Abstract
Aim
To present our 10-year clinical experience with brucellosis patients at the University Clinic for Infectious Diseases and Febrile Conditions in Skopje, Republic of Macedonia.
Methods
A total of 550 patients with brucellosis treated between 1998 and 2007 were retrospectively assessed for their demographic, epidemiological, and clinical characteristics and outcomes.
Results
Of the 550 patients, 395 (72%) were male. The median age was 34.5 years (range, 1-82). Direct contact with infected animals was recorded in 333 (61%) patients and positive family history in 310 (56%). The most frequently seen symptoms were arthralgia (438, 80%), fever (419, 76%), and sweating (394, 72%). The most common signs were fever and hepatomegaly, which were verified in 357 (65%) and 273 (50%) patients, respectively. Focal brucellosis was found in 362 patients (66%) and osteoarticular in 299 (54%). Therapeutic failures were registered in 37 (6.7%) patients. Of the 453 (82%) patients who completed a follow-up period of at least 6 months, relapses occurred in 60 (13%).
Conclusion
Due to non-specific clinical manifestation and laboratory parameters, brucellosis should be considered one of the differential diagnoses of any patient suffering from obscure involvement of various organs in a brucellosis-endemic region. High percentage of relapses and therapeutic failures in spite of the use of currently recommended therapeutic regimens indicates the seriousness of this zoonosis and the need to control it.
Brucellosis is a zoonosis caused by intracellular bacteria of the genus Brucella (1). The disease is widespread in many countries of the Mediterranean basin, and together with hydatidosis, trichinellosis, and leishmaniasis, it is considered to be a typical Mediterranean zoonosis (2). Human brucellosis is a multisystem disease whose patients present with nonspecific symptoms (3) and a high risk of complications, a protracted clinical course, and relapses (4).
The main clinical characteristics of human brucellosis have been well known for a long time. Marston had been the first to give an accurate description of brucellosis as a disease entity even before the etiological agent was detected (5). The monographs published by Hughes in 1897 (6) and Spink in 1956 (7) contain perhaps the most detailed and still accurate data on this topic. Today, there is a lot of information about the characteristics of human brucellosis available from various parts of the world, and the description of its characteristics varies widely.
For almost 30 years, brucellosis has been a dominant zoonosis in the Republic of Macedonia, causing a high morbidity and huge economic losses. However, the main reasons for persistence of the disease are not only husbandry practices and traditional food and living habits (2), but also an inadequate strategy of brucellosis control (8). The aim of our study was to present more detailed insights into the predominant demographic, epidemiological, clinical, and laboratory features of brucellosis patients, and their outcomes, during a 10-year period in the endemic region of the Republic of Macedonia.
Methods
This retrospective study included 550 patients who were diagnosed with brucellosis and treated at the University Clinic for Infectious Diseases and Febrile Conditions in Skopje between January 1998 and December 2007. The diagnosis was based on clinical findings compatible with brucellosis (arthralgia, fever, sweating, malaise, hepatomegaly, splenomegaly, signs of focal disease), supported by detection of specific antibodies at significant titers and/or demonstration of at least a 4-fold rise in antibody titer in serum samples obtained 3-4 weeks apart. Antibody titers were determined by standard tube agglutination (STA), Brucella Coombs test (9,10), or the Brucellacapt assay (11). The corresponding titers considered positive were ≥1/160, ≥1/320, and >1/320, respectively. During the study period, bacteriological isolation was not a routine practice in the Republic of Macedonia.
Demographic and epidemiological data, clinical symptoms and signs, laboratory characteristics and outcome of the patients were analyzed. If a focal form of the disease was suspected after clinical examination, further investigations were performed, such as x-rays, electrocardiography, ultrasound investigations, lumbar puncture, radionuclide bone scan, computerized tomography, magnetic resonance imaging, and electromyography.
The patients were treated with various combinations of two or three of the following drugs: (a) oral doxycycline at 100-200 mg/d in patients ≥8 years of age; (b) oral rifampin at 600-900 mg/d in adults or 15-20 mg/kg/d in children; (c) oral trimethoprim (TMP)-sulfamethoxazole (SMZ) combination therapy at TMP doses of 160-320 mg/d and SMZ doses of 800-1600 mg/d in adults, or TMP doses of 10-12 mg/kg/d and SMZ doses of 50-60 mg/kg/d in children; (d) oral ciprofloxacin at 1000 mg/d in adults; (e) intramuscular gentamicin at 240 mg/d in adults or 5 mg/kg/d in children, and (f) intravenous ceftriaxone at 4 g/d in adults or 80 mg/kg/d in children. Doxycycline, TMP-SMZ, rifampin, and ciprofloxacin were administered for 45-60 days; gentamicin, for the first 7-14 days. Ceftriaxone was part of the antimicrobial therapy in patients suffering from neurobrucellosis and was administered for 14-30 days. In patients with spondylitis, neurobrucellosis, endocarditis and those with therapeutic failures, antimicrobial treatment was prolonged for at least 3 months.
Osteoarticular involvement was considered to be present if there were any inflammatory signs in peripheral osteoarticular locations (swelling, pain, functional disability, heat, and redness of the joints), or inflammatory pain in deep osteoarticular locations concurrently with pathologic findings on x-rays, radionuclide bone scans, computerized tomography, or magnetic resonance imaging (12). Orchitis and epididymitis were diagnosed by the presence of swelling and tenderness of the testis and epididymis, respectively. Hepatic involvement was defined as more than a 2-fold increase in alanine aminotransferase levels above the reference values (13). Neurobrucellosis was defined as presence of neurological dysfunction, not otherwise explainable, in combination with pathologic laboratory findings in the cerebrospinal fluid, ie, >10 cells/mL or protein concentrations >0.45 g/L and detection of anti-Brucella antibodies (14,15). Endocarditis was confirmed by auscultation of a cardiac murmur and detection of valvular vegetations using trans-thoracic echocardiography (15). Respiratory complications were defined by the protocol described by Pappas et al (16). Hematological parameters were described according to the criteria published by Troy et al (17). However, hematological involvement was considered to be present only in patients with manifestations due to hematological discrasia.
