Abstract
It is intriguing that during human cultural evolution man has detected plant natural products that appear to target key protein receptors of important physiological systems rather selectively. Plants containing such secondary metabolites usually belong to unique chemotaxa, induce potent pharmacological effects and have typically been used for recreational and medicinal purposes or as poisons. Cannabis sativa L. has a long history as a medicinal plant and was fundamental in the discovery of the endocannabinoid system. The major psychoactive Cannabis constituent Δ9-tetrahydrocannabinol (Δ9-THC) potently activates the G-protein-coupled cannabinoid receptor CB1 and also modulates the cannabinoid receptor CB2. In the last few years, several other non-cannabinoid plant constituents have been reported to bind to and functionally interact with CB receptors. Moreover, certain plant natural products, from both Cannabis and other plants, also target other proteins of the endocannabinoid system, such as hydrolytic enzymes that control endocannabinoid levels. In this commentary we summarize and critically discuss recent findings.
This article is part of a themed issue on Cannabinoids. To view the editorial for this themed issue visit http://dx.doi.org/10.1111/j.1476-5381.2010.00831.x
Keywords: phytocannabinoid, cannabinoid, plant natural products, Cannabis, endocannabinoid system
Today we perceive the endocannabinoid system (ECS) as a rather complex lipid signalling network in which different proteins play distinct roles in the control or in the modulation of numerous physiological and pathophysiological processes (Pertwee, 2005; Di Marzo, 2008). The ECS comprises classical cannabinoid receptors (CB1 and CB2), potentially also the orphan receptor GPR55, and arachidonic acid-derived ligands, which, however, also promiscuously target other receptors like, e.g. TRPV1 and PPAR-gamma (O'Sullivan, 2007; De Petrocellis and Di Marzo, 2010; Ross, 2009; Pertwee, 2010). Importantly, the enzymes degrading the endocannabinoids anandamide and 2-arachidonoyl glycerol, namely fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL), have been shown to be promising therapeutic targets (Di Marzo, 2008). Finally, there appears to be an anandamide cellular reuptake mechanism that can be blocked by specific inhibitors (Di Marzo, 2008). Both cannabinoid receptor agonists and antagonists have actual or potential therapeutic applications (Di Marzo, 2008; Oesch and Gertsch, 2009; Pertwee, 2009). Cannabinoids are defined as the terpenophenolic constituents of Cannabis sativa L and until recently, the phenylterpenoid Δ9-THC and some of its naturally occurring derivatives were the only plant natural products known to directly interact with cannabinoid receptors. However, in the last few years, several non-cannabinoid plant natural products have been reported to act as cannabinoid receptor ligands. This prompts us to define ‘phytocannabinoids’ as any plant-derived natural product capable of either directly interacting with cannabinoid receptors or sharing chemical similarity with cannabinoids or both. Direct cannabinoid receptor ligands are compounds that show high binding affinities (in the lower nM range) for cannabinoid receptors and exert discrete functional effects (i.e. agonism, neutral antagonism or inverse agonism). By contrast, indirect ligands target either key proteins within the ECS that regulate tissue levels of endocannabinoids or allosteric sites on the CB1 receptor. Certain plant natural products, including some cannabinoids, possess at least some of these properties. Given the often high variability of molecular pharmacological data obtained in different laboratories and the distinct degrees of scrutiny of the experimental setup, molecular pharmacological data on natural products should always be interpreted with care (Gertsch, 2009). For example, the availability of CB receptor KO mice provides a powerful means of investigating the actual cannabimimetic nature of a particular compound in vivo. This commentary focuses on natural products from medicinal and dietary plants which have been reported to interact with the ECS.
