Abstract
Temporal coordination of meiosis with spermatid morphogenesis is crucial for successful generation of mature sperm cells. We identified a recessive male sterile Drosophila melanogaster mutant, mitoshell, in which events of spermatid morphogenesis are initiated too early, before meiotic onset. Premature mitochondrial aggregation and fusion lead to an aberrant mitochondrial shell around premeiotic nuclei. Despite successful meiotic karyokinesis, improper mitochondrial localization in mitoshell testes is associated with defective astral central spindles and a lack of contractile rings, leading to meiotic cytokinesis failure. We mapped and cloned the mitoshell gene and found that it encodes a novel protein with a bromodomain-related region. It is conserved in some insect lineages. Bromodomains typically bind to histone acetyl-lysine residues and therefore are often associated with chromatin. The Mitoshell bromodomain-related region is predicted to have an alpha helical structure similar to that of bromodomains, but not all the crucial residues in the ligand-binding loops are conserved. We speculate that Mitoshell may participate in transcriptional regulation of spermatogenesis-specific genes, though perhaps with different ligand specificity compared to traditional bromodomains.
Introduction
Spermatogenesis in insects and mammals begins with germ line stem cell divisions that produce spermatogonia, which divide mitotically to generate spermatocytes. These cells undergo meiosis, and the resulting haploid spermatids then execute dramatic morphogenetic changes to generate mature sperm. This process is a suitable context for molecular dissection of cell biological phenomena, such as cytokinesis and mitochondrial morphogenesis, as well as events of developmental control and coordination. Spermatogenesis in Drosophila melanogaster involves precise coordination between two semi-independent pathways, meiosis and spermiogenesis, the latter involving the morphogenetic changes of various organelles as early spermatids become mature sperm cells (reviewed in Fuller, 1993). In wild-type males, meiotic onset occurs only when spermatocytes have enlarged and accumulated most transcripts needed after meiosis; additional regulatory mechanisms then ensure completion of meiosis before spermiogenesis occurs. Easily detectable hallmarks of spermiogenesis include aggregation of mitochondria in early haploid spermatids and subsequent fusion into the two giant mitochondrial derivatives that intertwine to form the spherical Nebenkern. The two derivatives within the Nebenkern then unfurl and elongate beside the growing flagellar axoneme (reviewed in Fuller, 1993).
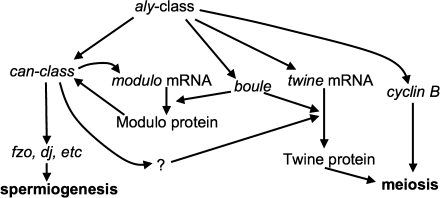
Complex transcriptional and translational cascades coordinate meiosis with spermiogenesis (Fig. 1). The always early (aly) class of transcriptional regulators encode components of a chromatin-associated complex that activates genes required both for meiosis (twine, boule, and cyclin B) and for spermiogenesis (cannonball [can]-class genes) (reviewed in White-Cooper, 2010). Mutations in aly-class genes lead to a complete arrest at primary spermatocyte stages. Downstream genes often function within one pathway or the other but not both. The exceptions to this rule provide the needed crosstalk to delay spermiogenesis until meiosis is complete.
FIG. 1.
Regulatory pathways coordinating meiosis and spermiogenesis in Drosophila melanogaster. The always early (aly)–class genes (including comr, tomb, topi, and achi-vis) control transcription of cell cycle regulators twine and cyclin B as well as spermiogenesis regulators from the can class of genes (including mia, sa, nht, and mip40) (reviewed in White-Cooper, 2010). The can-class gene products activate transcription of genes such as fzo and dj that is essential for the morphological changes of spermatid development. Crosstalk between the meiosis and spermiogenesis pathways includes the activity of boule, modulo, and an unidentified downstream target of can-class genes that facilitates twine translation (Maines and Wasserman, 1999; Mikhaylova et al., 2006; White-Cooper, 2010).
Within the meiosis pathway, Twine, the meiotic Cdc25 phosphatase, is a central mediator of meiotic onset but is not required for sperm tail growth; males homozygous for twine mutations make spermatids that have large 4n nuclei and that proceed through spermiogenesis (Alphey et al., 1992; Courtot et al., 1992; White-Cooper et al., 1993; Sigrist et al., 1995). Within the spermiogenesis pathway, the can-class genes, encoding testis-specific TATA binding protein–associated factors (TAFs), are crucial for the transcription of many genes required for sperm tail growth (Hiller et al., 2004). Testis TAFs help to coordinate meiosis with spermiogenesis through transcriptional activation of an yet-unidentified mediator of translation of twine RNA (reviewed in White-Cooper, 2010). Translation of Twine also requires Boule, an RNA-binding protein whose expression is under the control of aly-class but not can-class genes (Maines and Wasserman, 1999). Twine, Boule, and other cell cycle regulators like Cyclin A and Roughex depend upon the testis-specific translation elongation factor eIF4G for proper timing of expression, as do unidentified spermiogenesis mediators; in eIF4G mutants, meiosis fails entirely, and only an initial aggregation of mitochondria forms beside the undivided nucleus before spermiogenesis arrest (Baker and Fuller, 2007; Franklin-Dumont et al., 2007). Fine-tuning of the coordination between meiotic onset and sperm tail growth involves Modulo, a nucleolin homolog that, through a feedback loop, amplifies expression of can-class genes and that is itself dependent upon the meiotic activator Boule to be translated (Mikhaylova et al., 2006).
Here we add to the roster of gene products that coordinate developmental pathways in the Drosophila testis. We characterize the recessive male sterile D. melanogaster mutant mitoshell that shows faulty coordination of meiosis and spermiogenesis, with mitochondria aggregating and fusing before completion of meiosis. We identify the associated gene and show that a protein containing a bromodomain-related region is required for proper developmental control of these events. We also explore mechanisms underlying meiotic cytokinesis failure in this mutant strain.
Materials and Methods
Fly husbandry, stocks, and fertility tests
Stocks and crosses were maintained at 25°C on an instant Drosophila medium from Ward's Natural Science. Fertility tests were performed by allowing test males to mate freely with three to five virgin females for 10 days, and the presence or absence of larvae was scored. Oregon R was the wild-type strain. The mtshZ2-2620/CyO, mtshZ2-3484/CyO, and fzoZ3-4436/TM6 stocks were from the Zuker collection, as was the Z2-2588 line used as the background chromosome for the mtsh Zuker alleles (Koundakjian et al., 2004). We obtained P{RS3}CB-5520-3 (Ryder et al., 2004) from the Szeged Drosophila Stock Center. The GFP-anillin stock (Goldbach et al., 2010) was a gift from Philip Goldbach and Julie Brill (Hospital for Sick Children, Toronto, ON). The β tubulin-EGFP stock was created by Hiroki Oda and Yasuko Akiyama-Oda (JT Biohistory Research Hall). The Sep2-GFP (Silverman-Gavrila et al., 2008) and fzo2/TM3 (Hales and Fuller, 1997) stocks are as described. The following kit and stocks were from the Bloomington Drosophila Stock Center, each listed with FlyBase identifier:
Chromosome 2 Deficiency Kit (2002 version, including 81 stocks)
In(1)wm4h, y1; Df(2L)TE29Aa-11, dp/CyO (FBst0000179)
w1118; Df(2L)BSC111/CyO (FBst0008836)
w1118; Df(2L)Exel7034/CyO (FBst0007807)
w1118; Df(2L)ED611, P{3′.RS5 + 3.3′}ED611/SM6a (FBst0009298)
y1 w; CyO, H{PΔ2-3}HoP2.1/Bc1 (FBst0002078)
w; KrIf-1/CyO; D1/TM6C, Sb1 Tb1 (FBst0007199)
w; nocSco/CyO, S bw1 (FBst0003198)
A w; Kr/CyO stock was derived from w; KrIf-1/CyO; D1/TM6C, Sb1 Tb1 and w; nocSco/CyO, S bw1 using appropriate crosses.