Therapeutic failure was defined as persistence of symptoms and signs attributable to the disease after two months of antibiotic treatment, and relapse as the reappearance of symptoms and signs after completion of antibrucellar treatment. Relapses were evaluated only in patients who had a follow-up period of at least 6 months post-therapy, whereas therapeutic failures were estimated in all treated patients irrespective of the follow-up period.
Patients were hospitalized until clinical improvement was achieved. Laboratory and serological controls were conducted on the 15th and 40th day of the treatment. In the follow-up period, these controls were done once a month during the first three months, and then every two to six months until the patient stopped coming for check-ups. Controls were conducted earlier if signs and symptoms aggravated or re-appeared after clinical cure. In the case of a relapse, the same diagnostic and therapeutic procedures were performed as in the initial episode of the disease.
Age, illness duration prior to diagnosis, treatment duration and follow-up period are presented using median and range values. All other parameters are presented as frequencies and percentages. Statistical analysis was performed using the SPSS, version 8.0 (SPSS, Inc., Chicago, IL, USA).
Results
The patients were 1 to 82 years old (median, 34.5). Most of them belonged to the age group 15-40 years (n = 252 patients, 46%), followed by the age group 41-60 years (n = 132, 24%). The number of patients younger than 14 (n = 86, 16%) was nearly the same as the number of those older than 60 years (n = 80, 15%). The male/female ratio was 395/155 (72/28%). Four female patients were pregnant at the time when the diagnosis was established.
An occupational exposure was reported by 333 (61%) patients. The ingestion of potentially contaminated food was mentioned by 155 (28%) patients. In 62 (11%) patients, the mode of transmission remained obscure. Three hundred and ten patients (56%) belonging to 103 families, had a confirmed family history of brucellosis in at least one of their members. Patients presented at the hospital most frequently during the spring (n = 216, 39%) and summer (n = 183, 33%). The nadir of brucellosis was in winter (n = 95, 17%) and fall (n = 56, 10%).
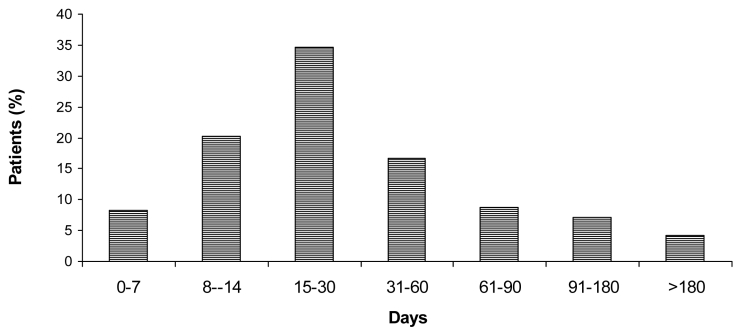
The median illness duration prior to diagnosis was 30 days (range, 3 to 360). More than 60% of patients were diagnosed within 30 days, and 80% within 60 days after the onset of symptomatic disease (Figure 1). The clinical characteristics, laboratory findings, and initial serological titers at the time of admission are shown in Table 1 to Table 3, respectively. Osteoarticular involvement was the most frequent focal complication, found in 299 (54%) patients. Patients presented with peripheral arthritis (n = 187, 34%), sacroiliitis (n = 68, 12%), spondylitis (n = 78, 14%), and miscellaneous osteoarticular forms (n = 11, 2%). The genitourinary, respiratory, hematological, nervous, hepatobiliary, cardiovascular, and other organ systems were affected, respectively, in 36 (6.5%), 34 (6.2%), 32 (5.8%), 19 (3.4%), 15 (2.7%), 10 (1.8%), and 6 (1.1%) patients.
Figure 1.
Illness duration before diagnosis in 550 patients with brucellosis diagnosed and treated during 1998-2007 at the University Clinic for Infectious Diseases and Febrile Conditions in Skopje
Table 1.
Frequency of clinical characteristics in patients with brucellosis, as reported in our and other studies
| Percent of patients reporting condition |
|||||||
|---|---|---|---|---|---|---|---|
| Literature reference | fever (symptom) | malaise | night per-spiration | arthralgia | fever | hepato-megaly | spleno-megaly |
| Aygen et al (14) |
80 |
90 |
84 |
82 |
39 |
21 |
14 |
| Yinnon (18) |
96 |
33 |
ND* |
35 |
ND |
55 |
69 |
| Memish et al (19) |
91 |
ND |
19 |
66 |
84 |
6 |
7 |
| Andriopoulos et al (20) |
100 |
97 |
96 |
87 |
ND |
25 |
51 |
| Shehabi et al (21) |
100 |
ND |
88 |
60 |
ND |
54 |
61 |
| Demiroglu et al (22) |
80 |
86 |
78 |
87 |
ND |
ND |
ND |
| Tasbakan et al (23) |
94 |
96 |
92 |
82 |
97 |
38 |
60 |
| Pourbagher et al (24) |
55 |
76 |
53 |
85 |
ND |
6 |
8 |
| Trujillo et al (25) |
100 |
77 |
96 |
81 |
ND |
10 |
12 |
| Buchanan et al (26) |
ND |
95 |
93 |
17 |
95 |
ND |
ND |
| Kokoglu et al (27) |
78 |
ND |
72 |
78 |
41 |
27 |
36 |
| Present study, No. (%) | 419 (76) | 377 (68) | 394 (72) | 438 (80) | 357 (65) | 273 (50) | 159 (29) |
*Abbreviation: ND – not determined.
Table 3.
Number (and percent) of patients showing the indicated anti-Brucella antibody titers as measured using the serum tube agglutination (STA) test, Coombs test, or Brucellacapt at the time of admission to hospital*
| Titer | STA† | Brucella Coombs† | Brucellacapt‡§ |
|---|---|---|---|
| ≤1/80 |
24 (7) |
0 |
0 |
| 1/160 |
34 (10) |
28 (8) |
4 (2) |
| 1/320 |
51 (15) |
43 (12) |
8 (4) |
| 1/640 |
82 (24) |
67 (20) |
20 (10) |
| 1/1280 |
153 (44) |
206 (60) |
20 (10) |
| 1/2560 |
ND |
ND |
29 (14) |
| 1/5120 | ND | ND | 125 (61) |
*Abbreviation: ND – not determined.
