SUMMARY
Presenilin (PS) is the catalytic moiety of the γ-secretase complex. PS and other γ-secretase components are well conserved among metazoa, but their presence and function in more-distant species are not resolved. Because inappropriate γ-secretase processing of amyloid precursor protein (APP) in humans is associated with familial Alzheimer’s disease, understanding essential elements within each γ-secretase component is crucial to functional studies. Diverged proteins have been identified in primitive plants but experiments have failed to demonstrate γ-secretase activity. We have identified highly diverged orthologs for each γ-secretase component in the ancient eukaryote Dictyostelium, which lacks equivalents of APP, Notch and other characterized PS/γ-secretase substrates. We show that wild-type (WT) Dictyostelium is capable of amyloidogenic processing of ectopically expressed human APP to generate amyloid-β peptides Aβ40 and Aβ42; strains deficient in γ-secretase cannot produce Aβ peptides but accumulate processed intermediates of APP that co-migrate with the C-terminal fragments α- and β-CTF of APP that are found in mammalian cells. We further demonstrate that Dictyostelium requires PS for phagocytosis and cell-fate specification in a cell-autonomous manner, and show that regulation of phagocytosis requires an active γ-secretase, a pathway suggested, but not proven, to occur in mammalian and Drosophila cells. Our results indicate that PS signaling is an ancient process that arose prior to metazoan radiation, perhaps independently of Notch. Dictyostelium might serve to identify novel PS/γ-secretase signaling targets and provide a unique system for high-throughput screening of small-molecule libraries to select new therapeutic targets for diseases associated with this pathway.
INTRODUCTION
The presenilin gene (PSEN) was first identified from an autosomal dominant mutation associated with early-onset familial Alzheimer’s disease (FAD) (Sherrington et al., 1995). The presenilin protein (PS) is the evolutionarily conserved catalytic moiety of γ-secretase (Wolfe and Kopan, 2004; Wolfe et al., 1999), an unusual membrane aspartyl protease that cleaves a wide variety of type 1 (single-pass) integral membrane proteins (Steiner et al., 2008) within their transmembrane (TM) domains to modulate cell signaling (De Strooper et al., 1999; De Strooper et al., 1998; Kim et al., 2005). γ-secretase is thought to function as a high molecular weight, multiprotein complex comprising three other components in addition to PS: Nicastrin (Nct; named from Nicastro, an Italian village with a high incidence of FAD), Aph1 (anterior pharynx-defective 1, first identified in Caenorhabditis elegans) and Pen2 (presenilin enhancer 2, first identified in Drosophila melanogaster), which are all well conserved among the metazoa (Edbauer et al., 2003; Francis et al., 2002; Yu et al., 2000). These accessory proteins do not possess an inherent catalytic function, but regulate assembly, trafficking and proteolytic activity of the complex (Francis et al., 2002; Steiner et al., 2008; Yu et al., 2000). The presence and function of PS/γ-secretase in more-distantly related and unicellular species have not been demonstrated. PS-like components have been identified in moss (Khandelwal et al., 2007), but γ-secretase activity could not be detected.
Full-length PS/γ-secretase substrates, such as Notch and β-amyloid precursor protein (APP), have long, extracellular N-terminal domains and are generally not subject to direct processing by γ-secretase. An extracellular proteolyic cleavage must first release a soluble N-terminal, extramembranous fragment to generate the bona fide PS/γ-secretase target (De Strooper et al., 1999; Vassar et al., 1999), which is a membrane-anchored C-terminal intermediate.
In the Notch pathway, γ-secretase cleavage of the TM intermediate produces the Notch intracellular domain (NICD), which functions as a transcriptional co-factor that directs developmental gene activation and subsequent cell differentiation (Tien et al., 2009). Notch signaling can be enhanced by increasing either ectodomain processing or NICD stability. Because Psen1;Psen2-double-null mutations in mice cause embryonic lethality as a result of loss-of-function defects in the Notch pathway (Donoviel et al., 1999), definitive de novo functions of other PS/γ-secretase cleavage products have been difficult to assess.
For APP, the process of ectodomain shedding involves proteolytic cleavage by either α- or β-secretases to generate α- and β-CTF intermediates (C-terminal fragments of 83 and 99 residues, respectively). Cleavage of β-CTF by γ-secretase creates two product classes: an APP intracellular domain (AICD) and an amyloid-β(Aβ) peptide. Cleavage of α-CTF by γ-secretase releases the identical AICD, but a smaller intramembrane peptide, p3 (Selkoe, 1998; Suzuki and Nakaya, 2008). Additionally, PS/γ-secretase can cleave APP CTFs at several TM sites to generate Aβpeptides ranging from 37 to 49 residues (Wang et al., 1996; Zhao et al., 2005). FAD-type mutations in PSEN are associated with aberrant processing of APP that does not alter the size or abundance of the AICD but leads to changes in the relative levels of the various Aβ peptides. For example, >90% of Aβ peptides in the normal brain are of the 40-residue type, Aβ40; the ‘aggregation-prone’ Aβ42 moiety is highly under-represented (Walsh and Selkoe, 2007). By contrast, cells with FAD mutations in PSEN have increased ratios of Aβ42:Aβ40 (Borchelt et al., 1996). Still, a biological significance of PS/γ-secretase cleavage of APP in normal individuals is not fully established. A function for Aβ40 has not been described, but the AICD is suggested to participate in intracellular signaling and transcriptional regulation (Cao and Sudhof, 2001; Gao and Pimplikar, 2001).
In an effort to further elucidate molecular mechanisms intrinsic to PS function or dysfunction, we have examined PS signaling in Dictyostelium discoideum, a small, ancient eukaryote that has proven to be an effective model system to study unique aspects of signaling mechanisms that are conserved in the metazoan – in the control of nutrient sensing, chemotactic migration, and development, among others (Kimmel and Firtel, 2004). Dictyostelium grows unicellularly in the presence of abundant nutrients, but upon starvation it enters an ordered developmental sequence. Developing cells secrete and chemotax towards cAMP and form multicellular aggregates (McMains et al., 2008); these differentiate into patterned organisms with spatially segregated precursor populations of prespore and prestalk cells, the progenitors of the terminally differentiated cell types.
We now show that Dictyostelium has highly diverged orthologs for each γ-secretase component but, interestingly, seems to lack endogenous equivalents of APP, Notch and other characterized PS/γ-secretase substrates. We created a series of single and double mutants for genes encoding the γ-secretase components of Dictyostelium and demonstrated that wild-type (WT) Dictyostelium is capable of amyloidogenic processing of ectopically expressed human APP to generate Aβ40 and Aβ42 peptides. By contrast, PS-null strains and strains deficient in otherγ-secretase components accumulate α- and β-CTF APP intermediates but are unable to process these APP intermediate fragments further to release Aβ peptides. We conclude that Dictyostelium has a PS complex comprising highly diverged components that possesses bona fide γ-secretase activity. We further demonstrate that Dictyostelium requires PS/γ-secretase signaling for phagocytic capture and for cell-fate specification during development. PS signaling regulates cell-fate specification in a cell-autonomous manner. We conclude that PS signaling preceded metazoan expansion and perhaps arose independently of Notch. Finally, the extreme sequence divergence of the Dictyostelium orthologs might present a unique model for focus on a more highly restricted set of amino acid sequences required for PS/γ-secretase function than previously appreciated.
RESULTS
Dictyostelium has highly diverged subunits of the PS/γ-secretase complex
Dictyostelium orthologs for PS, Nct, Aph1 and Pen2 – the subunits of the PS/γ-secretase complex – were identified in a bioinformatic search using sequences of mammalian, Drosophila and C. elegans proteins (supplementary material Fig. S1A-D). Dictyostelium has single-copy genes encoding Nct, Aph1 and Pen2, but two genes for PS. As all the Dictyostelium proteins are highly diverged (sequence identities ∼25–35%) in comparison with their mammalian counterparts (supplementary material Fig. S1A-D), we evaluated if they were functionally equivalent (see below).
The predicted Dictyostelium PS proteins possess the multiple-TM organization and two essential aspartyl residues that are characteristic of all members of the membrane protease superfamily (Hutton and Hardy, 1997; Steiner et al., 2008). Using the Dictyostelium PS sequences, we identified two additional, more-distant protein relatives. Nonetheless, in a phylogenetic comparison [http://www.genebee.msu.su/services/phtree_reduced.html (Brodskii et al., 1995; Chumakov and Iushmanov, 1988; Iushmanov and Chumakov, 1988)] of all these putative Dictyostelium membrane aspartyl proteases with human PS and signal-peptidyl proteases (SPPs), the identified Dictyostelium PS proteins cluster most closely with the human PS proteins, whereas the other Dictyostelium proteins group with the SPPs (Fig. 1A). These alignments are seen more clearly when the three most highly conserved sequence domains of PS are analyzed. Strong PS identity is observed in the regions that encompass the two essential catalytic aspartyl residues and the PALP domain, a proline-alanine-leucine-proline motif found in all PSs (Fig. 1B,C,E); although somewhat related, the SPPs are clearly more diverged (Xia and Wolfe, 2003). These data suggest that we have identified two bona fide PS orthologs. Furthermore, ∼70% of the residues mutated in the human PSEN1 gene that are associated with early-onset FAD (Kim and Kim, 2008) are similar between WT human PS1 and Dictyostelium PS1 proteins (supplementary material Fig. S2).