Fatty acid derivatives
Despite the fact that N-acylethanolamines (NAEs) (Table 1) from plants do not interact with CB receptors (plants do not generally produce arachidonic acid, which is the acyl scaffold favoured for CB interaction) they have been shown to inhibit FAAH, thus leading to an increase in endocannabinoid tone. N-linoleoylethanolamide and N-oleoylethanolamide, which are found not only in chocolate (Theobroma cacao L.) but also other plants (Di Marzo et al., 1998), and the widespread NAE palmitoylethanolamide, inhibit anandamide breakdown (Maurelli et al., 1995; Di Tomaso et al., 1996). Certain N-alkylamides (alkamides) from Echinacea spp. (Table 2) have been shown to interact functionally with the human CB2 receptor with low nM to µM Ki values (Gertsch et al., 2006). These N-isobutylamides selectively act at the CB2 receptor over the CB1 receptor, leading to an increase in intracellular calcium which could be blocked by the selective CB2 receptor inverse agonist SR144528, but they do not modulate the Gαi signalling pathway. Intriguingly, CB2 receptor-binding N-alkylamides show similar anti-inflammatory effects as anandamide (e.g. inhibition of TNF-α) at low nM concentrations (Raduner et al., 2006). Certain Echinacea N-alkylamides inhibit anandamide reuptake in vitro (Chicca et al., 2009). Like anandamide, N-alkylamides also target PPAR-gamma (Spelman et al., 2009). Different Echinacea N-isobutylamides are orally bioavailable resulting in nM plasma levels in humans (Woelkart et al., 2008). The polyacetylenic polyyne falcarinol, which is found in different plants of the Apiaceae family (e.g. in carrots) shows significant binding interactions with both cannabinoid receptors, but appears to selectively undergo an alkylation reaction with the CB1 receptor (Ki value <1 µM), leading to relatively potent inverse agonistic and pro-inflammatory effects in human skin (Leonti et al., 2010). Finally, it has been proposed that certain dietary fatty acids, which can also be found in plants, can modulate the ECS by influencing the availability of phospholipid biosynthetic precursors of endocannabinoids (Banni and Di Marzo, 2009).
Table 1.
Plant natural products that have been suggested to exert cannabimimetic effects but do not interact directly with cannabinoid (CB) receptors
| Structure | Name | Origin | CB receptor affinity | Function | In vivo efficacy | Other targets (ECS) | References |
|---|---|---|---|---|---|---|---|
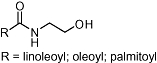 |
N-acylethanolamines | Widespread in plants | No affinity | FAAH inhibitors | Validated in CB1 and CB2 KO mice | GPR55 | Maurelli et al., 1995; Di Tomaso et al., 1996; Di Marzo, 2008 |
| Indirect cannabimimetics | |||||||
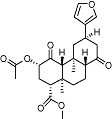 |
Salvinorin A | Salvia divinorum | Insignificant affinity to CB receptors | Indirect cannabimimetic effects at CB1 (mechanism unknown) | No data | KOP agonist | Capasso et al., 2008; Fichna et al., 2009; |
| Epling & Jativa-M | |||||||
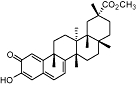 |
Pristimerin | Relatively widespread in the Celastraceae | No data | Potent reversible | No data | No data | King et al., 2009 |
| MAGL inhibitor (IC50 value <100 nM) | |||||||
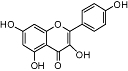 |
Kaempferol | Widespread in plants | No affinity | FAAH inhibitor (IC50 value <1 µM) | No data | No data | Thors et al., 2007; 2008; |
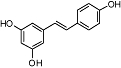 |
Trans-resveratrol | Relatively widespread in plants (e.g. Vitis vinifera L.) | Insignificant affinity | Insignificant effects | No data | No data | Prather et al., 2009 |
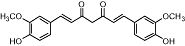 |
Curcumin | Curcuma spp. | Insignificant affinity | Insignificant effects | No data | No data | Prather et al., 2009 |
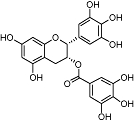 |
Epigallocatechin-3-O-gallate | Relatively widespread in plants (e.g. Camellia sinensis L.) | Insignificant affinity | No data | No data | No data | Korte et al., 2010 |
ECS, endocannabinoid system; FAAH, fatty acid amide hydrolase; MAGL, monoacylglycerol lipase.