†Performed in 344 patients.
‡Performed in 206 patients.
§The numbers do not add up to 100% because of rounding.
Eighty-six patients (16%) were treated with a combination of two drugs; in the remaining 464 (84%) patients three drugs were used. Three hundred and nineteen (58%) patients were treated with a combination of doxycycline, rifampin, and trimethoprim/sulfamethoxazole; 62 (11%) with a combination of doxycycline, rifampin, and gentamicin; 53 (10%) with a combination of doxycycline and rifampin; and 31 (6%) with a combination of rifampin and TMP/SMZ. In the remaining 85 (15%) patients, other therapeutic regimens were used. Treatment duration was median 45 days (range 45-360). In 102 (18%) patients, treatment duration was prolonged for at least 90 days. Corticosteroids were used in 15, 12, 11, and 4 patients presenting with meningo/myelo/radiculo/neuritis, thrombocytopenia/ pancytopenia, orchitis/orchoepididymitis, and vasculitis as a consequence of brucellosis, respectively.
The outcome of the pregnancy in the 4 expecting patients was spontaneous deliveries in 3 (1 premature, 2 in-term) and 1 abortion. One patient with brucellar endocarditis underwent a cardiac surgery and valve replacement. Two patients died within the treatment period: the first fatal outcome was a result of a myocardial infarction, and the second of renal and respiratory complications due to brucellosis. Therapeutic failures occurred in 37 (6.7%) patients. The median follow-up of our patients was 10 months (range, 0-84) post-therapy. A follow-up period of ≥6 months was recorded in 453 (82%) cases, 60 (13%) of which relapsed.
Discussion
Most of the patients with brucellosis in our study were men and belonged to the age group 15-40 years. Age distribution of the study population is important because it may influence the clinical expression of brucellosis, including the frequency and type of signs, symptoms, complications (18,28,29,33).
The age distribution of the patients in our study did not differ significantly from that of patients in other studies (19,28). The frequency of brucellosis among children (≤14 years) in our study was similar to that reported in other studies (20,28); in fact, in several other studies, nearly one of every four patients fell into this age group (3,18,19,21,34). In an Iranian study, 56% of the examined patients were younger than 14 years (35). The frequency of patients older than 60 years varied from 2% (30,36,37) to 32% (38). A high frequency in older age groups was also found in a study conducted in northwestern Greece (39).
Consistent with our results, the majority of studies indicate that men are infected more often, ie, in 55% (15,19,31,40-43) or even 96% of the cases (36). Nevertheless, sex distribution of patients with brucellosis varies widely: several studies have indicated either equal distribution (21,28,32,44) or, in some cases, predominance of female patients (14,22,29,45).
Gotuzzo et al detected a more severe course of the disease in women, especially those with brucellar arthritis (46). However, the complication rate seems to be higher in men (15). Headaches and lethargy are more frequently observed in women and splenomegaly in males (3).
Age and sex distribution found in this study is a result of regional habits, mostly due to husbandry practices that make working-age men most vulnerable to brucellosis.
Nevertheless, brucellosis can be found in women, as well as marginal age groups due to consumption of food products of animal origin (eg, young cheese from sheep or goats) that are not adequately thermally processed.
The epidemiology of brucellosis often remains obscure and is not well defined. In patients with professional exposure, besides the skin and conjunctival contact, the infection can be acquired both by airborne transmission and by ingestion of contaminated animal products (45). Hence, risk assessment is often subjective. Some authors think that occupational exposure risk is the most important (28,42), others favor the transmission by animal food (described in 63%-92% of the patients) (20,23,24,30,31,45), whereas in several studies the mode of transmission remains unknown for up to 57% of the patients (15,47). It is of particular importance to understand the mode of brucellosis transmission since this knowledge helps to apply appropriate countermeasures.
Our study confirmed that screening the family members of a brucellosis patient is an important issue. Family history of brucellosis was reported in 9% to 51% of patients (15,24,46,48). Family screening leads to early diagnosis of the disease, which may prevent complications (49,50).
Brucellosis duration prior to diagnosis is an important parameter in assessing the clinical course and outcome. It has been recognized that brucellosis duration without adequate treatment is directly correlated with the complication rate and unfavorable outcome (42). A long period of symptomatic disease before therapeutic intervention was significantly more frequent in patients with brucellar spondylitis than in patients without spondylitis (12,48). The percentage of relapses among patients suffering from the disease for more than a month before therapy was higher than that among patients suffering from the disease for less than a month (29). Contradictory to these findings, other studies demonstrated that treatment beginning less than 10 days after disease onset led to higher relapse rates (51,52). In endemic countries, brucellosis may be diagnosed earlier because physicians are familiar with the clinical signs and symptoms and take it more often into consideration as a differential diagnosis. However, this is not always the case. In some series, 16% of patients were diagnosed with a delay of more than three months after the onset of symptoms (42,47). The diagnostic delay could be a result of either misdiagnosis or postponed visit to a medical professional (8,53).
Clinical and laboratory features are often not pathognomonic for human brucellosis and differ widely (Table 1 and Table 2). In addition, the number of patients suffering from focal involvement ranges from 6% to 92% (20,21,43,54) and is usually about 30% (14,15,31,42,55). In our study, two-thirds of the enrolled patients had focal manifestations and osteoarticular forms predominated, similar to other reports (1,15,20,28,44). The wide variation in the frequencies of clinical manifestations may reflect the characteristics of the examined population, the nature of the causative agent, geographic variations of the disease, the stage of disease, the diversity of case definition criteria, the diagnostic procedures, and the type of study (retrospective or prospective) (12).
Table 2.