Imprecise P element excision
Flies homozygous for the P{RS3}CB-5520-3 insertion were crossed to y w; CyO, H{PΔ2-3}HoP2.1/Bc1. Resulting w; P{RS3}CB-5520-3/CyO, H{PΔ2-3}HoP2.1 flies were crossed to w; Kr/CyO, and male offspring carrying potential excision chromosomes over Kr were identified by loss of P{RS3}CB-5520-3-associated eye color. These males were crossed individually to w; Df(2L)BSC111/CyO females. When viable, the w; P{RS3}CB-5520-3 putative excision/Df(2L)BSC111 males were subjected to fertility tests. If sterility was detected, sibling w; P{RS3}CB-5520-3 putative excision/CyO males and females were crossed to create a balanced stock.
Generation of mtsh flies marked with GFP-tagged transgenes
Flies of genotype mtshZ2-2620/CyO were crossed to w; Kr/CyO; D/TM6C, Sb. The w; mtshZ2-2620/CyO; D/+ offspring were crossed to w/w+; mtshZ2-2620/CyO; TM6C, Sb/+ from which flies were selected to generate a w; mtshZ2-2620/CyO; D/TM6C, Sb stock. Simultaneously, w; β tubulin-EGFP flies (insert on the 3rd) were crossed to w; Kr/CyO; D/TM6C, Sb. The w; Kr/+; β tubulin-EGFP/TM6C, Sb and w; β tubulin-EGFP/TM6C, Sb offspring were crossed, from which w; Kr/CyO; β tubulin-EGFP/TM6C, Sb flies were used to make a stock. Then, w; mtshZ2-2620/CyO; D/TM6C, Sb flies were crossed to w; Kr/CyO; β tubulin-EGFP/TM6C, Sb, and w; mtshZ2-2620/CyO; β tubulin-EGFP/TM6C, Sb flies were used to make a stock. From that stock, w; mtshZ2-2620/mtshZ2-2620; β tubulin-EGFP/TM6C, Sb males (and their heterozygous siblings as controls) were subjected to testis dissection and fluorescence microscopy. The Sep2-GFP and GFP-anillin stocks were subjected to analogous crosses as described for β tubulin-EGFP to generate w; mtshZ2-2620/CyO; Sep2-GFP/TM6C and w; mtshZ2-2620/CyO; GFP-anillin/TM6C stocks from which mtshZ2-2620 homozygotes carrying each transgene could be identified. These stocks were also crossed to mtshZ2-3484/CyO and mtsh55207-12/CyO to create mtsh transheterozygotes carrying the GFP-anillin or β tubulin-EGFP transgenes.
Generation of mtsh; fzo double mutants
Males carrying the fzo2 (Hales and Fuller, 1997) and fzoZ3-4436 (Koundakjian et al., 2004) alleles balanced over CyO were crossed to w; Kr/CyO; D/TM6C, Sb virgins. Male w; Kr/+; fzo/TM6C offspring were crossed to w+/w; CyO/+; fzo/TM6C females, and w; Kr/CyO; fzo/TM6C progeny were collected to create a stock for each fzo allele. These flies were then crossed to w; mtshZ2-2620/CyO; Sep2-GFP/TM6C, Sb flies. The w; mtshZ2-2620/CyO; fzo/TM6C progeny were collected to create balanced stocks for each fzo allele. We crossed these two stocks to generate w; mtshZ2-2620/mtshZ2-2620; fzo2/fzoZ3-4436 males for testis dissection. Siblings of genotype w; mtshZ2-2620/mtshZ2-2620; fzo/TM6C and w; mtshZ2-2620/CyO; fzo2/fzoZ3-4436 were also dissected and examined.
Microscopy of live squashed testis preparations
We dissected testes in TB1 buffer (7 mM K2HPO4, 7 mM KH2PO4 [pH 6.7], 80 mM KCl, 16 mM NaCl, 5 mM MgCl2, and 1% PEG-6000), sometimes including 4 μg/mL Hoechst DNA stain (Sigma Life Science), and opened testes with forceps to allow cells to form a monolayer after introduction of a cover slip. Samples were examined with phase-contrast optics and/or under fluorescence with a Nikon Eclipse E600W or Olympus B201 microscope. Images were captured with a Nikon Coolpix 4500 camera or Spot Camera.
Sequencing of alleles
Using standard methods we purified genomic DNA from flies homozygous for each mtsh allele, as well as from homozygotes of the nonallelic Z2-2588 male sterile Zuker collection strain as the background chromosome for mtshZ2-2620 and mtshZ2-3484. We amplified CG7795 in two parts using the following primer pairs purchased from Integrated DNA Technologies: 5′-ACACGGGCTACATCCCATTATCTC-3′ and 5′-TCATTCCCAGGTACTTCTGTGCCA-3′, and 5′-CGGATGGCGTGCTGCCAAATATAA-3′ and 5′-CATGCTATTGGCGCAGGACATTGA-3′. Additional sequencing primers included the following: 5′-TCTGCTCCCTAGGGTTTCAGAACA-3′, 5′-TTTGGATCGCAATCGCATGGTCAC-3′, 5′-ACAGTCGAAACTGTCGCGGTATGA-3′, 5′-AGCCAAGACCAGAAAGGTAAGGCT-3′, 5′-CTGCGCCGCTTTCCAATGAACTAA-3′, 5′-CAACGCCATGTAGATGCCAATGGT-3′, 5′-TAATGGTCATTTGGCTTGGCTGGC-3′, 5′-GGATCATCTTTACGGTGCGCTGTT-3′, 5′-TGTGAACGACATGCTTTCCAAGCC-3′, 5′-CTGCAGATAACAGCGCACCGTAA-3′, 5′-GATAATGTGGCGGCTTGGAAAGC-3′, 5′-CTCCCGTTCTTTGGTCGCAACATT-3′, and 5′-AATGTTGCGACCAAAGAACGGGAG-3′.
Retrogen Inc. performed the sequencing reactions. Sequence differences in mtshZ2-2620 and mtshZ2-3484 compared to the background chromosome in Z2-2588 were confirmed with sequence reads on both strands, using template DNA amplified separately in two independent reactions.
Sequence analysis
Homology searches were with BLAST (Altschul et al., 1990) at the National Center for Biotechnology Information and FlyBase (Tweedie et al., 2009) Web sites. Multiple sequence alignment was performed with ClustalW (Larkin et al., 2007). In sequence similarity calculations, the ClustalW conserved but not semi-conserved residues were counted. Protein secondary structure prediction used the PROF algorithm (Rost and Sander, 1993) at the PredictProtein Web site (Rost et al., 2004). Protein localization prediction was with WoLF PSORT (Horton et al., 2007) and LOCtree (Nair and Rost, 2005). Nuclear localization signal prediction was with predictNLS (Cokol et al., 2000).
