SUMMARY
Classic galactosemia is a potentially lethal disorder that results from profound impairment of galactose-1-phosphate uridylyltransferase (GALT). Despite decades of research, the underlying pathophysiology of classic galactosemia remains unclear, in part owing to the lack of an appropriate animal model. Here, we report the establishment of a Drosophila melanogaster model of classic galactosemia; this is the first whole-animal genetic model to mimic aspects of the patient phenotype. Analogous to humans, GALT-deficient D. melanogaster survive under conditions of galactose restriction, but accumulate elevated levels of galactose-1-phosphate and succumb during larval development following galactose exposure. As in patients, the potentially lethal damage is reversible if dietary galactose restriction is initiated early in life. GALT-deficient Drosophila also exhibit locomotor complications despite dietary galactose restriction, and both the acute and long-term complications can be rescued by transgenic expression of human GALT. Using this new Drosophila model, we have begun to dissect the timing, extent and mechanism(s) of galactose sensitivity in the absence of GALT activity.
INTRODUCTION
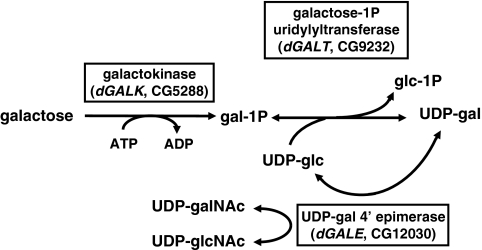
In higher eukaryotes, galactose and its derivatives serve as key constituents of glycoproteins, glycolipids and complex carbohydrates. As a component of lactose, galactose also provides close to half of the sugar calories in mammalian milk. Galactose is metabolized in mammals and other species by the enzymes of the Leloir pathway: galactokinase (GALK, EC 2.7.1.6), galactose-1-phosphate uridylyltransferase (GALT, EC 2.7.7.12) and uridine diphosphate (UDP)-galactose 4′ epimerase (GALE, EC 5.1.3.2) (Fig. 1) (Holden et al., 2003). In humans, profound impairment of GALT results in the potentially lethal disorder classic galactosemia [OMIM #230400, reviewed in Fridovich-Keil and Walter (Fridovich-Keil and Walter, 2008)].
Fig. 1.
The Leloir pathway of galactose metabolism. Classic galactosemia results from profound impairment of the second enzyme in the Leloir pathway, galactose-1P uridylyltransferase (GALT). GALT catalyzes the synthesis of glucose-1P (glc-1P) and UDP-galactose (UDP-gal) from UDP-glucose (UDP-glc) and galactose-1P (gal-1P).
Classic galactosemia is an autosomal recessive disorder that affects around one in 60,000 live births. Following exposure to milk, affected infants experience a rapid clinical demise, with symptoms that escalate from vomiting, diarrhea and a failure to thrive, to jaundice, Escherichia coli sepsis and neonatal death. Untreated infants with classic galactosemia also accumulate dramatically elevated levels of galactose-1-phosphate (gal-1P) in their cells and tissues. Although careful dietary galactose restriction prevents or reverses the acute and potentially lethal symptoms of classic galactosemia, and also largely resolves the abnormal accumulation of gal-1P, significant long-term complications are common; these include cognitive impairment, speech difficulties, neuromuscular problems and premature ovarian insufficiency, among others (for a review, see Fridovich-Keil and Walter, 2008).
Despite decades of study, the underlying bases of the pathophysiology of both the acute and long-term complications of classic galactosemia remain unclear (for a review, see Fridovich-Keil and Walter, 2008) (Tyfield and Walter, 2002; Leslie, 2003). One of the principal factors limiting research progress has been the lack of an animal model that recapitulates either the acute or the long-term complications of the disorder. Studies conducted by ourselves and others using yeast and mammalian tissue culture models have provided biochemical insights into the consequences of GALT activity loss (e.g. Fridovich-Keil and Jinks-Robertson, 1993; Fridovich-Keil et al., 1995a; Fridovich-Keil et al., 1995b; Elsevier and Fridovich-Keil, 1996; Elsevier et al., 1996; Lai et al., 1996; Lai and Elsas, 2000; Riehman et al., 2001; Christacos and Fridovich-Keil, 2002; Lai et al., 2003; Ross et al., 2004), but the absence of an appropriate multicellular model has precluded studies into the timing and impact of GALT deficiency on organismal development, or on intact tissues and organ systems. Of note, more than a decade ago, Leslie and colleagues (Leslie et al., 1996) reported a Galt knockout mouse that demonstrated a complete loss of GALT activity and that accumulated gal-1P; however, these mice unexpectedly remained healthy and fertile despite dietary exposure to large quantities of galactose (Leslie et al., 1996; Ning et al., 2000; Leslie and Bai, 2001; Ning et al., 2001; Leslie et al., 2005). This outcome disparity between GALT-deficient humans and mice remains unexplained.
As an alternative to mice, we turned to the fruit fly Drosophila melanogaster. Fruit flies have facilitated genetic experiments for more than a century and, in recent years, have emerged as an extremely powerful system to model human genetic diseases (Bier, 2005), including complex metabolic disorders, such as diabetes and obesity (Lasko, 2002; Bharucha, 2009; Zhang et al., 2009). Sequence alignments show that greater than 75% of recognized human disease genes, including all three Leloir pathway genes (http://superfly.ucsd.edu/homophila/), have related sequences in the D. melanogaster genome (Bier, 2005).
Here, we report the establishment and first application of a D. melanogaster model of classic galactosemia. Like human patients, and unlike mice, GALT-deficient Drosophila survive if maintained on food that contains only glucose (glc), but die as larvae if exposed to food that contains both glucose and galactose. This galactose-dependent lethality is dose dependent, sugar specific and can be rescued by the expression of a human GALT transgene. GALT-deficient animals are also rescued from death by the initiation of a galactose-restricted diet early in development. Of note, larval animals transferred from a glucose diet to a glucose-plus-galactose diet die within days of the transfer, but adult flies do not, arguing for stage-specific or dose-dependent consequences to impaired galactose metabolism. As expected, metabolite studies of GALT-deficient larvae and adults exposed to galactose show an abnormal accumulation of gal-1P. Finally, GALT-deficient flies that are raised and maintained exclusively on a galactose-restricted diet demonstrate a clear deficiency in the normal negative geotaxic response seen in controls (Benzer, 1967; Akai, 1979). As with the acute galactose-dependent phenotype, this galactose-independent muscular or neuromuscular deficit is rescued by the expression of a human GALT transgene. These data confirm that GALT-deficient D. melanogaster mimic aspects of both the acute and long-term outcomes of classic galactosemia, paving the way for future studies to explore the genetic and environmental factors that underlie and modify these phenotypes.