Frequency of hematological and biochemical features in patients with brucellosis, as reported in our and other studies*
| Percent of patients with the given feature |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Literature reference | erythrocyte sedimentation rate >20 mm/h | C-reactive protein >5 mg/L | alanine aminotransferase >40 U/L | leucopenia | leucocytosis | lymphocytes >45% | thrombocytopenia | anemia | pancytopenia |
| 1 |
ND |
ND |
24 |
2 |
ND |
40 |
5 |
ND |
2 |
| 13 |
ND |
ND |
31 |
27 |
ND |
ND |
15 |
31 |
2 |
| 14 |
59 |
ND |
ND |
8 |
6 |
68 |
ND |
55 |
ND |
| 18 |
ND |
ND |
40 |
31 |
3 |
55 |
3 |
51 |
ND |
| 24 |
49 |
23 |
10 |
ND |
ND |
ND |
ND |
30 |
ND |
| 28 |
ND |
74 |
39 |
14 |
8 |
32 |
ND |
70 |
ND |
| 29 |
42 |
48 |
18 |
6 |
4 |
ND |
ND |
ND |
ND |
| 30 |
77 |
ND |
40 |
19 |
9 |
41 |
8 |
7 |
3.5 |
| Present study, No. (%) | 337 (63)† | 389 (79)‡ | 180 (33) | 50 (9.1) | 45 (8.2) | 132 (24) | 58 (12)§ | 148 (27) | 3 (0.6)§ |
*Abbreviations: ND – not determined.
†Measured in 535 patients.
‡Measured in 490 patients.
§Measured in 500 patients.
The serum agglutination test is an important diagnostic tool, bearing in mind that bacterial isolation methods are time-consuming, show lack sensitivity, and pose risk to laboratory personnel (56). The combination of the serum agglutination test and Coombs tests in diagnosing the disease may help to overcome the problem of false-negative results (1,57). Recently, the Brucellacapt test has started to replace other serological tests (58,59). Serological evaluation of our results at the time of admission to hospital generally showed high anti-Brucella antibody titers, especially in the Coombs test and the Brucellacapt. In the case of inconclusive serological results but a high clinical suspicion of brucellosis, the patients were retested after 2-4 weeks to assess possible seroconversion (19).
The primary goal of brucellosis therapy is to control the illness and prevent complications, relapses, and sequels (60). The currently recommended treatment regimens are based upon the recommendations of the World Health Organization in 1986 (61), updated by experts in the field at the Conference for Treatment of Human Brucellosis held in Ioannina, Greece, in 2006 (62). Nevertheless, a great diversity of therapeutic protocols exists. The selection of antimicrobial agents depends on the clinical presentation, age of the patient, pregnancy, drug side effects, and co-morbidity; in addition, in regions with scarce resources, therapeutic decisions are also determined by treatment costs and drug availability. The triple antimicrobial combination is rarely implemented and its use is traditionally restricted to patients with neurobrucellosis, endocarditis, or abscesses (3,23,63,64). Nevertheless, the highest cure rate can be achieved with a triple therapy (3) and adding a third drug to the standard regimen seems to be beneficial (63). In two reports, children treated with three drugs had a better outcome than those treated with two drugs (65,66). Almost 84% of the patients in the present study were treated with a combination of three antimicrobial agents, based on our previous experience that three-drug therapy was better than two-drug therapy (unpublished data).
In the first epidemiological study on human brucellosis in Malta during the period 1901-1907, when understanding of the disease was very limited and treatment was inadequate, Eyre reported that the mortality rate among the civilian population was approximately 10%, and 2.3% among soldiers and marines (7,67). In the recent decades with the use of appropriate antimicrobial treatment, the mortality rate has been lower than 1% (4,14,29,42,68). However, there are exceptions, in non-endemic countries as a result of the decreasing knowledge about the disease and in endemic regions of the developing world due to lack of medical care. For instance, in Germany, mortality rates increased from 0.4% (1978-1981) to a maximum of 6.5% (1998-2001) (69). In Nigeria, a mortality rate of 5.4% was reported (70). Of course, mortality rates might be biased by the selection of patients with specific clinical manifestations; for example, the rate was 2.1% in a series of patients with vertebral osteomyelitis in Spain (71). Brucella endocarditis is the main cause of death attributable to this disease (72-74), but many other causes have been described, eg, endotoxic shock with disseminated intravascular coagulation (75), rupture of a mycotic aneurysm (76), aortitis with mesenteric thrombosis (42), myocarditis (77), dissemination with multifocal liver and lung nodules (78), thrombocytopenia (79), and neurobrucellosis (14,29,42). We had only one mortality case as a direct consequence of brucellosis. The main reason for fatal outcome was development of renal and respiratory failure, which can be added to the above list of rare causes of fatal outcome in patients with brucellosis.
Therapeutic failures and relapses are inevitable characteristics of the disease. Unfavorable outcomes usually have to be ascribed to the inability to eradicate the bacteria from their intracellular niche. In adequately treated patients, relapses occur in 0 to 16% of the cases (14,30,31,34,42,45,80-82). The frequency of relapses found in our patients is similar to those mentioned in other reports (27,37,83-85). In our study, only patients with a follow-up period of at least 6 months post-therapy were included when assessing the relapse rate. If we included the patients with a follow-up period shorter than 6 months, eg, those who had never showed up for a check-up or who had come to only one, we would have underestimated the relapse rate, given that relapses most frequently appear in brucellosis during the first 6 months after the end of treatment (52,86). Possible reasons for relapse are inadequate choice of antibiotics, short treatment duration, and a lack of compliance. However, the relapse rate may be biased by the inability to distinguish re-infections from relapses and short follow-up periods (12). Therapeutic failures, which occurred in 6.7% of patients in our study, are mostly associated with brucellar spondylitis (12,42,48,87). According to literature data, the frequency of therapeutic failures varies from 0 to 15% (17,22,23,30,31,42,43,55,80).
Our study has two main limitations. First, we did not use Brucellae isolation, which is important for disease diagnosis, determination of Brucella species, detection of relapses, and follow-up of antimicrobial sensitivity. Second, this study was retrospective and data were sometimes missing about biochemical tests or other diagnostic findings such as magnetic resonance imaging, which has since then become the preferred procedure for patients with spondylitis, but which was performed in our study in only 29 of 78 (37%) patients with this condition. In addition, since the study was retrospective, the study group included many different therapy combinations and longer follow-up for some of the patients was not possible.