Results
Mitochondria aggregate and fuse aberrantly early in mitoshell mutants
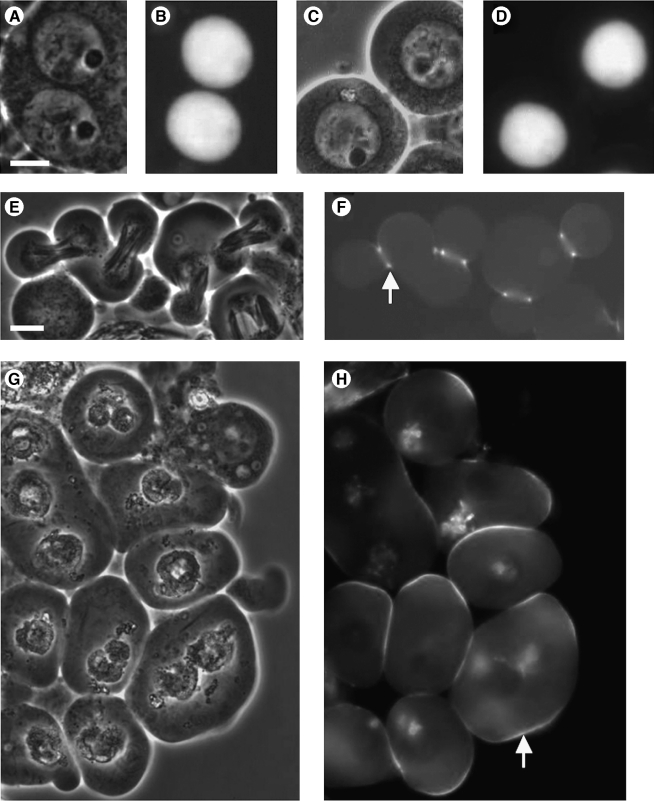
During spermatogenesis in wild-type Drosophila males, mitochondria are diffuse in the cytoplasm of mature primary spermatocytes (Fig. 2A, B) and gather in the central region of the meiotic spindle (Fig. 2C), subsequently aggregating and fusing in early round spermatids (Fig. 2D) into two giant derivatives that interwrap to form the Nebenkern, a phase-dark body adjacent to the phase-light nucleus (Fig. 2D–F) (Fuller, 1993). The Nebenkern unfurls and disentangles in a process that requires mitochondrial fission (Aldridge et al., 2007). Mitochondria then elongate beside the flagellar axoneme (Fig. 2G, H). We identified from the Zuker collection of ethyl methane sulfate (EMS)–induced recessive nonlethal mutants (Koundakjian et al., 2004; Wakimoto et al., 2004) two allelic male sterile strains, mtshZ2-2620 and mtshZ2-3484, that showed aberrant aggregation of mitochondria in spermatocyes and spermatids. Homozygous adults were fully viable, and homozygous females were fertile.
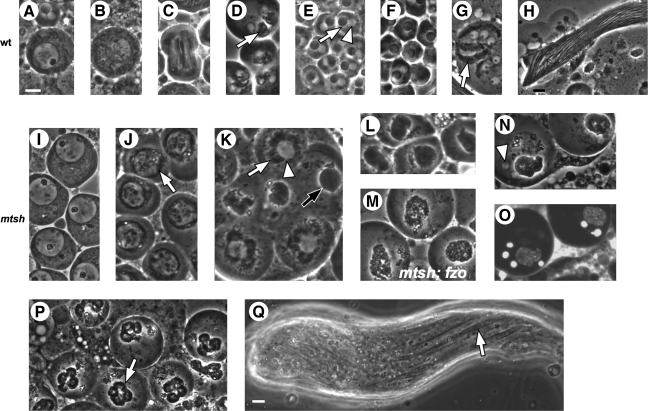
FIG. 2.
In mtsh spermatogenesis, mitochondria aggregate and fuse prematurely, and meiotic cytokinesis fails. Phase-contrast (A–N and P–Q) and fluorescence (O) micrographs of live squashed preparations of testes from wild-type (A–H), mtshZ2-2620/mtshZ2-2620 (I–L and N–Q), and mtshZ2-2620/mtshZ2-2620; fzo2/fzoZ3-4436 (M) males. Wild-type primary spermatocyte (A) includes a large phase-light nucleus with phase-dark nucleolus; small, phase-dark mitochondria are diffuse in the cytoplasm. In spermatocytes approaching meiosis (B), the nucleolus becomes less distinct, and mitochondria remain diffuse. In wild-type meiosis I (C) phase-dark mitochondria are aligned on the spindle. (D) After meiosis, mitochondria aggregate (arrow) and fuse beside each daughter nucleus. In early round spermatids (E, F), mitochondrial derivatives form a spherical phase-dark Nebenkern (arrow) beside each phase-light nucleus (arrowhead); the size of the Nebenkern is not affected in preparations not fully squashed (F). Mitochondria unfurl and elongate (G, arrow) and later stretch the full length of each cell within a spermatid bundle (H, partial view). Spermatocytes from mtsh males appear normal (I) but show aberrant and premature mitochondrial aggregation (J, arrow) in cells approaching the first meiotic division. (K) Cyst of mtsh spermatocytes in which mitochondrial aggregation has progressed further in some cells than in others. Aggregating mitochondria (white arrow) appear to form a shell (black arrow) eclipsing each premeiotic nucleus (white arrowhead). (L) Live testis preparation not fully squashed; mtsh nuclei appear surrounded by mitochondrial shells. Compare to panel (F). (N, O) Fully squashed and Hoescht-stained mtsh postmeiotic cells under phase-contrast optics (N) and fluorescence (O); four smaller nuclei (e.g., arrowhead) of roughly the size found in normal haploid spermatids (see panel E) have escaped one mitochondrial shell. (P) Later-stage mtsh spermatids squashed after mitochondrial fission and unfurling; each mitochondrial aggregate separates into four nucleus-associated lobes (arrow) under cover slip pressure. (Q) Elongating spermatid bundles in mtsh, with wide parallel arrays of elongating mitochondria (arrow). Transheterozygotes showed identical phenotypes. Scale bars 10 μm. Panels (A–G) and (I–P) at same magnification; (H) and (Q) at same magnification.
In homozygous or transheterozygous mtsh males, primary spermatocytes appeared normal (Fig. 2I). Mitochondria aggregated abnormally early, in late primary spermatocytes before meiotic divisions, and in a single shell surrounding each premeiotic spermatocyte nucleus (Fig. 2J–L) instead of a separate body beside each postmeiotic nucleus as in wild type. Mutant spermatocytes underwent karyokinesis, with the resulting four nuclei staying adjacent to each other within the single mitochondrial shell. Under pressure from a cover slip, postmeiotic nuclei were visibly excluded from the intact mitochondrial shell and detectable by phase-contrast microscopy and Hoescht staining (Fig. 2N, O). However, Hoescht staining of nuclei completely surrounded by an intact mitochondrial shell (in a preparation not fully squashed) was undetectable, with no nuclei seen elsewhere (not shown), apparently an artifactual result of mitochondria inhibiting entry of the stain or transmission of fluorescence. In cells at a slightly later stage, presumably after undergoing mitochondrial fission and dissociation, cover slip pressure forced the single mitochondrial aggregate into four lobes (Fig. 2P). Hoescht staining indicated that nuclei sometimes, but not always, remained associated these lobes when under cover slip pressure (not shown). Meiotic cytokinesis was never observed in mtsh testes. Spermatid bundle elongation and other subsequent events of spermiogenesis occurred in mtsh mutant flies (Fig. 2Q). Spermatid tails appeared irregularly packed (Fig. 2Q, arrow; compare to Fig. 2H), consistent with the presence of multinucleate spermatids, as in fwd mutant males (Brill et al., 2000). The resulting sperm cells were not individualized, a common phenotype in male sterile strains with sperm structural defects (reviewed in Fuller, 1993). The mtshZ2-2620 and mtshZ2-3484 mutant phenotypes were fully penetrant.