Table 2.
Plant natural products that have been shown to interact directly with cannabinoid (CB) receptors
| Structure | Name | Origin | CB receptor affinity | Function | In vivo efficacy | Other targets (ECS) | References |
|---|---|---|---|---|---|---|---|
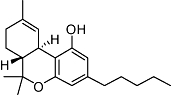 |
Δ9-THC | Cannabis sativa L. | Non-selective CB1 and CB2 affinity (Ki values <50 nM) (human) | Parial agonist Gi/o | Validated in CB1 and CB2 KO mice | GPR55 | Mechoulam, 1986; Pertwee, 2006 |
| Inhibition by SR141716 and SR144528 | PPARs | ||||||
| Different ion channels | |||||||
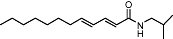 |
N-alkylamide | Echinacea spp. | Selective CB2 affinity (Ki value <100 nM) (human) | Parial agonist | No data | PPAR-γ | Raduner et al., 2006; Chicca et al., 2009 |
| [Ca2+]iInhibition by SR144528 | Inhibition of AEA transport | ||||||
| Partial FAAH inhibtion | |||||||
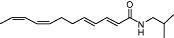 |
N-alkylamide | Echinacea spp. | Selective CB2 affinity (Ki value <100 nM) (human) | Parial agonist [Ca2+] i | No data | PPAR-γ | Raduner et al., 2006; Chicca et al., 2009 |
| Inhibition by SR144528 | Inhibition of AEA transport | ||||||
| Parial FAAH inhibition | |||||||
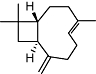 |
β-caryophyllene | Widespread in plants | Selective CB2 affinity (Ki value <200 nM) (human) | Full agonist | Validated in CB2 KO mice | No data | Gertsch et al., 2008 |
| Gi/o | |||||||
| [Ca2+]i | |||||||
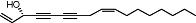 |
Falcarinol | Relatively widespread in Apiaceae (e.g. Daucus carota L.) | Non-selective CB1 affinity (Ki value <1 µM) (human) | CB1 receptor-selective inverse (covalent) agonist | No data | No data | Leonti et al., 2010 |
| Inhibition of AEA/WIN55212-2 | |||||||
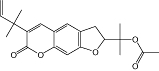 |
Rutamarin | Ruta graveolens L. | Selective CB2 affinity (Ki value <10 µM) (human) | No data | No data | No data | Rollinger et al., 2009 |
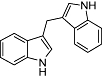 |
DIM 3,3-diindolylmethane metabolite from indole-3-carbinol | Relatively widespread in the Brassica genus | Selective CB2 affinity (Ki value ≅1 µM) (human) | Partial agonist at CB2 receptor | No data | No data | Yin et al., 2009 |
Δ9-THC is shown as the major phytocannabinoid from Cannabis sativa L. but there are several other structurally related cannabinoids that interact with CB receptors.
Δ9-THC, Δ9-tetrahydrocannabinol; DIM, 3,3′-diindolylmethane; ECS, endocannabinoid system; FAAH, fatty acid amide hydrolase; PPAR, peroxisome proliferator-activated protein.