In summary, brucellosis is still a serious public health problem in the Republic of Macedonia. For early diagnosis, considering family history of the disease is important and special emphasis should be placed on asking about occupational exposure and family members suffering from brucellosis. Keeping in mind that clinical and laboratory characteristics of the disease are heterogeneous and non-specific, brucellosis has to be considered in the differential diagnoses of patients with complex organ involvement referred to hospitals in endemic regions and in travelers returning from endemic regions. The high relapse and therapeutic failure rates, despite the use of an adequate therapy, show the seriousness of this zoonosis and the absolute need to control it in the animal reservoir.
References
- 1.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. N Engl J Med. 2005;352:2325–36. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A. General epidemiological aspects of major zoonoses in the Mediterranean Region. Proceedings of the MZCP/Workshop on Zoonoses Surveillance and Control in the Mediterranean Region; 1998 Mar 30-31; Cephalonia Island, Greece. Athens: WHO Mediterranean Zoonoses Control Centre; 1998. p. 4-7.
- 3.Mousa AR, Elhag KM, Khogali M, Marafie AA. The nature of human brucellosis in Kuwait: study of 379 cases. Rev Infect Dis. 1988;10:211–7. doi: 10.1093/clinids/10.1.211. [DOI] [PubMed] [Google Scholar]
- 4.Bosilkovski M, Krteva L, Dimzova M, Kondova I. Brucellosis in 418 patients from the Balkan Peninsula: exposure-related differences in clinical manifestations, laboratory test results, and therapy outcome. Int J Infect Dis. 2007;11:342–7. doi: 10.1016/j.ijid.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Madkour MM. Historical aspect of brucellosis. In: Madkuor MM, editor. Brucellosis. Cambridge (UK): Butterworths, University Press; 1989. p. 1-10. [Google Scholar]
- 6.Hughes ML. Mediterranean, Malta or undulant fever. London: Macmillan and Co; 1897. [Google Scholar]
- 7.Spink WW. The nature of brucellosis. Minneapolis (MN): University of Minnesota Press; 1956. [Google Scholar]
- 8.Bosilkovski M, Dimzova M, Grozdanovski K. Natural history of brucellosis in an endemic region in different time periods. Acta Clin Croat. 2009;48:41–6. [PubMed] [Google Scholar]
- 9.Kerr WR, McCaughey WJ, Coghlan JD, Payne DJ, Quaife RA, Robertson L, et al. Techniques and interpretations in the serological diagnosis of brucellosis in man. J Med Microbiol. 1968;1:181–93. doi: 10.1099/00222615-1-2-181. [DOI] [PubMed] [Google Scholar]
- 10.Alton GG, Jones LM, Pietz DE. Laboratory techniques in brucellosis. 2nd ed. Geneva (Switzerland): WHO Monograph Series; 1975. [PubMed] [Google Scholar]
- 11.Serra J, Velasco J, Godoy P, Mendoza J. Can the Brucellacapt test be substituted for the Coombs test in the diagnosis of human brucellosis? Enferm Infecc Microbiol Clin. 2001;19:202–5. doi: 10.1016/s0213-005x(01)72613-4. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 12.Bosilkovski M, Krteva L, Caparoska S, Dimzova M. Osteoarticular involvement in brucellosis: study of 196 cases in the Republic of Macedonia. Croat Med J. 2004;45:727–33. [PubMed] [Google Scholar]
- 13.Dokuzoguz B, Ergonul O, Baykam N, Esener H, Kilic S, Celikbac A, et al. Characteristics of B. melitensis versus B. abortus bacteraemias. J Infect. 2005;50:41–5. doi: 10.1016/j.jinf.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Aygen B, Doganay M, Sumerkan B, Yildiz O, Kayaba U. Clinical manifestations, complications and treatment of brucellosis: a retrospective evaluation of 480 patients. Med Mal Infect. 2002;32:485–93. doi: 10.1016/S0399-077X(02)00403-1. [DOI] [Google Scholar]
- 15.Hasanjani Roushan MR, Mohrez M, Smailnejad Gangi SM, Soleimani Amiri MJ, Hajiahmadi M. Epidemiological features and clinical manifestations in 469 adult patients with brucellosis in Babol, Northern Iran. Epidemiol Infect. 2004;132:1109–14. doi: 10.1017/S0950268804002833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pappas G, Bosilkovski M, Akritidis N, Mastora M, Krteva L, Tsianos E. Brucellosis and the respiratory system. Clin Infect Dis. 2003;37:e95–9. doi: 10.1086/378125. [DOI] [PubMed] [Google Scholar]
- 17.Troy SB, Rickman LS, Davis CE. Brucellosis in San Diego: epidemiology and species-related differences in acute clinical presentations. Medicine. 2005;84:174–87. doi: 10.1097/01.md.0000165659.20988.25. [DOI] [PubMed] [Google Scholar]
- 18.Yinnon AM, Morali GA, Goren A, Rudensky B, Isacsohn M, Michel J, et al. Effect of age and duration of disease on the clinical manifestations of brucellosis. A study of 73 consecutive patients in Israel. Isr J Med Sci. 1993;29:11–6. [PubMed] [Google Scholar]
- 19.Memish Z, Mah MW, Al Mahmoud S, Al Shaalan M, Khan MY. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect. 2000;40:59–63. doi: 10.1053/jinf.1999.0586. [DOI] [PubMed] [Google Scholar]
