Abstract
Alzheimer, Parkinson and other neurodegenerative diseases involve a series of brain proteins, referred to as ‘amyloidogenic proteins’, with exceptional conformational plasticity and a high propensity for self-aggregation. Although the mechanisms by which amyloidogenic proteins kill neural cells are not fully understood, a common feature is the concentration of unstructured amyloidogenic monomers on bidimensional membrane lattices. Membrane-bound monomers undergo a series of lipid-dependent conformational changes, leading to the formation of oligomers of varying toxicity rich in β-sheet structures (annular pores, amyloid fibrils) or in α-helix structures (transmembrane channels). Condensed membrane nano- or microdomains formed by sphingolipids and cholesterol are privileged sites for the binding and oligomerisation of amyloidogenic proteins. By controlling the balance between unstructured monomers and α or β conformers (the chaperone effect), sphingolipids can either inhibit or stimulate the oligomerisation of amyloidogenic proteins. Cholesterol has a dual role: regulation of protein–sphingolipid interactions through a fine tuning of sphingolipid conformation (indirect effect), and facilitation of pore (or channel) formation through direct binding to amyloidogenic proteins. Deciphering this complex network of molecular interactions in the context of age- and disease-related evolution of brain lipid expression will help understanding of how amyloidogenic proteins induce neural toxicity and will stimulate the development of innovative therapies for neurodegenerative diseases.
The discovery that a significant proportion of cellular proteins do not have a well-defined structure under physiological conditions is one of the most fascinating concepts in modern biology (Ref. 1). This iconoclastic finding has shaken the broadly accepted dogma that a protein is predisposed to fold into a unique structure that is specified by its amino acid sequence.
Proteins that lack a well-defined 3D structure have been referred to as ‘intrinsically disordered proteins’ (IDPs), and they constitute the part of the proteome called the ‘unfoldome’ (Ref. 2). IDPs perform a wide range of regulatory functions related to signal transduction, molecular recognition and molecular assembly (Ref. 3), and several have been implicated in fatal human pathologies (Ref. 4). One representative member of IDPs is α-synuclein (encoded by SNCA), a brain protein associated with Parkinson disease. Depending on its environment, α-synuclein can adopt a variety of structurally unrelated conformations (Fig. 1), from a natively unfolded (mostly disordered) state to different α-helical or β-structural species folded to a different degree (Ref. 5). The protein can also self-aggregate and form various types of supramolecular assemblies with different morphology, including oligomers, amorphous aggregates and amyloid-like fibrils (Ref. 5). This incredible level of conformational plasticity is also a hallmark of other amyloidogenic proteins, such as amyloid β (Aβ; encoded by APP) involved in Alzheimer disease and the prion protein (PrP; encoded by PRNP) involved in Creutzfeldt–Jakob disease and prion infections (Ref. 6). Some amyloidogenic proteins (Alzheimer Aβ peptides, α-synuclein) belong to the IDP family; in other cases (e.g. PrP), the protein has a 3D structure but can misfold into an alternative structure that is prone to aggregation. There are several mechanisms by which an amyloidogenic protein can acquire a disease-related 3D structure: overexpression, mutations or other genetic alterations, and exposure to harmful environmental conditions (Ref. 2). These mechanisms have been extensively studied during the past two decades. However, how amyloidogenic proteins induce cell death in these neurodegenerative diseases is still a mystery. Given the high prevalence and the fatal outcome of these pathologies in humans, this is clearly one of the most important challenges of biomedical research for the 21st century.
Figure 1.
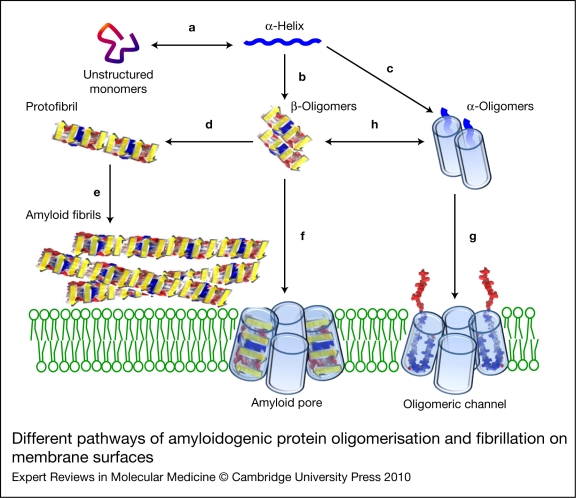
Different pathways of amyloidogenic protein oligomerisation and fibrillation on membrane surfaces. Upon interaction with neuronal membranes, unstructured soluble monomers of amyloidogenic proteins undergo an α-helix shift of their conformation (a). Further accumulation of the proteins on the surface of the membrane induces oligomerisation into β-sheet aggregates (b) or α-oligomers (c). Oligomers with a β-sheet structure can form protofibrils (d), amyloid fibrils (e) and amyloid pores (annular protofibrils) with ion-channel properties (f). In some instances (e.g. for α-synuclein), transmembrane channels can also be generated by oligomers with an α-helix structure (g). In all cases, the formation of functional ion channels requires the insertion and assembly of the oligomers in the neuronal membrane (green lipid bilayer). Note the possibility of conversion between α- and β-oligomers (h).
Among the different research strategies in this field, those aiming at identifying common features in distinct amyloidogenic proteins have been particularly fruitful. Obviously, it is somewhat paradoxical that the similar behaviour of amyloidogenic proteins – that is, their conformational plasticity and aggregation properties – does not rely on any kind of homology in their amino acid sequence. Instead, these common features might involve the 3D structures of these proteins. A major breakthrough was the identification of a common epitope in oligomers formed by various amyloidogenic proteins (Ref. 7). This discovery suggested a universal mode of oligomerisation underlying a common mechanism of toxicity. Moreover, conformation-dependent antibodies that recognise generic epitopes have been used as a structural basis to distinguish amyloid prefibrillar and fibrillar oligomers (Ref. 8). A second breakthrough was the demonstration that amyloidogenic proteins can assemble into annular structures forming amyloid pores with ion channel properties (Refs 9, 10) (Fig. 1). This finding suggested that amyloid oligomers could perturb membrane permeability (e.g. cellular calcium fluxes) and thus kill neuronal cells more efficiently than amyloid fibrils. This mechanism, originally proposed by Arispe et al. (Refs 11, 12) for explaining the toxicity of Aβ, is now commonly referred to as the β-amyloid calcium channel hypothesis (Ref. 13). However, despite their fruitful contribution to the field, these important discoveries did not really inform us on the molecular mechanisms involved in the different modes of amyloid oligomerisation and aggregation. To progress towards this knowledge, we have to consider another common feature tightly linked to the biology of amyloids in the brain, which lies in their immediate environment – that is, biological membranes (Refs 13, 14). Indeed, Aβ peptides are generated by the proteolytic cleavage of a transmembrane precursor (the amyloid protein precursor, APP) (Ref. 15), PrP is a membrane protein anchored in the external leaflet of the plasma membrane by a glycosylphosphatidylinositol anchor (Ref. 16), and α-synuclein is involved in the traffic of synaptic vesicles (Ref. 17).
Amyloidogenic proteins reside in a lipid world constituted by a myriad of lipid-based structures such as internal membrane networks, plasma membrane nano- and microdomains, lipoproteins and exosomes. This is not totally surprising if one considers that lipids are the most abundant organic compounds found in the brain, accounting for up to 50% of its dry weight (Ref. 18). As in other mammalian tissues, brain lipids consist of three major categories in roughly equimolar proportions (Ref. 19): glycerophospholipids, sphingolipids and cholesterol. Sphingolipids and cholesterol self-aggregate into specific membrane domains referred to as nanodomains, microdomains, caveolae, lipid shells or lipid rafts (Refs 20, 21). These sphingolipid–cholesterol-enriched domains (the so-called lipid rafts) are in a liquid-ordered (Lo) phase floating in the more liquid glycerophospholipid-rich and cholesterol-poor bulk (Ld phase) of the plasma membrane. Lipid rafts are chiefly involved in cellular trafficking and signalling functions (Ref. 22) and are also preferential sites for host interactions with pathogens and toxins (Ref. 23).
The aim of this review is to emphasise the specific role of cholesterol and sphingolipids, alone and in combination, in the interaction between amyloidogenic proteins and neural membranes, and to figure out how these interactions can affect the structural and functional properties of these proteins. We also discuss the impact of age- and disease-related changes in brain lipid content on the pathophysiology of amyloid-associated neurodegenerative diseases. We do not intend to present a separate section for each amyloidogenic protein (Aβ, α-synuclein, PrP, etc.). Instead, it is our goal to identify common membrane-based mechanisms that, at least at the molecular level, might apply to any type of amyloidogenic protein and thus could serve as a solid biochemical background for studying amyloid toxicity in the brain.
Mechanisms of concentration of amyloidogenic proteins on neuronal membranes
Reduction of dimensionality
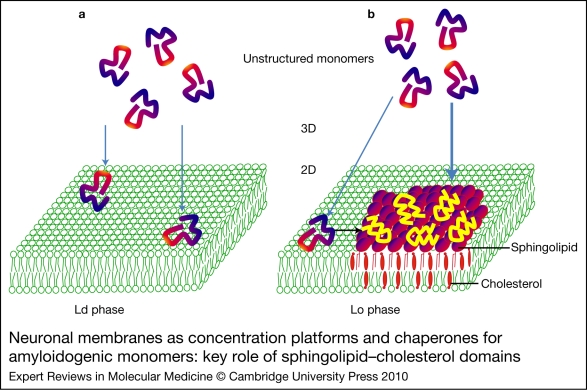
There are two main mechanisms ensuring the efficient transfer of amyloidogenic proteins from the cytosolic or extracellular milieu to neuronal plasma membranes. The first one is the reduction of dimensionality (from 3D to 2D) that occurs when a soluble protein dissolved in the bulk solvent binds to a membrane surface (Ref. 24). The underlying idea is that the membrane 2D space can concentrate proteins far more efficiently than the 3D space of the bulk solvent. As a consequence, the average distance between two proteins is shorter in 2D than in 3D, and this effect is more pronounced for higher protein concentrations (Ref. 25). This means that increased concentration of a protein lowers the average protein–protein distance more efficiently on a 2D surface than in a 3D space. Consequently, protein–protein interactions are favoured in the 2D space. This phenomenon is particularly important for amyloidogenic proteins that tend to self-aggregate after a conformational transition (Fig. 2). Thus, the simple fact of the concentration of monomers in a restricted membrane area is, by itself, a crucial step in the complex process of amyloid oligomerisation/aggregation.