Terpenes
The bicyclic sesquiterpene, β-caryophyllene (trans-isomer) (Table 2), which is a plant volatile very frequently found in plants, has been shown to selectively target the CB2 receptor at nM concentrations (Ki= 155 nM) and to act as a full agonist (Gertsch et al., 2008). Remarkably, β-caryophyllene is also a major compound in Cannabis sativa L. essential oil. Thus, Cannabis produces two entirely different chemical scaffolds able to differentially target CB receptors. While studies on the pharmacokinetics of β-caryophyllene are still ongoing, it is already clear that this cyclobutane-ring containing terpene is readily bioavailable, and, unlike many polyphenolic natural products, is not metabolized immediately but shows a Tmax >1 h after one single oral administration (J.G., unpublished data). Orally administed β-caryophyllene (<5 mg·kg−1) produces strong anti-inflammatory and analgesic effects in wild-type mice but not in CB2 receptor knockout mice, which is a clear indication that it may be a functional CB2 ligand. Ongoing studies show that β-caryophyllene is effective at reducing neuropathic pain in a CB2 receptor-dependent manner (Zimmer et al., 2009). Therefore, the FDA approved food additive β-caryophyllene has the potential to become an attractive candidate for clinical trials targeting the CB2 receptor (Gertsch, 2008). Interestingly, the diterpene salvinorin A from Salvia divinorum Epling & Jativa-M (Table 1) has been reported to be a selective high-affinity kappa-opioid receptor (KOP) agonist, but recent data also suggest that it may interact with a putative CB receptor/KOP heterodimer which may be formed during inflammatory conditions (Fichna et al., 2009). To date, binding experiments have shown that salvinorin A has very low affinity for homomeric cannabinoid receptors and does not inhibit endocannabinoid degradation (Capasso et al., 2008). Consequently, further research is needed to establish whether salvinorin A interacts with a putative cannabinoid/KOP heterodimeric receptor or whether the cannabimimetic effects reported are indirectly mediated via KOP. More recently, two naturally occurring quinonoid triterpenoids, pristimerin (Table 1) and euphol, were found to inhibit MAGL with high potency (IC50= 93 nM and 315 nM respectively) through a reversible mechanism (King et al., 2009). As this class of triterpenes is relatively frequent in nature, it may not be unusual to find ‘indirect’ rather than ‘direct’ agonists of cannabinoid receptors among plant secondary metabolites. Several distinct triterpenes are known to modulate immune functions through yet unknown mechanisms (Rios, 2010) and it will thus be interesting to see in a more systematic study whether other similar triterpenoids are also able to inhibit MAGL.
Polyphenols
The dietary polyphenols trans-resveratrol and curcumin (Table 1) were reported to bind selectively to the human CB1 cannabinoid receptor with low nM Ki values (5.9 nM and 45 nM respectively) and to exert potent pharmacological effects in mice similar to those induced by the CB1 receptor inverse agonist rimonabant (Seely et al., 2009). Intrigued by this unexpected finding, our research groups independently measured the binding affinities of these compounds for CB1 and CB2 receptors in our laboratories. In our experiments, trans-resveratrol and curcumin only displaced [3H]CP55 940 from cannabinoid receptors at high µM concentrations, suggesting that they lack significant affinity for these receptors. Also polydatin, a glycosilated form of resveratrol, was inactive in these binding assays. Recently, the senior author of the original report retracted the paper (Prather et al., 2009). Hence, neither trans-resveratrol nor curcumin interact functionally with the CB1 receptor, despite the fact that these compounds appear to share the ability of the CB1 receptor inverse agonist, rimonabant, to induce weight loss in mice.
More recently, catechin-derivatives were shown to bind to human cannabinoid receptors rather non-selectively at high µM concentrations (Korte et al., 2010). Among these, epigallocatechin 3-gallate and (-)-epigallocatechin (Table 1) were reported to bind to the CB1 receptor with Ki values of 33.6 and 35.7 µM respectively. However, these Ki values may not be correct. For the calculation of the Ki values the Cheng-Prussof equation (Ki= IC50/1+([S]/Kd) was not applied correctly. The EC50 values used to calculate the Ki values were approximations as neither compound produced more than 60% radioligand displacement even at the highest concentration used. Catechins are very widespread plant secondary metabolites which may provide nutritional health benefits. The same group has recently reported similar CB1 and CB2 receptor Ki values for delphinidin and cyanidin, two hydrophilic anthocyanidins (Korte et al., 2009). In both reports, no functional data were shown. In our hands, flavonoid-type compounds (catechins, anthocyanidins, flavones) lead to negligible or very high Ki values, which likely reflect a nonspecific molecular denaturation of the protein surface rather than a functional binding interaction. Similar potentially artefactual effects would most likely be observed with other GPCRs.