To determine whether mitochondrial fusion accompanies premature mitochondrial aggregation in mtsh mutants, we generated flies homozygous for mtshZ2-2620 and transheterozygous for two alleles of fzo. Fzo is a testis-specific mediator of mitochondrial fusion that in wild type is detectable only during and after telophase of meiosis II (Hales and Fuller, 1997). The mtsh; fzo double mutants differed from mtsh flies, with the double mutants showing aggregated but fragmented mitochondria (Fig. 2M), compared to the smooth and cohesive mitochondrial shell in mtsh/mtsh testes (Fig. 2K, L). These results suggest that in flies homozygous for only mtsh, premature Fzo-mediated mitochondrial fusion accompanies premature mitochondrial aggregation.
mitoshell corresponds to CG7795
To map mitoshell, we crossed mtshZ2-2620/CyO flies to the 81 strains that comprised the 2002 Chromosome 2 Deficiency Kit from the Bloomington Drosophila Stock Center and tested the mtshZ2-2620/deficiency offspring for fertility. The mtshZ2-2620 allele failed to complement Df(2L)TE29Aa-11, which lacks polytene region 28E4; 29C1 (Tweedie et al., 2009). Df(2L)TE29Aa-11/mtshZ2-2620 flies had an identically severe phenotype to the mtshZ2-2620 or mtshZ2-3484 homozygotes, suggesting that the EMS alleles from the Zuker collection were either null alleles or very strong hypomorphs. We obtained additional deficiencies in the region and found that Df(2L)BSC111 (28F5; 29B1), also failed to complement mtshZ2-3484, while Df(2L)Exel7034 (28E1; 28F1) and Df(2L)ED611 (29B4; 29C3) complemented the mutation. Df(2L)BSC111 lacks the molecularly defined interval 2L: 8,240,266-8,362,842 (FlyBase release 2010_02) (Tweedie et al., 2009), encompassing ∼23 genes. Four of these genes, CG7795, CG8086, CG8292, and CG8349, were recorded in the FlyAtlas database (Chintapalli et al., 2007) as having markedly enriched expression only in the testis. We viewed these genes as strong candidates since mutant mitoshell flies are male sterile but fully viable and female fertile. We amplified and sequenced CG7795 from mtshZ2-2620 and mtshZ2-3484 homozygotes as well as from homozygotes of a different, nonallelic Zuker collection second chromosome stock (Z2-2588) as the background chromosome. We found that mtshZ2-2620 and mtshZ2-3484 each contained a nonsense mutation at a different position in CG7795 (Fig. 3A), while the Z2-2588 background chromosome contained an open reading frame that matched the CG7795 reference sequence in the genome database (Tweedie et al., 2009). Sequence analysis of a different candidate gene, CG8292, revealed no differences between mtsh alleles and the background chromosome. We did not obtain sequence data for CG8086 and CG8349.
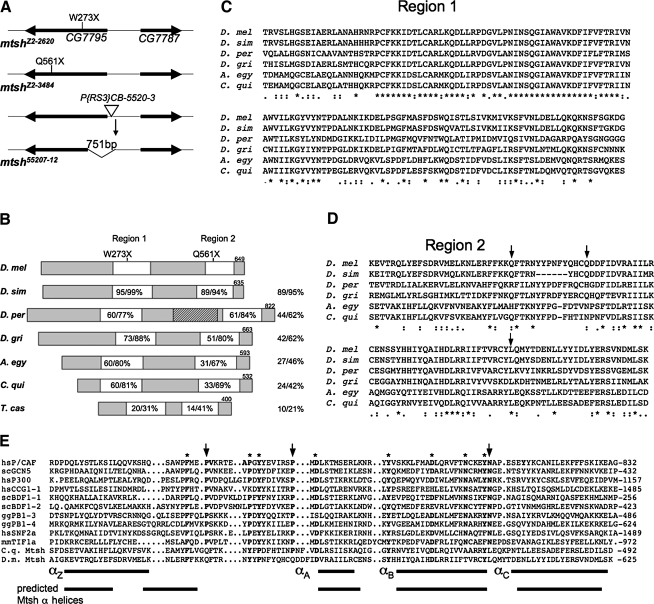
FIG. 3.
The mitoshell gene corresponds to CG7795 and encodes a protein containing a bromodomain-related region. (A) Schematic depiction of the CG7795 region in polytene interval 29A on chromosome 2, showing the mutations in three mtsh alleles. The Zuker collection alleles mtshZ2-2620 and mtshZ2-3484 each have a unique nonsense mutation in CG7795. Mobilization of the P{RS3}CB-5520-3 homozygous viable and fertile transposable element (triangle) led to the mtsh55207-12 allele that lacks 751 bp, including the transcriptional and translational start sites of CG7795, but not affecting the adjacent gene CG7787. (B) Schematic diagram of Mtsh and its orthologs in 3 of the 11 other sequenced Drosophila species (D. simulans representing the closest neighbor, D. persimilis representing the obscura subgroup, and D. grimshawi representing the most distantly related), as well as Aedes egyptii, Culex quinquefasciatus, and Tribolium castaneum. Protein sizes are indicated at top right of each. The locations of nonsense mutations in mtshZ2-2620 and mtshZ2-3484 are indicated in regions 1 and 2 respectively. Amino acid identity/similarity to D. melanogaster Mtsh is shown at right for each full protein and within the white boxes for the highly conserved regions 1 and 2. The stippled box is a region found only in the obscura group of Drosophila species that resembles a domain of DNA polymerase III subunits tau and gamma. (C) and (D) ClustalW sequence lineups of the highly conserved regions 1 and 2 in Mtsh and Dipteran orthologs. Asterisks indicate identical residues, and double and single dots indicate the strongly and weakly similar residues, respectively. Arrows indicate sites corresponding to those most crucial for binding to acetylated lysine in typical bromodomains; also indicated with arrows in panel (E). (E) Sequence lineup comparing the bromodomain-related region (region 2) in Mtsh and the C. quinquefasciatus ortholog (bottom two rows) with 10 known bromodomains. Adapted from Dhalluin et al. (1999), with bromodomain-containing proteins as described therein. Arrows indicate the most highly conserved bromodomain residues. Asterisks indicate other highly conserved bromodomain residues. At bottom, the known four alpha helical regions of bromodomains are indicated, juxtaposed with the predicted alpha helical regions of Mtsh.
To generate new alleles by imprecise P element excision (Voelker et al., 1984) and to confirm that CG7795 represented mitoshell, we mobilized the homozygous viable and fertile P{RS3}CB-5520-3 insertion (Ryder et al., 2004; Tweedie et al., 2009), located just upstream (13 and 36 bp, respectively) from the two predicted CG7795 transcriptional start sites (Fig. 3A), by crossing in the PΔ2-3 transgene encoding transposase (Tweedie et al., 2009). Of 79 independent lines showing loss of the P{RS3}CB-5520-3-associated eye color, 2 were recessive male sterile and failed to complement mtshZ2-2620 and mtshZ2-3484. Homozygotes for one of the P element excision alleles, mtsh55207-12, showed the same severe phenotype as the Zuker collection alleles, while mtsh55201-3 was hypomorphic, with some normal-sized Nebenkerns observed. To determine the molecular lesion in the severe P element excision allele mtsh55207-12, we amplified and sequenced the region surrounding the former P element insertion site. We found that mtsh55207-12 contained a 751 bp deletion, which removed the predicted CG7795 transcription start site, the 5′ untranslated region, and 434 bp of the coding region (Fig. 3A). Twenty base pairs of the P element were left behind. The deletion left intact the region upstream from the P element insertion site toward the next closest gene CG7787, whose transcriptional start site is predicted to be about 400 bp away. We were unable to amplify the region surrounding the P element insertion site in the hypomorphic allele mtsh55201-3 allele, suggesting that a significant portion of the P element may be retained/and or at least one primer-binding site may be deleted. The nature of the lesions in the three molecularly characterized mitoshell alleles together indicates that CG7795 represents mitoshell.