Figure 2.
Neuronal membranes as concentration platforms and chaperones for amyloidogenic monomers: key role of sphingolipid–cholesterol domains. (a) Amyloidogenic monomers have a low affinity for the liquid-disordered (Ld) phase of plasma membranes enriched in phosphatidylcholine. (b) By contrast, the monomers have a high affinity for sphingolipid–cholesterol domains in the liquid-ordered (Lo) phase. In this case, monomers are not only concentrated on the membrane surface, but also undergo a major conformational change induced by the sphingolipids. This property is referred to as the chaperone effect of sphingolipids on amyloidogenic proteins.
Specific binding to membrane lipids
The second mechanism explaining the affinity of amyloidogenic proteins for neuronal membranes is lipid specificity. Converging data obtained from various laboratories with a broad range of experimental approaches have shown that amyloidogenic proteins interact with the lipids found in the Lo phase – that is, sphingolipids and cholesterol (Fig. 2). Alzheimer Aβ peptides recognise several glycosphingolipids, including neutral species such as asialo-GM1 or galactosylceramide (GalCer) as well as gangliosides such as GM1 (Refs 26, 27, 28, 29); α-synuclein binds to gangliosides GM1 and GM3 (Refs 30, 31); PrP interacts with sphingomyelin, GalCer, GM1 and GM3 (Refs 27, 32, 33) and is associated with sphingolipid signalling platforms (Ref. 34). Overall, this high affinity for sphingolipids determines the concentration of amyloid proteins in lipid raft areas of the extracellular leaflet of the plasma membrane. Several amyloidogenic proteins can also bind to negatively charged glycerophospholipids (Refs 35, 36), which are enriched in the cytoplasmic leaflet of the plasma membrane (Ref. 19). This type of interaction has been extensively studied for α-synuclein, which recognises anionic phosphatidylserine and phosphatidylglycerol (Refs 36, 37, 38, 39). In this review, we restrict our analysis to the role of cholesterol and sphingolipids in the interaction of amyloidogenic proteins and membranes. As we will see, these lipids play a more active role in the conformation, oligomerisation and aggregation of amyloidogenic proteins than just acting as a concentration platform.
Molecular mechanisms of sphingolipid–amyloidogenic-protein interactions
Structural diversity of membrane sphingolipids
That the same amyloidogenic protein could interact with several distinct sphingolipids (Refs 26, 27, 31) could be viewed as paradoxical and difficult to reconcile with a real specificity of interaction. A biochemical analysis of sphingolipid structures is necessary to get a clearer view on this issue. Sphingolipids are formed by the condensation of a fatty acid chain with a sphingoid base (which is generally sphingosine although five different sphingoid bases have been detected in mammalian cells). The biochemical diversity of sphingolipids is first generated by the numerous fatty acids (more than 20, varying in chain length, degree of saturation and degree of hydroxylation) that can be attached to the sphingoid base to form a ceramide (Ref. 40). Sphingolipids are then classified into sphingomyelin and glycosphingolipids, according to the biochemical nature of the polar part attached to ceramide: phosphorylcholine for sphingomyelin, and a glycan moiety for glycosphingolipids. Several hundred different carbohydrate structures have been described in glycosphingolipids, with various combinations of neutral sugars, sulfated sugars and sialic acids (Ref. 19).
Cracking the code governing glycosphingolipid–protein interactions
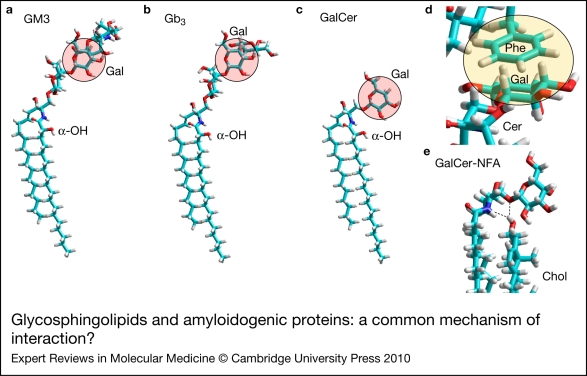
Despite this huge structural diversity, glycosphingolipids can share common structural fingerprints that render them ‘readable’ by the same protein. This is the case for the glycosphingolipids GalCer, globotriaosylceramide (Gb3) and GM3, which have a distinct sugar moiety but a common galactosyl residue with the same orientation (Fig. 3a–c). As a logical consequence of this common structural feature, a protein that recognises one of these glycosphingolipids (e.g. GalCer), through binding to this galactose residue, might also interact with the other two (in this case, Gb3 and GM3). This means that the biochemical code governing glycosphingolipid–protein interactions is degenerate. An example of this striking property is the V3 loop domain of the human immunodeficiency virus 1 (HIV-1) surface-envelope glycoprotein gp120, which was shown to recognise first GalCer (Refs 41, 42, 43), and then, several years later, both Gb3 and GM3 (Refs 44, 45). However, there is a unique molecular mechanism that accounts for the interaction of each of these glycosphingolipids with gp120 (Ref. 46), and this mechanism also applies for amyloidogenic proteins.
Figure 3.
Glycosphingolipids and amyloidogenic proteins: a common mechanism of interaction? Despite their high level of biochemical diversity, some glycosphingolipids share a common galactosyl ring with an orientation compatible with the establishment of stacking CH-π interactions between the sugar and an aromatic residue. Such galactosyl rings are indicated in shaded circles in the sugar moiety of ganglioside GM3 (a), globotriaosylceramide Gb3 (b) and galactosylceramide GalCer (c). In these cases, the apolar side of the galactosyl ring fits particularly well with the aromatic-ring side chain of an aromatic residue (Phe, in this case) exposed at the surface of an amyloid protein (d). The electronic cloud of the π system stacks onto the CH groups of the galactosyl ring. This interaction is favoured by the common geometric structure of both rings, which allows a coordinated binding process between CH groups and π electrons. Cholesterol, which has a strong affinity for sphingolipids, can further improve the accessibility of the galactosyl group through fine tuning of glycosphingolipid conformation (e). This effect involves the establishment of an H-bond network between the OH group of cholesterol, the NH group of the sphingolipid and the oxygen atom of the glycosidic bond linking the ceramide and the sugar part of the sphingolipid. GalCer-NFA, GalCer with a nonhydroxylated fatty acid in the ceramide moiety.
Let us first consider the way in which proteins bind to GalCer, which is both the major lipid of myelin sheaths (Ref. 47) and the simplest possible brain-expressed glycosphingolipid, consisting of a ceramide linked to a single galactosyl group (i.e. a ceramide monohexoside). Most of the GalCer molecule is dipped into the membrane, so that the galactosyl moiety is the principal region of the glycolipid available for protein binding. In this respect, there is a functional similarity between proteins that recognise GalCer and galactose-specific lectins. However, the local concentration of galactosyl groups in a nanodomain of GalCer is extremely high compared with the maximal concentration of free galactose in biological fluids (another illustration of the reduction-in-dimensionality concept discussed above). This explains why even high concentrations of free galactose cannot inhibit GalCer–gp120 interactions (Ref. 41). Moreover, the conformation of the galactose head group in the glycolipid is restricted by the ceramide backbone, which allows only limited motion of the sugar (Ref. 48). By contrast, galactose in water solution is free to adopt a wide range of conformations. Finally, the most polar part of ceramide, which includes both amide and hydroxyl (OH) groups, as well as the β-glycosidic bond linking ceramide to galactose, can also participate in the binding reaction and further stabilise the GalCer–protein complex. Taken together, these biochemical properties of the galactosyl head group of GalCer explain why GalCer–protein interactions are distinct from galactose–lectin interactions (Ref. 49).
Key role of aromatic residues in protein–glycosphingolipid interactions
At the molecular level, numerous proteins that bind to GalCer have a solvent-exposed aromatic residue that provides a complementary surface for a stacking interaction with the galactose ring (Fig. 3d). As a result of the distribution of the OH groups linked to the pyranosyl ring of galactose, this sugar has two chemically distinct faces: one apolar with hydrocarbyl (CH) groups and the other polar with OH groups. Therefore, the apolar face of galactose is particularly suited for a stacking interaction with an aromatic structure (Ref. 50). This type of interaction between CH groups and a π-electron cloud system has been described and referred to as CH–π interaction by Nishio and co-workers (Ref. 51). The CH–π stacking mechanism is involved in the interaction between glycosphingolipids and several amyloidogenic proteins, including PrP (Ref. 49), Aβ (Ref. 52) and amylin (Ref. 53). In both Gb3 and GM3, the apolar side of the central galactosyl group has a similar orientation to that in GalCer (Fig. 3a–c); thus, GalCer-binding proteins are predicted to also bind to Gb3 and GM3 through CH–π stacking. Note that the conformation of the sugar moiety of glycosphingolipids is restricted by a network of H-bonds (Ref. 52) that involves either an OH group in the acyl chain of the ceramide (intramolecular network) or the OH group of cholesterol (intermolecular network, Fig. 3e), as further discussed below (see the section ‘The outside and inside effects of cholesterol’).
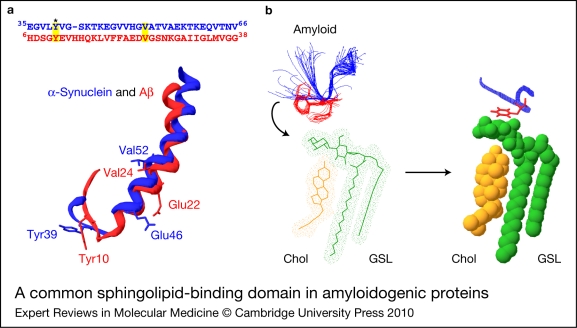
The notion that unrelated proteins, displaying a solvent-accessible aromatic residue in a hairpin domain, might bind these glycosphingolipids through a similar stacking mechanism has motivated the search for V3-like domains in various proteins, including amyloidogenic proteins (Ref. 27). This is the concept of the glycosphingolipid-binding domain or, more generally, of the sphingolipid-binding domain (Ref. 49). Such domains are characterised by the presence, in a flexible region of the surface of a protein, of an aromatic residue with its side chain oriented towards the solvent, as illustrated in Figure 4a with the superimposition of the glycosphingolipid-binding domains of Aβ and α-synuclein (Ref. 31). The recognition of the glycosphingolipid by this region of the protein is thought to proceed through an induced-fit mechanism involving the conformational mobility of the aromatic side chain and a concomitant adjustment of the orientation of the galactosyl head group (Fig. 4b).
Figure 4.