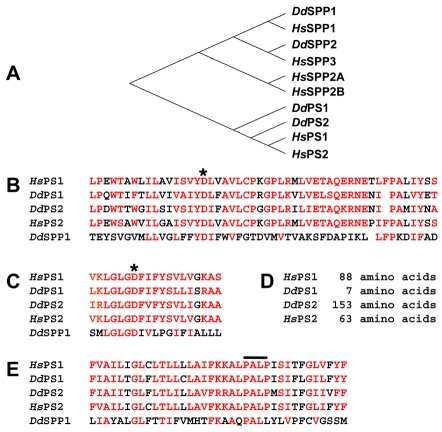
Fig. 1.
Dictyostelium PS proteins align with human PS proteins. (A) Phylogenic comparison of amino acid sequences of Dictyostelium proteins and the human PS and SPP proteins. Sequence alignments excluded the highly variable cytosolic loops of PSs. The human SPPs and PSs are separated on different branches of the tree. The Dictyostelium PS proteins cluster closely with human PSs. The more distantly related Dictyostelium proteins are more closely related to human SPPs; we term them SPP1 and SPP2. (B) Residue alignment surrounding the N-terminal enzymatic aspartate (asterisk); similar amino acid residues are indicated in red. (C) Residue alignment surrounding the C-terminal enzymatic aspartate (asterisk); similar amino acid residues are indicated in red. (D) Amino acid length of the nonconserved cytoplasmic loop. Length is determined between conserved residues PAXIYXS (in B) and VKLGLGD (in C). (E) Residue alignment within the conserved, overlined PALP domain region; similar amino acid residues are indicated in red.
Dictyostelium PS1 and PS2 share only 40% sequence identity (60% similarity; supplementary material Fig. S1D). The most significant structural difference between PS1 and PS2 is the length of the nonconserved region within the cytoplasmic loop located between the essential aspartyl-domain regions (Fig. 1D; supplementary material Fig. S2). The nonconserved loop region of PS2 (between residues PAXIYXS and VKLGLGD; see Fig. 1B–D) is 153 amino acids, similar to that of all metazoan PS proteins. By contrast, the nonconserved ‘loop’ of PS1 is only seven amino acids (supplementary material Fig. S2).
Dictyostelium discoideum Nct is 74 kDa, with a single-pass TM domain at amino acids 596–618, and Dictyostelium Aph1 is a predicted 38 kDa, seven-TM-domain protein. The Dictyostelium Nct and Aph1 proteins, respectively, possess the GxxxGxxxG- and YIGSS-like motifs characteristic of their metazoan counterparts. The final component, Dictyostelium Pen2, is the smallest, with a predicted size of 8 kDa and two TM domains.
Dictyosteliumγ-secretase subunits are expressed during growth and throughout development
To characterize the function of the ps, nct, aph1 and pen2 genes, we first examined their expression patterns through the Dictyostelium 24-hour developmental cycle. RNA samples were isolated from growing cells and cells developed at 5-hour intervals, and probed by either northern blot hybridization or by semi-quantitative reverse-transcriptase polymerase chain reaction (RT-PCR). ps1, nct, aph1 and pen2 are all expressed in growing cells and at every developmental stage examined (Fig. 2). By contrast, ps2 was expressed minimally during times of growth and early development, but expression was highly upregulated at 10 hours of development, in parallel with the initiation of cell-fate determination. These data suggest that γ-secretase has cellular functions throughout development and that PS2 might play a separate role from PS1.
Fig. 2.

Developmental expression of ps1, ps2, nct, aph1 and pen2. RNA was isolated from WT Dictyostelium during growth or at 5-hour increments during development. Gene expression patterns were analyzed by either northern blotting (PS1, Nct, Aph1, Ig7) or semi-quantitative RT-PCR (PS2, Pen2, Ig7). Ig7 is a nonregulated mRNA control.
To study γ-secretase in Dictyostelium, we used homologous recombination to create strains carrying single or multiple gene disruptions. ps1- and ps2-null constructions have been previously described (Faix et al., 2004), but the strains had not been characterized biologically. We have now generated aph1- and nct-nulls, as well as ps1;ps2-, aph1;ps2-, nct;ps2- and aph;nct-nulls. All the mutant strains were verified by Southern blot analysis and by RT-PCR (data not shown). We were unable to isolate a cell deficient for pen2.
Dictyostelium has an endogenous PS-dependent, γ-secretase activity that processes human APP
The extensive sequence differences between the Dictyostelium PS1, PS2, Aph1, Nct and Pen2 in comparison with their human orthologs (see supplementary material Fig. S1) provide controversy regarding their definitive roles as γ-secretase components. γ-secretase activity can be assayed by measuring the production of Aβ peptides, using either full-length APP or N-terminal truncations of APP as substrates (McLendon et al., 2000; McPhie et al., 1997; Oster-Granite et al., 1996; Yang et al., 2008). Since there is no endogenous APP (or Notch) equivalent in Dictyostelium, we applied a similar approach using expressed human APP.
In Dictyostelium, full-length human APP is expressed very poorly but a truncated version comprising the C-terminal 240 amino acids (Fig. 3A) is expressed to relatively high levels. This N-terminal deletion construct (N-APP) of human APP includes the sites cleaved by both α- and β-secretase (Fig. 3A), and allowed us to detect the generation and accumulation of Aβ peptides as well as the α- and β-CTFs – the direct γ-secretase substrates in mammalian cells (Fig. 3A).
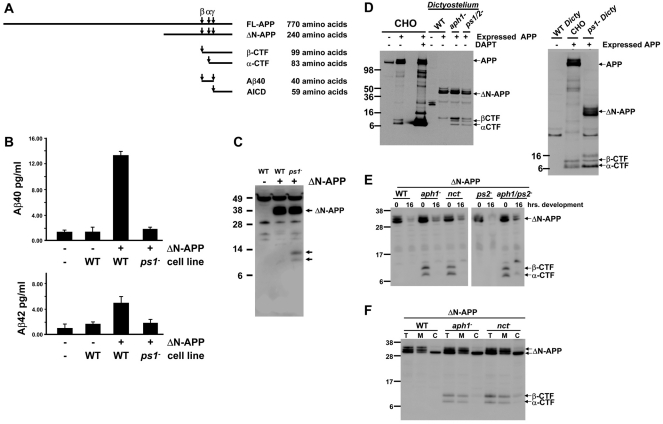
Fig. 3.
Dictyostelium has PS-dependent γ-secretase activity that processes human APP to release Aβ peptides. (A) Diagrams of human APP α, β and γ proteolytic cleavage sites, and processed fragments; shown are FL (full-length), ΔN (N-terminal deletion), α- and β-CTFs, Aβ40 peptide, and AICD regions. (B) Media conditioned by growing WT and ps1-null cells that express ΔN-APP (a truncated human APP) were analyzed for levels of Aβ40 and Aβ42 peptides by quantitative ELISA. Fresh media and media conditioned by native WT cells were used as negative controls. Bars indicate standard errors that are derived from two independent experiments, each with two replicates. (C)Protein samples were collected from growing native WT Dictyostelium, or WT and ps1-null Dictyostelium that express ΔN-APP. ΔN-APP expression and processed fragments (arrows) were determined by immunoblot assay using α-APP C-terminus. (D) Protein samples were collected from native and APP-expressing CHO cells untreated or incubated with DAPT, and from growing native WT Dictyostelium or WT, aph1-null, ps1;ps2-null and ps1-null Dictyostelium that express ΔN-APP. APP expression and processing was determined by immunoblot assay using α-APP C-terminus. (E) Protein samples were collected from growing or 16-hour-developed WT, aph1-null, nct-null, ps2-null, and aph1;ps2-null Dictyostelium that express ΔN-APP. ΔN-APP expression and processing was determined by immunoblot assay using α-APP C-terminus. (F) Total cell fractions (T), membrane fractions (M) and cytosolic fractions (C) were prepared from growing WT, aph1-null and nct-null Dictyostelium that express ΔN-APP. ΔN-APP and α- and β-CTFs in WT and γ-secretase-null mutants were detected by immunoblotting with α-APP C-terminus. Positions of molecular weight markers, APP, ΔN-APP and α- and β-CTFs are indicated.
We evaluated the ability of WT cells expressing N-APP to process and secrete Aβ40 and Aβ42 – the γ-secretase products of APP – using an ELISA approach. Background levels of both Aβ40 and Aβ42 are minimal in fresh axenic growth media and in media conditioned with growing native WT Dictyostelium that does not express APP (Fig. 3B,C). However, we detect a significant increase in Aβ40 levels in media conditioned with growing WT Dictyostelium that expresses ΔN-APP (Fig. 3B,C); Aβ42 levels are also increased to a statistically significant degree, but at a lower absolute concentration (Fig. 3B). We deduce that growing WT Dictyostelium possesses an endogenous γ-secretase activity capable of processing human APP.