mitoshell/CG7795 encodes a novel protein with a bromodomain-related region, conserved in some insect lineages
The mitoshell/CG7795 gene is annotated to encompass bases 8,316,889–8,319,920 on the minus strand of D. melanogaster chromosome 2 (FlyBase release 2010_02) (Tweedie et al., 2009). Two transcripts of five and six exons are predicted, respectively, encoding 649 and 604 amino acid polypeptides. The shorter predicted protein isoform lacks carboxy terminal residues due to retention of a 27 bp exon containing a stop codon. The single testis expressed sequence tag (EST) in GenBank (accession BE976148) corresponding to CG7795 matches the transcript encoding the longer protein isoform, while a single EST from embryonic tissue (accession CK131376) matches the transcript encoding the trucated isoform. Mtsh is conserved only in Diptera, Lepidoptera, and Coleoptera; all predicted orthologs match the longer predicted D. melanogaster protein isoform. Among orthologs in the other 11 sequenced Drosophila species, amino acid identity/similarity to D. melanogaster Mtsh ranges from 89%/95% in Drosophila simulans to 42%/62% in Drosophila grimshawi (Fig. 3B). In the obscura subgroup of Drosophila species (but not in D. melanogaster), the Mtsh orthologs also include a domain common to DNA polymerase III subunits tau and gamma (Jarvis et al., 2005); the tau and gamma subunits are necessary for oligomerization of the DnaX complex, which is required for assembling the two DNA polymerase III core complexes on the leading and lagging strands (Gao and McHenry, 2001; Glover et al., 2001; McHenry, 2003). D. melanogaster Mtsh is 27% identical/46% similar and 24% identical/42% similar, respectively, to orthologs in the mosquitoes Aedes egyptii and Culex quinquefasciatus. An ortholog in the flour beetle Tribolium castaneum shares 14% identity and 27% similarity to Mtsh. The genomes of the mosquito Anopheles gambiae and the silkworm Bombyx mori include unannotated sequences predicted to encode Mtsh homologs; however, no homolog is detected in sequenced genomes from Hymenoptera or more distantly related insect orders.
Sequence alignments indicate two conserved domains, of 137 and 105 amino acids, respectively (Fig. 3B–D), that are both predicted to be highly alpha helical (PROF algorithm; PredictProtein) (Rost et al., 2004). Region 1 contains no recognizable motifs. Region 2 in the A. egyptii and C. quinquefasciatus orthologs is annotated in the Conserved Domain Database (Marchler-Bauer et al., 2009) to be similar to bromodomains, a motif with four alpha helices and two loops that typically confers chromatin association via binding to acetylated histones (reviewed in Loyola and Almouzni, 2004). The bromodomain-like region 2 shows identical predicted alpha helical profiles among the Mtsh orthologs despite some sequence divergence. The predicted alpha helical profile also matches that of known bromodomains except at the amino terminal region where Mtsh and orthologs have two shorter predicted helices in the region of the single longer bromodomain Z helix (Fig. 3E). Of the 11 highly conserved residues found in most bromodomains, Mtsh and orthologs include 5 exact matches plus 2 others that are very close in location and switched in position (Fig. 3E). However, none of the three residues that are absolutely conserved in bromodomains and important for binding to acetylated lysines (arrows in Fig. 3D, E) are conserved in Mtsh and orthologs (Mujtaba et al., 2007). At the amino acid position between the B and C helices where a crucial bromodomain asparagine (rightmost arrow, Fig. 3E) forms a hydrogen bond with acetylated lysine (reviewed in Zeng and Zhou, 2002), Mtsh and orthologs contain an invariant leucine (lower arrow, Fig. 3D). Mtsh is predicted to be a soluble nuclear protein by WoLF PSORT (Horton et al., 2007) and a nuclear DNA-binding protein by LOCtree (Nair and Rost, 2005), though no nuclear localization signal is detected by PredictNLS (Cokol et al., 2000).
The mitoshell cytokinesis failure results from a spindle defect and aberrant localization of contractile ring components
To determine the basis of the meiotic cytokinesis failure in mtsh mutants, we crossed into mtshZ2-2620 flies transgenes encoding GFP- or EGFP-tagged versions of β tubulin, anillin, and Sep2, and examined the appropriate structures in mtsh homozygous and transheterozygous mutant testes under fluorescence microscopy. Cytokinesis depends in part upon formation and contraction of a contractile ring, whose cortical position is determined by signals from an equatorial region of overlapping antipolar spindle microtubules (reviewed in Barr and Gruneberg, 2007). We asked whether mtsh meiotic spindles in were intact by observing β tubulin-GFP localization in cells from dissected testes. In most primary spermatocytes whose mitochondria had aggregated but not yet fused, centrosomes were in the process of separating (not shown). Aberrant meiosis I spindles were detectable in the subset of cells with aggregated mitochondria just before mitochondrial fusion: mtsh cells in meiosis I lacked the overlapping astral microtubules in the central spindle region normally seen in wild type (Fig. 4A–D). The region corresponding to the anastral spindle described by Rebollo et al. (2004) was still intact in mtsh, and some nonoverlapping astral microtubules were present. The most advanced meiotic spindle we observed, as determined by Hoescht costaining (not shown), was at metaphase I. Mitochondrial fusion at that time led to an artifact of the phenotype, namely that neither spindle fluorescence nor DNA fluorescence could be detected from within fully formed mitochondrial shells (with these structures visible nowhere else in the cell), so no further spindle assessment could be made after metaphase I. The fact that meiotic karyokinesis gave rise to four nuclei of roughly normal size (Fig. 2N–P) indicated that meiosis is not fully arrested and that parts of the spindle, such as perhaps the anastral spindle, are functioning normally within the mitochondrial shell through meiosis II.
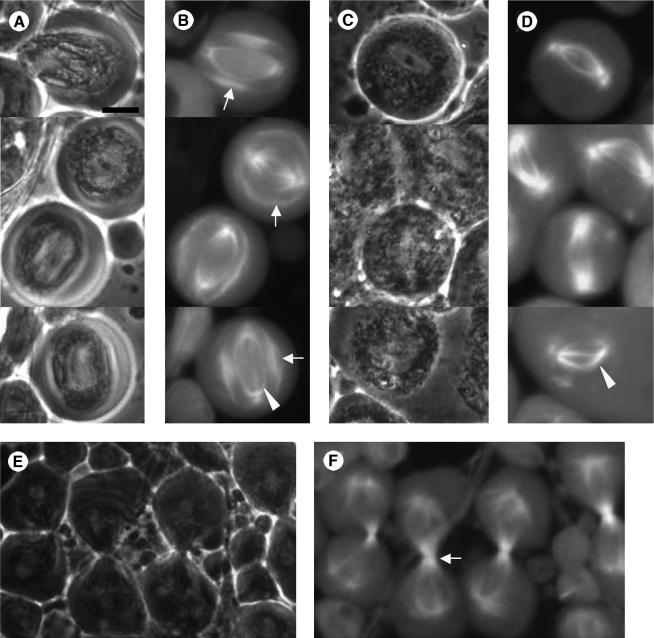
FIG. 4.
Meiotic spindles do not form properly in mtsh. Phase-contrast (A, C, E) and fluorescence (B, D, F) micrographs of male meiotic cells from wild-type (A, B, E, F) and mtshZ2-2620/mtshZ2-2620 (C, D) flies carrying a β tubulin-EGFP transgene (gift from H. Oda and Y. Akiyama-Oda). Wild-type cells in meiosis I (A, B) have strongly fluorescent regions of overlapping microtubules from opposite asters (B, arrows), along with a region of the spindle (arrowhead) thought to be anastral and nucleated from the chromosomal region (Rebollo et al., 2004). Cells from mtsh mutant males (C, D) do not have the overlapping astral spindle region and appear to contain only the anastral central spindle (D, arrowhead) plus some nonoverlapping astral microtubules. In wild-type cells at telophase I (E, F), strong fluorescence from the astral central spindle is detected in the region of the cleavage furrow and contractile ring (arrow). No such furrowing cells are ever observed in mtsh flies. The identical phenotype is seen in mtshZ2-2620/mtshZ2-3484 and mtshZ2-2620/mtsh55207-12 transheterozygotes. Scale bar 10 μm.
In wild-type cells, the anillin protein cycles from the nucleus at interphase to the cell cortex during mitosis or meiosis; at the cortex, anillin gradually becomes more tightly localized to the cleavage furrow and contractile ring, where it helps orchestrate the recruitment of other ring proteins like septins, actin, and myosin (reviewed in Hickson and O'Farrell, 2008). The tight localization of anillin at the equatorial cortex in anaphase and telophase depends upon Pebble GEF-mediated Rho GTPase activation signals from the central spindle (reviewed in Hickson and O'Farrell, 2008). GFP-anillin has normal nuclear localization in mtsh primary spermatocytes (Fig. 5A–D) but is aberrantly localized in male meiotic cells, with only diffuse cortical localization instead of focusing into a tight ring (Fig. 5E–H). Sep2-GFP, another contractile ring component, localized in the ring in wild-type male meiotic cells but was not detected at the cell cortex in mtsh meiotic cells (not shown).