A common sphingolipid-binding domain in amyloidogenic proteins. (a) Amyloidogenic proteins share a common sphingolipid-binding domain, which can be shown by superimposing the structure of micelle-bound Alzheimer Aβ peptide [PDB ID: 1BA4, in red] (Ref. 150) onto the structure of micelle-bound α-synuclein [PDB ID: 1XQ8, in blue] (Ref. 142). The motif corresponds to a helix–turn–helix structure displaying an aromatic residue (Tyr10 for Aβ; Tyr39 for α-synuclein) oriented towards the solvent and located at a similar position in the loop. The location of Glu residues associated with Glu to Lys mutations in genetic forms of Alzheimer and Parkinson diseases is indicated (Glu22 for Aβ and Glu46 for α-synuclein, respectively). The amino acid sequence of both proteins (upper panel) show very little homology, apart from the above-mentioned Tyr residues (asterisk) and a common Val residue (Val52 for α-synuclein and Val24 for Aβ). (b) Due to the high conformational plasticity of amyloidogenic proteins, the sphingolipid-binding domain can adopt several distinct conformations, as shown for the core amyloid-forming motif of amylin [NFGAILSS octapeptide, PDB ID: 1KUW] (Ref. 68). The α-carbon chain of the peptide is coloured in blue, and the Phe residue in red. Twenty conformers obtained by nuclear magnetic resonance (NMR) analysis are superposed. Once bound to the glycosphingolipid (GSL, coloured in green), the amyloid peptide is locked in a unique conformation. Cholesterol (Chol) is coloured in orange. This drawing is based on data in Ref. 53 obtained with lactosylceramide (LacCer).
In summary, a glycosphingolipid-binding domain is a flexible domain containing turn-inducing (Gly, Pro), basic (Arg, Lys, His) and aromatic residues (Phe, Tyr, Trp) (Refs 27, 54, 55, 56, 57). The critical involvement of aromatic residues in the interaction between proteins and glycosphingolipids has been convincingly demonstrated by using synthetic peptides in which these residues were replaced by Ala or Leu (Refs 53, 54, 55): correspondingly, the interaction with the glycosphingolipid has been dramatically decreased or even totally lost. Reciprocally, conservative aromatic substitutions (e.g. Phe to Trp) did not affect binding to glycosphingolipids (Ref. 54). Taken together, these data show that the sphingolipid-binding domain mediates specific protein–glycosphingolipid interactions driven by CH–π stacking of a sugar and aromatic residue (Refs 52, 58).
Glycosphingolipids exert a chaperone effect on amyloidogenic proteins
Conformational changes mediated by sphingolipid-binding domains
One important consequence of protein–sphingolipid interactions is that the conformation of the protein can be significantly affected on binding to sphingolipids (Fig. 4b). This chaperone effect of sphingolipids is a direct consequence of the unique modalities of protein–sphingolipid interactions (Ref. 49). As soon as binding begins, the structure of the sphingolipid changes and the protein gradually adapts its shape to this moving target. A stable anchoring of the protein to the sphingolipids can be obtained by optimising protein–sphingolipid contacts, and this requires significant conformational changes of the protein. The flexibility of the sphingolipid-binding domain is particularly well suited to trigger structural rearrangements in the protein (Ref. 49). This unexpected consequence of protein–sphingolipid interactions has been extensively studied with HIV-1. Briefly, on binding to raft glycosphingolipids through its V3 domain, HIV-1 gp120 undergoes a series of conformational changes that are required for virus fusion (Refs 19, 45). Because the sphingolipid-binding domain is evolutionarily conserved (it has been found in virus, bacteria, insect and mammalian proteins), it is likely that sphingolipids can modulate the conformation and regulate the function of a wide range of proteins (Refs 52, 53, 54, 55, 56, 57).
Chaperone effect of ganglioside GM1 on Alzheimer Aβ peptides
How could this concept of a sphingolipid-mediated chaperone effect apply to membrane-bound amyloids? Seminal work by Matsuzaki, Yanagasiwa and co-workers has shown that lipid rafts act as surface catalysts able to accelerate the aggregation of Aβ (Refs 29, 59, 60, 61, 62, 63). Most interestingly, GM1-bound Aβ has been identified as a pre-amyloid form acting as an endogenous seed for the formation of neurotoxic amyloid fibrils in Alzheimer disease (Refs 29, 61, 62, 63). These data have not only strengthened the role of lipid rafts as preferential binding sites for unstructured amyloidogenic proteins on neuronal membranes but have also suggested that these membrane domains behave as conformational catalysts for amyloid formation. In other words, lipid rafts can control the conformation of membrane-bound amyloid through a chaperone effect (Refs 49, 59, 64). Yet, conflicting data have suggested that binding of Aβ to GM1 induces a conformational transition from random coil to a protective α-helical structure (Ref. 65), as also observed for α-synuclein (Ref. 30), or to a fibrillation-prone β-structure (Refs 61, 66). The structure of amyloidogenic proteins bound to GM1 depends on several physicochemical parameters, including pH, membrane fluidity, GM1-carrier lipid ratios, protein concentration and the absence or presence of cholesterol, which could explain the discrepancies reported in the literature. In any case, the available data suggest that lipid rafts could (1) modulate the conformational changes of unstructured monomers from the unfolded state to more-ordered α or β structures, and (2) control the balance between α and β structures, which in turn determine the oligomerisation/aggregation status of amyloidogenic proteins.
Kakio et al. (Ref. 59) have shown that the oligomerisation and aggregation of Aβ peptides occur in ganglioside-enriched domains of the plasma membrane. A possible sequence of events could be as follows: (1) soluble Aβ monomers bind to the sugar head group of gangliosides in cholesterol–sphingolipid-enriched domains such as lipid rafts; (2) on binding to gangliosides, Aβ is constrained to adopt an α-helical structure; (3) as the protein density on the membrane increases, Aβ undergoes a conformational transition from an α-helix-rich structure to a β-strand-rich one; and (4) the ganglioside-bound Aβ complex with acquired secondary structures serves as a seed for amyloid fibril formation. Indeed, Aβ fibrils with high toxicity have been successfully generated in an acellular system using GM1-containing raft-like liposomes (Ref. 67). Altogether, these data strongly support the concept that the ganglioside cluster can act as a chaperone able to lower the activation energy for the conformational changes of Aβ (Ref. 59). This scenario is also consistent with the involvement of the glycosphingolipid-binding domain in membrane–amyloid interactions (Refs 53, 64).
How glycosphingolipids could stabilise an α-helix and prevent amyloid formation
Unstructured amyloidogenic proteins can adopt a wide range of conformations in water and in biological membranes (Fig. 1). The conformational flexibility of the amyloid core peptide of amylin (NFGAILSS) has been studied by nuclear magnetic resonance (NMR) (Ref. 68). In a membrane-like micellar environment, this peptide can adopt three distinct classes of conformations owing to the wide flexibility of the peptide chain (Fig. 4b). Correspondingly, the unique Phe residue of the motif has three main possible orientations (Ref. 53). On binding to a glycosphingolipid bearing an appropriately oriented galactosyl group (lactosylceramide in Fig. 4b), the Phe residue stacks onto the sugar so that only one of the numerous peptide conformations is selected and locked.
This illustrates the potency of CH–π stacking interactions to stabilise a thermodynamically unstable conformation at a minimal energetic cost, just like a wedge can efficiently block a car on a sloping street (Ref. 49). This simple but efficient mechanism could allow raft glycosphingolipids to act as molecular chaperones inducing α-helix-rich structures in amyloidogenic proteins (Fig. 5). In the case of PrP, the stabilisation of an α-helix structure is beneficial, because this can prevent protein misfolding and aggregation (Ref. 49). However, the α-helix conformation could also facilitate membrane insertion and channel/pore formation, leading to dramatic perturbations of membrane permeability, as shown for α-synuclein (Ref. 69). In addition, the chaperone effect of glycosphingolipids is not irreversible. Changing the physicochemical properties of the raft–protein complex, for example through oxidative damage to the proteins or lipids (Refs 70, 71), could theoretically induce the dissociation of the amyloid peptides from glycosphingolipids such as GalCer. Then, the sudden exposure of aromatic side chains would allow a tight packing (through π–π interactions) between adjacent helices. This would lead to the formation of a dimer, a key step in the generation of amyloid fibrils (Refs 72, 73), which is thought to proceed through an ordered polymerisation of β structures (Ref. 74).
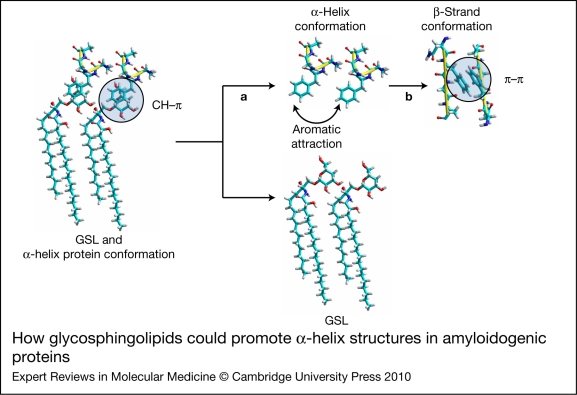
Figure 5.
How glycosphingolipids could promote α-helix structures in amyloidogenic proteins. When bound to glycosphingolipids (GSLs) through the sphingolipid-binding domain, the aromatic residues of two vicinal amyloidogenic proteins cannot interact with each other and the protein is forced to adopt an α-helix conformation. The molecular mechanism of this interaction is the CH–π stacking between the galactosyl ring of GalCer and the aromatic residue of the protein. The α-helix structure of amyloidogenic proteins is not only present in membrane-bound monomers (as illustrated in Fig. 2, right panel), but also in oligomeric α-synuclein channels (Fig. 1g). The β-amyloid aggregation process is triggered when the glycosphingolipid–protein complex is destabilised, resulting in the release of the protein from its glycosphingolipid chaperone (a). In this new glycosphingolipid-free environment, the aromatic residues of two vicinal amyloidogenic proteins can interact together (b), inducing a drastic conformational change from an initial α-helix to a β-strand structure. Amyloid aggregation then results from the π–π-driven assembly of aromatic side chains (for a review, see Ref. 79), forming amyloid dimers that in turn associate to form amyloid fibrils (as illustrated in Fig. 1e).