Next, we analyzed media conditioned with growing ps1-null cells that express ΔN-APP (Fig. 3B,C); Aβ40 or Aβ42 levels were not enhanced in comparison with background controls (Fig. 3B,C). The absence of Aβ peptides in the ps1-null cells indicates the loss of γ-secretase activity. We conclude that although the identified γ-secretase component of Dictyostelium is highly diverged from its human counterpart, it nonetheless retains γ-secretase proteolytic activity.
We also detect novel, C-terminal APP fragments that accumulate in growing ps1-null cells but not in WT cells that express ΔN-APP (Fig. 3C). These new fragments are the processed TM intermediates of APP (see below) that are generated by proteolytic ectodomain shedding of full-length APP and are the substrates cleaved by PS/γ-secretase to release the Aβ40 or Aβ42 products. Accordingly, these TM intermediates of APP accumulate only in cells that lack PS/γ-secretase activity.
Cells lacking PS, Nct or Aph1 are unable to process APP
APP processing in mammalian cells involves two cleavage processes. The first cleavage by either α- or β-secretase is an extramembranous, N-terminal ectodomain proteolytic shedding event, which generates C-terminal TM intermediates (α- and β-CTFs) that are the ultimate substrates for TM cleavage by γ-secretase; γ-secretase cleavage of APP is absolutely dependent upon prior ectodomain processing. Both β- and γ-secretase cleavages must be precise to allow production and detection of the Aβ40 peptide.
To understand the biochemical bases of the novel C-terminal APP fragments that accumulate in ps1-null cells (Fig. 3C), we examined APP processing in WT and PS/γ-secretase-deficient Dictyostelium in comparison with normal APP processing in mammalian Chinese hamster ovary (CHO) cells (Fig. 3D). Endogenous expression of APP in native CHO cells is low. A full-length APP is detected but the CTFs are difficult to detect by immunoblotting (Fig. 3D), as they are efficiently processed to release the N-terminal Aβ40 or Aβ42 products and the unstable APP C-terminal 6 kDa AICD, which is rapidly degraded and also not easily detected (Fig. 3D). Stable expression of full-length human APP leads to low-level accumulation of the α- and β-CTFs but, again, the AICD is degraded rapidly and not detected by immunoblot (Fig. 3D). Treatment of the APP-expressing CHO cells with 250 nM DAPT, a specific γ-secretase inhibitor, leads to a much greater accrual of the α- and β-CTFs (Fig. 3D) and of inhibition of Aβ40 and Aβ42 production (data not shown).
We further examined CTF production in growing WT and mutant Dictyostelium that express ΔN-APP (Fig. 3C,D). The various cells express similar levels of ΔN-APP as a triplet, indicative of expected glycosylated variants. WT cells express full-length ΔN-APP, but only minimal levels of any C-terminal processed fragment (Fig. 3C,D). However, cells lacking PS1, Aph1, Nct, both PS1 and PS2, or both PS2 and Aph1 accumulate CTFs that co-migrate with mammalian α- and β-CTFs (Fig. 3C–E). These data demonstrate that the identified Dictyostelium genes encode functional γ-secretase components. Remarkably, the sites for α- and β-secretase cleavage of APP, N-terminal to the transmembrane region, in Dictyostelium correspond to those in mammalian cells (Fig. 3D). As the extracellular proteases of Dictyostelium and mammalian cells are not well conserved, perhaps structural elements within APP regulate proteolytic processing by ectodomain shedding.
Since growing cells that lack PS2 still express high levels of PS1, ps2-nulls do not accumulate CTFs (Fig. 3E), but accumulate similar Aβ40 and Aβ42 levels to those of WT cells (data not shown). As ΔN-APP expression levels declined significantly during later developmental stages, CTF intermediates are virtually undetectable in the various mutants (Fig. 3E). Thus, we could not evaluate the impact of PS2 function on APP processing in later development when PS2 expression levels rise.
Given that γ-secretase processing activity is membrane-specific, we wanted to determine the localization of full-length ΔN-APP and of the α- and β-CTF intermediates in Dictyostelium. Full-length, glycosylated ΔN-APP was found predominantly in the membrane fraction, whereas the presumed immature, lower molecular weight ΔN-APP form was also found in the cytosolic fraction (Fig. 3F). As expected, α- and β-CTFs are primarily membrane-associated.
We also studied the sensitivity of APP processing in Dictyostelium to a series of γ-secretase inhibitors, such as DAPT3 (Fig. 4). Although DAPT inhibited APP processing in Dictyostelium, the concentrations (>50 μM) required were only partially effective, even at one-to-two orders of magnitude greater than needed in mammalian cells. We suggest that this reflects either the sequence divergence of Dictyostelium PS1 or its minimal ‘loop’ domain. We note that there is a mechanistic preference for DAPT interaction with the C-terminus of endoproteolyzed PS (Morohashi et al., 2006).
Fig. 4.
DAPT inhibits Dictyostelium processing of APP. Growing native and ΔN-APP-expressing WT Dictyostelium were untreated or incubated with DMSO ±50 μM DAPT for 18 hours. Protein samples were collected and analyzed by immunoblot using α-APP C-terminus. Positions of molecular weight markers, ΔN-APP and α- and β-CTFs are indicated.
Although we were able to detect secreted Aβ40 and Aβ42 in the media of WT cells, we wished to observe the corresponding, but unstable, AICD in cytosolic fractions. We were unable to stabilize the AICD using proteasomal inhibitors calpain I (at 10–50 μM), epoxomicin (at 9–45 μM) or lactacystin (10–100 μM), or with 100 μM 1,10-phrenanthroline (supplementary material Fig. S3). We also tried to stabilize the AICD with a C-terminal yellow fluorescent protein (YFP) tag (ΔN-APP-YFP); although we detected accumulation of α- and β-CTF-YFP in the γ-secretase mutants, we did not observe a corresponding AICD-YFP in WT or ps2-null cells (data not shown). Finally, we replaced the entire ICD of ΔN-APP with YFP. Not only did we not detect cytosolic YFP in WT cells, but also we no longer observed accumulation of α- and β-CTFs in the γ-secretase mutants (supplementary material Fig. S4). These data suggest that structural elements within the AICD are essential for ectodomain processing of APP in Dictyostelium.
γ-secretase activity is required for phagocytosis in Dictyostelium
Although PS1 and the other γ-secretase components are expressed in growing cells, we did not observe significant differences of growth in axenic liquid-nutrient culture among the various mutant strains as compared with WT. In addition, loss of PS or other γ-secretase components had no effect on migration towards folate, a growth chemoattractant (data not shown). However, in the course of analyzing the growth-to-development transition of Dictyostelium growing on bacteria, we observed an interesting dependency on γ-secretase components. In the absence of a chemotactic defect, the areas (plaques) cleared by Dictyostelium that is growing on bacterial lawns are generally proportional to the rates of bacterial engulfment and uptake by phagocytosis. Consistently, the ps1-, aph1;ps2-, nct;ps2- and ps1;ps2-nulls all form significantly smaller plaques than do WT, within identical time frames. These data suggest a role for PS/γ-secretase in bacterial engulfment. The double mutants have a more severe defect in bacterial clearing than do cells carrying single mutations, as nct-, and aph1-single nulls show growth plaque sizes that are similar to WT (Fig. 5A,B); growing cells express only low levels of PS2 and ps2-nulls show no growth phenotype.
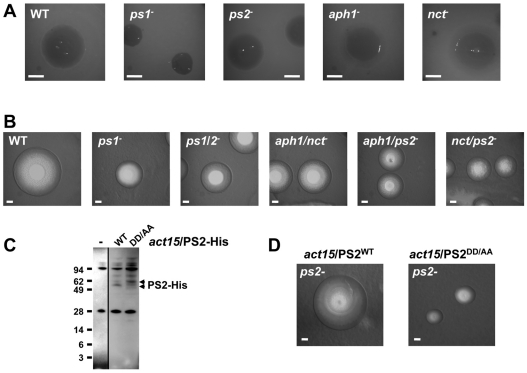
Fig. 5.
PS/γ-secretase mutants exhibit defects in growth on bacteria. (A) Single cells of WT and mutant strains of Dictyostelium were grown in the presence of bacteria for identical times. Growth zones were illuminated from below and photographed. Bars: 53cm. (B) Single cells of WT and mutant strains of Dictyostelium were grown in the presence of bacteria for identical times. Growth zones were illuminated from above and photographed. Bar: 5 cm. (C) Protein samples were collected from growing WT controls (–) or ps2-null cells expressing either PS2WT or PS2DD/AA carrying His-epitope tags. PS2 expression was determined by immunoblot assay using α-His. Similar mobility differences between PSWT and PSDD/AA variants have been observed previously. (D) Single cells of ps2-null cells expressing either PS2WT or PS2DD/AA were grown in the presence of bacteria for identical times. Growth zones were illuminated from above and photographed. Bars: 5 cm.