RESULTS
D. melanogaster metabolize galactose via the Leloir pathway
To explore the role of galactose metabolism in D. melanogaster, we first confirmed that flies encode and express orthologs of human GALK, GALT and GALE (Fig. 1). The D. melanogaster genes whose predicted protein products show the greatest amino acid sequence homology to the human Leloir enzymes are CG5288 (on chromosome III, designated here as dGALK), CG9232 (on chromosome II, designated here as dGALT), and CG12030 (on chromosome III, designated here as dGALE) (http://superfly.ucsd.edu/homophila/) (Chien et al., 2002). Both dGALT and dGALE show strong conservation with their corresponding human orthologs, having 57% sequence identity with 72% sequence similarity, and 60% sequence identity with 76% sequence similarity, respectively. dGALK demonstrates slightly lower conservation, with 27% amino acid sequence identity and 44% sequence similarity.
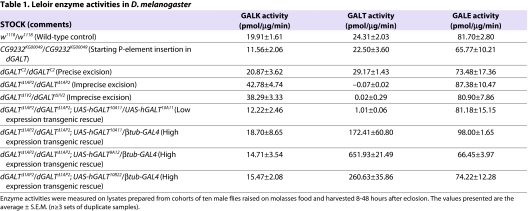
Publicly available in situ RNA hybridization data from the Berkeley Drosophila Genome Project (BDGP; http://www.fruitfly.org/) confirm that all three fly Leloir genes are expressed during embryogenesis. Assays of lysates prepared from wild-type (w1118) D. melanogaster adults also reveal the active presence of all three enzymes (Table 1). Finally, studies of animals carrying disruptions or deletions of each of the three fly genes (Table 1) (Sanders et al. 2010) (P.J.L., K. Garza and J.L.F.-K., unpublished) demonstrate loss of the corresponding enzymatic activity, thereby confirming a functional connection between each gene sequence and the enzymatic activity attributed to its predicted protein product.
Table 1.
Leloir enzyme activities in D. melanogaster
The dGALT gene is approximately 2 kb in length and includes four exons, the first of which is noncoding (supplementary material Fig. S1); the predicted mRNA is ∼1.5 kb. The fly dGALE and dGALK genes will be described in detail elsewhere (Sanders et al., 2010) (P.J.L., K. Garza and J.L.F.-K., unpublished). Of note, the dGALT gene is located entirely within the second intron of another gene, cup (Keyes and Spradling, 1997). cup is required for ovarian function and egg production in flies; female flies that are deficient in cup are infertile and fail to lay embryos (Keyes and Spradling, 1997) (for a review, see Piccioni et al., 2005).
Creation of a dGALT-deficient allele within a functional allele of cup
We created an impaired allele of dGALT by imprecise excision of an existing P-element insertion, KG00049, in the 5′-untranslated region (UTR) of the CG9232 gene (supplementary material Fig. S1A). This CG9232KG00049 allele fully complemented a strong cup allele (cup01355), suggesting that it has little if any effect on cup expression; female cup01355/dGALTKG00049 compound heterozygotes crossed to w1118 males produced viable embryos that developed into phenotypically normal flies. The dGALTKG00049 allele also failed to impair dGALT expression or function, as indicated by the wild-type level of GALT activity detected in lysates from homozygotes (Table 1).
Excision of the P-element was achieved by transient exposure to the Δ2-3 transposase in the germ line of male flies (Ryder and Russell, 2003), as described in the Methods. From the resulting excision alleles, we identified two, designated dGALTΔ1AP2 and dGALTΔ1V2, that each carried the same 1647-bp deletion removing almost the entire dGALT gene (supplementary material Fig. S1B). Flies carrying a precise excision of the KG00049 P-element insertion, designated dGALTC2, were also identified and characterized (supplementary material Fig. S1C). As expected, dGALTΔ1AP2 and dGALTΔ1V2 homozygotes demonstrated a complete lack of detectable GALT activity, whereas dGALTC2 homozygotes demonstrated wild-type GALT activity (Table 1). Finally, all three excision alleles demonstrated normal GALE activity (Table 1) and complemented the cup01355 allele, indicating that cup remained functional on these chromosomes. Of note, a small increase in GALK activity was apparent when GALT activity was deficient (Table 1). The basis for this apparent increase remains unknown but may reflect either a true compensatory increase in GALK expression or activity, or alternatively an artifact of the assay, perhaps resulting from increased stability of the GALK reaction product, gal-1P, when GALT is not present to metabolize it.
Dietary galactose is lethal to dGALT-deficient flies
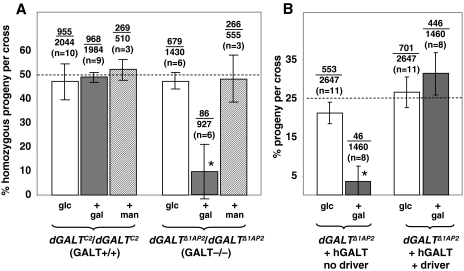
To test the impact of galactose exposure on dGALT-deficient Drosophila, we assessed the survival of F1 progeny from crosses of homozygous dGALTΔ1AP2 or dGALTΔ1V2 virgin females to heterozygous dGALTΔ1AP2/CyO or dGALTΔ1V2/CyO males. Crosses were performed on fly food containing 555 mM glucose, or 555 mM glucose plus 222 mM galactose (Fig. 2; Table 2). Surviving adult progeny were genotyped by the presence or absence of the balancer chromosome (CyO), which conferred a dominant curly wing phenotype. Viability of the dGALT-deficient progeny resulting from each cross was quantified in terms of the percentage of surviving F1 animals that were unbalanced (exhibiting straight wings).
Fig. 2.