From this discussion, we would like to underscore (1) the key role of aromatic residues in the self-assembly of amyloid fibrils and (2) the chaperone effect of raft glycosphingolipids that could induce otherwise thermodynamically unstable α-helix structures of amyloid fibril-forming proteins. Indeed, most amyloidogenic proteins (including Aβ and PrP) have an amino acid sequence that is expected to form β but not α structures (Ref. 75). These proteins are referred to as ‘α-helix/β-strand discordant’. Lipid rafts can prevent those proteins from adopting the conformation they prefer, stabilising their structure in a ‘forced’ α conformation. This stabilisation can occur in the early biosynthetic pathway as demonstrated for PrP (Ref. 76). Inhibition of sphingolipid biosynthesis logically increases PrP misfolding into disease-associated β structures (Ref. 77). This mechanism seems to also apply for several neurotransmitter receptors (e.g. the nicotinic acetylcholine receptor) whose transport and assembly in the plasma membrane require the assistance of sphingolipids (Ref. 78). Mutations in amyloidogenic proteins that are associated with inherited forms of neurodegenerative diseases have been shown to directly affect sphingolipid binding. This is the case for the Creutzfeldt–Jakob-linked E200K mutation of PrP, which affects PrP–sphingomyelin interactions (Ref. 27).
In summary, by providing a complementary surface for amyloidogenic proteins, cellular glycosphingolipids might constitutively stabilise their α conformation and inhibit their aggregation, as recently demonstrated for amylin (Ref. 53). Replacing these glycosphingolipid–protein CH–π interactions with protein–protein π–π interactions (Refs 18, 50) would then trigger amyloid growth and deposition (Refs 49, 79). To what extent glycosphingolipids could also stimulate β-strand-mediated amyloid aggregation (Ref. 59) remains to be described and explained at the molecular scale. The mutual effect of GM1 and Aβ on their respective conformations is likely to displace the equilibrium of the reaction towards the formation of amyloid aggregates, as observed in vitro (Ref. 80).
Finally, it should be noted that noncovalent interactions distinct from CH–π stacking might also contribute to sphingolipid–amyloid interactions. These could include hydrophobic interactions (Ref. 81), H-bonds and electrostatic bridges (especially for sulfated glycosphingolipids, gangliosides and sphingomyelin) (Ref. 82). Indeed, in addition to aromatic side chains (Tyr10), several aliphatic (Ref. 81) and basic residues such as His13 have been implicated in GM1–Aβ interactions (Ref. 83). Anionic phospholipids such as phosphatidylserine, which bind to α-synuclein through electrostatic interactions (Ref. 38) and stimulate the formation of amyloid fibres (Ref. 84), could, in the cytoplasmic leaflet of the plasma membrane, have a role equivalent to the one played by gangliosides in the external leaflet.
The outside and inside effects of cholesterol
Cholesterol as a risk factor and a therapeutic target for neurodegenerative diseases
High cholesterol has been identified as a major risk factor for both Alzheimer (Ref. 85) and Parkinson (Ref. 86) diseases, and dysregulation of cholesterol homeostasis is a hallmark of neurodegenerative diseases (Ref. 87). Unfortunately, the effects of cholesterol on amyloid fibrillogenesis and toxicity are not well understood and the results reported so far are controversial (Ref. 88). Cholesterol directly binds to APP (Ref. 89) and stimulates its insertion into phospholipid monolayers (Ref. 90). It also binds to Aβ protofibrils (Ref. 91). However, whether cholesterol accelerates (Ref. 92) or decreases (Refs 93, 94) Aβ polymerisation is still uncertain. Moreover, the generation of Aβ peptides through APP proteolysis occurs within lipid rafts and is sensitive to inhibitors of cholesterol biosynthesis (Ref. 95), so that the involvement of cholesterol homeostasis in Alzheimer disease cannot be simply ascribed to the regulation of Aβ fibrillogenesis.
In the case of Parkinson disease, data obtained with both cultured neuronal cells and transgenic mice have shown that the cholesterol-depleting agent methyl-β-cyclodextrin decreased the level of α-synuclein in membrane fractions (Ref. 96). Moreover, metabolic inhibition of cholesterol biosynthesis with statins reduced the levels of α-synuclein accumulation in neuronal membranes, whereas cholesterol supplementation of cultured neurons increased α-synuclein aggregation (Ref. 97). Consistent with these studies, it was recently reported that lovastatin treatment of α-synuclein transgenic mice was associated with a marked reduction of α-synuclein aggregation and abrogation of neuronal pathology (Ref. 98). This suggests that treatment with cholesterol-lowering agents might be beneficial for patients with Parkinson disease. It has been suggested that oxidised cholesterol metabolites could accelerate α-synuclein aggregation (Ref. 71), which provides a mechanistic link between oxidative stress and Parkinson disease. Yet the molecular mechanisms by which cholesterol and/or its metabolites could affect the oligomerisation/aggregation status of amyloidogenic proteins have not been fully deciphered. Recent physicochemical data could shed some light on this complex issue.
Effects of cholesterol on sphingolipid conformation
First, cholesterol has a major impact on the conformation of sphingolipids. The apolar part of sphingolipids (i.e. the most important part of the ceramide) interacts with the smooth face of cholesterol, whereas the OH group of cholesterol is accessible to the polar part of the sphingolipid (Ref. 18). In the case of glycosphingolipids, this OH group is involved in an H-bond network that restricts the conformation of the sugar moiety in a parallel orientation with respect to the membrane. This effect is particularly important when the ceramide contains a nonhydroxylated fatty acid (NFA, Fig. 3e). Because this conformation of the sphingolipid is particularly suited for a sphingolipid-binding domain, cholesterol usually accelerates protein binding to sphingolipid (Ref. 99) and this also applies for amyloidogenic proteins (Ref. 52). By contrast, when the sphingolipid contains a hydroxylated fatty acid (HFA), the OH group of cholesterol is excluded from the H-bond network and it cannot exert its conformational effect on the sphingolipid. In this case, cholesterol does not improve but rather perturbs the organisation of sphingolipids and can even inhibit glycosphingolipid binding to amyloidogenic proteins (Ref. 52). Thus, according to the distribution of HFAs versus NFAs in sphingolipids, an increase of membrane cholesterol can lead to opposite effects on sphingolipid-mediated amyloidogenic protein binding and aggregation. In any case, these effects are due to a fine tuning of sphingolipid conformation induced by cholesterol, and do not involve any kind of physical interaction between amyloidogenic proteins and cholesterol.
Amyloidogenic proteins interact first with sphingolipids and then with cholesterol
By contrast, the second mechanism involves a direct interaction between the amyloidogenic protein and cholesterol. Therefore, this effect requires the insertion of a part of the amyloid protein in the membrane; otherwise the protein would not be in physical contact with cholesterol. Devanathan et al. (Ref. 100) have shown that Aβ peptide aggregation on the bilayer surface requires a sphingomyelin-rich environment but can occur in the absence of cholesterol. This is in line with our hypothesis that sphingolipids are fully responsible for the initial binding of amyloidogenic proteins to lipid rafts, and that cholesterol is not directly involved at this stage. However, at the second step the presence of cholesterol may greatly facilitate peptide insertion into the bilayer. The preferential interaction of amyloidogenic proteins with lipid raft domains ensures that cholesterol is indeed present underneath the sphingolipids that have attracted the unstructured monomer (Fig. 6). Once inserted in the membrane, the amyloidogenic protein will immediately find cholesterol and interact with its free side – that is, the face that is not in contact with sphingolipids (for a detailed explanation of cholesterol topology, see Ref. 18).
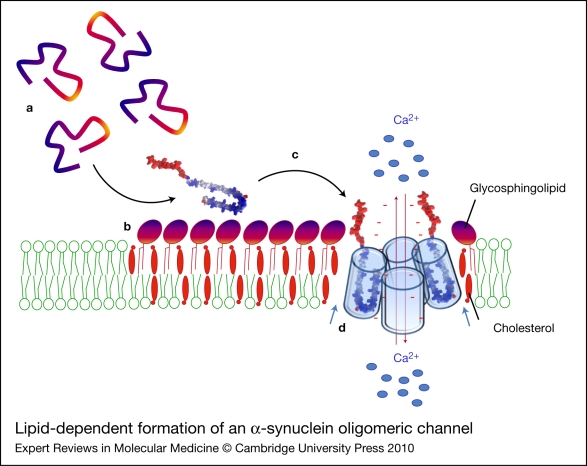
Figure 6.
Lipid-dependent formation of an α-synuclein oligomeric channel. Unfolded α-synuclein monomers (a) are attracted by the polar heads of glycosphingolipids and concentrated on the surface of sphingolipid–cholesterol membrane domains (b). Sphingolipids, complexed with cholesterol, induce a conformational change (unstructured to α-helix transition) of α-synuclein. The newly formed α-helices can insert into the plasma membrane (c) where they can further oligomerise under the control of cholesterol molecules (blue arrows) (d) and eventually form an oligomeric ion channel. Such channels are thought to disturb membrane permeability to calcium ions (red arrows), resulting in neuronal dysfunction and toxicity. The minus signs (in red) refer to the negative charges of α-synuclein.
How cholesterol could regulate the formation of oligomeric pores and channels
By stimulating the oligomerisation of membrane-inserted amyloidogenic proteins, cholesterol could facilitate the formation of a pore-like assembly displaying ion channel properties (Fig. 1). Quist et al. (Ref. 10) have hypothesised that amyloid pore/channels provide the most direct pathway for inducing neurodegenerative effects, through loss of ionic homeostasis increasing cell calcium to toxic levels. The structure of amyloid pores, which have been observed by atomic force microscopy (Refs 9, 10), remains to be experimentally elucidated at the atomic level. Computational models have suggested that most amyloid pores are probably formed by a complex assembly of β-rich protofibrils or oligomers (Ref. 130), as shown in Figure 1. However, in the case of α-synuclein, Tsigelny et al. (Ref. 101) have elegantly modelled a realistic oligomeric channel formed by the assembly of α-helical monomers. The possible events leading to the formation of an α-synuclein oligomeric channel under the dual control of sphingolipids and cholesterol are summarised in Figure 6. There is a striking similarity between α-synuclein (Refs 69, 31) and colicin E1, a bacterial toxin that also inserts into anionic areas of the plasma membrane, forming channel-like structures consisting of an α-helix bundle (Ref. 102). Moreover, it has been hypothesised that amyloid pores might be similar to β-barrel pore-forming bacterial toxins (Ref. 103). Since cholesterol controls the oligomerisation and insertion of these bacterial toxins (Ref. 104) in the target membrane, it is likely that it is also required for the assembly of amyloid pores.