To address this more directly, we quantified phagocytic rates for several strains in comparison with WT. Dictyostelium was mixed with TRITC-labeled yeast, and internalized fluorescence was used to determine particle uptake (Khurana et al., 2005). WT, ps2-null and nct-null cells exhibit similar linear rates of phagocytosis during the time course (Fig. 6A), consistent with their similar growth rates on bacteria (Fig. 5A). By contrast, ps1-null, ps1;ps2-null, and aph1;nct-null cells had highly reduced rates of phagocytosis in comparison with WT controls (Fig. 6A,B).
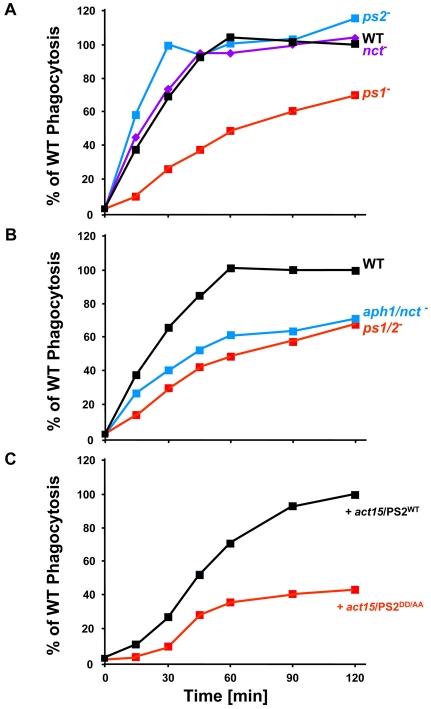
Fig. 6.
PS/γ-secretase mutants exhibit defects in growth on bacteria. WT and mutant strains of Dictyostelium were mixed with TRITC-labeled, heat-killed yeast particles; samples were removed at the times indicated and monitored by fluorimetric analyses. Arbitrary fluorescence units were used to normalize each strain relative to the maximum obtained for WT within the same experiment. Each of the cell lines was examined at least three times. ps1-, ps1;ps2- and aph1:nct-null differences to WT were statistically significant (P<0.05); differences of PS2DD/AA-expressing cells to PS2WT-expressing cells were statistically significant (P<0.05).
To determine whether γ-secretase activity was required for phagocytosis, we examined cells expressing a PS variant (PS2DD/AA) in which the essential catalytic aspartates (see Fig. 1B,C) were mutated to alanines; in other systems, these PSDD/AA variants functionally deplete endogenous γ-secretase activity and act as dominant-negatives (Berezovska et al., 2000; Kimberly et al., 2000; Wolfe et al., 1999). PS2WT and PS2DD/AA were expressed with His-epitope tags at similar levels in growing ps2-null cells (Fig. 5C). The growth on bacteria of cells expressing PS2WT was similar to that of WT and ps2-null cells, whereas PS2DD/AA-expressing cells phenocopied the growth of ps1-nulls (Fig. 5D). Furthermore, PS2DD/AA-expressing cells had significantly reduced rates of phagocytosis compared with controls expressing PS2WT (Fig. 6C). These data suggest strongly that an active γ-secretase, not just a PS complex, is required to regulate phagocytosis in Dictyostelium.
Loss of γ-secretase components in Dictyostelium causes developmental delay and abnormal morphogenesis
Since PS/γ-secretase complexes are essential for metazoan development (Donoviel et al., 1999), we were interested in determining if there were similar dependencies in Dictyostelium in the absence of equivalents of Notch, APP and other known γ-secretase substrates. Dictyostelium development is characterized by a transition from a unicellular growth state to a multicellular organism patterned by chemotactic movement, cellular differentiation, and morphogenesis. As single cells aggregate they initiate processes for differentiation into the progenitor prestalk and prespore cell types. As the multicelluar aggregates transition to the pseudoplasmodia or migrating slug stage, the precursor cells sort from one another and, during culmination, ultimately give rise to the terminally differentiated spore and stalk cells of the fruiting body, a mature terminal structure.
The ps1-, ps2-, aph1- and nct-null mutants aggregated efficiently and did not exhibit early developmental or chemotactic defects (data not shown). Interestingly, ps2-, aph1- and nct-null cells exhibited significant post-aggregation defects, a phenotype not observed with ps1-nulls (Fig. 7). The defects were primarily manifested in extending the length of the slug stage. Characteristics of this ‘slugger’ phenotype are developmental asynchrony, inefficient culmination, and poor terminal differentiation. Whereas WT and ps1-null cells complete development within ∼24 hours, ps2-, aph1- and nct-null mutants formed few intact fruiting bodies and mostly arrested as abnormal intermediates in this time frame (Fig. 7). These results suggest that the γ-secretase components PS2, Nct and Aph1 share a common developmental function.
Fig. 7.
PS/γ-secretase mutants exhibit developmental delays and morphological defects. WT and γ-secretase mutant strains were developed for 24 hours on filter matrices. Mutant strains fail to form terminal developmental structures.
The double-mutant ps1;ps2-, aph1;ps2- and nct;ps2-null cells exhibited a late developmental delay similar to that of the individual ps2-, nct- and aph1-nulls; however, consistently, the double mutants formed even fewer fruiting bodies and more aberrant structures than did any of the single mutants, even when developed over extended periods. Since the ps1;ps2-double-null mutant was more defective than the ps2-, nct- or aph1-single nulls, we suggest that PS1 contributes partly to γ-secretase signaling during late development. In addition, PS/γ-secretase function might not be fully inactivated by loss-of-function mutation of either nct or aph1, an effect similarly observed during growth on bacteria.
γ-secretase mutants have primary defects in prespore-spore differentiation
Given that the γ-secretase mutants form fewer fruiting bodies than do WT, we wanted to analyze their ability to differentiate into spore and stalk cells in low-cell-density monolayer culture (Kay, 1987) –conditions that minimize cell-cell interactions and intercellular signaling. Sporulation was assayed in the presence of the sporulation-inducer 8Br-cAMP, a cell-permeable analog of cAMP. At the concentrations used, 8Br-cAMP binds and activates the cell-surface cAMP receptors and the intracellular protein kinase A (PKA), which are essential for spore-cell differentiation. In this assay, sporulation in the mutants generally was delayed ∼24 hours compared with WT. Apart from ps1-nulls, the γ-secretase-null mutants collectively formed spore cells at ∼50% efficiency compared with WT controls after three days of differentiation (Fig. 8A); routinely, there was less than 20% sporulation of γ-secretase-mutant cells after 24 hours.
Fig. 8.
PS/γ-secretase mutants have cell-autonomous defects in prespore-spore cell differentiation. (A) WT and γ-secretase-mutant strains were starved in monolayer culture in the presence of 8Br-cAMP to induce sporulation. The percentage of differentiated spore cells was calculated from total cells after three days. Bars indicate standard errors that are derived from three independent experiments, each with three replicates. (B) Real-time quantitative RT-PCR was used to determine relative gene expression levels of the prespore genes cotB (black) and pspA (gray) at 15 hours of development in γ-secretase-mutant strains compared with WT. Expression levels were normalized to H3a. Bars indicate standard errors that are derived from two independent experiments, each with two replicates. (C) PS/γ-secretase signaling regulates prespore differentiation in a cell-autonomous manner. WT and ps2-null cells that express lacZ were mixed with a ninefold excess of unmarked WT, ps2-null or ps1;ps2-null cells as indicated. Cell mixes were developed to the slug stage and stained by β-galactosidase activity. Slugs are positioned with their indicated anterior prestalk (pst) zones to the left and posterior prespore (psp) zones to the right.
Since all γ-secretase mutants exhibited developmental delay and defects in sporulation, we examined prespore and prestalk gene expression patterns. RNA samples were collected from cells that had been developed on filters for various times, and mRNA expression was quantified by real-time RT-PCR, using H3a, the gene for histone 3A, as a normalizing control. cotB and pspA prespore gene expression was markedly reduced by loss of γ-secretase components; ps1;ps2-, aph1;ps2-, nct;ps2- and aph1;nct-nulls express prespore mRNAs at ∼20% abundance level compared with WT (Fig. 8B). Prespore mRNA levels are also reduced in ps2-, aph1- and nct-null cells (Fig. 8B), although the impact is less severe. This is also true for the ps1-nulls, again suggesting a role for PS1 during late development. Thus, and consistent with the developmental data, we suggest that PS/γ-secretase signaling regulates prespore-spore differentiation. No reduction in ecmA prestalk gene expression was observed (data not shown), further suggesting that the γ-secretase complex has a primary function in the pathway that regulates spore differentiation.
The double nulls routinely have a more severe developmental defect than do the ps2-, aph1- and nct-single nulls. Again, these data indicate that in the absence of either Nct or Aph1, but not both, some PS/γ-secretase function might persist.
PS/γ-secretase functions in a cell-autonomous manner to regulate prespore-spore differentiation
The ICDs released upon γ-secretase substrate cleavage are generally considered to be the primary effectors of PS function. Thus, Notch signaling downstream of PS/γ-secretase is viewed as a cell-autonomous event. For other pathways, cell autonomy is not so obvious. Secretion of novel protein fragments following intramembrane γ-secretase cleavage of other PS substrates might potentially generate a functional extracellular, nonautonomous paracrine-like signal. Although there is minimal cell-cell communication during the monolayer sporulation experiments, the data are insufficient to address this role of PS function in Dictyostelium. To directly address issues of cell autonomy and nonautonomy during prespore differentiation, we followed cell lineages of WT and PS/γ-secretase-deficient cells during chimeric development of mixed cell cultures.