Plant polyphenols, such as phenylpropanoids (e.g. epigallocatechin 3-O-gallate, curcumin, resveratrol) possess chemical scaffolds which at µM concentrations bind to protein targets in vitro with limited specificity. This is clearly reflected by numerous reports on protein binding interactions that such compounds undergo in the µM range (Anand et al., 2008; Bisht et al., 2009). At the macroscopic level, polyphenols (i.e. tannins) have been used to tan leather by denaturing of proteins, and at the microscopic level µM concentrations of polyphenols interact with multiple protein binding sites (via their hydroxyl groups) non-specifically and therefore such compounds score as frequent hitters in vitro. The great majority of established cannabinoid receptor ligands are highly lipophilic, which reflects the nature of the active site within cannabinoid receptors. Thus, hydrophilic polyphenols like catechins and anthocyanidins would clearly be atypical cannabinoid receptor ligands.
More interesting are findings that certain flavonoids inhibit fatty acid amide hydrolase (FAAH), which is the enzyme responsible for the breakdown of the endogenous CB receptor ligand anandamide (Thors et al., 2007; 2008;). Both the isoflavonoid genistein and the flavonoids kaempferol (Table 1), 7-hydroxyflavone and 3,7-dihydroxyflavone have been shown to concentration-dependently inhibit anandamide hydrolysis in rat brain homogenates, albeit at relatively high concentrations (IC50 values between 2 and 10 µM). Nevertheless, the authors of these studies showed a preliminary structure-activity relationship with 7-hydroxyflavone being the most potent inhibitor (IC50 value <1 µM).
An abundant literature is devoted to mechanisms underlying the biological activity of plant polyphenols (Landis-Piwowar and Dou, 2008; Bisht et al., 2009). However, although most beneficial and potentially therapeutic effects of trans-resveratrol, curcumin, catechins and kaempferol-type flavonoids are typically detected in the low µM range in vitro, all such compounds show limited bioavailability and poor pharmacokinetics in vivo with reported plasma concentrations in the low nM range (DuPont et al., 2004; Garcea et al., 2004; Boocock et al., 2007).
Other plant natural products with binding affinity to the CB2 receptor
Other plant natural products have been shown to bind weakly to the CB2 receptor. These include the coumarin derivative rutamarin from the medicinal plant Ruta graveolens L. (Rollinger et al., 2009) and 3,3′-diindolylmethane (DIM) (Table 2), which is an anticarcinogenic metabolite generated by ingestion of indole-3-carbinol. Indole-3-carbinol is commonly found in Brassica vegetables. DIM has been shown to be a weak CB2 receptor partial agonist (Yin et al., 2009).