- 20.Andriopoulos P, Tsironi M, Deftereos S, Aessopos A, Assimakopoulos G. Acute brucellosis: presentation, diagnosis, and treatment of 144 cases. Int J Infect Dis. 2007;11:52–7. doi: 10.1016/j.ijid.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Shehabi A, Shakir K, el-Khateeb M, Qubain H, Fararjeh N, Shamat AR. Diagnosis and treatment of 106 cases of human brucellosis. J Infect. 1990;20:5–10. doi: 10.1016/S0163-4453(90)92214-6. [DOI] [PubMed] [Google Scholar]
- 22.Demiroglu YZ, Turunc T, Aliskan H, Colakoglu S, Arslan H. Brucellosis: retrospective evaluation of the clinical, laboratory and epidemiological features of 151 cases. Mikrobiyol Bul. 2007;41:517–27. [in Turkish] [PubMed] [Google Scholar]
- 23.Tasbakan MI, Yamazhan T, Gökengin D, Arda B, Sertpolat M, Ulusoy S, et al. Brucellosis: a retrospective evaluation. Trop Doct. 2003;33:151–3. doi: 10.1177/004947550303300310. [DOI] [PubMed] [Google Scholar]
- 24.Pourbagher MA, Pourbagher A, Savas L, Turunc T, Demiroglu YZ, Erol I, et al. Clinical pattern and abdominal sonographic findings in 251 cases of brucellosis in southern Turkey. AJR Am J Roentgenol. 2006;187:W191-4. doi: 10.2214/AJR.05.0241. [DOI] [PubMed] [Google Scholar]
- 25.Trujillo IZ, Zavala AN, Caceres JG, Miranda CQ. Brucellosis. Infect Dis Clin North Am. 1994;8:225–41. [PubMed] [Google Scholar]
- 26.Buchanan TM, Faber LC, Feldman RA. Brucellosis in the United States, 1960-1972. An abattoir-associated disease. Part I. Clinical features and therapy. Medicine (Baltimore) 1974;53:403–13. doi: 10.1097/00005792-197411000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Kokoglu OF, Hosoglu S, Geyik MF, Ayaz C, Akalin S, Buyukbese MA, et al. Clinical and laboratory features of brucellosis in two university hospitals in Southeast Turkey. Trop Doct. 2006;36:49–51. doi: 10.1258/004947506775598752. [DOI] [PubMed] [Google Scholar]
- 28.Gur A, Geyik MF, Dikici B, Nas K, Cevik R, Sarac J, et al. Complications of brucellosis in different age groups: a study of 283 cases in southeastern Anatolia of Turkey. Yonsei Med J. 2003;44:33–44. doi: 10.3349/ymj.2003.44.1.33. [DOI] [PubMed] [Google Scholar]
- 29.Hizel K, Guzel O, Dizbay M, Karakus R, Senol E, Arman D, et al. Age and duration of disease as factors affecting clinical findings and sacroiliitis in brucellosis. Infection. 2007;35:434–7. doi: 10.1007/s15010-007-6361-z. [DOI] [PubMed] [Google Scholar]
- 30.Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, Fenech FF. Human brucellosis in Kuwait: a prospective study of 400 cases. Q J Med. 1988;66:39–54. [PubMed] [Google Scholar]
- 31.Demirturk N, Demirdal T, Erben N, Demir S, Asci Z, Kilit TP, et al. Brucellosis: a retrospective evaluation of 99 cases and review of brucellosis treatment. Trop Doct. 2008;38:59–62. doi: 10.1258/td.2006.006266. [DOI] [PubMed] [Google Scholar]
- 32.Namiduru M, Gungor K, Dikensoy O, Baydar I, Ekinci E, Karaoglan I, et al. Epidemiological, clinical and laboratory features of brucellosis: a prospective evaluation of 120 adult patients. Int J Clin Pract. 2003;57:20–4. [PubMed] [Google Scholar]
- 33.Colmenero JD, Reguera JM, Cabrera FP, Cisneros JM, Orjuela DL, Fernandez-Crehuet J. Serology, clinical manifestations and treatment of brucellosis in different age groups. Infection. 1990;18:152–6. doi: 10.1007/BF01642103. [DOI] [PubMed] [Google Scholar]
- 34.Ayatollahi J. Epidemiological, clinical, diagnostic and therapeutic survey of 686 cases of brucellosis. Ann Saudi Med. 2004;24:398–9. doi: 10.5144/0256-4947.2004.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feiz J, Sabbaghian H. Miralai. Brucellosis due to Brucella melitensis in children. Clin Pediatr. 1978;17:904–7. doi: 10.1177/000992287801701210. [DOI] [PubMed] [Google Scholar]
- 36.Pfischner WC, Jr, Ishak KG, Neptune EM, Jr, Fox SM, III, Farid Z, El Din GN. Brucellosis in Egypt; a review of experience with 228 patients. Am J Med. 1957;22:915–29. doi: 10.1016/0002-9343(57)90027-X. [DOI] [PubMed] [Google Scholar]
- 37.Fallatah SM, Oduloju AJ, Al-Dusari SN, Fakunle YM. Human brucellosis in Northern Saudi Arabia. Saudi Med J. 2005;26:1562–6. [PubMed] [Google Scholar]
- 38.Bikas C, Jelastopulu E, Leotsinidis M, Kondakis X. Epidemiology of human brucellosis in a rural area of north-western Peloponnese in Greece. Eur J Epidemiol. 2003;18:267–74. doi: 10.1023/A:1023368420840. [DOI] [PubMed] [Google Scholar]
- 39.Avdikou I, Maipa V, Alamanos Y. Epidemiology of human brucellosis in a defined area of Northwestern Greece. Epidemiol Infect. 2005;133:905–10. doi: 10.1017/S0950268805003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivero-Puente A, Maravi Poma E, Garcia Carasusan M, Gamboa J, Perez C, Eguaras J, et al. Brucellosis: study of 222 cases. II. Clinical aspects of acute brucellosis. Rev Clin Esp. 1982;166:59–63. [in Spanish] [PubMed] [Google Scholar]
- 41.Minas M, Minas A, Gourgulianis K, Stournara A. Epidemiological and clinical aspects of human brucellosis in Central Greece. Jpn J Infect Dis. 2007;60:362–6. [PubMed] [Google Scholar]
- 42.Colmenero JD, Reguera JM, Martos F, Sanchez-De-Mora D, Delgado M, Causse M, et al. Complications associated with Brucella melitensis infection: a study of 530 cases. Medicine. 1996;75:195–211. doi: 10.1097/00005792-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Sit D, Kadiroglu AK, Kayabasi H, Hosoglu S. Retrospective evaluation of brucellosis cases inhabiting in Mus province. Mikrobiyol Bul. 2006;40:289–90. [in Turkish] [PubMed] [Google Scholar]