FIG. 5.
Anillin is not properly localized to a contractile ring in mtsh meiotic cells. Phase-contrast (A, C, E, G) and fluorescence (B, D, F, H) micrographs of cells from wild-type (A, B, E, F) and mtshZ2-2620/mtshZ2-2620 (C, D, G, H) flies carrying a transgene encoding GFP-Anillin (Goldbach et al., 2010). GFP-anillin is nuclear in wild-type (A, B) and mtsh (C, D) primary spermatocytes. In wild-type meiotic cells (E, F), GFP-anillin localizes to the contractile ring (arrow). In mtsh meiotic cells (G, H), no GFP-anillin-containing contractile rings are seen, though GFP-anillin has redistributed from the nucleus diffusely to the cell cortex (arrow). The identical phenotype is seen in mtshZ2-2620/mtshZ2-3484 and mtshZ2-2620/mtsh55207-12 transheterozygotes. Scale bars 10 μm in (A–D), (E–H).
Discussion
The predicted bromodomain-related protein Mtsh is required for coordination of events in Drosophila spermatogenesis. We identified two alleles of mtsh from the Zuker collection of EMS-induced nonlethal mutants (Koundakjian et al., 2004) and generated two additional P element excision alleles. The alleles have molecular lesions in CG7795, with the two EMS-induced alleles each containing a different nonsense mutation not seen in the background chromosome. The null P element excision allele mtsh55207-12 is a small deletion of the 5′ portion of CG7795, including the transcriptional and translational start sites, and does not affect neighboring genes. The less severe phenotype of the P element excision allele mtsh55201-34 may result from retention of a part of the P element and/or deletion of an upstream regulatory element, based on preliminary results. The mtsh gene appears to be required only in the testis, as homozygous individuals are completely viable, and homozygous null females are fully fertile. This observation is consistent with expression data in the FlyAtlas database (Chintapalli et al., 2007), indicating that the testis is the only tissue in which mtsh/CG7795 shows significantly enriched expression.
In the absence of functional Mtsh protein, mitochondrial aggregation (an early event of spermiogenesis) occurred at or before meiotic onset instead of at the end of meiosis. We demonstrated that mitochondria also fused prematurely, as the presence of nonfunctional testis mitofusin Fzo in double mutant mtsh; fzo flies resulted in fragmented mitochondrial shells compared to those seen in mtsh alone. In wild type, Fzo is first detectable at the end of telophase II (Hales and Fuller, 1997). In mtsh, mitochondria fused into a shell around the time of metaphase I, as the fully formed shell subsequently obscured observation of interior fluorescence from spindles or DNA at later stages. The abnormally early function of Fzo in mtsh indicated early Fzo expression and confirmed that multiple events of spermiogenesis occur prematurely.
Meiotic karyokinesis eventually occurred in mtsh homozygous males, but meiotic cytokinesis failed. The aberrant postmeiotic spermatids each with four haploid nuclei showed axoneme growth. Sperm individualization did not occur, as is typical when sperm morphology is defective (Fuller, 1993). This phenotype differs from other mutants known to affect meiosis/spermiogenesis coordination: in aly- and can-class mutant males, all aspects of sperm development are arrested at primary spermatocyte stages (reviewed in White-Cooper, 2010), and in twine mutants, meiosis fails but spermiogenesis proceeds, leading to a single 4n nucleus per cell associated with a large growing sperm tail (Alphey et al., 1992; Courtot et al., 1992; White-Cooper et al., 1993; Sigrist et al., 1995). In males mutant for eIF4G, meiosis is arrested, and only an initial mitochondrial cloud forms before spermiogenesis arrests as well (Baker and Fuller, 2007; Franklin-Dumont et al., 2007). The mtsh mutant is the first identified in which both meiosis and spermiogenesis proceed though with lack of proper coordination.
All mtsh orthologs are predicted to encode a single polypeptide that matches the longer 649 amino acid predicted isoform of D. melanogaster Mtsh. Evolutionary conservation of the bromodomain-related region (which is predicted to be truncated in the shorter predicted D. melanogaster isoform) is consistent with functional relevance of the longer isoform. The prediction of the shorter isoform, which would result from inclusion of an additional 27-bp exon containing a stop codon, may be spurious, but it may alternatively reflect divergence of the Drosophila ortholog to a situation of regulation by alternative splicing. Indeed, a D. melanogaster testis EST matches the transcript encoding the isoform with the full bromodomain-related region, while an embryo EST matches the transcript encoding the truncated isoform.
The mtsh gene is detectably conserved only in Diptera, Coleoptera, and Lepidoptera. Mtsh and its orthologs have diverged fairly quickly overall in these insect lineages, perhaps reflecting mechanisms of reproductive isolation, as is common with genes involved in gametogenesis (Dorus et al., 2006). Two regions of the protein are highly conserved, region 1 (highly alpha helical though with no recognizable motifs) in the amino terminal half of the protein, and region 2 near the carboxy terminus, which resembles a bromodomain both in sequence and in predicted alpha helical profile (Fig. 3B–E). Mtsh orthologs all show strikingly similar predicted alpha helical profiles in this region.
Bromodomains bind to acetylated lysine residues, typically on histones, and are found in some transcriptional regulators, histone acetyltransferases, and other chromatin remodeling factors (reviewed in Loyola and Almouzni, 2004). These domains are typically around 110 amino acids and encompass four alpha helices, αZ, αA, αB, and αC, with the loop between helices Z and A interacting with the loop between helices B and C. Some bromodomains contain a small fifth helix within the ZA loop. Ligands bind in a hydrophobic zone within the ZA–BC loop interaction. Region 2 in Mtsh is reminiscent of a bromodomain in size, alpha helical structure, and sequence, though only a subset of the highly conserved residues seen in bromodomains are retained (Fig. 3E). Notably, the three residues important for acetylated histone binding (arrows in Fig. 3D, E) (Mujtaba et al., 2007), including the BC loop asparagine that forms a crucial hydrogen bond with the acetyl-lysine carbonyl group (reviewed in Zeng and Zhou, 2002), are not conserved in Mtsh. Mtsh orthologs instead include an invariant leucine at the position corresponding to the BC loop asparagine, suggesting the possibility of different ligand sensitivity.
The nonconservation of acetyl-lysine binding residues is consistent with the possibility that Mtsh may serve a role other than chromatin binding. Alternatively, Mtsh may, like other bromodomain family proteins, participate in chromatin binding and transcriptional regulation but with different ligand selectivity. A possible role for Mtsh as a chromatin-binding protein would be consistent with its coordination of meiosis and spermiogenesis in spermatogenesis, as many of the known genes involved in that process function in transcriptional activation; Mtsh may activate transcription of meiotic cell cycle regulators, such that meiotic progression is delayed in mtsh flies, or it may control expression of translational repressors of spermiogenesis genes, such that spermiogenesis is activated early in mtsh testes. Mtsh may instead be part of the yet-uncharacterized link between the can-class TAFs and translational activation of the testis cdc25 homolog twine. Assessment of mtsh expression in can-class mutants and Twine in mtsh mutants will in the future test this hypothesis. Exploration of these possibilities will elucidate whether the mtsh phenotype can be defined precisely as premature spermiogenesis, delayed meiosis, or both.