Conclusions
There is no doubt that phytocannabinoids from Cannabis have greatly influenced research on the ECS and without the milestone discovery that Δ9-THC is the main psychoactive principle (reviewed in Mechoulam, 1986) many of the subsequent discoveries in the field of cannabinoid research would probably not have been made. Furthermore, with the development of therapeutic Cannabis extracts, as with Sativex™, this plant is also likely to provide new pharmaceutical applications in the future. The question remains as to why Cannabis sativa L. appears to be the only plant that produces a metabolite (Δ9-THC acid) that readily leads to its decarboxylation product Δ9-THC, which is the most potent phytocannabinoid activator of the CB1 receptor. Interestingly enough, while nature may have been rather parsimonious in its provision of botanical secondary metabolites that activate the CB1 receptor, there is an increasing number of plant-derived natural products reported to target the CB2 receptor to varying degrees. Flavonoids, which belong to natural polyphenols that readily interact with proteins, may target some of the proteins within the ECS, such as FAAH. However, no convincing evidence has been provided that polyphenols modulate cannabinoid receptors with significant potency. The finding that certain triterpenes potently inhibit MAGL further adds to the repertoire of plant-produced ‘indirect’ cannabinoid receptor agonists. Although higher plants do not contain endocannabinoids and lack the classical G-protein-coupled cannabinoid receptors, they do express enzyme isoforms that resemble some of the enzymes known to be important in the processing of endocannabinoids (Shrestha et al., 2006). Plants produce fatty acid amides, some of which are able to inhibit the degradation of anandamide but do not generally bind with significant affinity to CB receptors (Gertsch et al., 2006; Di Marzo et al., 2007). At present, the only phytocannabinoid that has been discovered to also exist in plants other than Cannabis is β-caryophyllene, which is among the most abundant plant essential oil components. Although Δ9-THC is a partial agonist at both CB1 and CB2 receptors, it has significant lower efficacy at the CB2 receptor. Another phytocannabinoid, Δ9-tetrahydrocannabivarin, has also recently been shown to be a CB2 receptor partial agonist, but is also a CB1 receptor antagonist (Bolognini et al., 2010). Therefore, β-caryophyllene, which is also one of the most abundant secondary metabolites in Cannabis essential oil, could be considered as a true CB2 receptor-selective Cannabis constituent. During mammalian evolution contacts with ‘direct’ CB2 receptor active plant metabolites like β-caryophyllene or ‘indirect’ cannabinoid receptor agonists (FAAH and MAGL inhibitors) in diet may have led to hitherto unrecognized physiological effects. Although it is tempting to believe that these compounds exert beneficial effects in humans, clinical evidence is lacking. Future studies will have to determine whether there are additional apparently nontoxic CB2 receptor-selective ligands in plants other than Cannabis and whether they could in fact be exploited therapeutically.
Glossary
Abbreviations:
- CB1
type-1 cannabinoid receptor
- CB2
type-2 cannabinoid receptor
- CP55940
(–)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- Δ9-THC
Δ9-tetrahydrocannabinol
- DIM
3,3′-diindolylmethane
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolase
- FDA
US Food and Drug Administration
- Gi/o
G-protein alpha subunit
- GPR55
orphan receptor G-protein-coupled receptor 55
- MAGL
monoacylglycerol lipase
- NAEs
N-acylethanolamines
- PPAR
peroxisome proliferator-activated protein
- SR144528
(1S-endo)-5-(4-Chloro-3-methylphenyl)-1-((4-methylphenyl)methyl)-N-(1,3,3-trimethylbicyclo(2.2.1)hept-2-yl)-1H-pyrazole-3-carboxamide
- SR141716A
N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride
- Tmax
time to maximal concentration in plasma (pharmacokinetic parameter)
- TRPV1
transient receptor potential vanilloid-1 receptor
- WIN55212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphtaleneylmethanone
Conflict of interest