- 44.Aydoslu B, Celik AD, Kuloglu F, Tansel O, Akata F, Tugrul M. Evaluation of brucellosis patients in Trakya University Hospital. Mikrobiyol Bul. 2006;40:257–63. [in Turkish] [PubMed] [Google Scholar]
- 45.Malik GM. A clinical study of brucellosis in adults in the Asir region of southern Saudi Arabia. Am J Trop Med Hyg. 1997;56:375–7. doi: 10.4269/ajtmh.1997.56.375. [DOI] [PubMed] [Google Scholar]
- 46.Gotuzzo E, Seas C, Guerra JG, Carrillo C, Bocanegra TS, Calvo A, et al. Brucellar arthritis: a study of 39 Peruvian families. Ann Rheum Dis. 1987;46:506–9. doi: 10.1136/ard.46.7.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samra Y, Shaked Y, Hertz M, Altman G. Brucellosis: difficulties in diagnosis and a report on 38 cases. Infection. 1983;11:310–2. doi: 10.1007/BF01641353. [DOI] [PubMed] [Google Scholar]
- 48.Solera J, Lozano E, Martinez-Alfaro E, Espinosa A, Castillejos ML, Abad L. Brucellar spondylitis: review of 35 cases and literature survey. Clin Infect Dis. 1999;29:1440–9. doi: 10.1086/313524. [DOI] [PubMed] [Google Scholar]
- 49.Tabak F, Hakko E, Mete B, Ozaras R, Mert A, Ozturk R. Is family screening necessary in brucellosis? Infection. 2008;36:575–7. doi: 10.1007/s15010-008-7022-6. [DOI] [PubMed] [Google Scholar]
- 50.Mantur BG, Amarnath SK. Brucellosis in India – a review. J Biosci. 2008;33:539–47. doi: 10.1007/s12038-008-0072-1. [DOI] [PubMed] [Google Scholar]
- 51.Solera J, Martínez-Alfaro E, Espinosa A, Castillejos ML, Geijo P, Rodriguez-Zapata M. Multivariate model for predicting relapse in human brucellosis. J Infect. 1998;36:85–92. doi: 10.1016/S0163-4453(98)93342-4. [DOI] [PubMed] [Google Scholar]
- 52.Ariza J, Corredoira J, Pallares R, Viladrich PF, Rufi G, Pujol M, et al. Characteristics of and risk factors for relapse of brucellosis in humans. Clin Infect Dis. 1995;20:1241–9. doi: 10.1093/clinids/20.5.1241. [DOI] [PubMed] [Google Scholar]
- 53.Kunda J, Fitzpatrick J, Kazwala R, French NP, Shirima G, MacMillan A, et al. Health-seeking behavior of human brucellosis cases in rural Tanzania. BMC Public Health. 2007;7:315. doi: 10.1186/1471-2458-7-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mantur BG, Amarnath SK, Shinde RS. Review of clinical and laboratory features of human brucellosis. Indian J Med Microbiol. 2007;25:188–202. doi: 10.4103/0255-0857.34758. [DOI] [PubMed] [Google Scholar]
- 55.Zancada Díaz de Entre-Sotos F, Roldan Montaud A, Fernandez Ballesteros A, Jimenez Jimenez FJ, Agulla Budino A. Brucellosis: a clinico-serological study in a rural health area. Rev Clin Esp. 1992;191:8–12. [in Spanish] [PubMed] [Google Scholar]
- 56.Mantur BG, Biradar MS, Bidri RC, Mulimani MS, Veerappa K, Kariholu PJ, et al. Protean clinical manifestations and diagnostic challenges of human brucellosis in adults: 16 years' experience in an endemic area. Med Microbiol. 2006;55:897–903. doi: 10.1099/jmm.0.46097-0. [DOI] [PubMed] [Google Scholar]
- 57.Young EJ. Serologic diagnosis of human brucellosis: analysis of 214 cases by agglutination tests and review of the literature. Rev Infect Dis. 1991;13:359–72. doi: 10.1093/clinids/13.3.359. [DOI] [PubMed] [Google Scholar]
- 58.Casao MA, Navarro E, Solera J. Evaluation of Brucellacapt for the diagnosis of human brucellosis. J Infect. 2004;49:102–8. doi: 10.1016/j.jinf.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Serra J, Velasco J, Godoy P, Mendoza J. Can the Brucellacapt test be substituted for the Coombs test in the diagnosis of human brucellosis? Enferm Infecc Microbiol Clin. 2001;19:202–5. doi: 10.1016/s0213-005x(01)72613-4. [in Spanish] [DOI] [PubMed] [Google Scholar]
- 60.Solera J. Treatment of human brucellosis. J Med Liban. 2000;48:255–63. [PubMed] [Google Scholar]
- 61.Joint Food and Agriculture Organization (FAO), World Health Organization (WHO). FAO-WHO expert committee on brucellosis (sixth report). WHO technical report series, no 740. Geneva: World Health Organization; 1986. [PubMed] [Google Scholar]
- 62.Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 2007;4:e317. doi: 10.1371/journal.pmed.0040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ranjbar M, Keramat F, Mamani M, Kia AR, Khalilian FO, Hashemi SH, et al. Comparison between doxycycline-rifampin-amikacin and doxycycline-rifampin regimens in the treatment of brucellosis. Int J Infect Dis. 2007;11:152–6. doi: 10.1016/j.ijid.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Bayindir Y, Sonmez E, Aladag A, Buyukberber N. Comparison of five antimicrobial regimens for the treatment of brucellar spondylitis: a prospective, randomized study. J Chemother. 2003;15:466–71. doi: 10.1179/joc.2003.15.5.466. [DOI] [PubMed] [Google Scholar]
- 65.Mantur BG, Akki AS, Mangalgi SS, Patil SV, Gobbur RH, Peerapur BV. Childhood brucellosis – a microbiological, epidemiological and clinical study. J Trop Pediatr. 2004;50:153–7. doi: 10.1093/tropej/50.3.153. [DOI] [PubMed] [Google Scholar]