Meiotic cytokinesis does not occur in mtsh mutant males. Microtubule observation in mtsh testes showed a meiosis I spindle defect in a region of overlapping antipolar microtubules. Such a region of interdigitating microtubules is often called the central spindle, but that term is imprecise in that it does not differentiate whether the microtubules originate from the asters or from the distinct anastral portion of the spindle nucleated in the chromosomal region (Rebollo et al., 2004). The mtsh mutant appears to lack interdigitating astral microtubules in male meiotic cells, while the anastral spindle appears intact. It is well established that in many cell types, positional signals from the central spindle to the cell cortex determine the location of the contractile ring. Conflicting reports on Drosophila male meiosis indicate, alternately, that the central region of the astral spindle (Rebollo et al., 2004) or the anastral spindle (Bonaccorsi et al., 1998) is essential for determining contractile ring placement. Our results support the hypothesis that the aster-derived central spindle helps position the contractile ring, since in mtsh only the anastral central spindle forms, but the contractile ring components anillin and Sep2 are not properly localized.
We speculate that the failure of meiotic cytokinesis in mtsh homozygous males, via aberrant spindle and contractile ring construction, is ultimately a secondary effect of faulty developmental coordination. Previous work from other researchers suggests a role for mitochondria in establishing or stabilizing the meiotic central spindle. The Infertile crescent (Ifc) protein, a sphingolipid desaturase (Ternes et al., 2002), is associated both with mitochondria during meiosis as well as later with the meiotic contractile ring (Basu and Li, 1998). In the absence of Ifc, central spindles are not properly assembled or stabilized, and meiotic cytokinesis fails (Basu and Li, 1998). Mitochondria are thought to travel on microtubule tracks in Drosophila spermatogenesis (reviewed in Hales, 2004; Aldridge et al., 2007). In wild type, mitochondria associate in the midzone of the meiotic spindle, presumably in association with plus-end-directed microtubule motors; later, in early round spermatids, mitochondria aggregate toward the minus ends of microtubules (reviewed in Fuller, 1993). If the signal for mitochondrial aggregation occurs prematurely, before spindle formation, the shell-like fused mitochondrial structure surrounding mtsh spermatocyte nuclei may reflect the premeiotic pattern of microtubules. Improper mitochondrial localization in mtsh mutant cells may therefore prevent Ifc from helping to stabilize the astral central spindle and establish the equatorial plane where the contractile ring would otherwise form. (The causal relationship could instead be reversed, though this is less likely given the chronology of events and the normal centrosome migration in mtsh.) Another possibility is that the mitochondrial shell prevents the transfer of chromosomal passenger proteins to the cell cortex, where they would normally trigger early events of cytokinesis (Ruchaud et al., 2007). Consistent with either hypothesis, GFP-anillin and Sep2-GFP fail to localize properly in mtsh meiotic cells. Our observation of some anillin cortical localization fits with previous observations that anillin associates diffusely with the cell cortex in the absence of central spindle-related signals like Pebble (reviewed in Hickson and O'Farrell, 2008).
Conclusions
Proper coordination of meiosis and spermiogenesis in D. melanogaster spermatogenesis requires the function of a novel gene, mtsh, which encodes a protein with a predicted bromodomain-related region. Mtsh is conserved in some insect lineages. In the absence of functional Mtsh, events of sperm tail growth such as mitochondrial aggregation and fusion precede and accompany meiotic onset. Meiotic nuclear division subsequently occurs; however, meiotic cytokinesis fails in mtsh as a result of faulty central spindle and contractile ring formation, both likely secondary effects of aberrant mitochondrial localization.
Acknowledgments
The authors thank the 119 undergraduate genetics students at Davidson College from the Fall 2002, 2003, 2004, and 2007 semesters for their participation in deficiency mapping and P element excision screens. The authors are grateful to Julie Brill, Phil Goldbach, and other members of the Brill Lab for hospitality, fly stocks, and comments on the project. The authors thank H. Oda, Y. Akiyama-Oda, C. Zuker, B. Wakimoto, D. Lindsley, and the Bloomington and Szeged Drosophila Stock Centers for fly stocks. The FlyBase and FlyAtlas databases provided crucial information. Members of the Biology Department at Davidson College, including M. Campbell, S. Sarafova, K. Bernd, D. Wessner, B. Lom, and C. Healey, contributed help with equipment and general advice. Work in the Hales lab has been supported by National Science Foundation CAREER Grant 0133335, National Institutes of Health AREA Grant R15GM080689, and Davidson College.
Disclosure Statement
No competing financial interests exist.
References
- Aldridge A.C. Benson L.P. Siegenthaler M.M. Whigham B.T. Stowers R.S. Hales K.G. Roles for Drp1, a dynamin-related protein, and milton, a kinesin-associated protein, in mitochondrial segregation, unfurling and elongation during Drosophila spermatogenesis. Fly (Austin) 2007;1:38–46. doi: 10.4161/fly.3913. [DOI] [PubMed] [Google Scholar]
- Alphey L. Jimenez J. White-Cooper H. Dawson I. Nurse P. Glover D.M. Twine, a cdc25 homolog that functions in the male and female germline of Drosophila. Cell. 1992;69:977–988. doi: 10.1016/0092-8674(92)90616-k. [DOI] [PubMed] [Google Scholar]
- Altschul S.F. Gish W. Miller W. Myers E.W. Lipman D.J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baker C.C. Fuller M.T. Translational control of meiotic cell cycle progression and spermatid differentiation in male germ cells by a novel eIF4G homolog. Development. 2007;134:2863–2869. doi: 10.1242/dev.003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F.A. Gruneberg U. Cytokinesis: placing and making the final cut. Cell. 2007;131:847–860. doi: 10.1016/j.cell.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Basu J. Li Z. The Des-1 protein, required for central spindle assembly and cytokinesis, is associated with mitochondria along the meiotic spindle apparatus and with the contractile ring during male meiosis in Drosophila melanogaster. Mol Gen Genet. 1998;259:664–673. doi: 10.1007/s004380050861. [DOI] [PubMed] [Google Scholar]
- Bonaccorsi S. Giansanti M.G. Gatti M. Spindle self-organization and cytokinesis during male meiosis in asterless mutants of Drosophila melanogaster. J Cell Biol. 1998;142:751–761. doi: 10.1083/jcb.142.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill J.A. Hime G.R. Scharer-Schuksz M. Fuller M.T. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. doi: 10.1242/dev.127.17.3855. [DOI] [PubMed] [Google Scholar]
- Chintapalli V.R. Wang J. Dow J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Cokol M. Nair R. Rost B. Finding nuclear localization signals. EMBO Rep. 2000;1:411–415. doi: 10.1093/embo-reports/kvd092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtot C. Fankhauser C. Simanis V. Lehner C.F. The Drosophila cdc25 homolog twine is required for meiosis. Development. 1992;116:405–416. doi: 10.1242/dev.116.2.405. [DOI] [PubMed] [Google Scholar]
- Dhalluin C. Carlson J.E. Zeng L. He C. Aggarwal A.K. Zhou M.M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- Dorus S. Busby S.A. Gerike U. Shabanowitz J. Hunt D.F. Karr T.L. Genomic and functional evolution of the Drosophila melanogaster sperm proteome. Nat Genet. 2006;38:1440–1445. doi: 10.1038/ng1915. [DOI] [PubMed] [Google Scholar]
