [PubMed] [Google Scholar]
- 12.Garin-Bastuji B, Hars J, Calvez D, Thiebaud M, Artois M. Brucellosis in domestic pigs and wild boar caused by Brucella suis biovar 2 in France. Epidémiologie & Santé Animale. 2000;38:1–5. [in French] [Google Scholar]
- 13.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6:91–9. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Mazuelos E, Nogales MC, Florez C, Gomez-Mateos JM, Lozano F, Sanchez A. Outbreak of Brucella melitensis among microbiology laboratory workers. J Clin Microbiol. 1994;32:2035–6. doi: 10.1128/jcm.32.8.2035-2036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brew SD, Perrett LL, Stack JA, MacMillan AP, Staunton NJ. Human exposure to Brucella recovered from a sea mammal. Vet Rec. 1999;144:483. [PubMed] [Google Scholar]
- 16.Sohn AH, Probert WS, Glaser CA, Gupta N, Bollen AW, Wong JD, et al. Human neurobrucellosis with intracerebral granuloma caused by a marine mammal Brucella spp. Emerg Infect Dis. 2003;9:485–8. doi: 10.3201/eid0904.020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald WL, Jamaludin R, Mackereth G, Hansen M, Humphrey S, Short P, et al. Characterization of a Brucella sp. strain as a marine-mammal type despite isolation from a patient with spinal osteomyelitis in New Zealand. J Clin Microbiol. 2006;44:4363–70. doi: 10.1128/JCM.00680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godfroid J, Cloeckaert A, Liautard JP, Kohler S, Fretin D, Walravens K, et al. From the discovery of the Malta fever's agent to the discovery of a marine mammal reservoir, brucellosis has continuously been a re-emerging zoonosis. Vet Res. 2005;36:313–26. doi: 10.1051/vetres:2005003. [DOI] [PubMed] [Google Scholar]
- 19.Le Fleche P, Jacques I, Grayon M, Al Dahouk S, Bouchon P, Denoeud F, et al. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 2006;6:9. doi: 10.1186/1471-2180-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maquart M, Le Flčche P, Foster G, Tryland M, Ramisse F, Djřnne B, et al. MLVA-16 typing of 295 marine mammal Brucella isolates from different animal and geographic origins identifies 7 major groups within Brucella ceti and Brucella pinnipedialis. BMC Microbiol. 2009;9:145. doi: 10.1186/1471-2180-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whatmore AM. Current understanding of the genetic diversity of Brucella, an expanding genus of zoonotic pathogens. Infect Genet Evol. 2009;9:1168–84. doi: 10.1016/j.meegid.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Bricker BJ, Halling SM. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J Clin Microbiol. 1994;32:2660–6. doi: 10.1128/jcm.32.11.2660-2666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bricker BJ. PCR as a diagnostic tool for brucellosis. Vet Microbiol. 2002;90:435–46. doi: 10.1016/S0378-1135(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 24.Bricker BJ, Ewalt DR, Olsen SC, Jensen AE. Evaluation of the Brucella abortus species-specific polymerase chain reaction assay, an improved version of the Brucella AMOS polymerase chain reaction assay for cattle. J Vet Diagn Invest. 2003;15:374–8. doi: 10.1177/104063870301500413. [DOI] [PubMed] [Google Scholar]
- 25.Baddour MM, Alkhalifa DH. Evaluation of three polymerase chain reaction techniques for detection of Brucella DNA in peripheral human blood. Can J Microbiol. 2008;54:352–7. doi: 10.1139/W08-017. [DOI] [PubMed] [Google Scholar]
- 26.Ouahrani-Bettache S, Soubrier MP, Liautard JP. IS6501-anchored PCR for the detection and identification of Brucella species and strains. J Appl Bacteriol. 1996;81:154–60. doi: 10.1111/j.1365-2672.1996.tb04493.x. [DOI] [PubMed] [Google Scholar]