WT and ps2-null strains that express lacZ in all cells, using the ubiquitous act15 promoter, were mixed with unmarked cells at different ratios in every combination, developed to various stages, and stained in situ for β-galactosidase expression. At the slug stage, prestalk cells primarily define the anterior region, with the prespore cells restricted to the slug posterior (Williams, 2006). In both homologous controls, β-galactosidase staining is observed throughout the entire organism (Fig. 8C). Similarly, WT cells do not preferentially populate a specific differentiated region in the presence of a predominant ps2-null population. However, the ps2-nulls are restricted to the anterior prestalk zone in developmental chimeras with WT, with some enrichment in the PstO region, a spatially restricted subregion of the prestalk zone. Some ps2-null cells are also enriched at the very posterior, but these cells also are fated to mark the prestalk-like basal disc structure at later developmental stages. These data are consistent with our previous conclusions that PS2 signaling is not essential for prestalk fates but regulates prespore-spore differentiation. As data suggest that PS1 contributes to PS/γ-secretase signaling during late development, we examined WT fate specification during chimeric development in the presence of excess ps1;ps2-null cells, which would completely lack γ-secretase activity. In these chimeras, WT cells are only able to populate the prespore region. WT cells are preferentially recruited to a prespore fate when developed with cells defective for PS/γ-secretase signaling. These data establish PS/γ-secretase signaling as a required cell-autonomous pathway for prespore differentiation in Dictyostelium. We were unable to obtain sufficient levels of ectopically expressed PS2WT and PS2DD/AA proteins to currently assess a direct role for γ-secretase activity in this process.
DISCUSSION
Dictyostelium PS/γ-secretase and APP processing
Although Dictyostelium does not contain an obvious ortholog of APP, its highly diverged PS/γ-secretase complex is fully competent to process ectopically expressed human APP correctly. Perhaps more surprising is the ability of Dictyostelium to produce APP-derived α- and β-CTFs that co-migrate with those of mammalian cells. Dictyostelium secretes many proteases during growth and development, but a functionally equivalent candidate with β-CTF-generating BACE (β-secretase) activity is not simply identified in a bioinformatic comparison. The presence of β-CTFs in Dictyostelium allows analyses of APP cleavage by PS/γ-secretase and the detection of Aβ moieties – processes not easily studied in other models. Despite the presence of endogenous APP-like proteins in both Drosophila and C. elegans, Aβ-like peptides do not readily accumulate in these organisms. Nonetheless, Aβ peptides can be produced by Drosophila cells that express human APP, suggesting the presence of a β-CTF-generating BACE activity (Carmine-Simmen et al., 2009; Takasugi et al., 2002).
We have applied numerous and varied experimental manipulations, but have been unable to detect the presence of any AICD molecules in Dictyostelium. AICD is also not easily detected in mammalian cells, but can be stabilized through interaction with Fe65 and X11 protein family members (Kimberly et al., 2001; Suzuki and Nakaya, 2008); neither has been identified in Dictyostelium.
Interestingly, we consistently observe more-severe phenotypic defects in strains carrying mutations in any two γ-secretase component genes than in singly mutant cells. Data in the metazoa suggest that each component of the PS complex is essential for γ-secretase function, but loss of either Nct or Aph1 might not be sufficient to completely eliminate γ-secretase activity in Dictyostelium. While it remains to be determined if this is a unique aspect, a mutation at S438 has been identified in human PS1 that renders its γ-secretase activity functionally independent of Nct when expressed in either mammalian cells or in yeast (Futai et al., 2009). Although S438 is fully conserved in both PS1 and PS2 of Dictyostelium, we note the high divergence from human PSs at other sites in PS1 and PS2, as well as in Nct.
The phenotypes of the ps1;ps2-null strain are more simply consistent with partial redundancy of PS1 and PS2. ps1 is expressed during growth and throughout the Dictyostelium developmental cycle. ps1 mutants have defects in phagocytic particle capture, but less overt developmental effects. By contrast, ps2 is expressed at low levels during growth and upregulated during cytodifferentiation. ps2 mutants exhibit defects in prespore and/or spore differentiation, a cell-fate defect that is extremely enhanced in ps1;ps2-null cells. During late development PS1 might, in part, substitute for PS2 and provide some γ-secretase activity. It is not possible to determine if the partial compensation of PS2 by PS1 reflects differences in protein levels, substrate preference, specific activity, or structure. We note that Dictyostelium PS1 lacks the large cytoplasmic loop present in Dictyostelium PS2 and the presenilins thus far described in other systems.
Phagocytosis
We present compelling evidence for a required function for γ-secretase activity in the regulation of phagocytosis. This conclusion is consistent with the postulates of others (Jutras et al., 2005), although previous functional linkages had been less clear. All four components of the PS/γ-secretase complex have been identified on phagosomes purified from murine macrophages and from Drosophila S2 cells (Jutras et al., 2005). Furthermore, two PS/γ-secretase substrates – CD44 and the low-density-lipoprotein receptor-related protein 1 (LRP1) – mediate phagocytosis in macrophages and other cells (Lammich et al., 2002; Leemans et al., 2003; Lillis et al., 2008). In particular, the cytoplasmic domain of LRP1 is implicated as the primary effector for this process (Patel et al., 2003), potentially supporting a role for phagocytic activation mediated by γ-secretase cleavage of LRP1.
Phagocytosis in Dictyostelium is essential for nutrient capture during growth on bacterial and fungal sources, but is also required for additional functions. During development, a small population of phagocytic cells serves a protective roll to sequester toxic components. These ‘sentinel’ cells exhibit immune-like properties (Chen et al., 2007), and this might suggest a shared function of PS/γ-secretase in these cells and in mammalian macrophages.
Development
Loss-of-function mutants in PS/γ-secretase phenocopy Notch deficiencies and cause embryonic lethality in mammals, Drosophila and C. elegans. Since development in Dictyostelium occurs after growth, we do not observe a similar lethality in cells lacking PS/γ-secretase activity; however, as with mutant mammalian organisms, mutant Dictyostelium also exhibits a cell-fate switch. Nonetheless, Dictyostelium does not possess a classical Notch substrate as defined by a Notch-domain sequence or potential NICD and we have not identified any of the known metazoan PS/γ-secretase substrates in Dictyostelium, suggesting that Dictyostelium has unique PS/γ-secretase substrate pathways that regulate development. Although there is not a consensus amino acid sequence that defines a PS/γ-secretase substrate (Dries and Yu, 2008; Kopan and Ilagan, 2004), substrates all share certain structural features, including relative location of the TM domain and a cluster of basic amino acids that are C-terminal to the TM domain. Numerous type 1 TM proteins can be identified in Dictyostelium. Several of these possess epidermal growth factor (EGF)-like motifs, are known to be cleaved extracellularly, and have other characteristics shared by the known PS/γ-secretase substrates. Studies are in progress to determine if any of these might be bona fide PS/γ-secretase substrates in Dictyostelium.
Although most PS/γ-secretase pathways require the release of an ICD to propagate a signal, it has been suggested that γ-secretase might have originally functioned as a ‘membrane proteasome’ to recycle membrane components (Kopan and Ilagan, 2004; Selkoe and Wolfe, 2007; Wolfe and Kopan, 2004), releasing protein fragments for complete degradation by the 26S proteasome or other proteases. Signaling functions that are dependent upon cleavage by the γ-secretase might have become secondarily associated with released Aβ-like peptides and ICDs. Regardless, PS/γ-secretase has a definitive cell-autonomous function during Dictyostelium development, suggesting that cell-autonomous PS signaling is an ancient process that might have preceded the evolutionary appearance of the definitive Notch pathway (Selkoe and Wolfe, 2007).
PS/γ-secretase might also regulate signaling by targeting specific membrane proteins for degradation and endosomal recycling (Zhang et al., 2006). Our data do not make a simple mechanistic pathway prediction for PS function in Dictyostelium. Regulatory ICDs in Dictyostelium could function as activators of prespore differentiation or as inhibitors of prestalk fate. In either situation, loss of PS function would suppress prespore-fate choice. Alternatively, a membrane protein(s) might be the active regulatory component that is inhibited through targeted degradation by PS/γ-secretase. Such a factor would act reciprocally to that predicted for an ICD, functioning as a cell-autonomous activator of prestalk differentiation or inhibitor of prespore fate. Nonetheless, loss of PS/γ-secretase function would still promote prestalk differentiation of cells during chimeric development with an excess WT population.