How could cholesterol control the oligomerisation process of membrane-inserted amyloidogenic proteins and facilitate the formation of amyloid pores? The recent high-resolution crystal structure of an engineered human adrenergic β2 receptor, suggesting a molecular mechanism by which cholesterol mediates receptor dimerisation (Ref. 105), might give some clues to this fundamental issue. On the basis of these structural data, one could hypothesise that cholesterol binds to membrane-embedded fragments of amyloidogenic proteins, facilitates their recruitment and coordinates their oligomerisation. Overall, this would mean that amyloid pore formation and ion channel function are under the control of both sphingolipids and cholesterol, in agreement with recent data obtained in model membranes with α-synuclein (Ref. 31) and amylin (Ref. 106). Finally, it has been reported that spherical amyloid oligomers or protofibrils can also increase membrane conductance without forming ion channels or pores (Ref. 107). It is not known if cholesterol and/or sphingolipids are involved in this nonspecific permeabilising activity.
Changes in lipid content of the brain: what impact on neurodegenerative diseases?
The involvement of gangliosides in several neurodegenerative diseases is not totally surprising if one considers that these sphingolipids are critical for neuronal integrity and plasticity (Ref. 108) and synaptic function (Ref. 109). Since the probability of acquiring these diseases gradually increases with age, it is important to evaluate how ganglioside expression varies during the lifetime of individuals, and whether these diseases can alter this process (Ref. 110). In vitro, the differentiation of PC12 cells into neuron-like cells caused a marked increase in both gangliosides and cholesterol, and thereby greatly potentiated the accumulation and cytotoxicity of Aβ (Ref. 111). GM1 seems to control the oligomerisation and aggregation process of Aβ and α-synuclein (Refs 29, 30, 31). GM3 has been shown to control α-synuclein channel formation and to correct the channelopathy induced in planar lipid membranes by the Parkinson-disease-linked E46K mutant of the protein (Ref. 31). In the human brain, GM3 is a minor ganglioside, which, in marked contrast with the major ganglioside species GM1 and GD1A, shows a regular increase with age (Ref. 112). The expression of GM3 is highly regulated during brain development (Ref. 113), and this ganglioside has been implicated in the regulation of neuronal cell death (Ref. 114). Moreover, the homozygous loss-of-function mutation of GM3 synthase, which totally suppresses the expression of GM3 and all GM3-derived gangliosides, has been linked to an infantile symptomatic epilepsy syndrome (Ref. 115). Taken together, these data indicate that ganglioside GM3 probably plays a more important role in brain physiology and pathology than its low expression levels in adult brain could have suggested in the past.
Several studies support the view that deregulation of lipid metabolism is an important feature of neurodegenerative diseases. The three main lipid categories – glycerophospholipids, sphingolipids and cholesterol – are affected (Refs 116, 117, 118, 119, 120, 121). A large body of data has established a link between cholesterol homeostasis, apolipoprotein E polymorphism and APP processing (Ref. 122). Correspondingly, cholesterol-lowering strategies with statins have acquired potential therapeutic importance in treating Alzheimer disease (Ref. 123), although success has been variable (Ref. 124). Statins have also proved to be efficient in decreasing α-synuclein aggregation and neuronal toxicity in animal models of Parkinson disease (Ref. 98). Specific changes in ganglioside content have been detected in the brains of patients with Alzheimer disease (Refs 116, 125): a decrease or even loss of the major gangliosides GM1, GD1a, GD1b and GT1b, and an increase in GM2, GM3 and GD3. Similar alterations of ganglioside expression were observed in the brains of transgenic mouse models of Alzheimer disease (Ref. 126). Regional variations in ganglioside content have been shown in Alzheimer brains (Ref. 127). Moreover, the balance between NFA and HFA ceramides is altered in animal models (Ref. 128). Interestingly, the authors of the latter study showed that there is a gender-dependent accumulation of ceramides in the cerebral cortex: female mice exhibited a strong increase in HFA species, and males in NFA species. This observation could be linked to the increased risk of women versus men for developing Alzheimer disease (Ref. 129). Because cholesterol can exert distinct effects on Aβ–membrane interactions according to the NFA:HFA content of brain sphingolipids (Ref. 52), it will be interesting to determine how cholesterol impacts on Aβ–glycosphingolipid interaction in men and women suffering from Alzheimer disease.
Perspectives
How amyloidogenic proteins kill neurons is still a mystery. In particular, as recently discussed by Butterfield and Lashuel (Ref. 130), ‘there remains a knowledge gap regarding the molecular-level details by which amyloid-forming proteins act on the membrane and induce membrane permeabilization’. Deciphering the complex interplay between amyloidogenic proteins and membrane lipids, especially sphingolipids and cholesterol, will certainly help to achieve a clearer view on this enigma. Several milestones have already been reached, which have inspired an important part of today's research efforts worldwide. Despite their lack of amino acid sequence homology, amyloidogenic proteins share a number of intriguing properties: (1) an important conformational plasticity, allowing the same protein to remain unordered or to form highly ordered α and β structures (Refs 4, 5); (2) self-oligomerising capacities that revealed unexpected common antigenic properties (Ref. 7); (3) the ability to form pore-like structures with ion channel properties (Refs 9, 10); (4) self-aggregating activity leading to fibrillation and plaque deposition (Ref. 131); and (5) common structural motifs allowing specific interactions with sphingolipids (Ref. 49) and cholesterol (yet uncharacterised) in lipid raft domains of the plasma membrane. It is intriguing that HIV-1, which interacts with glycosphingolipids through the V3 domain of its surface glycoprotein gp120, can induce major neurological disabilities, identified as HIV-1-associated dementia (Ref. 110). In this respect, it is also worth noting that the protein CLN3, involved in Batten disease (the juvenile form of neuronal ceroid lipofuscinosis), also binds to GalCer through a V3-like sphingolipid-binding domain (Ref. 132). This neural disease is caused by a mutation in this domain, namely E295K (Ref. 133), which is analogous to mutations E22K in Aβ, E46K in α-synuclein and E200K in PrP, all associated with inherited forms of neurodegenerative disease and located in the sphingolipid-binding domains of these proteins. At least for two of these proteins (PrP and α-synuclein), the substitution of an anionic glutamic acid side chain by a cationic lysine resulted in altered binding to membrane sphingolipids (Refs 27, 31). This further emphasises the key role of the sphingolipid-binding domain in neuronal diseases.
Future studies will be necessary to better evaluate the neurotoxicity of the various forms of amyloidogenic proteins, including monomers, oligomers and aggregates, and to understand how these different species cooperate to accelerate (or slow down) the onset of neurodegenerative symptoms. This will help to decide on a rational basis which therapeutic strategy should be used at the different stages of the diseases. This will be possible with the finding of nontoxic drugs specifically affecting amyloid channels (Ref. 134) or amyloid aggregation (Ref. 135). Combining molecular (Refs 31, 52), biophysical (Refs 14, 31, 69, 136, 137), cellular (Refs 93, 114, 138) and animal (Refs 126, 128, 139) studies will allow a better characterisation of the sphingolipids that regulate amyloid oligomerisation/aggregation. Biophysical properties of the membrane such as membrane curvature should be taken into consideration (Ref. 130). Indeed, depending on the surface curvature of the model membrane, membrane-bound α-synuclein can adopt an extended helix (Ref. 140), a bent helix (Ref. 141) or an antiparallel helix–turn–helix conformation (Ref. 142) (for a review see Ref. 130). Interestingly, GM1 and GM3 are concentrated in membrane areas that markedly differ in membrane curvature (Ref. 143), so that on binding to distinct gangliosides, the same amyloid protein could adopt distinct conformations. This could be the case for α-synuclein, which recognises both GM1 (Ref. 30) and GM3 (Ref. 31). Antiganglioside antibodies could interfere with normal ganglioside function and could thus play a role in disease pathogenesis, as anti-GM1 antibodies probably do in some Parkinson patients (Refs 110, 144). Careful determinations of the titres of these antibodies in various biological fluids, including cerebrospinal fluid, will be particularly informative. Beneficial effects of ganglioside supplementation have been reported in animal models of Parkinson disease (Ref. 145). This warrants further investigation. Enzymes involved in glycosphingolipid metabolism might represent targets that inhibit both the production and amyloid aggregation of Alzheimer Aβ peptides (Ref. 146). Correspondingly, lipid raft disruption has been shown to protect neurons against amyloid oligomer toxicity in vitro (Ref. 147). We need to better understand how cholesterol interacts with amyloidogenic proteins, regulates their supramolecular structures and is involved in the pathophysiology of neurodegenerative diseases (Refs 86, 87). We should also determine how mutations of amyloidogenic proteins affect their interaction with neural membranes (Refs 27, 69, 148). A decisive breakthrough will be to understand why neurodegenerative diseases involve specific brain areas, and how local lipid composition may account for such geographic restrictions (Refs 127, 135). Finally, we have to identify the environmental molecules (such as food contaminants, pesticides and mycotoxins) that could modulate amyloid formation through direct binding to amyloid proteins and could represent important risk factors for neurodegenerative diseases (Ref. 149).
Acknowledgements and funding
The authors thank the peer reviewers for their interest in this work and their very constructive and thoughtful comments, which have stimulated us to improve our manuscript.