- 27.Dauphin LA, Hutchins RJ, Bost LA, Bowen MD. Evaluation of automated and manual commercial DNA extraction methods for recovery of Brucella DNA from suspensions and spiked swabs. J Clin Microbiol. 2009;47:3920–6. doi: 10.1128/JCM.01288-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leyla G, Kadri G, Umran O. Comparison of polymerase chain reaction and bacteriological culture for the diagnosis of sheep brucellosis using aborted fetus samples. Vet Microbiol. 2003;93:53–61. doi: 10.1016/S0378-1135(02)00442-X. [DOI] [PubMed] [Google Scholar]
- 29.Marianelli C, Martucciello A, Tarantino M, Vecchio R, Iovane G, Galiero G. Evaluation of molecular methods for the detection of Brucella species in water buffalo milk. J Dairy Sci. 2008;91:3779–86. doi: 10.3168/jds.2008-1233. [DOI] [PubMed] [Google Scholar]
- 30.Hinić V, Brodard I, Thomann A, Holub M, Miserez R, Abril C. IS711-based real-time PCR assay as a tool for detection of Brucella spp. in wild boars and comparison with bacterial isolation and serology. BMC Vet Res. 2009;5:22. doi: 10.1186/1746-6148-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Yoldi D, Marin CM, de Miguel MJ, Munoz PM, Vizmanos JL, Lopez-Goni I. Multiplex PCR assay for the identification and differentiation of all Brucella species and the vaccine strains Brucella abortus S19 and RB51 and Brucella melitensis Rev1. Clin Chem. 2006;52:779–81. doi: 10.1373/clinchem.2005.062596. [DOI] [PubMed] [Google Scholar]
- 32.Clavareau C, Wellemans V, Walravens K, Tryland M, Verger JM, Grayon M, et al. Phenotypic and molecular characterization of a Brucella strain isolated from a minke whale (Balaenoptera acutorostrata). Microbiology. 1998;144:3267–73. doi: 10.1099/00221287-144-12-3267. [DOI] [PubMed] [Google Scholar]
- 33.Cloeckaert A, Verger JM, Grayon M, Grepinet O. Restriction site polymorphism of the genes encoding the major 25 kDa and 36 kDa outer-membrane proteins of Brucella. Microbiology. 1995;141:2111–21. doi: 10.1099/13500872-141-9-2111. [DOI] [PubMed] [Google Scholar]
- 34.Emmerzaal A, de Wit JJ, Dijkstra T, Bakker D, van Zijderveld FG. The Dutch Brucella abortus monitoring programme for cattle: the impact of false-positive serological reactions and comparison of serological tests. Vet Q. 2002;24:40–6. doi: 10.1080/01652176.2002.9695123. [DOI] [PubMed] [Google Scholar]
- 35.Greiner M, Verloo D, de Massis F. Meta-analytical equivalence studies on diagnostic tests for bovine brucellosis allowing assessment of a test against a group of comparative tests. Prev Vet Med. 2009;92:373–81. doi: 10.1016/j.prevetmed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 36.McGiven JA, Tucker JD, Perrett LL, Stack JA, Brew SD, MacMillan AP. Validation of FPA and cELISA for the detection of antibodies to Brucella abortus in cattle sera and comparison to SAT, CFT, and iELISA. J Immunol Methods. 2003;278:171–8. doi: 10.1016/S0022-1759(03)00201-1. [DOI] [PubMed] [Google Scholar]
- 37.Saegerman C, De Waele L, Gilson D, Godfroid J, Thiange P, Michel P, et al. Evaluation of three serum i-ELISAs using monoclonal antibodies and protein G as peroxidase conjugate for the diagnosis of bovine brucellosis. Vet Microbiol. 2004;100:91–105. doi: 10.1016/j.vetmic.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Nielsen KH, Kelly L, Gall D, Nicoletti P, Kelly W. Improved competitive enzyme immunoassay for the diagnosis of bovine brucellosis. Vet Immunol Immunopathol. 1995;46:285–91. doi: 10.1016/0165-2427(94)05361-U. [DOI] [PubMed] [Google Scholar]