- Franklin-Dumont T.M. Chatterjee C. Wasserman S.A. Dinardo S. A novel eIF4G homolog, off-schedule, couples translational control to meiosis and differentiation in Drosophila spermatocytes. Development. 2007;134:2851–2861. doi: 10.1242/dev.003517. [DOI] [PubMed] [Google Scholar]
- Fuller M.T. Spermatogenesis. In: M. Bate., editor; A. Martinez-Arias., editor. The Development of Drosophila melanogaster. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1993. pp. 71–147. [Google Scholar]
- Gao D. McHenry C.S. Tau binds and organizes Escherichia coli replication proteins through distinct domains. Domain III, shared by gamma and tau, binds delta delta′ and chi psi. J Biol Chem. 2001;276:4447–4453. doi: 10.1074/jbc.M009827200. [DOI] [PubMed] [Google Scholar]
- Glover B.P. Pritchard A.E. McHenry C.S. Tau binds and organizes Escherichia coli replication proteins through distinct domains: domain III, shared by gamma and tau, oligomerizes DnaX. J Biol Chem. 2001;276:35842–35846. doi: 10.1074/jbc.M103719200. [DOI] [PubMed] [Google Scholar]
- Goldbach P. Wong R. Sarpal R. Brill J.A. Stabilization of the actomyosin ring enables spermatocyte cytokinesis in Drosophila. Mol Biol Cell. 2010. (In press). [DOI] [PMC free article] [PubMed]
- Hales K.G. The machinery of mitochondrial fusion, division, and distribution, and emerging connections to apoptosis. Mitochondrion. 2004;4:285–308. doi: 10.1016/j.mito.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hales K.G. Fuller M.T. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–129. doi: 10.1016/s0092-8674(00)80319-0. [DOI] [PubMed] [Google Scholar]
- Hickson G.R. O'Farrell P.H. Anillin: a pivotal organizer of the cytokinetic machinery. Biochem Soc Trans. 2008;36:439–441. doi: 10.1042/BST0360439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller M. Chen X. Pringle M.J. Suchorolski M. Sancak Y. Viswanathan S. Bolival B. Lin T.Y. Marino S. Fuller M.T. Testis-specific TAF homologs collaborate to control a tissue-specific transcription program. Development. 2004;131:5297–5308. doi: 10.1242/dev.01314. [DOI] [PubMed] [Google Scholar]
- Horton P. Park K.J. Obayashi T. Fujita N. Harada H. Adams-Collier C.J. Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35:W585–W587. doi: 10.1093/nar/gkm259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis T.C. Beaudry A.A. Bullard J.M. Janjic N. McHenry C.S. Reconstitution of a minimal DNA replicase from Pseudomonas aeruginosa and stimulation by non-cognate auxiliary factors. J Biol Chem. 2005;280:7890–7900. doi: 10.1074/jbc.M412263200. [DOI] [PubMed] [Google Scholar]
- Koundakjian E.J. Cowan D.M. Hardy R.W. Becker A.H. The Zuker collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics. 2004;167:203–206. doi: 10.1534/genetics.167.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin M.A. Blackshields G. Brown N.P. Chenna R. McGettigan P.A. McWilliam H. Valentin F. Wallace I.M. Wilm A. Lopez R. Thompson J.D. Gibson T.J. Higgins D.G. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Loyola A. Almouzni G. Bromodomains in living cells participate in deciphering the histone code. Trends Cell Biol. 2004;14:279–281. doi: 10.1016/j.tcb.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Maines J.Z. Wasserman S.A. Post-transcriptional regulation of the meiotic Cdc25 protein Twine by the Dazl orthologue Boule. Nat Cell Biol. 1999;1:171–174. doi: 10.1038/11091. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A. Anderson J.B. Chitsaz F. Derbyshire M.K. DeWeese-Scott C. Fong J.H. Geer L.Y. Geer R.C. Gonzales N.R. Gwadz M. He S. Hurwitz D.I. Jackson J.D. Ke Z. Lanczycki C.J. Liebert C.A. Liu C. Lu F. Lu S. Marchler G.H. Mullokandov M. Song J.S. Tasneem A. Thanki N. Yamashita R.A. Zhang D. Zhang N. Bryant S.H. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 2009;37:D205–D210. doi: 10.1093/nar/gkn845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHenry C.S. Chromosomal replicases as asymmetric dimers: studies of subunit arrangement and functional consequences. Mol Microbiol. 2003;49:1157–1165. doi: 10.1046/j.1365-2958.2003.03645.x. [DOI] [PubMed] [Google Scholar]
- Mikhaylova L.M. Boutanaev A.M. Nurminsky D.I. Transcriptional regulation by Modulo integrates meiosis and spermatid differentiation in male germ line. Proc Natl Acad Sci U S A. 2006;103:11975–11980. doi: 10.1073/pnas.0605087103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujtaba S. Zeng L. Zhou M.M. Structure and acetyl-lysine recognition of the bromodomain. Oncogene. 2007;26:5521–5527. doi: 10.1038/sj.onc.1210618. [DOI] [PubMed] [Google Scholar]
- Nair R. Rost B. Mimicking cellular sorting improves prediction of subcellular localization. J Mol Biol. 2005;348:85–100. doi: 10.1016/j.jmb.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Rebollo E. Llamazares S. Reina J. Gonzalez C. Contribution of noncentrosomal microtubules to spindle assembly in Drosophila spermatocytes. PLoS Biol. 2004;2:E8. doi: 10.1371/journal.pbio.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rost B. Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- Rost B. Yachdav G. Liu J. The PredictProtein server. Nucleic Acids Res. 2004;32:W321–W326. doi: 10.1093/nar/gkh377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchaud S. Carmena M. Earnshaw W.C. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Ryder E. Blows F. Ashburner M. Bautista-Llacer R. Coulson D. Drummond J. Webster J. Gubb D. Gunton N. Johnson G. O'Kane C.J. Huen D. Sharma P. Asztalos Z. Baisch H. Schulze J. Kube M. Kittlaus K. Reuter G. Maroy P. Szidonya J. Rasmuson-Lestander A. Ekstrom K. Dickson B. Hugentobler C. Stocker H. Hafen E. Lepesant J.A. Pflugfelder G. Heisenberg M. Mechler B. Serras F. Corominas M. Schneuwly S. Preat T. Roote J. Russell S. The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics. 2004;167:797–813. doi: 10.1534/genetics.104.026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigrist S. Ried G. Lehner C.F. Dmcdc2 kinase is required for both meiotic divisions during Drosophila spermatogenesis and is activated by the Twine/cdc25 phosphatase. Mech Dev. 1995;53:247–260. doi: 10.1016/0925-4773(95)00441-3. [DOI] [PubMed] [Google Scholar]
- Silverman-Gavrila R.V. Hales K.G. Wilde A. Anillin-mediated targeting of peanut to pseudocleavage furrows is regulated by the GTPase Ran. Mol Biol Cell. 2008;19:3735–3744. doi: 10.1091/mbc.E08-01-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternes P. Franke S. Zahringer U. Sperling P. Heinz E. Identification and characterization of a sphingolipid delta 4-desaturase family. J Biol Chem. 2002;277:25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- Tweedie S. Ashburner M. Falls K. Leyland P. McQuilton P. Marygold S. Millburn G. Osumi-Sutherland D. Schroeder A. Seal R. Zhang H. FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res. 2009;37:D555–D559. doi: 10.1093/nar/gkn788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R.A. Greenleaf A.L. Gyurkovics H. Wisely G.B. Huang S.M. Searles L.L. Frequent imprecise excision among reversions of a P element-caused lethal mutation in Drosophila. Genetics. 1984;107:279–294. doi: 10.1093/genetics/107.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto B.T. Lindsley D.L. Herrera C. Toward a comprehensive genetic analysis of male fertility in Drosophila melanogaster. Genetics. 2004;167:207–216. doi: 10.1534/genetics.167.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Cooper H. Molecular mechanisms of gene regulation during Drosophila spermatogenesis. Reproduction. 2010;139:11–21. doi: 10.1530/REP-09-0083. [DOI] [PubMed] [Google Scholar]
- White-Cooper H. Alphey L. Glover D.M. The cdc25 homologue twine is required for only some aspects of the entry into meiosis in Drosophila. J Cell Sci. 1993;106:1035–1044. doi: 10.1242/jcs.106.4.1035. [DOI] [PubMed] [Google Scholar]
- Zeng L. Zhou M.M. Bromodomain: an acetyl-lysine binding domain. FEBS Lett. 2002;513:124–128. doi: 10.1016/s0014-5793(01)03309-9. [DOI] [PubMed] [Google Scholar]