References
- 1.Wright P.E., Dyson H.J.. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. Journal of Molecular Biology. 1999;293:321–331. doi: 10.1006/jmbi.1999.3110. [DOI] [PubMed] [Google Scholar]
- 2.Uversky V.N.. The mysterious unfoldome: structureless, underappreciated, yet vital part of any given proteome. Journal of Biomedicine and Biotechnology. 2010;2010:568068. doi: 10.1155/2010/568068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunker A.K., Uversky V.N.. Signal transduction via unstructured protein conduits. Nature Chemical Biology. 2008;4:229–230. doi: 10.1038/nchembio0408-229. [DOI] [PubMed] [Google Scholar]
- 4.Uversky V.N.. et al. Unfoldomics of human diseases: linking protein intrinsic disorder with diseases. BMC Genomics. 2009;10:S7. doi: 10.1186/1471-2164-10-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uversky V.N.. A protein-chameleon: conformational plasticity of alpha-synuclein, a disordered protein involved in neurodegenerative disorders. Journal of Biomolecular Structure and Dynamics. 2003;21:211–234. doi: 10.1080/07391102.2003.10506918. [DOI] [PubMed] [Google Scholar]
- 6.Ghahghaei A., Faridi N.. Structure of amyloid fibrils in diseases. Journal of Biomedical Science Engineering. 2009;2:345–358. [Google Scholar]
- 7.Kayed R.. et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 8.Glabe C.C.. Structural classification of toxic amyloid oligomers. Journal of Biological Chemistry. 2008;283:29639–29643. doi: 10.1074/jbc.R800016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lashuel H.A.. et al. Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 10.Quist A.. et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arispe N., Rojas E., Pollard H.B.. Alzheimer's disease amyloid β-protein forms channels in bilayer membranes: blockage by tromethamine and aluminium. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arispe N., Rojas E., Pollard H.B.. β-amyloid Ca2+-channel hypothesis for neuronal death in Alzheimer's disease. Molecular and Cellular Biochemistry. 1994;140:119–125. doi: 10.1007/BF00926750. [DOI] [PubMed] [Google Scholar]
- 13.Vestergaard M., Hamada T., Takagi M.. Using model membranes for the study of amyloid beta:lipid interactions and neurotoxicity. Biotechnology and Bioengineering. 2008;99:753–763. doi: 10.1002/bit.21731. [DOI] [PubMed] [Google Scholar]
- 14.Munishkina L.A., Fink A.L.. Fluorescence as a method to reveal structures and membrane-interactions of amyloidogenic proteins. Biochimica et Biophysica Acta. 2007;1768:1862–1885. doi: 10.1016/j.bbamem.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J.A., Higgins G.A.. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 16.Prusiner S.. Prions. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabin D.E.. et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. Journal of Neuroscience. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantini J., Barrantes F.J.. Sphingolipid/cholesterol regulation of neurotransmitter receptor conformation and function. Biochimica et Biophysica Acta. 2009;1788:2345–2361. doi: 10.1016/j.bbamem.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Fantini J.. et al. Lipid rafts: structure, function and role in HIV, Alzheimer's and prion diseases. Expert Reviews in Molecular Medicine. 2002;4:1–22. doi: 10.1017/S1462399402005392. [DOI] [PubMed] [Google Scholar]
- 20.Simons K., Ikonen E.. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 21.Lingwood D., Simons K.. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 22.Drevot P.. et al. TCR signal initiation machinery is pre-assembled and activated in a subset of membrane rafts. EMBO Journal. 2002;21:1899–1908. doi: 10.1093/emboj/21.8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taïeb N., Yahi N., Fantini J.. Rafts and related glycosphingolipid-enriched microdomains in the intestinal epithelium: bacterial targets linked to nutrient absorption. Advances in Drug Delivery Reviews. 2004;56:779–794. doi: 10.1016/j.addr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Adam G., Delbrück M. Rich A., Davidson N. Structural Chemistry and Molecular Biology. W.H. Freeman & Co; 1968. Reduction of dimensionality in biological diffusion processes; pp. 198–215. , eds), pp. , San Francisco, USA. [Google Scholar]
- 25.Aisenbrey C.. et al. How is protein aggregation in amyloidogenic diseases modulated by biological membranes? European Biophysical Journal. 2008;37:247–255. doi: 10.1007/s00249-007-0237-0. [DOI] [PubMed] [Google Scholar]
- 26.Valdes-Gonzalez T., Inagawa J., Ido T.. Neuropeptides interact with glycolipid receptors. A surface plasmon resonance study. Peptides. 2001;22:1099–1106. doi: 10.1016/s0196-9781(01)00432-6. [DOI] [PubMed] [Google Scholar]
- 27.Mahfoud R.. et al. Identification of a common sphingolipid-binding domain in Alzheimer, prion, and HIV-1 proteins. Journal of Biological Chemistry. 2002;277:11292–11296. doi: 10.1074/jbc.M111679200. [DOI] [PubMed] [Google Scholar]
- 28.Hebbar S.. et al. A fluorescent sphingolipid binding domain peptide probe interacts with sphingolipids and cholesterol-dependent raft domains. Journal of Lipid Research. 2008;49:1077–1089. doi: 10.1194/jlr.M700543-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Yanagisawa K.. et al. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer's disease. Nature Medicine. 1995;1:1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- 30.Martinez Z.. et al. GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 31.Di Pasquale E.. et al. Altered ion channel formation by the Parkinson's-disease-linked E46 K mutant of alpha-synuclein is corrected by GM3 but not by GM1 gangliosides. Journal of Molecular Biology. 2010;397:202–218. doi: 10.1016/j.jmb.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 32.Mattei V.. et al. Prion protein is a component of the multimolecular signaling complex involved in T cell activation. FEBS Letters. 2004;560:14–18. doi: 10.1016/S0014-5793(04)00029-8. [DOI] [PubMed] [Google Scholar]
- 33.Sanghera N., Pinheiro T.J.. Binding of prion protein to lipid membranes and implications for prion conversion. Journal of Molecular Biology. 2002;315:1241–1256. doi: 10.1006/jmbi.2001.5322. [DOI] [PubMed] [Google Scholar]
- 34.Schmalzbauer R.. et al. Evidence for an association of prion protein and sphingolipid-mediated signaling. Journal of Neurochemistry. 2008;106:1459–1470. doi: 10.1111/j.1471-4159.2008.05498.x. [DOI] [PubMed] [Google Scholar]
- 35.Jha S.. et al. Amyloidogenic propensities and conformational properties of ProIAPP and IAPP in the presence of lipid bilayer membranes. Journal of Molecular Biology. 2009;389:907–920. doi: 10.1016/j.jmb.2009.04.077. [DOI] [PubMed] [Google Scholar]
- 36.Jo E.. et al. Alpha-synuclein membrane interactions and lipid specificity. Journal of Biological Chemistry. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 37.Ramakrishnan M., Jensen P.H., Marsh D.. Alpha-synuclein association with phosphatidylglycerol probed by lipid spin labels. Biochemistry. 2003;42:12919–12926. doi: 10.1021/bi035048e. [DOI] [PubMed] [Google Scholar]
- 38.Kubo S.. et al. A combinatorial code for the interaction of alpha-synuclein with membranes. Journal of Biological Chemistry. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- 39.Stöckl M.. et al. Alpha-synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. Journal of Molecular Biology. 2008;375:1394–1404. doi: 10.1016/j.jmb.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Futerman A.H., Hannun Y.A.. The complex life of simple sphingolipids. EMBO Reports. 2004;5:777–782. doi: 10.1038/sj.embor.7400208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harouse J.M.. et al. Inhibition of entry of HIV-1 in neural cell lines by antibodies against galactosyl ceramide. Science. 1991;253:320–323. doi: 10.1126/science.1857969. [DOI] [PubMed] [Google Scholar]
- 42.Yahi N.. et al. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. Journal of Virology. 1992;66:4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook D.G.. et al. Binding of human immunodeficiency virus type I (HIV-1) gp120 to galactosylceramide (GalCer): relationship to the V3 loop. Virology. 1994;201:206–214. doi: 10.1006/viro.1994.1287. [DOI] [PubMed] [Google Scholar]
- 44.Hammache D.. et al. Specific interaction of HIV-1 and HIV-2 surface envelope glycoproteins with monolayers of galactosylceramide and ganglioside GM3. Journal of Biological Chemistry. 1998;273:7967–7971. doi: 10.1074/jbc.273.14.7967. [DOI] [PubMed] [Google Scholar]
- 45.Hammache D.. et al. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3) Journal of Virology. 1999;73:5244–5248. doi: 10.1128/jvi.73.6.5244-5248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delézay O.. et al. SPC3, a V3 loop-derived synthetic peptide inhibitor of HIV-1 infection, binds to cell surface glycosphingolipids. Biochemistry. 1996;35:15663–15671. doi: 10.1021/bi961205g. [DOI] [PubMed] [Google Scholar]
- 47.Curatolo W.. Glycolipid function. Biochimica et Biophysica Acta. 1987;906:137–160. doi: 10.1016/0304-4157(87)90009-8. [DOI] [PubMed] [Google Scholar]
- 48.Nyholm P.G., Pascher I., Sundell S.. The effect of hydrogen bonds on the conformation of glycosphingolipids. Methylated and unmethylated cerebroside studied by X-ray single crystal analysis and model calculations. Chemistry and Physics of Lipids. 1990;52:1–10. doi: 10.1016/0009-3084(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 49.Fantini J.. How sphingolipids bind and shape proteins: molecular basis of lipid-protein interactions in lipid shells, rafts and related biomembrane domains. Cellular and Molecular Life Sciences. 2003;60:1027–1032. doi: 10.1007/s00018-003-3003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maresca M.. et al. Controlled aggregation of adenine by sugars: physicochemical studies, molecular modelling simulations of sugar-aromatic CH-pi stacking interactions, and biological significance. Physical Chemistry and Chemical Physics. 2008;10:2792–2800. doi: 10.1039/b802594k. [DOI] [PubMed] [Google Scholar]
- 51.Nishio M.. et al. The CH/pi interaction: significance in molecular recognition. Tetrahedron. 1995;51:8665–8701. [Google Scholar]
- 52.Yahi N., Aulas A., Fantini J.. How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer's beta amyloid peptide (Abeta1-40) PLoS One. 2010;5:e9079. doi: 10.1371/journal.pone.0009079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levy M.. et al. The minimal amyloid-forming fragment of the islet amyloid polypeptide is a glycolipid-binding domain. FEBS Journal. 2006;273:5724–5735. doi: 10.1111/j.1742-4658.2006.05562.x. [DOI] [PubMed] [Google Scholar]
- 54.Fantini J., Garmy N., Yahi N.. Prediction of glycolipid-binding domains from the amino acid sequence of lipid raft-associated proteins: application to HpaA, a protein involved in the adhesion of Helicobacter pylori to gastrointestinal cells. Biochemistry. 2006;45:10957–10962. doi: 10.1021/bi060762s. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabandhu K.. et al. The extracellular glycosphingolipid-binding motif of Fas defines its internalization route, mode and outcome of signals upon activation by ligand. Cell Death and Differentiation. 2008;15:1824–1837. doi: 10.1038/cdd.2008.115. [DOI] [PubMed] [Google Scholar]