The γ-secretase inhibitor DAPT is weakly effective in Dictyostelium even at 50 μM and can partially suppress sporulation as compared with DMSO controls (data not shown). Still, we recognize that the PS complex might have functions that are not fully dependent upon its proteolytic activity. Mammalian PS can interact with glycogen synthase kinase 3 (GSK3) and potentially function as a scaffold (Kang et al., 2002; Prager et al., 2007; Tesco and Tanzi, 2000). Although in Dictyostelium both PS and GSK3 promote prespore differentiation (Kim et al., 2002; Kim et al., 1999; Plyte et al., 1999), we have been unable to detect a physical interaction of Dictyostelium PS1 with either Dictyostelium or mammalian GSK3 in vivo or in vitro (data not shown). Recent data in moss suggest a function for a PS-like protein that is independent of γ-secretase activity (Khandelwal et al., 2007), and an inherent γ-secretase function in the moss protein could not be demonstrated. Loss-of-function PS mutants compromise Ca2+ signaling in mammalian cells (Tu et al., 2006; Yoo et al., 2000) but, conversely, in Dictyostelium, it is increases in intracellular Ca2+ that are associated with stalk-cell differentiation (Kubohara et al., 2007).
We conclude that Dictyostelium possesses a bona fide PS/γ-secretase pathway that is required, in a cell-autonomous manner, for both phagocytosis and cell-fate specification. Thus, PS signaling and γ-secretase activity are ancient processes that arose prior to metazoan radiation. The extreme sequence divergence of the Dictyostelium orthologs might now direct focus to a more restricted set of PS/γ-secretase structural elements and also to novel targets and effectors. Dictyostelium can also offer a unique system for applying novel genomic and chemical approaches. By expressing FAD mutations in Dictyostelium, we might be able to recapitulate aberrant cleavage of APP. Such cell lines might present a relatively simple and inexpensive system for the high-throughput screening of small-molecule libraries to identify novel pharmaceutical agents. Expression of mutant PS in Dictyostelium might also allow the study of causative effects of γ-secretase misregulation on phagocytosis and developmental pathways. Phagocytes provide the immediate defense against invading microorganisms, so these data might also provide a new understanding to immune system function.
METHODS
Growth and development of Dictyostelium
Dictyostelium discoideum axenic strains were grown in D3-T or HL-5 media with or without selection as required (Kim et al., 1999), or cultivated as single cells on SM agar plates with Klebsiella aerogenes. Dictyostelium was developed on nitrocellulose filters as described (Kim et al., 1999). For chimeric development, marked and unmarked cells were mixed at varying percentages prior to development on filters. To trace cell lineages, Dictyostelium was transfected with the act15-lacZ expression vector and total populations were selected with 20 μg/ml G418. Detection of in situ β-galactosidase expression during development was as described (Richardson et al., 1994).
Spore differentiation (Kim et al., 1999) was induced in monolayer culture through the administration of 8Br-cAMP (Sigma-Aldrich, St Louis, MO, USA) and visualized in bright-field microscopy (Zeiss Axiovert 200M, Thornwood, NY, USA). To maximize differentiation of γ-secretase-mutant strains, experiments were monitored after three days of culture. After two days, there was routinely less than 20% sporulation of γ-secretase-mutant cells. Assays were performed three independent times, each with three replicates.
CHO cell culture
Naive CHO cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4.5 mg/ml D-glucose supplemented with 10% fetal bovine serum and 100 units/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine (Sigma-Aldrich) at 37°C in a 5% CO2 atmosphere. CHO cells stably expressing human WT APP751 (CHO-7W) were grown as described above but supplemented with hygromycin.
Construction of γ-secretase mutant strains
Dictyostelium has two presenilin genes – ps1 (DDB_G0292310) and ps2 (DDB_G0291352) – which, respectively, encode PS1 and PS2. Single genes exist for aph1 (DDB_G0267976), nct (DDB_G0287801) and pen2 (DDB_G0293484). We also identify two SPP genes: SPP1 (DDB_G0292836) and SPP2 (DDB_G0287521). ps1- and ps2-null strains have been described previously (Faix et al., 2004).
A 1.3 kb genomic fragment of aph1 was amplified using the forward primer 5′-GGACAAGCTGGAGAGAATAAAGTACCAGG-3′ and the reverse primer 5′-CAGGTTTTATTTTATGGTTGTCTTTTTATTAC-3′, and subcloned into the TOPO-pCR4 vector (TOPO TA Cloning Kit; Invitrogen, Carlsbad, CA, USA). This vector was linearized with NdeI (530 bp) within aph1, blunt-ended using the Klenow fragment of DNA polymerase I and dNTPs, and ligated to the SmaI fragment of pLPBLP containing the floxed blasticidin-resistant cassette (Faix et al., 2004). The resulting aph1-LoxP-BSR vector was linearized with EcoRI and transfected into WT Dictyostelium, and aph1-null cells were clonally selected with blasticidin.
A 0.5 kb genomic fragment from the 5′-end of nct was amplified using the forward primer 5′-GAATTTTCCTTTCCCAATGTTTGCTTTGG-3′ and the reverse primer 5′-CCCAACATAACCCCATCTCTCTGAATTCC-3′; a 0.8 kb genomic fragment from the 3′-end of nct was amplified using the forward primer 5′-GGAATTCAGAGAGATGGGGTTATGTTGGG-3′ and the reverse primer 5′-GGGGTAATATGATTTACTGGGCTTACACC-3′. The fragments were subcloned separately into the TOPO-pCR4 vector. The 5′ nct fragment was excised with XbaI and BglII and the 3′ nct fragment was excised with SalI and HindIII. The fragments were individually ligated into pLPBLP and the resulting nct-LoxP-BSR vector was linearized with PvuII and transfected into WT Dictyostelium, and nct-null cells were clonally selected with blasticidin.
ps1;ps2-, aph1;nct-, nct;ps2- and aph1;ps2-null strains were created using the Cre-LoxP BLAST recycling system to generate multiple insertions (Faix et al., 2004).
Mutant strains were confirmed using Southern blot hybridization and RT-PCR analysis.
Quantitative RT-PCR
Prespore- and prestalk-cell gene expression levels were measured by quantitative real-time RT-PCR. WT and γ-secretase-mutant cells were developed for 15 and 20 hours on nitrocellulose filters (Kim et al., 1999). RNA was isolated from cells by the TRIzol method (Invitrogen), treated with DNase (Ambion, Austin, TX, USA), and reverse transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). We performed PCRs using Bio-Rad’s iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA) and the ABI Prism 7900 (Applied Biosystems, Foster City, CA, USA). Each cell line was developed in three independent experiments; each reaction was in duplicate. Primer-pair efficiencies were compared with the amplification of genomic DNA. Prespore and prestalk gene expression levels were normalized to those of the histone H3a mRNA. The data analysis threshold point was set for linear amplification. Sequences of the primers (IDT, Inc., Coralville, IA, USA) are as follows: (1) H3a, 5′-GGTTCTAAACAAGCCCATAAACAAACTCCA G-3′ (forward) and 5′-CTCTAAGAGCGACAGTACCTGGTCTG-3′ (reverse); (2) PspA, 5′-CAACAGTTACACCAACAGTTACACCAACAG-3′ (forward) and 5′-GCAACAACAGTTGAAGCAGAACCAGTTG-3′ (reverse); (3) CotB, 5′-GCTCACATACTACTACTGGTGGTTCAACTAC-3′ (forward) and 5′-GTCAAATTCATCGGCAACACAAACAGC-3′ (reverse); (4) EcmA, 5′-GCCTGTACAGATGATTCCTGCTCAC-3′ (forward) and 5′-GATTGG-GGTATAACAGCAACCAGTTGAATTG-3′ (reverse); (5) EcmB, 5′-CTGAAGATAAATGTACTCAATCAGGTGGTGTAACTC-3′ (forward) and 5′-GTATGGCAACAGCCAGTTGAGTTTGAAC-3′ (reverse).
Northern blot analysis
Total RNA was isolated from growing cells or cells developed on filters, separated by gel electrophoresis (Kim et al., 1999), and transferred to Amersham Hybond N+ membrane (GE Healthcare, Buckinghamshire, UK) using Turboblotter (Schleicher and Schuell, Dassel, Germany). DNA radiolabeled probes were synthesized using Amersham Rediprime II Random Prime Labelling System (GE Healthcare). Membranes were hybridized at 42°C overnight in ULTRAhyb (Ambion). Hybridizations were imaged using the Fujifilm FLA-5000 (Japan).
ΔN-APP vectors
PCR primers were designed to amplify the C-terminal 240 amino acids of human APP, containing the predicted sites for glycosylation, and for cleavage by α-secretase, β-secretase and γ-secretase. Restriction sites for BamHI and XhoI were incorporated into the primer pairs: 5′-TTTGGATCCATGACACACCTCCGTGTGATTTATGAGCG-3′ and 5′-TTCTCGAGCTAGTTCTGCATCTGCTCAAAGAACTTGTAGG-3′.
The N-APP fragment was amplified using Expand High Fidelity PCR kit (Roche Molecular Biochemicals) and the GeneAmp PCR System 9600 (PerkinElmer Life Sciences, Waltham, MA, USA), cloned into Dictyostelium extrachromosomal vector pTX-FLAG (Levi et al., 2000) by using BamHI and XhoI, and transformed into E. coli DH5α. The N-APP recombinant plasmid, designated pTX-FLAG- N-APP, was confirmed by sequencing.