Loss of dGALT results in galactose sensitivity of D. melanogaster. (A) Percentage of viable dGALT-deficient progeny resulting from crosses of dGALT –/– virgin females with dGALT +/– males on the food sources indicated (glc, 555 mM glucose food; + gal, 555 mM glucose plus 222 mM galactose food; + man, 555 mM glucose plus 222 mM mannose). The fractions over the bars indicate the combined numbers of homozygous adult flies over the total numbers of progeny counted in each category; n=the number of crosses from which those progeny were derived. Each cross produced at least 75 viable F1 adults. Flies carrying a precise excision (dGALTC2) rather than an imprecise excision (dGALTΔ1AP2) allele of dGALT served as the positive control. (B) Expression of a human GALT transgene rescued the viability of dGALTΔ1AP2/dGALTΔ1AP2 D. melanogaster exposed to galactose. Note that these data were generated using the hGALT transgene alleles 9A12, 10A12 and 10B22, which were minimally, if at all, leaky. Asterisks (*) indicate statistical significance, P<0.0001 for a two-tailed t-test at the 95% confidence interval.
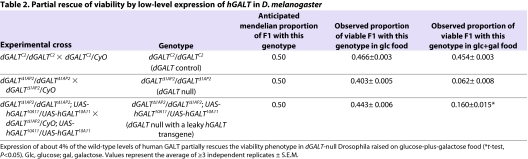
Table 2.
Partial rescue of viability by low-level expression of hGALT in D. melanogaster
The result was striking: on food that contained glucose as the sole sugar, unbalanced dGALT-deficient flies accounted for close to the expected 50% of viable offspring, but on food supplemented with 222 mM galactose, dGALT-deficient flies accounted, on average, for <10% of viable offspring (Fig. 2A; Table 2). When the galactose concentration was raised to 278 mM, the number of viable dGALT-deficient animals plummeted nearly to zero (data not shown). This same result was also obtained using the dGALTΔ1V2 allele, as well as using dGALTΔ1AP2/dGALTΔ1V2 trans-heterozygotes.
As a control for galactose specificity of the apparent food sensitivity, we repeated each cross on food containing 555 mM glucose plus 222 mM mannose rather than galactose; all dGALT-deficient crosses yielded close to 50% unbalanced offspring (Fig. 2A). We also performed crosses using flies that were homozygous for the precise excision allele (dGALTC2); these crosses also yielded close to 50% unbalanced offspring regardless of the sugar composition of the food (Fig. 2A).
Finally, to test whether dGALTΔ1AP2 behaved as a genetic null allele, we crossed homozygous dGALTΔ1AP2 virgin females to males that were heterozygous for a large genomic deletion that removes the entire dGALT locus (Df(2L)Exel7027) and assessed the effect of this dGALTΔ1AP2/Df(2L)Exel7027 genotype on galactose sensitivity. The outcomes of these crosses (data not shown) were comparable to the outcomes of crosses using dGALTΔ1AP2/dGALTΔ1AP2 or dGALTΔ1V2/dGALTΔ1V2 flies, indicating that dGALTΔ1AP2 and dGALTΔ1V2 behave as genetic nulls.
Transgenic expression of human GALT rescues the viability of dGALT-deficient D. melanogaster exposed to galactose
To confirm that the galactose sensitivity of dGALT-deficient Drosophila resulted from the loss of GALT activity, we attempted transgenic rescue using a UAS-driven human GALT transgene (UAS-hGALT). The rationale for using a human rather than a fly GALT transgene was to test whether dGALT and hGALT are true functional orthologs. Male flies that are homozygous for dGALTΔ1AP2 and heterozygous for a β-tubulin-GAL4 driver were crossed to virgin females that were heterozygous for dGALTΔ1AP2 and homozygous for one of the three UAS-hGALT transgenes, UAS-hGALT9A12, UAS-hGALT10A12 or UAS-hGALT10B22. These crosses were conducted in parallel on foods containing 555 mM glucose or 555 mM glucose plus 222 mM galactose. As illustrated in Fig. 2B and Table 1, the presence of an hGALT transgene together with the β-tubulin-GAL4 driver resulted in overexpression of hGALT activity and fully rescued the viability of dGALTΔ1AP2 homozygous Drosophila that were exposed to galactose. The transgenic rescue data further demonstrated that overexpression of hGALT appears benign, although we have not ruled out subtle effects.
To test the ability of low-level expression of human GALT to rescue the viability of dGALT-null Drosophila, we generated animals that were homozygous for dGALTΔ1AP2 and also homozygous for a leaky hGALT transgene, UAS-hGALT10A11. These animals expressed just over 4% of the wild-type levels of GALT activity despite the absence of any GAL4 driver (Table 1), and even this low level was sufficient for partial rescue of the viability phenotype on glucose-plus-galactose food (Table 2).
Timing and dose-dependent lethality of GALT-deficient D. melanogaster exposed to galactose
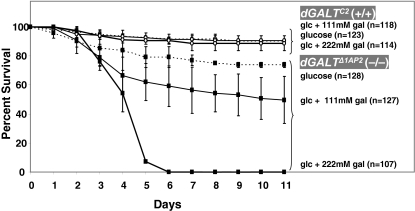
To identify the stage of development at which GALT-deficient D. melanogaster succumb to galactose toxicity, we followed the fates of cohorts of >100 individual progeny derived from crosses of dGALTΔ1AP2 homozygous virgin females and males. In brief, embryos less than 12-hours old were harvested from egg-laying plates and transferred to the wells of 96-well plates that were preloaded with each of three types of fly food: food containing 555 mM glucose, food containing 555 mM glucose plus 111 mM galactose, and food containing 555 mM glucose plus 222 mM galactose. Each day the numbers of live and dead animals were recorded; the results (Fig. 3) revealed four important points.
Fig. 3.
Timing of death in dGALT-deficient Drosophila exposed to galactose. The age of animals, in days, is plotted on the x-axis; the percentage of animals remaining viable in each cohort is plotted on the y-axis. Animals homozygous for the dGALTC2 precise excision allele served as a positive control; these animals remained predominantly viable throughout the 11-day course of the experiment, regardless of the food content. By contrast, animals homozygous for the dGALTΔ1AP2 imprecise excision allele remained predominantly viable in the absence of galactose, but died in increasing numbers during larval development when exposed to increasing levels of dietary galactose.
First, wild-type dGALT animals that were homozygous for the precise excision allele (dGALTC2) remained viable and developed normally throughout the 11-day course of the experiment, regardless of the presence or absence of galactose in their food. By day 11, close to 90% of these animals had eclosed as viable adults, demonstrating that the 96-well plate format was not itself a major cause of delayed development or death.
Second, even in the absence of dietary galactose, animals that were homozygous for the imprecise excision allele (dGALTΔ1AP2) were slightly less robust than their precise excision counterparts. By day 11, only 74% of these homozygotes had eclosed as viable adults, compared with 90% of controls. Of note, this difference did not appear to be as pronounced in standard culture vials, suggesting that the 96-well plate format may have disproportionately stressed the dGALT-deficient animals, although this possibility remains unexplained.