- 56.Taieb N.. et al. The first extracellular domain of the tumour stem cell marker CD133 contains an antigenic ganglioside-binding motif. Cancer Letters. 2009;278:164–173. doi: 10.1016/j.canlet.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Hamel S., Fantini J., Schweisguth F.. Notch ligand activity is modulated by glycosphingolipid membrane composition in Drosophila melanogaster. Journal of Cell Biology. 2010;188:581–594. doi: 10.1083/jcb.200907116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Snook C.F., Jones J.A., Hannun Y.A.. Sphingolipid-binding proteins. Biochimica et Biophysica Acta. 2006;1761:927–946. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Kakio A.. et al. Formation of a membrane-active form of amyloid β-protein in raft-like model membranes. Biochemical and Biophysical Research Communications. 2003;303:514–518. doi: 10.1016/s0006-291x(03)00386-3. [DOI] [PubMed] [Google Scholar]
- 60.Matsuzaki K.. Physicochemical interactions of amyloid beta-peptide with lipid bilayers. Biochimica et Biophysica Acta. 2007;1768:1935–1942. doi: 10.1016/j.bbamem.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 61.Kakio A.. et al. Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry. 2002;41:7385–7390. doi: 10.1021/bi0255874. [DOI] [PubMed] [Google Scholar]
- 62.Okada T.. et al. Formation of toxic fibrils of Alzheimer's amyloid β-protein-(1-40) by monosialoganglioside GM1, a neuronal membrane component. Journal of Molecular Biology. 2007;371:481–489. doi: 10.1016/j.jmb.2007.05.069. [DOI] [PubMed] [Google Scholar]
- 63.Matsuzaki K., Kato K., Yanagisawa K.. Abeta polymerization through interaction with membrane gangliosides. Biochimica et Biophysica Acta. 2010;1801:868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Fantini J.. Interaction of proteins with lipid rafts through glycolipid-binding domains: biochemical background and potential therapeutic applications. Current Medicinal Chemistry. 2007;14:2911–2917. doi: 10.2174/092986707782360033. [DOI] [PubMed] [Google Scholar]
- 65.McLaurin J.A.. et al. Structural transitions associated with the interaction of Alzheimer β-amyloid peptides with gangliosides. Journal of Biological Chemistry. 1998;273:4506–4515. doi: 10.1074/jbc.273.8.4506. [DOI] [PubMed] [Google Scholar]
- 66.Choo-Smith L.P., Surewicz W.K.. The interaction between Alzheimer amyloid beta(1-40) peptide and ganglioside GM1-containing membranes. FEBS Letters. 1997;402:95–98. doi: 10.1016/s0014-5793(96)01504-9. [DOI] [PubMed] [Google Scholar]
- 67.Okada T.. et al. Formation of toxic Abeta(1-40) fibrils on GM1 ganglioside-containing membranes mimicking lipid rafts: polymorphisms in Abeta(1-40) fibrils. Journal of Molecular Biology. 2008;382:1066–1074. doi: 10.1016/j.jmb.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 68.Mascioni A.. et al. High-resolution structure and localization of amylin nucleation site in detergent micelles. Biopolymers. 2003;69:29–41. doi: 10.1002/bip.10305. [DOI] [PubMed] [Google Scholar]
- 69.Zakharov S.D.. et al. Helical alpha-synuclein forms highly conductive ion channels. Biochemistry. 2007;46:14369–14379. doi: 10.1021/bi701275p. [DOI] [PubMed] [Google Scholar]
- 70.Ruf R.A.S.. et al. alpha-synuclein conformation affects its tyrosine-dependent oxidative aggregation. Biochemistry. 2008;47:13604–13609. doi: 10.1021/bi801884z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bosco D.A.. et al. Elevated levels of oxidized cholesterol metabolites in Lewy bdy disease brains accelerate alpha-synuclein fibrilization. Nature Chemical Biology. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- 72.Knaus K.J.. et al. Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nature Structural Biology. 2001;8:770–774. doi: 10.1038/nsb0901-770. [DOI] [PubMed] [Google Scholar]
- 73.Krishnan S.. et al. Oxidative dimer formation is the critical rate-limiting step for Parkinson's disease alpha-synuclein fibrillogenesis. Biochemistry. 2003;42:829–837. doi: 10.1021/bi026528t. [DOI] [PubMed] [Google Scholar]
- 74.Wille H.. et al. Structural studies of the scrapie prion protein by electron crystallography. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3563–3568. doi: 10.1073/pnas.052703499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kallberg Y.. et al. Prediction of amyloid fibril-forming proteins. Journal of Biological Chemistry. 2002;276:12945–12950. doi: 10.1074/jbc.M010402200. [DOI] [PubMed] [Google Scholar]
- 76.Sarnataro D.. et al. PrPc association with lipid rafts in the early secretory pathway stabilizes its cellular conformation. Molecular and Cellular Biology. 2004;15:4031–4042. doi: 10.1091/mbc.E03-05-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naslavski N.. et al. Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. Journal of Biological Chemistry. 1999;274:20763–20771. doi: 10.1074/jbc.274.30.20763. [DOI] [PubMed] [Google Scholar]
- 78.Baier C.J., Barrantes F.J.. Sphingolipids are necessary for nicotinic acetylcholine receptor export in the early secretory pathway. Journal of Neurochemistry. 2007;101:1072–1084. doi: 10.1111/j.1471-4159.2007.04561.x. [DOI] [PubMed] [Google Scholar]
- 79.Gazit E.. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB Journal. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 80.Mao Y.. et al. Surface-induced phase separation of a sphingomyelin/cholesterol/ganglioside GM1-planar bilayer on mica surfaces and microdomain molecular conformation that accelerates Abeta oligomerization. Biochimica et Biophysica Acta. 2010;1798:1090–1099. doi: 10.1016/j.bbamem.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Utsumi M.. et al. Up-and-down topological mode of amyloid beta-peptide lying on hydrophilic/hydrophobic interface of ganglioside clusters. Glycoconjugate Journal. 2009;26:999–1006. doi: 10.1007/s10719-008-9216-7. [DOI] [PubMed] [Google Scholar]
- 82.Ikeda K., Matsuzaki K.. Driving force of amyloid-β protein to lipid bilayers. Biochemical and Biophysical Research Communications. 2008;370:525–529. doi: 10.1016/j.bbrc.2008.03.130. [DOI] [PubMed] [Google Scholar]
- 83.Williamson M.P.. et al. Binding of beta-peptide to ganglioside micelles is dependent on histidine-13. Biochemical Journal. 2006;397:483–490. doi: 10.1042/BJ20060293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao H., Tuominen E.K., Kinnunen P.K.. Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry. 2004;43:10302–10307. doi: 10.1021/bi049002c. [DOI] [PubMed] [Google Scholar]
- 85.Martins I.J.. et al. Cholesterol metabolism and transport in the pathogenesis of Alzheimer's disease. Journal of Neurochemistry. 2009;111:1275–1308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 86.Hu G.. et al. Total cholesterol and the risk of Parkinson disease. Neurology. 2008;70:1972–1979. doi: 10.1212/01.wnl.0000312511.62699.a8. [DOI] [PubMed] [Google Scholar]
- 87.Liu J.P.. et al. Cholesterol involvement in the pathogenesis of neurodegenerative disease. Molecular and Cellular Neuroscience. 2010;43:33–42. doi: 10.1016/j.mcn.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 88.Yanagisawa K.. Cholesterol and amyloid β fibrillogenesis. Subcellular Biochemistry. 2005;38:179–202. doi: 10.1007/0-387-23226-5_9. [DOI] [PubMed] [Google Scholar]
- 89.Beel A.J.. et al. Direct binding of cholesterol to the amyloid precursor protein: an important interaction in lipid-Alzheimer's disease relationships? Biochimica et Biophysica Acta. 2010;1801:975–982. doi: 10.1016/j.bbalip.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lahdo R., de La Fournière-Bessueille L.. Insertion of the amyloid precursor protein into lipid monolayers: effects of cholesterol and apolipoprotein E. Biochemical Journal. 2004;382:987–994. doi: 10.1042/BJ20040777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harris J.R.. Cholesterol binding to amyloid fibrils: a TEM study. Micron. 2008;39:1192–1196. doi: 10.1016/j.micron.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 92.Harris J.R.. In vitro fibrillogenesis of the amyloid β1-42 peptide; cholesterol potentiation and aspirin inhibition. Micron. 2002;33:609–626. doi: 10.1016/s0968-4328(02)00029-x. [DOI] [PubMed] [Google Scholar]
- 93.Arispe N., Doh M.. Plasma membrane cholesterol controls the cytotoxicity of Alzheimer's disease AβP (1– 40) and (1– 42) peptides. FASEB Journal. 2002;16:1526–1536. doi: 10.1096/fj.02-0829com. [DOI] [PubMed] [Google Scholar]
- 94.Yip C.M.. et al. Cholesterol, a modulator of membrane-associated Aβ-fibrillogenesis and neurotoxicity. Journal of Molecular Biology. 2001;311:723–734. doi: 10.1006/jmbi.2001.4881. [DOI] [PubMed] [Google Scholar]
- 95.Hartmann T.. et al. Alzheimer's disease: the lipid connection. Journal of Neurochemistry. 2007;103:159–170. doi: 10.1111/j.1471-4159.2007.04715.x. [DOI] [PubMed] [Google Scholar]
- 96.Bar-On P.. et al. Effects of cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synuclein. Journal of Neurochemistry. 2006;98:1032–1045. doi: 10.1111/j.1471-4159.2006.04017.x. [DOI] [PubMed] [Google Scholar]
- 97.Bar-On P.. et al. Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson's disease. Journal of Neurochemistry. 2008;105:1656–1667. doi: 10.1111/j.1471-4159.2008.05254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koob A.O.. et al. Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Experimental Neurology. 2010;221:267–274. doi: 10.1016/j.expneurol.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mahfoud R.. et al. A novel soluble analog of the HIV-1 fusion cofactor, globotriaosylceramide (Gb(3)), eliminates the cholesterol requirement for high affinity gp120/Gb(3) interaction. Journal of Lipid Research. 2002;43:1670–1679. doi: 10.1194/jlr.m200165-jlr200. [DOI] [PubMed] [Google Scholar]
- 100.Devanathan S.. et al. Effects of sphingomyelin, cholesterol and zinc ions on the binding, insertion and aggregation of the amyloid Abeta(1-40) peptide in solid-supported lipid bilayers. FEBS Journal. 2006;273:1389–1402. doi: 10.1111/j.1742-4658.2006.05162.x. [DOI] [PubMed] [Google Scholar]
- 101.Tsigelny I.F.. et al. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. FEBS Journal. 2007;274:1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- 102.Stroud R.M.. et al. Ion-channel-forming colicins. Current Opinion in Structural Biology. 1998;8:525–533. doi: 10.1016/s0959-440x(98)80132-2. [DOI] [PubMed] [Google Scholar]
- 103.Lashuel H.A., Lansbury P.T. Jr. Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins. Quarterly Reviews of Biophysics. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 104.Palmer M.. Cholesterol and the activity of bacterial toxins. FEMS Microbiology Letters. 2004;238:281–289. doi: 10.1016/j.femsle.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 105.Cherezov V.. et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2008;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cho W.J., Trikha S., Jeremic A.M.. Cholesterol regulates assembly of human islet amyloid polypeptide on model membranes. Journal of Molecular Biology. 2009;393:765–775. doi: 10.1016/j.jmb.2009.08.055. [DOI] [PubMed] [Google Scholar]