To generate a Flag-N-APP-YFP construct, we amplified N-APP from pTX-FLAG- N-APP using primers flanked with KpnI and BamHI: 5′-GGTACCATGACACACCTCCGTGTGATTTATGAG-3′ and 5′-GGATCCGTTCTGCATCTGCTCAAAGAACTT-3′.
Amplified Flag- N-APP was subcloned into TOPO-pCR4, and used for KpnI- and BamHI-mediated cloning into pDXA-msc-YFP.
Transfections into WT and mutant Dictyostelium and population selections were as described (Kim et al., 1999).
Detection and quantification of secreted levels of Aβ40 and Aβ42
The levels of secreted Aβ were quantified by sandwich ELISA. Growing Dictyostelium cells (5×105) were deposited into six-well tissue culture dishes and allowed to grow as adherent cells overnight at 21°C in HL-5. Cells transformed with pTX-FLAG-N-APP were cultured in HL-5 supplemented with 10 μg/ml of G418. The next day, the media was replaced with 1.4 ml of fresh HL-5. After 24 hours, the conditioned media (1.2 ml) was collected and centrifuged at 14,000 rpm for 15 minutes at 4°C to remove any cells and debris. The concentration of Aβ40 and Aβ42 was detected and quantified in triplicate using the β-Amyloid 1–40 or 1–42 Colorimetric ELISA Kit (Biosource International, Camarillo, CA) according to the manufacturer’s instructions. The level of Aβ represents the mean value from three independent experiments performed on separate days.
Immunoblot detection of APP
Dictyostelium and CHO cells were lysed in ice-cold RIPA buffer [20 mM Tris–HCl (pH 7.4) containing 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40 (NP-40), 50 mM NaF, 1 mM Na3VO4, 1 mM Na2MoO4, 10 mg/ml aprotinin and 10 mg/ml leupeptin], supplemented with Complete Mini Protease Inhibitor Cocktail Tablets (Roche) and briefly sonicated. Insoluble material was removed by centrifugation at 14,000 rpm for 15 minutes at 4°C. In some instances cell pellets were resuspended in NuPAGE 1× LDS Sample Buffer (Invitrogen) and phosphate buffer (7.4 mM NaH2PO4-H2O, 0.4 mM NaHPO4-7H2O) containing protease inhibitor tablets (Roche). Proteins were separated using pre-cast SDS-PAGE (12% Bis-Tris polyacrylamide gels or 8% and 14% Tris-glycine; Invitrogen) and transferred to PVDF membranes using Novex mini-cell XCell Surelock and XCell II Blot Module (Invitrogen).
The membranes were blocked with 5% fat-free powdered milk, then incubated with the α-APP-C-terminal (1:6000; Sigma-Aldrich) or α-GFP (1:5000 dilution; Sigma-Aldrich) primary antibodies, and followed with the HRP-labeled goat α-rabbit (1:10,000 dilution; BD Biosciences, San Jose, CA) or goat α-mouse HRP (1:10,000 dilution; BD Biosciences) secondary antibodies. Reactivity was visualized using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Scientific, Rockford, IL) ECL reagent and imaged with the FujiFilm LAS-3000 (Japan), or using the enhanced chemiluminescence (ECL) system (GE Healthcare) and imaged with XOMAT Kodak film.
PS2 expression
PS2 cDNA was amplified from mRNA isolated from 20-hour-developed cells using primers 5′-GGATCCTACAAATATTTGATTTTCACCCAAAAAGTAAATG-3′ and 5′-GAGCTCATGAAAGAAAATGAAGATGATACTAATAAAAC-3′, with added SacI and BamHI restriction sites. The cDNA was cloned into the TOPO pCR4 vector (Invitrogen). QuikChange Multi Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA) was used to introduce point mutations to convert GAT aspartate codons to GCT alanine codons: mutagenized PS2DD/AA had D237A and D429A residues. WT and mutated cDNA was cloned into pDXA-3H-Hygro and co-transfected with the replication plasmid pREP into WT and ps2-null cells and selected with 100 μg/μl hygromycin.
Quantitative phagocytosis
Dictyostelium was mixed with TRITC-labeled heat-killed yeast cells in shaking suspension at room temperature as described (Khurana et al., 2005). 1 ml samples were withdrawn at various times and added to Trypan Blue solution (2 mg/ml dissolved in 20 mM sodium citrate containing 150 mM NaCl) to quench the fluorescence of noninternalized yeast. Cell pellets were collected and fluorescence was measured using 544 nm light for excitation and 574 nm for emission in a luminescence spectrometer LS50B (PerkinElmer). Arbitrary fluorescence units were normalized to the maximal value obtained for WT cells within the same experiment. Experiments were performed three times.
Supplementary Material
Acknowledgments
We thank Xiuli Huang, Xin-Hua Liao, Collette Young, Marielle Young, Jonathan Buggey, Joseph Brzostowski and Taruna Khurana for insightful discussions and expert technical advice. We also thank Konstantin Chumakov for guidance on phylogenic analyses, and appreciate the generosity of Wilma Wasco. This research was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
None of the authors has any competing interests.
AUTHOR CONTRIBUTIONS
V.C.M. developed the concepts and approach, performed experiments, analyzed data, and prepared and edited the manuscript prior to submission; M.M. developed the concepts and approach, performed experiments, analyzed data, and prepared and edited the manuscript prior to submission; L.K. developed the concepts and performed experiments; A.R.K. developed the concepts and approach, performed experiments, analyzed data, and prepared and edited the manuscript prior to submission.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.004457/-/DC1
REFERENCES
- Berezovska O, Jack C, McLean P, Aster JC, Hicks C, Xia W, Wolfe MS, Kimberly WT, Weinmaster G, Selkoe DJ, et al. (2000). Aspartate mutations in presenilin and gamma-secretase inhibitors both impair notch1 proteolysis and nuclear translocation with relative preservation of notch1 signaling. J Neurochem. 75, 583–593 [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, et al. (1996). Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 17, 1005–1013 [DOI] [PubMed] [Google Scholar]
- Brodskii LI, Ivanov VV, Kalaidzidis Ia L, Leontovich AM, Nikolaev VK, Feranchuk SI, Drachev VA. (1995). GeneBee-NET: An Internet based server for biopolymer structure analysis. Biokhimiia 60, 1221–1230 [PubMed] [Google Scholar]
- Cao X, Sudhof TC. (2001). A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science 293, 115–120 [DOI] [PubMed] [Google Scholar]
- Carmine-Simmen K, Proctor T, Tschape J, Poeck B, Triphan T, Strauss R, Kretzschmar D. (2009). Neurotoxic effects induced by the Drosophila amyloid-beta peptide suggest a conserved toxic function. Neurobiol Dis. 33, 274–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhuchenko O, Kuspa A. (2007). Immune-like phagocyte activity in the social amoeba. Science 317, 678–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov KM, Iushmanov SV. (1988). A principle of maximum topological similarity in molecular systematics. Mol Gen Mikrobiol Virusol. 3, 3–9 [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. (1998). Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, et al. (1999). A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A. (1999). Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 13, 2801–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dries DR, Yu G. (2008). Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Curr Alzheimer Res. 5, 132–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. (2003). Reconstitution of gamma-secretase activity. Nat Cell Biol. 5, 486–488 [DOI] [PubMed] [Google Scholar]
- Faix J, Kreppel L, Shaulsky G, Schleicher M, Kimmel AR. (2004). A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32, e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, et al. (2002). aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell 3, 85–97 [DOI] [PubMed] [Google Scholar]
- Futai E, Yagishita S, Ishiura S. (2009). Nicastrin is dispensable for gamma-secretase protease activity in the presence of specific presenilin mutations. J Biol Chem. 284, 13013–13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Pimplikar SW. (2001). The gamma-secretase-cleaved C-terminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci USA 98, 14979–14984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Hardy J. (1997). The presenilins and Alzheimer’s disease. Hum Mol Genet. 6, 1639–1646 [DOI] [PubMed] [Google Scholar]
- Iushmanov SV, Chumakov KM. (1988). Algorithms for constructing phylogenetic trees of maximum topological similarity. Mol Gen Mikrobiol Virusol. 3, 9–15 [PubMed] [Google Scholar]
- Jutras I, Laplante A, Boulais J, Brunet S, Thinakaran G, Desjardins M. (2005). Gamma-secretase is a functional component of phagosomes. J Biol Chem. 280, 36310–36317 [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. (2002). Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell 110, 751–762 [DOI] [PubMed] [Google Scholar]
- Kay RR. (1987). Cell differentiation in monolayers and the investigation of slime mold morphogens. Methods Cell Biol. 28, 433–448 [DOI] [PubMed] [Google Scholar]