Third, in the presence of increasing concentrations of galactose, we saw a decreasing percentage of dGALT-deficient animals that survived to adulthood. Specifically, on food containing 111 mM galactose, only 50% of the animals eclosed as viable adults, whereas on food containing 222 mM galactose, none of the dGALT-deficient animals eclosed as viable adults.
Finally, the timing of death of the dGALT-deficient Drosophila that were exposed to galactose occurred during mid- to late-larval stages. Essentially, all the mutant embryos visibly hatched into viable first instar (L1) larvae and began to eat food, as shown by the presence of coloring from the food in their gut, but none of these larvae survived to pupation. It is difficult to distinguish the precise stage of lethality, because the dying animals were stunted relative to controls (supplementary material Fig. S2). Whether this small organismal size reflected a true developmental delay or simply a growth defect remains unclear.
Window of galactose sensitivity of GALT-deficient D. melanogaster
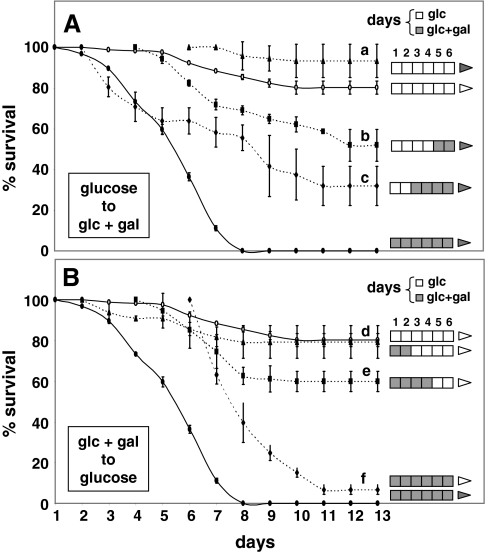
To define the stage of development at which GALT-deficient D. melanogaster are most sensitive to galactose, we performed dietary ‘cross-over’ experiments (Fig. 4) in which cohorts of dGALTΔ1AP2 homozygotes (GALT deficient) were transferred, as embryos or larvae, from food containing only 555 mM glucose to food containing 555 mM glucose plus 222 mM galactose, or from food containing 555 mM glucose plus 222 mM galactose to food containing only 555 mM glucose. As a control for the transfer process, animals were also transferred each day from glucose food to glucose food, and from glucose-plus-galactose food to glucose-plus-galactose food. The transfers were performed for six days, which covered the span of larval development (L1 to L3), after which all animals were allowed to complete their development without further interference.
Fig. 4.
Window of galactose sensitivity of dGALTΔ1AP2 imprecise excision homozygotes. The day of development is plotted on the x-axis; the percentage survival in each cohort is plotted on the y-axis. dGALT-deficient embryos and larvae were transferred daily in cohorts of >20 animals each from 555 mM glucose-only food to 222 mM galactose-supplemented food (dashed lines in A), or from 222 mM galactose-supplemented food to 555 mM glucose-only food (dashed lines in B), for six days, after which the animals in each cohort were allowed to complete development without further interference. To the right of each plotted curve is a key illustrating the food exposure of the corresponding cohort of animals; each open box represents one day of development on glucose food and each shaded box represents one day of development on food supplemented with 222 mM galactose. The shading of the arrow to the right of each key indicates whether subsequent development occurred on glucose (open arrow) or glucose-plus-galactose (shaded arrow) food. Of note, only live animals were transferred, which led to an apparent but artificial upward shift in the survival curves for some cohorts of animals, as explained in the Results. As a control, animals were also transferred from glucose-only to glucose-only food (the upper solid line in each panel), and from galactose-supplemented food to galactose-supplemented food (the lower solid line in each panel). In both experiments, animals demonstrated a reversible response to galactose exposure during early larval development.
The results clearly showed that GALT-deficient animals that started developing on glucose-only food nonetheless succumbed if they were transferred to glucose-plus-galactose food early in larval development (e.g. on day 2, which corresponded to late L1 or early L2 stage) (Fig. 4). Transfer on subsequent days (e.g. on day 4, which corresponded to late L2 or early L3 stage) resulted in diminished, albeit detectable, loss of viability. Significantly, GALT-deficient animals exposed to galactose late in larval development (e.g. on day 6, which corresponded to L3 stage), or as adults, demonstrated no significant loss of viability relative to their galactose-restricted counterparts.
Similarly, GALT-deficient animals that started life on food supplemented with galactose were rescued from death by transfer to glucose-only food, as long as the transfer occurred within the first two days of development. Transfer on later days conferred only a limited survival benefit. Of note, only live animals were transferred between foods, which led to an apparent but artificial upward shift in the survival curves of animals transferred from galactose-supplemented food to glucose-only food (Fig. 4B), and also to a slight upward shift in the survival curves of animals transferred between foods on later days of development (e.g. day 6) (Fig. 4A,B). Although cohorts of animals were transferred on each of the first six days of these experiments, for simplicity, the results from transfers on only the even-numbered days are presented in Fig. 4; however, the cohorts transferred on odd-numbered days showed survival profiles that were fully consistent with these patterns.
Finally, the vast majority of animals transferred from glucose-only food to fresh glucose-only food developed into viable adults, whereas all GALT-deficient animals transferred from galactose-supplemented food to fresh galactose-supplemented food died as larvae, demonstrating that the physical transfer process itself did not noticeably alter the outcomes observed.
GALT-deficient Drosophila accumulate gal-1P when exposed to galactose
To test whether GALT-deficient Drosophila that are exposed to dietary galactose accumulate abnormal levels of gal-1P, we exposed developing larvae and newly eclosed adults to food containing either 555 mM glucose alone or 555 mM glucose plus 222 mM galactose for four days. Extracts of these animals were analyzed as described in the Methods. In the absence of dietary galactose, dGALTΔ1AP2 homozygous larvae and adults had 0.75±0.13 (n=3) and 0.22±0.09 (n=3) pmol gal-1P/μg protein, respectively, and in the presence of dietary galactose, these animals accumulated 11.82 or 12.09 (n=2) and 7.95±4.25 (n=3) pmol gal-1P/μg protein, respectively. Parallel samples from dGALTC2 homozygous larvae and adults had only 0.37±0.09 and 0.10±0.04 (n=3/group, glucose-only food) and 0.85±0.12 and 0.30±0.10 (n=3/group, glucose-plus-galactose food) pmol gal-1P/μg protein, respectively.