The authors state no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 2008;76:S1590–S1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Banni S, Di Marzo V. Effect of dietary fat on endocannabinoids and related mediators: Consequences on energy homeostasis, inflammation and mood. Mol Nutr Food Res. 2009;54:S82–S92. doi: 10.1002/mnfr.200900516. [DOI] [PubMed] [Google Scholar]
- Bisht K, Wagner KH, Bulmer AC. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2009 doi: 10.1016/j.tox.2009.11.008. (doi: 10.1016/j.tox.2009.11.008). (ahead of print. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Costa B, Maione S, Comelli F, Marini P, Di Marzo V, et al. The plant cannabinoid Δ9-tetrahydrocannabivarin can decrease signs of inflammation and inflammatory pain in mice. Br J Pharmacol. 2010;160:677–687. doi: 10.1111/j.1476-5381.2010.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boocock DJ, Patel KR, Faust GE, Normolle DP, Marczylo TH, Crowell JA, et al. Quantitation of trans-resveratrol and detection of its metabolites in human plasma and urine by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:S182–S187. doi: 10.1016/j.jchromb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, et al. Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between kappa-opioid and cannabinoid CB(1) receptors. Br J Pharmacol. 2008;155:S681–S689. doi: 10.1038/bjp.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicca A, Raduner S, Pellati F, Strompen T, Altmann KH, Schoop R, et al. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharmacol. 2009;9:S850–S858. doi: 10.1016/j.intimp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Di Marzo V. Non-CB(1), Non-CB(2) receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat Rev Drug Discov. 2008;7:S438–S455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Sepe N, De Petrocellis L, Berger A, Crozier G, Fride E, et al. Trick or treat from food endocannabinoids. Nature. 1998;396:S636–S637. doi: 10.1038/25267. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14:S741–S756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Di Tomaso E, Beltramo M, Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382:S677–S678. doi: 10.1038/382677a0. [DOI] [PubMed] [Google Scholar]
- DuPont MS, Day AJ, Bennett RN, Mellon FA, Kroon PA. Absorption of kaempferol from endive, a source of kaempferol-3-glucuronide, in humans. Eur J Clin Nutr. 2004;58:S947–S954. doi: 10.1038/sj.ejcn.1601916. [DOI] [PubMed] [Google Scholar]
- Fichna J, Schicho R, Andrews CN, Bashashati M, Klompus M, McKay DM, et al. Salvinorin A inhibits colonic transit and neurogenic ion transport in mice by activating kappa-opioid and cannabinoid receptors. Neurogastroenterol Motil. 2009;21:S1326–Se128. doi: 10.1111/j.1365-2982.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:S1011–S1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J. Anti-inflammatory cannabinoids in diet: towards a better understanding of CB(2) receptor action? Commun Integr Biol. 2008;1:S26–S28. doi: 10.4161/cib.1.1.6568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J. How scientific is the science in ethnopharmacology: historical perspectives and epistemiological problems. J Ethnopharmacol. 2009;122:S177–S183. doi: 10.1016/j.jep.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 2008;105:S9099–S9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertsch J, Raduner S, Altmann KH. New natural noncannabinoid ligands for cannabinoid type-2 (CB2) receptors. J Recept Signal Transduct Res. 2006;26:S709–S730. doi: 10.1080/10799890600942674. [DOI] [PubMed] [Google Scholar]
- King AR, Dotsey EY, Lodola A, Jung KM, Ghomian A, Qiu Y, et al. Discovery of potent and reversible monoacylglycerol lipase inhibitors. Chem Biol. 2009;16:1045–1052. doi: 10.1016/j.chembiol.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte G, Dreiseitel A, Schreier P, Oehme A, Locher A, Goeran H, et al. An examination of anthocyanins' and anthocyanidins' affinity for cannabinoid receptors. J Med Food. 2009;12:S1407–S1410. doi: 10.1089/jmf.2008.0243. [DOI] [PubMed] [Google Scholar]
- Korte G, Dreiseitel A, Schreier P, Oehme A, Locher S, Geiger S, et al. Tea catechins' affinity for human cannabinoid receptors. Phytomedicine. 2010;17:S19–S22. doi: 10.1016/j.phymed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Landis-Piwowar KR, Dou QP. Polyphenols: biological activities, molecular targets, and the effect of methylation. Curr Mol Pharmacol. 2008;1:S233–S243. doi: 10.2174/1874467210801030233. [DOI] [PubMed] [Google Scholar]