- Khandelwal A, Chandu D, Roe CM, Kopan R, Quatrano RS. (2007). Moonlighting activity of presenilin in plants is independent of gamma-secretase and evolutionarily conserved. Proc Natl Acad Sci USA 104, 13337–13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana T, Brzostowski JA, Kimmel AR. (2005). A Rab21/LIM-only/CH-LIM complex regulates phagocytosis via both activating and inhibitory mechanisms. EMBO J. 24, 2254–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Ingano LA, Carey BW, Pettingell WH, Kovacs DM. (2005). Presenilin/gamma-secretase-mediated cleavage of the voltage-gated sodium channel beta2-subunit regulates cell adhesion and migration. J Biol Chem. 280, 23251–23261 [DOI] [PubMed] [Google Scholar]
- Kim L, Liu J, Kimmel AR. (1999). The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell 99, 399–408 [DOI] [PubMed] [Google Scholar]
- Kim L, Harwood A, Kimmel AR. (2002). Receptor-dependent and tyrosine phosphatase-mediated inhibition of GSK3 regulates cell fate choice. Dev Cell 3, 523–532 [DOI] [PubMed] [Google Scholar]
- Kim SD, Kim J. (2008). Sequence analyses of presenilin mutations linked to familial Alzheimer’s disease. Cell Stress Chaperones 13, 401–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, Xia W, Rahmati T, Wolfe MS, Selkoe DJ. (2000). The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. J Biol Chem. 275, 3173–3178 [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. (2001). The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 276, 40288–40292 [DOI] [PubMed] [Google Scholar]
- Kimmel AR, Firtel RA. (2004). Breaking symmetries: regulation of Dictyostelium development through chemoattractant and morphogen signal-response. Curr Opin Genet Dev. 14, 540–549 [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. (2004). Gamma-secretase: proteasome of the membrane? Nat Rev Mol Cell Biol. 5, 499–504 [DOI] [PubMed] [Google Scholar]
- Kubohara Y, Arai A, Gokan N, Hosaka K. (2007). Pharmacological evidence that stalk cell differentiation involves increases in the intracellular Ca(2+) and H(+) concentrations in Dictyostelium discoideum. Dev Growth Differ. 49, 253–264 [DOI] [PubMed] [Google Scholar]
- Lammich S, Okochi M, Takeda M, Kaether C, Capell A, Zimmer AK, Edbauer D, Walter J, Steiner H, Haass C. (2002). Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem. 277, 44754–44759 [DOI] [PubMed] [Google Scholar]
- Leemans JC, Florquin S, Heikens M, Pals ST, van der Neut R, Van Der Poll T. (2003). CD44 is a macrophage binding site for Mycobacterium tuberculosis that mediates macrophage recruitment and protective immunity against tuberculosis. J Clin Invest. 111, 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi S, Polyakov M, Egelhoff TT. (2000). Green fluorescent protein and epitope tag fusion vectors for Dictyostelium discoideum. Plasmid 44, 231–238 [DOI] [PubMed] [Google Scholar]
- Lillis AP, Greenlee MC, Mikhailenko I, Pizzo SV, Tenner AJ, Strickland DK, Bohlson SS. (2008). Murine low-density lipoprotein receptor-related protein 1 (LRP) is required for phagocytosis of targets bearing LRP ligands but is not required for C1q-triggered enhancement of phagocytosis. J Immunol. 181, 364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLendon C, Xin T, Ziani-Cherif C, Murphy MP, Findlay KA, Lewis PA, Pinnix I, Sambamurti K, Wang R, Fauq A, et al. (2000). Cell-free assays for gamma-secretase activity. FASEB J. 14, 2383–2386 [DOI] [PubMed] [Google Scholar]
- McMains VC, Liao XH, Kimmel AR. (2008). Oscillatory signaling and network responses during the development of Dictyostelium discoideum. Ageing Res Rev. 7, 234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhie DL, Lee RK, Eckman CB, Olstein DH, Durham SP, Yager D, Younkin SG, Wurtman RJ, Neve RL. (1997). Neuronal expression of beta-amyloid precursor protein Alzheimer mutations causes intracellular accumulation of a C-terminal fragment containing both the amyloid beta and cytoplasmic domains. J Biol Chem. 272, 24743–24746 [DOI] [PubMed] [Google Scholar]
- Morohashi Y, Kan T, Tominari Y, Fuwa H, Okamura Y, Watanabe N, Sato C, Natsugari H, Fukuyama T, Iwatsubo T, et al. (2006). C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester). J Biol Chem. 281, 14670–14676 [DOI] [PubMed] [Google Scholar]
- Oster-Granite ML, McPhie DL, Greenan J, Neve RL. (1996). Age-dependent neuronal and synaptic degeneration in mice transgenic for the C terminus of the amyloid precursor protein. J Neurosci. 16, 6732–6741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M, Morrow J, Maxfield FR, Strickland DK, Greenberg S, Tabas I. (2003). The cytoplasmic domain of the low density lipoprotein (LDL) receptor-related protein, but not that of the LDL receptor, triggers phagocytosis. J Biol Chem. 278, 44799–44807 [DOI] [PubMed] [Google Scholar]
- Plyte SE, O’Donovan E, Woodgett JR, Harwood AJ. (1999). Glycogen synthase kinase-3 (GSK-3) is regulated during Dictyostelium development via the serpentine receptor cAR3. Development 126, 325–333 [DOI] [PubMed] [Google Scholar]
- Prager K, Wang-Eckhardt L, Fluhrer R, Killick R, Barth E, Hampel H, Haass C, Walter J, Parisiadou L, Fassa A, et al. (2007). A structural switch of presenilin 1 by glycogen synthase kinase 3beta-mediated phosphorylation regulates the interaction with beta-catenin and its nuclear signaling. J Biol Chem. 282, 14083–14093 [DOI] [PubMed] [Google Scholar]
- Richardson DL, Loomis WF, Kimmel AR. (1994). Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development 120, 2891–2900 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. (1998). The cell biology of beta-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 8, 447–453 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. (2007). Presenilin: running with scissors in the membrane. Cell 131, 215–221 [DOI] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, et al. (1995). Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 375, 754–760 [DOI] [PubMed] [Google Scholar]
- Steiner H, Fluhrer R, Haass C. (2008). Intramembrane proteolysis by gamma-secretase. J Biol Chem. 283, 29627–29631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nakaya T. (2008). Regulation of amyloid beta-protein precursor by phosphorylation and protein interactions. J Biol Chem. 283, 29633–29637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasugi N, Takahashi Y, Morohashi Y, Tomita T, Iwatsubo T. (2002). The mechanism of gamma-secretase activities through high molecular weight complex formation of presenilins is conserved in Drosophila melanogaster and mammals. J Biol Chem. 277, 50198–50205 [DOI] [PubMed] [Google Scholar]
- Tesco G, Tanzi RE. (2000). GSK3 beta forms a tetrameric complex with endogenous PS1-CTF/NTF and beta-catenin. Effects of the D257/D385A and FAD-linked mutations. Ann NY Acad Sci. 920, 227–232 [DOI] [PubMed] [Google Scholar]
- Tien AC, Rajan A, Bellen HJ. (2009). A Notch updated. J Cell Biol. 184, 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nelson O, Bezprozvanny A, Wang Z, Lee SF, Hao YH, Serneels L, De Strooper B, Yu G, Bezprozvanny I. (2006). Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell 126, 981–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, et al. (1999). Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. (2007). A beta oligomers-a decade of discovery. J Neurochem. 101, 1172–1184 [DOI] [PubMed] [Google Scholar]
- Wang R, Sweeney D, Gandy SE, Sisodia SS. (1996). The profile of soluble amyloid beta protein in cultured cell media. Detection and quantification of amyloid beta protein and variants by immunoprecipitation-mass spectrometry. J Biol Chem. 271, 31894–31902 [DOI] [PubMed] [Google Scholar]
- Williams JG. (2006). Transcriptional regulation of Dictyostelium pattern formation. EMBO Rep. 7, 694–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Kopan R. (2004). Intramembrane proteolysis: theme and variations. Science 305, 1119–1123 [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. (1999). Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398, 513–517 [DOI] [PubMed] [Google Scholar]
- Xia W, Wolfe MS. (2003). Intramembrane proteolysis by presenilin and presenilin-like proteases. J Cell Sci. 116, 2839–2844 [DOI] [PubMed] [Google Scholar]
- Yang T, Arslanova D, Gu Y, Augelli-Szafran C, Xia W. (2008). Quantification of gamma-secretase modulation differentiates inhibitor compound selectivity between two substrates Notch and amyloid precursor protein. Mol Brain 1, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Cheng I, Chung S, Grenfell TZ, Lee H, Pack-Chung E, Handler M, Shen J, Xia W, Tesco G, et al. (2000). Presenilin-mediated modulation of capacitative calcium entry. Neuron 27, 561–572 [DOI] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, et al. (2000). Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and betaAPP processing. Nature 407, 48–54 [DOI] [PubMed] [Google Scholar]
- Zhang M, Haapasalo A, Kim DY, Ingano LA, Pettingell WH, Kovacs DM. (2006). Presenilin/gamma-secretase activity regulates protein clearance from the endocytic recycling compartment. FASEB J. 20, 1176–1178 [DOI] [PubMed] [Google Scholar]
- Zhao G, Cui MZ, Mao G, Dong Y, Tan J, Sun L, Xu X. (2005). gamma-Cleavage is dependent on zeta-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem. 280, 37689–37697 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.