- 107.Kayed R.. et al. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. Journal of Biological Chemistry. 2004;279:46363. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 108.Mocchetti I.. Exogenous gangliosides, neuronal plasticity and repair, and the neurotrophins. Cellular and Molecular Life Sciences. 2005;19-20:2283–2294. doi: 10.1007/s00018-005-5188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas P.D., Brewer G.J.. Gangliosides and synaptic transmission. Biochimica et Biophysica Acta. 1990;1031:277–289. doi: 10.1016/0304-4157(90)90013-3. [DOI] [PubMed] [Google Scholar]
- 110.Posse de Chaves E., Sipione S.. Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction. FEBS Letters. 2010;584:1748–1759. doi: 10.1016/j.febslet.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 111.Wakabayashi M., Matsuzaki K.. Formation of amyloids by Abeta-(1-42) on NGF-differentiated PC12 cells: roles of gangliosides and cholesterol. Journal of Molecular Biology. 2007;371:924–933. doi: 10.1016/j.jmb.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 112.Svennerholm L.. et al. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. Journal of Neurochemistry. 1994;63:1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- 113.Yu R.K., Nakatani Y., Yanagisawa M.. The role of glycosphingolipid metabolism in the developing brain. Journal of Lipid Research. 2009;50:S440–S445. doi: 10.1194/jlr.R800028-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sohn H.. et al. Ganglioside GM3 is involved in neuronal cell death. FASEB Journal. 2006;20:E525–E535. doi: 10.1096/fj.05-4911fje. [DOI] [PubMed] [Google Scholar]
- 115.Simpson M.A.. et al. Infantile-onset symptomatic epilepsy syndrome caused by homozygous loss-of-fucntion mutation of GM3 synthase. Nature Genetics. 2004;36:1225–1229. doi: 10.1038/ng1460. [DOI] [PubMed] [Google Scholar]
- 116.Svennerholm L., Gottfries C.G.. Membrane lipids, selectively diminished in Alzheimer brains, suggest synapse loss as a primary event in early-onset form (type I) and demyelination in late-onset form (type II) Journal of Neurochemistry. 1994;62:1039–1047. doi: 10.1046/j.1471-4159.1994.62031039.x. [DOI] [PubMed] [Google Scholar]
- 117.Grimm M.O.. et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nature Cell Biology. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 118.Bandaru V.V.R.. et al. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer's but not normal brain. Neurobiology of Aging. 2007;30:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ariga T., McDonald M.P., Yu R.K.. Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease–a review. Journal of Lipid Research. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He X.. et al. Deregulation of sphingolipid metabolism in Alzheimer's disease. Neurobiology of Aging. 2008;31:398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu Q., Huang Y.. Lipid metabolism in Alzheimer's and Parkinson's disease. Future Lipidology. 2006;1:441–453. [Google Scholar]
- 122.Chauhan N.B.. Membrane dynamics, cholesterol homeostasis, and Alzheimer's disease. Journal of Lipid Research. 2003;44:2019–2029. doi: 10.1194/jlr.R300010-JLR200. [DOI] [PubMed] [Google Scholar]
- 123.Kandiah N., Feldman H.H.. Therapeutic potential of statins in Alzheimer's disease. Journal of the Neurological Sciences. 2009;283:230–324. doi: 10.1016/j.jns.2009.02.352. [DOI] [PubMed] [Google Scholar]
- 124.Rea T.D.. et al. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Archives of Neurology. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 125.Kracun I.. et al. Cortical distribution of gangliosides in Alzheimer's disease. Neurochemistry International. 1992;20:433–438. doi: 10.1016/0197-0186(92)90058-y. [DOI] [PubMed] [Google Scholar]
- 126.Barrier L.. et al. Genotype-related changes of ganglioside composition in brain regions of transgenic mouse models of Alzheimer's disease. Neurobiology of Aging. 2007;28:1863–1872. doi: 10.1016/j.neurobiolaging.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Molander-Melin M.. et al. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development: increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane microdomains. Journal of Neurochemistry. 2005;92:171–182. doi: 10.1111/j.1471-4159.2004.02849.x. [DOI] [PubMed] [Google Scholar]
- 128.Barrier L.. et al. Gender-dependent accumulation of ceramides in the cerebral cortex of the APP(SL)/PS1Ki mouse model of Alzheimer's disease. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.10.011. Nov 24; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 129.Fratiglioni L.. et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurologic diseases in the ederly research group. Neurology. 2000;5:S10–S15. [PubMed] [Google Scholar]
- 130.Butterfield S.M., Lashuel H.A.. Amyloidogenic protein–membrane interactions: mechanistic insights from model systems. Angewandte Chemie 2010 doi: 10.1002/anie.200906670. . Jul 12; [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 131.Iversen L.L.. et al. The toxicity in vitro of beta-amyloid protein. Biochemical Journal. 1995;311:1–16. doi: 10.1042/bj3110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Persaud-Sawin D.A.. et al. A galactosylceramide binding domain is involved in trafficking of CLN3 from Golgi to rafts via recycling endosomes. Pediatric Research. 2004;56:449–463. doi: 10.1203/01.PDR.0000136152.54638.95. [DOI] [PubMed] [Google Scholar]
- 133.Zhong N.. et al. Molecular screening of Batten disease: identification of a missense mutation (E295 K) in the CLN3 gene. Human Genetics. 1998;102:57–62. doi: 10.1007/s004390050654. [DOI] [PubMed] [Google Scholar]
- 134.Diaz J.C.. et al. Small molecule blockers of the Alzheimer Abeta calcium channel potently protect neurons from Abeta cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3348–3353. doi: 10.1073/pnas.0813355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Panza F.. et al. Beyond the neurotransmitter-focused approach in treating Alzheimer's disease: drugs targeting beta-amyloid and tau protein. Aging Clinical and Experimental Research. 2009;21:386–406. doi: 10.1007/BF03327445. [DOI] [PubMed] [Google Scholar]
- 136.Waschuk S.A.. et al. Cellular membrane composition defines Aβ-lipid interactions. Journal of Biological Chemistry. 2001;276:33561–33568. doi: 10.1074/jbc.M103598200. [DOI] [PubMed] [Google Scholar]
- 137.Thakur G., Micic M., Leblanc R.M.. Surface chemistry of Alzheimer's disease: a Langmuir monolayer approach. Colloids and Surfaces B: Biointerfaces. 2009;74:436–456. doi: 10.1016/j.colsurfb.2009.07.043. [DOI] [PubMed] [Google Scholar]
- 138.Furukawa K.. et al. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. Journal of Neurochemistry. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 139.Oikawa N.. et al. Gangliosides determine the amyloid pathology of Alzheimer's disease. Neuroreport. 2009;20:1043–1046. doi: 10.1097/WNR.0b013e32832e4b9d. [DOI] [PubMed] [Google Scholar]
- 140.Georgieva E.R.. et al. Membrane-bound alpha-synuclein forms an extended helix: long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. Journal of American Chemical Association. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jao C.C.. et al. Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ulmer T.S.. et al. Structure and dynamics of micelle-bound human alpha-synuclein. Journal of Biological Chemistry. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 143.Chen Y., Qin J., Chen Z.W.. Fluorescence-topographic NSOM directly visualizes peak-valley polarities of GM1/GM3 rafts in cell membrane fluctuations. Journal of Lipid Research. 2008;49:2268–2275. doi: 10.1194/jlr.D800031-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zappia M.. et al. Anti-GM1 ganglioside antibodies in Parkinson's disease. Acta Neurologica Scandinavia. 2002;106:54–57. doi: 10.1034/j.1600-0404.2002.01240.x. [DOI] [PubMed] [Google Scholar]
- 145.Sautter J.. et al. Systemic treatment with GM1 ganglioside improves survival and function of cryopreserved embryonic midbrain grafted to the 6-hydroxydopamine-lesioned rat striatum. Experimental Neurology. 2000;164:121–129. doi: 10.1006/exnr.2000.7410. [DOI] [PubMed] [Google Scholar]
- 146.Tamboli I.Y.. et al. Inhibition of glycosphingolipid biosynthesis reduces secretion of the β-amyloid precursor protein and amyloid β-peptide. Journal of Biological Chemistry. 2005;280:28110–28117. doi: 10.1074/jbc.M414525200. [DOI] [PubMed] [Google Scholar]
- 147.Malchiodi-Albedi F.. et al. Lipid raft disruption protects mature neurons against amyloid oligomer toxicity. Biochimica et Biophysica Acta. 2010;1802:406–415. doi: 10.1016/j.bbadis.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 148.Bodner C.R.. et al. Differential phospholipid binding of alpha-synuclein variants implicated in Parkinson's disease revealed by solution NMR spectroscopy. Biochemistry. 2010;49:862–871. doi: 10.1021/bi901723p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Uversky V.N.. Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. Journal of Neurochemistry. 2007;103:17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 150.Coles M.. et al. Solution structure of amyloid beta-peptide(1-40) in a water-micelle environment. Is the membrane-spanning domain where we think it is? Biochemistry. 1998;37:11064–11077. doi: 10.1021/bi972979f. [DOI] [PubMed] [Google Scholar]
Further reading, resources and contacts
Publications
- Butterfield S.M., Lashuel H.A.. Amyloidogenic protein–membrane interactions: mechanistic insights from model systems. Angewandte Chemie 2010 doi: 10.1002/anie.200906670. . Jul 12; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; This is a very complete and up-to-date review of the available data on the interaction of amyloidogenic proteins with model membranes.
- Matsuzaki K., Kato K., Yanagisawa K.. Abeta polymerization through interaction with membrane gangliosides. Biochimica et Biophysica Acta. 2010;1801:868–877. doi: 10.1016/j.bbalip.2010.01.008. [DOI] [PubMed] [Google Scholar]; This excellent review explores the possibility that ganglioside binding is the initial and common step in the development of human diseases involving amyloidogenic proteins, including Alzheimer disease. Websites For a discussion on glycosphingolipid structure and conformation, see our GalCer website: http://www.galcer.u-3mrs.fr/ The Alzheimer Research Forum is a very informative free website dedicated to understanding Alzheimer disease and related disorders, with resources on amyloidogenic proteins and mutations, and research news: http://www.alzforum.org/ Useful resources on amyloid pores can be found at the following web page, held by Peter T. Lansbury, Jr: http://lansbury.bwh.harvard.edu/amyloid_pore.htm