- 39.Nielsen K, Gall D. Fluorescence polarization assay for the diagnosis of brucellosis: a review. J Immunoassay Immunochem. 2001;22:183–201. doi: 10.1081/IAS-100104705. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen K. Diagnosis of brucellosis by serology. Vet Microbiol. 2002;90:447–59. doi: 10.1016/S0378-1135(02)00229-8. [DOI] [PubMed] [Google Scholar]
- 41.Saegerman C, Vo TK, De Waele L, Gilson D, Bastin A, Dubray G, et al. Diagnosis of bovine brucellosis by skin test: conditions for the test and evaluation of its performance. Vet Rec. 1999;145:214–8. doi: 10.1136/vr.145.8.214. [DOI] [PubMed] [Google Scholar]
- 42.Weynants V, Gilson D, Cloeckaert A, Denoel PA, Tibor A, Thiange P, et al. Characterization of a monoclonal antibody specific for Brucella smooth lipopolysaccharide and development of a competitive enzyme-linked immunosorbent assay to improve the serological diagnosis of brucellosis. Clin Diagn Lab Immunol. 1996;3:309–14. doi: 10.1128/cdli.3.3.309-314.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland SS. Evaluation of the enzyme-linked immunosorbent assay in the detection of cattle infected with Brucella abortus. Vet Microbiol. 1984;10:23–32. doi: 10.1016/0378-1135(84)90053-1. [DOI] [PubMed] [Google Scholar]
- 44.Godfroid J, Saegerman C, Wellemans V, Walravens K, Letesson JJ, Tibor A, et al. How to substantiate eradication of bovine brucellosis when aspecific serological reactions occur in the course of brucellosis testing. Vet Microbiol. 2002;90:461–77. doi: 10.1016/S0378-1135(02)00230-4. [DOI] [PubMed] [Google Scholar]
- 45.Moriyon I, Grillo MJ, Monreal D, Gonzalez D, Marin C, Lopez-Goni I, et al. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res. 2004;35:1–38. doi: 10.1051/vetres:2003037. [DOI] [PubMed] [Google Scholar]
- 46.Schurig GG, Roop RM, II, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–88. doi: 10.1016/0378-1135(91)90091-S. [DOI] [PubMed] [Google Scholar]
- 47.Stevens MG, Hennager SG, Olsen SC, Cheville NF. Serologic responses in diagnostic tests for brucellosis in cattle vaccinated with Brucella abortus 19 or RB51. J Clin Microbiol. 1994;32:1065–6. doi: 10.1128/jcm.32.4.1065-1066.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.el Idrissi AH, Benkirane A, el Maadoudi M, Bouslikhane M, Berrada J, Zerouali A. Comparison of the efficacy of Brucella abortus strain RB51 and Brucella melitensis Rev. 1 live vaccines against experimental infection with Brucella melitensis in pregnant ewes. Rev Sci Tech. 2001;20:741–7. doi: 10.20506/rst.20.3.1305. [DOI] [PubMed] [Google Scholar]
- 49.Crawford RP, Huber JD, Adams BS. Epidemiology and surveillance. In: Nielsen K, Duncan JR, editors. Animal brucellosis. Boca Raton (FL): CRC Press; 1990. p. 131-51. [Google Scholar]
- 50.Palmer MV, Olsen SC, Gilsdorf MJ, Philo LM, Clarke PR, Cheville NF. Abortion and placentitis in pregnant bison (Bison bison) induced by the vaccine candidate, Brucella abortus strain RB51. Am J Vet Res. 1996;57:1604–7. [PubMed] [Google Scholar]
- 51.Godfroid J, Michel P, Uytterhaegen L, De Smedt C, Rasseneur F, Boelaert F, et al. Brucellosis caused by Brucella suis biovar 2 in wild boar (Sus scrofa) in Belgium. Ann Med Vet. 1994;138:263–8. [in French] [Google Scholar]
- 52.Gonzalez-Barrientos R, Morales JA, Hernandez-Mora G, Barquero-Calvo E, Guzman-Verri C, Chaves-Olarte E, et al. Pathology of striped dolphins (Stenella coeruleoalba) infected with Brucella ceti. J Comp Pathol. 2010;142:347–52. doi: 10.1016/j.jcpa.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 53.Whatmore AM, Dawson CE, Groussaud P, Koylass MS, King AC, Shankster SJ, et al. Marine mammal Brucella genotype associated with zoonotic infection. Emerg Infect Dis. 2008;14:517–8. doi: 10.3201/eid1403.070829. [DOI] [PMC free article] [PubMed] [Google Scholar]