Galactose-restricted GALT-deficient flies demonstrate an impaired negative geotaxic response
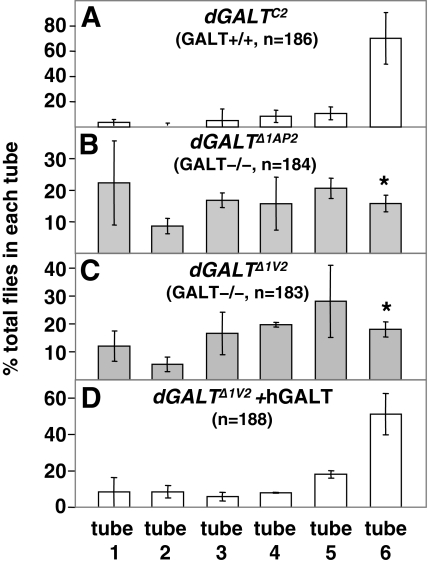
To address whether GALT-deficient D. melanogaster might, like their human counterparts, exhibit long-term complications despite lifelong dietary restriction of galactose, we assessed the negative geotaxic response of dGALTΔ1AP2 homozygous, dGALTΔ1V2 homozygous and dGALTC2 homozygous flies. All of these animals were raised on standard molasses food, which contains negligible if any galactose, and tested using a previously described ‘countercurrent’ apparatus (Benzer, 1967; Akai, 1979) that quantifies negative geotaxic response. Normal flies exhibit a strong negative geotaxic response and a quick response to stimulus, meaning they begin to move shortly after being tapped to the bottom of a tube, and they prefer to run ‘up’ the sides of a tube rather than stand still or run ‘down’.
The apparatus and procedure that we applied offered cohorts of approximately 60 male flies, aged 2–3 days, six sequential opportunities each to walk or run up the sides of a tube in a well-lit room within a specified period of time (15 seconds). Flies that moved to the top of inverted tube one within the first 15-second period were tapped to the bottom of tube two, and the next round was initiated. At the end of the six sequential rounds, the ‘fastest’ flies would be found in tube six and the ‘slowest’ flies would be found in tube one.
Control flies subjected to this countercurrent test presented a final distribution in which around 60% of the flies were in tube six, with the remainder distributed almost evenly between the other five tubes (Fig. 5A); this result is fully consistent with the original report of this behavior (Benzer, 1967). By striking contrast, both GALT-deficient stocks demonstrated an almost-random distribution of flies among the six tubes, with no enrichment in tube six (Fig. 5B,C). This differential distribution profile was highly statistically significant (P<0.01) and was rescued by the expression of the human GALT transgene (Fig. 5D), thereby demonstrating that this phenotype resulted from the absence of GALT activity.
Fig. 5.
dGALT-deficient flies demonstrate an impaired negative geotaxic response despite dietary restriction of galactose. Cohorts of 60 male flies, aged 2–3 days, of the indicated genotypes were subjected to ‘countercurrent’ analysis as a measure of negative geotaxic response. All flies began the experiment in the bottom of tube one. Those flies that walked to the top of tube one in 15 seconds were tapped to the bottom of tube two, and so forth. After six rounds of tapping and transfer, the fastest flies could be found in tube six and the slowest flies could be found in tube one. The values plotted represent averages ± standard deviation (n =3). Flies homozygous for the dGALTΔ1AP2 (B) and dGALTΔ1V2 (C) imprecise excision alleles demonstrated a statistically significant impairment of negative geotaxic response relative to flies homozygous for the dGALTC2 precise excision allele (A) [*P <0.01 in a one-way analysis of variance (ANOVA) with 95% confidence intervals]; this impairment was rescued by the expression of a human GALT transgene (D).
DISCUSSION
Here we report the generation and initial characterization of a D. melanogaster model of classic galactosemia. This is the first whole-animal genetic model of GALT deficiency to mimic any of the clinical manifestations of classic galactosemia and, as such, represents a major step forward for the field.
Galactose sensitivity of GALT-deficient flies
Our data establish two main points about galactose sensitivity. First, loss of dGALT in developing D. melanogaster results in a galactose-dependent lethality, and this outcome is rescued by transgenic expression of human GALT. Although obvious in its simplicity, this result is also profound because it largely puts to rest a nagging concern that has plagued the field since Leslie and colleagues first reported that loss of Galt in mice is apparently benign (Leslie et al., 1996). In short, the lack of a phenotype in Galt-deficient mice raised the unlikely, but disturbing, possibility that the clinical outcomes of patients with classic galactosemia might result from some cryptic impact of their mutations, rather than from the detected loss of GALT enzymatic activity. With the work presented here, the causal relationship between loss of GALT activity and galactosemia-related phenotypes in a multicellular organism is confirmed.
Our data presented here further establish that even trace expression (∼4%) of human GALT can at least partially rescue the galactose-sensitivity of dGALT-deficient Drosophila. This result is especially notable in light of patient data; humans who are homozygous for the S135L-hGALT allele, which is associated with about 5% residual GALT activity, tend to show a relatively mild clinical course (Lai et al., 1996) (for a review, see Fridovich-Keil and Walter, 2008). This result is also notable in contrast to GALE. The patients who are most severely affected with GALE-deficiency galactosemia express at least 5% residual GALE activity (for a review, see Fridovich-Keil and Walter, 2008), and Drosophila expressing approximately 4% residual GALE activity (from a hypomorphic dGALE allele) are nonviable (Sanders et al., 2010). The results observed in flies therefore parallel those seen in patients, namely that marginal levels of GALT activity are sufficient for life, whereas comparably marginal levels of GALE are not.
Second, the galactose-dependent mortality observed in dGALT-deficient D. melanogaster is both dose- and time-dependent. Exposure to a lower level of galactose, or exposure to galactose later in development, resulted in less severe outcomes. These data implicate a potential threshold, or series of thresholds, of galactose sensitivity in GALT-deficient Drosophila. Alternatively, or in addition, there may be a finite developmental window of sensitivity. As we did not quantify the exact amount of food (and therefore galactose) consumed by each animal on each day, we cannot distinguish between these possible options. The possibility therefore remains that younger animals appeared more sensitive to galactose because they consumed proportionally larger quantities of food and, therefore, probably accumulated proportionally larger quantities of galactose than their older counterparts.