- Leonti M, Casu L, Raduner S, Cottiglia F, Floris C, Altmann K-H, et al. Falcarinol is a covalent cannabinoid CB1 receptor antagonist and induces pro-allergic effects in skin. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.02.015. doi: 10.1016/j.bcp.2010.02.015 accepted. [DOI] [PubMed] [Google Scholar]
- Maurelli S, Bisogno T, De Petrocellis L, Di Luccia A, Marino G, Di Marzo V. Two novel classes of neuroactive fatty acid amides are substrates for mouse neuroblastoma ‘anandamide amidohydrolase. FEBS Lett. 1995;377:S82–S86. doi: 10.1016/0014-5793(95)01311-3. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Interview with Prof. Raphael Mechoulam, codiscoverer of THC. Interview by Stanley Einstein. Int J Addict. 1986;21:S579–S587. doi: 10.3109/10826088609083542. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch S, Gertsch J. Cannabis receptor ligands as potential anticancer agents – high hopes for new therapies? J Pharm Pharmacol. 2009;61:S839–S853. doi: 10.1211/jpp/61.07.0002. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol. 2006;147:S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br J Pharmacol. 2009;156:S397–S411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Receptors targeted by synthetic cannabinoid receptor agonists and antagonists. Curr Med Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather PL, Seely KA, Levi MS. Notice of retraction. J Pharmacol Exp Ther. 2009;331:1147. [PubMed] [Google Scholar]
- Raduner S, Majewska A, Chen JZ, Xie XQ, Hamon J, Faller B, et al. Alkylamides from Echinacea are a new class of cannabinomimetics. Cannabinoid type-2 receptor-dependent and –independent immunomodulatory effects. J Biol Chem. 2006;281:S14192–S1S206. doi: 10.1074/jbc.M601074200. [DOI] [PubMed] [Google Scholar]
- Rios JL. Effects of triterpenes on the immune system. J Ethnopharmacol. 2010;128:1–14. doi: 10.1016/j.jep.2009.12.045. [DOI] [PubMed] [Google Scholar]
- Rollinger JM, Schuster D, Danzl B, Schwaiger S, Markt P, Schmidtke M, et al. In silico fishing for rationalized ligand discovery exemplified on constituents of Ruta graveolens. Planta Med. 2009;75:S195–S204. doi: 10.1055/s-0028-1088397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci. 2009;30:S156–S163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Seely KA, Levi MS, Prather PL. The dietary polyphenols trans-resveratrol and curcumin selectively bind human CB1 cannabinoid receptors with nanomolar affinities and function as antagonists/inverse agonists. J Pharmacol Exp Ther. 2009;330:S31–S39. doi: 10.1124/jpet.109.151654. [DOI] [PubMed] [Google Scholar]
- Shrestha R, Kim SC, Dyer JM, Dixon RA, Chapman KD. Plant fatty acid (ethanol) amide hydrolases. Biochim Biophys Acta. 2006;1761:S324–S334. doi: 10.1016/j.bbalip.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Spelman K, Iiams-Hauser K, Cech NB, Taylor EW, Smirnoff N, Wenner CA. Role for PPARgamma in IL-2 inhibition in T cells by Echinacea-derived undeca-2E-ene-8,10-diynoic acid isobutylamide. Int Immunopharmacol. 2009;9:S1260–S1264. doi: 10.1016/j.intimp.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Thors L, Alajakku K, Fowler CJ. The ‘specific’ tyrosine kinase inhibitor genistein inhibits the enzymic hydrolysis of anandamide: implications for anandamide uptake. Br J Pharmacol. 2007;150:S951–S960. doi: 10.1038/sj.bjp.0707172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thors L, Belghiti M, Fowler CJ. Inhibition of fatty acid amide hydrolase by kaempferol and related naturally occurring flavonoids. Br J Pharmacol. 2008;155:S244–S252. doi: 10.1038/bjp.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelkart K, Dittrich P, Beubler E, Pinl F, Schoop R, Suter A, et al. Pharmacokinetics of the main alkamides after administration of three different Echinacea purpurea preparations in humans. Planta Med. 2008;74:S651–S656. doi: 10.1055/s-2008-1034284. [DOI] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284:S12328–S12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Racz I, Klauke AL, Markert A, Gertsch J. Beta-caryophyllene, a phytocannabinoid acting on CB2 receptors. 2009. IACM 5th Conference on cannabinoids in medicine, 2-3. October, Cologne, Germany. [DOI] [PubMed]