- 66.al-Eissa YA, Kambal AM, al-Nasser MN, al-Habib SA, al-Fawaz IM, al-Zamil FA. Childhood brucellosis: a study of 102 cases. Pediatr Infect Dis J. 1990;9:74–9. doi: 10.1097/00006454-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Williams E. The Mediterranean fever commission: its origin and achievements. In: Young EJ, Corbel MJ, editors. Brucellosis: clinical and laboratory aspects. Boca Raton (FL): CRC Press Ink; 1989. p.22. [Google Scholar]
- 68.Gul HC, Erdem H, Bek S. Overview of neurobrucellosis: a pooled analysis of 187 cases. Int J Infect Dis. Int J Infect Dis. 2009;13:e339–43. doi: 10.1016/j.ijid.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Dahouk SA, Neubauer H, Hensel A, Schoneberg I, Nockler K, Alpers K, et al. Changing epidemiology of human brucellosis, Germany, 1962-2005. Emerg Infect Dis. 2007;13:1895–900. doi: 10.3201/eid1312.070527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Onyemelukwe GC. Brucellosis in northern Nigerians. West Afr J Med. 1989;8:234–40. [PubMed] [Google Scholar]
- 71.Colmenero JD, Ruiz-Mesa JD, Plata A, Bermudez P, Martín-Rico P, Queipo-Ortuno MI, et al. Clinical findings, therapeutic approach, and outcome of brucellar vertebral osteomyelitis. Clin Infect Dis. 2008;46:426–33. doi: 10.1086/525266. [DOI] [PubMed] [Google Scholar]
- 72.Reguera JM, Alarcon A, Miralles F, Pachon J, Juarez C, Colmenero JD. Brucella endocarditis: clinical, diagnostic, and therapeutic approach. Eur J Clin Microbiol Infect Dis. 2003;22:647–50. doi: 10.1007/s10096-003-1026-z. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira P, Gama P, Correia J, Nunes L, Pipa J, Nascimento C, et al. Brucella endocarditis – case report and literature review. Rev Port Cardiol. 2008;27:1309–15. [PubMed] [Google Scholar]
- 74.Gunes Y, Tuncer M, Guntekin U, Akdag S, Ali Gumrukcuoglu H, Karahocagil M, et al. Clinical characteristics and outcome of Brucella endocarditis. Trop Doct. 2009;39:85–8. doi: 10.1258/td.2008.070436. [DOI] [PubMed] [Google Scholar]
- 75.Kress S, Klooker P, Kaufmann V, Sloot N, Riemann JF, Brass H. Brucellosis with fatal endotoxic shock. Med Klin (Munich) 1997;92:561–6. doi: 10.1007/BF03044933. [in German] [DOI] [PubMed] [Google Scholar]
- 76.McLean DR, Russell N, Khan MY. Neurobrucellosis: clinical and therapeutic features. Clin Infect Dis. 1992;15:582–90. doi: 10.1093/clind/15.4.582. [DOI] [PubMed] [Google Scholar]
- 77.Pandit VR, Seshadri S, Valsalan R, Bahuleyan S, Vandana KE, Kori P. Acute brucellosis complicated by fatal myocarditis. Int J Infect Dis. 2010;14:e358–60. doi: 10.1016/j.ijid.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 78.Park KW, Kim DM, Park CY, Kim HL, Jang SJ, Choi YS, et al. Fatal systemic infection with multifocal liver and lung nodules caused by Brucella abortus. Am J Trop Med Hyg. 2007;77:1120–3. [PubMed] [Google Scholar]
- 79.Young EJ, Tarry A, Genta RM, Ayden N, Gotuzzo E. Thrombocytopenic purpura associated with brucellosis: report of 2 cases and literature review. Clin Infect Dis. 2000;31:904–9. doi: 10.1086/318129. [DOI] [PubMed] [Google Scholar]
- 80.Roushan MR, Gangi SM, Ahmadi SA. Comparison of the efficacy of two months of treatment with co-trimoxazole plus doxycycline vs. co-trimoxazole plus rifampin in brucellosis. Swiss Med Wkly. 2004;134:564–8. doi: 10.4414/smw.2004.10665. [DOI] [PubMed] [Google Scholar]
- 81.Solera J, Rodríguez-Zapata M, Geijo P, Largo J, Paulino J, Saez L, et al. Doxycycline-rifampin versus doxycycline-streptomycin in treatment of human brucellosis due to Brucella melitensis. The GECMEI Group. Grupo de Estudio de Castilla-la Mancha de Enfermedades Infecciosas. Antimicrob Agents Chemother. 1995;39:2061–7. doi: 10.1128/aac.39.9.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agalar C, Usubutun S, Turkyilmaz R. Ciprofloxacin and rifampicin versus doxycycline and rifampicin in the treatment of brucellosis. Eur J Clin Microbiol Infect Dis. 1999;18:535–8. doi: 10.1007/s100960050344. [DOI] [PubMed] [Google Scholar]
- 83.Karabay O, Sencan I, Kayas D, Sahin I. Ofloxacin plus rifampicin versus doxycycline plus rifampicin in the treatment of brucellosis: a randomized clinical trial. BMC Infect Dis. 2004;4:18. doi: 10.1186/1471-2334-4-18. [ISRCTN11871179] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadadi A, Rasoulinejad M. HajiAbdolbaghi M, Mohraz M, Khashayar P. Clinical profile and management of brucellosis in Tehran – Iran. Acta Clin Belg. 2009;64:11–5. doi: 10.1179/acb.2009.004. [DOI] [PubMed] [Google Scholar]
- 85.Ersoy Y, Sonmez E, Tevfik MR, But AD. Comparison of three different combination therapies in the treatment of human brucellosis. Trop Doct. 2005;35:210–2. doi: 10.1258/004947505774938765. [DOI] [PubMed] [Google Scholar]
- 86.Roushan MR, Gangi SM, Ahmadi SA. Comparison of the efficacy of two months of treatment with co-trimoxazole plus doxycycline vs. co-trimoxazole plus rifampin in brucellosis. Swiss Med Wkly. 2004;134:564–8. doi: 10.4414/smw.2004.10665. [DOI] [PubMed] [Google Scholar]
- 87.Ariza J, Gudiol F, Valverde J, Pallares R, Fernandez-Viladrich P, Rufi G, et al. Brucellar spondylitis: a detailed analysis based on current findings. Rev Infect Dis. 1985;7:656–64. doi: 10.1093/clinids/7.5.656. [DOI] [PubMed] [Google Scholar]