Consistent with this conclusion, we observed that the numbers of dGALT-deficient flies that eclosed as viable adults when exposed to 222 mM galactose fell, on average, to below 10%; the precise proportion varied from experiment to experiment, even for crosses involving flies of the same genotype. Raising the level of galactose to 278 mM was sufficient to bring the number of dGALT-deficient survivors virtually to zero, but at lower concentrations of galactose, subtle factors such as the number of flies placed in a vial, or whether the animals were allowed to develop in vials or in the wells of a 96-well plate, seemed to impact the ability of some dGALT-deficient animals to escape early death. Future experiments will address the nature and extent of these cryptic modifying factors.
Also evident here was our finding that, if galactose exposure was early but transient, the potentially lethal ‘damage’ was reversible. This observation is not surprising given the decades of clinical experience reported from human populations under surveillance by newborn screening, where affected infants are routinely rescued from acute disease by rapid initiation of dietary galactose restriction (for a review, see Fridovich-Keil and Walter, 2008). The fly data are nonetheless compelling, because they suggest that future experiments using this model system may reveal, with greater precision, both the nature of the damage and the mechanism by which it is reversed or overcome when galactose is removed from the diet.
Despite the significance of these findings, it is important not to over-interpret the fly data. For example, although exposure of Drosophila adults or late-stage larvae to galactose was not lethal, we do not know that it was completely benign. It would be premature, therefore, to conclude that dGALT-deficient flies, or classic galactosemia patients, do not require lifelong dietary restriction of galactose. Nevertheless, there are intriguing anecdotal reports from the literature (Bosch et al., 2004; Panis et al., 2006) that describe a similar finding in patients; namely, that older children and adults with classic galactosemia appear to be able to consume dietary galactose with no acute negative clinical consequences.
Long-term complications
Although we have just begun to test our dGALT-deficient flies for long-term phenotypes, abnormalities clearly exist (Fig. 5) and these can be rescued by expression of a human GALT transgene. The galactose-independent phenotype that we report here, impaired negative geotaxic response, is a complex trait that could reflect any of a number of underlying muscular or neuromuscular abnormalities. Although many patients with classic galactosemia do experience apparent neuromuscular complications, the relationship between those patient outcomes and the fly phenotype reported here remains to be explored. Future studies will address the extent and nature of other potential abnormalities in dGALT-deficient flies, as well as the genetic and environmental factors that underlie them.
Metabolic abnormalities in GALT-impaired D. melanogaster
The finding that GALT-deficient Drosophila exposed to galactose accumulate elevated levels of gal-1P is fully consistent with metabolic abnormalities reported in patients (for a review, see Fridovich-Keil and Walter, 2008). What is striking, however, is that the levels of gal-1P accumulated from four days of galactose exposure in larvae and adults is fairly comparable [11.82 or 12.09 (n=2) and 7.95±4.25 (n=3) pmol gal-1P/μg protein, respectively], yet the larvae are progressing rapidly toward death, whereas the adults are not. There are many possible explanations for this apparent disparity, but we must also consider that gal-1P itself may not be the primary cause of symptoms. Rather, the symptoms may result from stage-specific ways in which the body responds to an elevated gal-1P level. This observation, in essence, parallels the lesson of the Galt knockout mouse, which also accumulated high levels of gal-1P in response to dietary galactose exposure, and which also did not die as a result (Leslie et al., 1996). Hence, regardless of age, mice do not succumb in response to galactose exposure, whereas flies only succumb to galactose-exposure within an apparent developmental window of sensitivity. Clearly, if we are to understand the underlying pathophysiology of galactosemia we must find out what occurs downstream of gal-1P accumulation in developing animals. With a fly genetic model that recapitulates aspects of the patient phenotype, at long last we have tools to begin the work.
METHODS
Fly stocks and maintenance
Details about the stocks of D. melanogaster used in this study are provided in supplementary material Table S1. Stocks were maintained at 25°C on a molasses-based food that contained 43.5 g/l cornmeal, 17.5 g/l yeast extract, 8.75 g/l agar, 54.7 ml/l molasses, 10 mls propionic acid and 14.4 ml/l tegosept mold inhibitor (10% w/v in ethanol). For experiments in which the levels and types of sugar were to be varied, we used a glucose-based food [5.5 g/l agar, 40 g/l yeast, 90 g/l cornmeal, 100 g/l glucose, 10 ml/l propionic acid and 14.4 ml/l tegosept mold inhibitor (10% w/v in ethanol)] (Honjo and Furukubo-Tokunaga, 2005) supplemented with galactose or mannose, as indicated. For some experiments, liquid food coloring was also added to facilitate confirmation that larvae were eating.
Creation and molecular characterization of the dGALTΔ1AP2, dGALTΔ1V2 and dGALTC2 alleles
Excision alleles of dGALT were generated by mobilizing an existing SUPor-P insertion in the 5′ -UTR of the CG9232 locus (KG00049) via transient expression of the Δ2–3 transposase enzyme in the male germ line, according to standard methods (Ryder and Russell, 2003). Flies carrying excision alleles were identified by loss of the associated mini-w+ marker (white eyes), and homozygous stocks derived from those flies were sorted according to the level of GALT enzymatic activity detected in soluble lysates. Of more than 50 excision stocks tested, five demonstrated a profound loss of GALT enzymatic activity; those stocks were designated as imprecise excision candidates, whereas stocks demonstrating wild-type GALT activity were designated as precise excision candidates.
Given the genomic location of dGALT within the second intron of cup, we further tested all excision alleles of interest for their ability to complement a strong mutant allele of cup (cup01355). Of note, the KG00049 P-element insertion allele was itself homozygous viable and female fertile, and also complemented the cup01355 allele. Similarly, female flies carrying the cup01355 allele in trans to either dGALTΔ1AP2, dGALTΔ1V2 or dGALTC2 remained viable and fertile, confirming that cup remained functional in each genotype.
To characterize the dGALTΔ1AP2, dGALTΔ1V2 and dGALTC2 alleles at the molecular level, we amplified the relevant genomic sequences using primers that annealed within the flanking exons of cup. The primer sequences were 5′-GCTGACTGCTGATCTCGCCGTTGT-3′ and 5′-CCAAGGAGAGCTTTGTGATGCCT-3′. Control PCR amplification of the corresponding region from w1118 flies revealed the anticipated 4-kb amplicon. The dGALTC2 stock also produced a 4-kb amplicon, whereas both the dGALTΔ1AP2 and dGALTΔ1V2 stocks produced ∼2.4-kb amplicons. Direct sequencing of the amplicons from all three excision templates revealed that the presumed precise excision allele was indeed precise (supplementary material Fig. S1C), and that each of the presumed imprecise excision alleles carried the same 1647-bp deletion removing virtually the entire coding region of the dGALT gene, with a small remnant of the P-element sequence left behind (supplementary material Fig. S1A,B).
Transgenic lines
A UAS-hGALT transgene was generated by subcloning the wild-type human GALT coding sequence, as an EcoRI-SalI fragment, into pUAST (Brand and Perrimon, 1993) using the EcoRI and XhoI sites in the pUAST polylinker region. The resulting plasmid was confirmed by sequence analysis. UAS-hGALT stocks were generated using standard transgenic techniques by the Fly Core of the Massachusetts General Hospital, Charlestown, MA. Candidate insertion lines were mapped and balanced. Four homozygous viable insertions (dGALT9B12, dGALT10A11, dGALT10A12 and dGALT10B22) on chromosome III were used in this study.
GALK, GALT and GALE enzyme assays
Lysates were prepared and assays (n≥3) were performed (for Table 1), as described in Sanders et al. (Sanders et al., 2010).
Measuring gal-1P in larvae and adults
Cohorts of newly hatched dGALTΔ1AP2 homozygous and dGALTC2 homozygous larvae and newly eclosed adults were transferred to cages or vials containing either 555 mM glucose only or 555 mM glucose plus 222 mM galactose food. After 4 days, pools of 10 adult flies or ≥20 larvae were anesthetized with CO2, suspended in 125 μl of ice-cold high-performance liquid chromatography (HPLC)-grade water, and ground on ice for 15 seconds using a Teflon micropestle and handheld micropestle motor (Kimble Chase Life Science and Research Products LLC, Vineland, NJ). Ten μl of each lysate was saved for protein quantification (using the BioRad DC assay with BSA as a standard). Intracellular metabolites were extracted from the remainder, as described previously (Ross et al., 2004; Openo et al., 2006). The extracted samples were then dried under vacuum with no heat (Eppendorf Vacufuge) until no liquid remained visible. Dried metabolite pellets were rehydrated with HPLC-grade water in volumes normalized for protein concentration, and centrifuged through 0.22-μm Costar Spin-X centrifuge tube filters (Corning Inc., Lowell, MA) at 4000 × g for four minutes to remove any insoluble matter. The soluble phase of each sample was transferred to a glass HPLC vial and metabolites were separated and quantified using a Dionex HPLC, as described previously (Ross et al., 2004). For each sample, 20 μl were injected into a 25-μl injection loop.
Time of death experiments
Embryos that were less than 24 hours old and homozygous for the dGALTΔ1AP2 (imprecise excision) or dGALTC2 (precise excision) alleles (n≥100 for each genotype) were harvested from egg-laying plates and deposited individually into the wells of 96-well plates, which had been preloaded to about three-quarters full with either 555 mM of glucose only, or 555 mM glucose plus 111 mM or 222 mM of galactose fly food. Once loaded, plates were tightly covered with a clear plastic wrap that was pricked with a needle to introduce a small hole over each well, to permit an exchange of air. All animals were monitored and scored for viability daily under a dissecting microscope until they either died or eclosed as adults.
Window of galactose sensitivity
Embryos homozygous for the dGALTΔ1AP2 (imprecise excision) and dGALTC2 (precise excision) alleles were deposited on plates containing either 555 mM glucose food or 555 mM glucose plus 222 mM galactose food. Every 24 hours, starting with first instar larvae (L1s), cohorts of more than 20 developing animals were transferred from glucose food individually into the wells of 96-well plates that had been preloaded with 555 mM glucose plus 222 mM galactose food, and similarly larvae were transferred from 555 mM glucose plus 222 mM galactose food into the wells of 96-well plates preloaded with 555 mM glucose-only food. To control for a potential impact of the transfer process itself, cohorts of larvae also were transferred from glucose-only food to wells containing glucose-only food, and from glucose plus galactose food to wells containing glucose plus galactose food. This transfer process was continued for the first six days of development, after which all animals were allowed to continue developing without further transfer.
Supplementary Material
Acknowledgments
We are grateful to Kerry Garza and members of the Moberg and Sanyal labs at Emory University for contributions and helpful discussions throughout the course of this project; to Jewels Chhay for contributions to the creation of the UAS-hGALT construct; and to Doug Rennie for embryo injection of the hGALT transgene. We are also grateful to Cheryl Strauss for helpful comments on the manuscript. This work was supported by National Institutes of Health grant DK046403 (to J.L.F.-K. and K.H.M.). Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
R.F.K. generated the dGALT excision alleles, and designed and performed the majority of the experiments presented in Figs 2–4 and Figs S1 and S2; E.L.R. generated some of the data for Fig. 2 and all of the data for Fig. 5; R.D.S. and J.M.I.S. generated most of the data presented in Table 1; P.J.L. generated some of the data for Tables 1 and 2; K.H.M. provided general oversight for experiments involving fly genetic manipulation; J.L.F.-K. conceived the project and provided oversight for much of its completion. All authors contributed to writing the final manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.005041/-/DC1
REFERENCES
- Akai S. (1979). Genetic variation in walking ability of Drosophila melanogaster. Jpn J Genet. 54, 317–324 [Google Scholar]
- Benzer S. (1967). Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc Natl Acad Sci USA 58, 1112–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha K. (2009). The epicurean fly: using Drosophila melanogaster to study metabolism. Pediatr Res. 65, 132–137 [DOI] [PubMed] [Google Scholar]
- Bier E. (2005). Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 6, 9–23 [DOI] [PubMed] [Google Scholar]
- Bosch A, Bakker H, Wenniger-Prick L, Wanders R, Wijburg F. (2004). High tolerance for oral galactose in classical galactosaemia: dietary implications. Arch Dis Child. 89, 1034–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 [DOI] [PubMed] [Google Scholar]
- Chien S, Reiter L, Bier E, Gribskov M. (2002). Homophila: human disease gene cognates in Drosophila. Nucleic Acids Res. 30, 149–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christacos NC, Fridovich-Keil JL. (2002). Impact of patient mutations on heterodimer formation and function in human galactose-1-P uridylyltransferase. Mol Genet Metab. 76, 319–326 [DOI] [PubMed] [Google Scholar]
- Elsevier JP, Fridovich-Keil JL. (1996). The Q188R mutation in human galactose-1-phosphate uridylyltransferase acts as a partial dominant negative. J Biol Chem. 271, 32002–32007 [DOI] [PubMed] [Google Scholar]
- Elsevier JP, Wells L, Quimby BB, Fridovich-Keil JL. (1996). Heterodimer formation and activity in the human enzyme galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci USA 93, 7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil JL, Jinks-Robertson S. (1993). A yeast expression system for human galactose-1-phosphate uridylyltransferase. Proc Natl Acad Sci USA 90, 398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil J, Walter J. (2008). Galactosemia. In The Online Metabolic and Molecular Bases of Inherited Disease - OMMBID (ed. Valle D, Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A.), Chapter 72. New York: McGraw-Hill; www.ommbid.com [Google Scholar]
- Fridovich-Keil JL, Langley SD, Mazur LA, Lennon JC, Dembure PP, Elsas LJ. (1995a). Identification and functional analysis of three distinct mutations in the human galactose-1-phosphate uridyltransferase gene associated with galactosemia in a single family. Am J Hum Gen. 56, 640–646 [PMC free article] [PubMed] [Google Scholar]
- Fridovich-Keil JL, Quimby BB, Wells L, Mazur LA, Elsevier JP. (1995b). Characterization of the N314D allele of human galactose-1-phosphate uridylyltransferase using a yeast expression system. Biochem Mol Med. 56, 121–130 [DOI] [PubMed] [Google Scholar]
- Holden HM, Rayment I, Thoden JB. (2003). Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 278, 43885–43888 [DOI] [PubMed] [Google Scholar]
- Honjo K, Furukubo-Tokunaga K. (2005). Induction of cAMP response element-binding protein-dependent medium-term memory by appetitive gustatory reinforcement in Drosophila larvae. J Neurosci. 25, 7905–7913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes L, Spradling A. (1997). The Drosophila gene fs(2)cup interacts with otu to define a cytoplasmic pathway required for the structure and function of germ-line chromosomes. Development 124, 1419–1431 [DOI] [PubMed] [Google Scholar]
- Lai K, Elsas L. (2000). Overexpression of human UDP-glucose pyrophosphorylase rescues galactose-1-phosphate uridyltransferase-deficient yeast. Biochem Biophys Res Commun. 271, 392–400 [DOI] [PubMed] [Google Scholar]
- Lai K, Langley SD, Singh RH, Dembure PP, Hjelm LN, Elsas LJ. (1996). A prevalent mutation for galactosemia among black Americans. J Pediatr. 128, 89–95 [DOI] [PubMed] [Google Scholar]
- Lai K, Langley S, Khwaja F, Schmitt E, Elsas L. (2003). GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 13, 285–294 [DOI] [PubMed] [Google Scholar]
- Lasko P. (2002). Diabetic flies? Using Drosophila melanogaster to understand the causes of monogenic and genetically complex diseases. Clin Genet. 62, 358–367 [DOI] [PubMed] [Google Scholar]
- Leslie ND. (2003). Insights into the pathogenesis of galactosemia. Annu Rev Nutr. 23, 59–80 [DOI] [PubMed] [Google Scholar]
- Leslie N, Bai S. (2001). Functional analysis of the mouse galactose-1-phosphate uridyl transferase (GALT) promoter. Mol Genet Metab. 72, 31–38 [DOI] [PubMed] [Google Scholar]
- Leslie ND, Yager KL, McNamara PD, Segal S. (1996). A mouse model of galactose-1-phosphate uridyl transferase deficiency. Biochem Mol Med. 59, 7–12 [DOI] [PubMed] [Google Scholar]
- Leslie N, Yager C, Reynolds R, Segal S. (2005). UDP-galactose pyrophosphorylase in mice with galactose-1-phosphate uridyltransferase deficiency. Mol Genet Metab. 85, 21–27 [DOI] [PubMed] [Google Scholar]
- Ning C, Reynolds R, Chen J, Yager C, Berry GT, McNamara PD, Leslie N, Segal S. (2000). Galactose metabolism by the mouse with galactose-1-phosphate uridyltransferase deficiency. Pediatr Res. 48, 211–217 [DOI] [PubMed] [Google Scholar]
- Ning C, Reynolds R, Chen J, Yager C, Berry G, Leslie N, Segal S. (2001). Galactose metabolism in mice with galactose-1-phosphate uridyltransferase deficiency: sucklings and 7-week-old animals fed a high-galactose diet. Mol Genet Metab. 72, 306–315 [DOI] [PubMed] [Google Scholar]
- Openo K, Schulz J, Vargas C, Orton C, Epstein M, Schnur R, Scaglia F, Berry G, Gottesman G, Ficicioglu C, et al. (2006). Epimerase-deficiency galactosemia is not a binary condition. Am J Hum Genet. 78, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panis B, Bakker JA, Sels JP, Spaapen LJ, van Loon LJ, Rubio-Gozalbo ME. (2006). Untreated classical galactosemia patient with mild phenotype. Mol Genet Metab. 89, 277–279 [DOI] [PubMed] [Google Scholar]
- Piccioni F, Zappavigna V, Verrotti A. (2005). A cup full of functions. RNA Biol. 2, 125–128 [DOI] [PubMed] [Google Scholar]
- Riehman K, Crews C, Fridovich-Keil JL. (2001). Relationship between genotype, activity, and galactose sensitivity in yeast expressing patient alleles of human galactose-1-phosphate uridylyltransferase. J Biol Chem. 276, 10634–10640 [DOI] [PubMed] [Google Scholar]
- Ross KL, Davis CN, Fridovich-Keil JL. (2004). Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol Gen Metab. 83, 103–116 [DOI] [PubMed] [Google Scholar]
- Ryder E, Russell S. (2003). Transposable elements as tools for genomics and genetics in Drosophila. Brief Funct Genomic Proteomic. 2, 57–71 [DOI] [PubMed] [Google Scholar]
- Sanders R, Sefton J, Moberg K, Fridovich-Keil JL. (2010). UDP-galactose 4’ epimerase (GALE) is essential for development of Drosophila melanogaster. Dis Model Mech. 3, (in press) [doi: 10.1242/dmm.005058]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyfield L, Walter J. (2002). Galactosemia. In The Metabolic and Molecular Bases of Inherited Disease (ed. Scriver C, Beaudet A, Sly W, Valle D, Childs B, Kinzler K, Vogelstein B.). New York: McGraw-Hill [Google Scholar]
- Zhang H, Liu J, Li C, Momen B, Kohanski R, Pick L. (2009). Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proc Natl Acad Sci USA 106, 19617–19622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.