Abstract
Background and purpose:
Intravenous injection of the endocannabinoid anandamide induces complex cardiovascular changes via cannabinoid CB1, CB2 and vanilloid TRPV1 receptors. Recently, evidence has been accumulating that in vitro, but not in vivo, anandamide relaxes blood vessels, via an as yet unidentified, non-CB1 vascular cannabinoid receptor, sensitive to O-1918 (1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene). We here examined whether the anandamide-induced hypotension in urethane-anaesthetized rats was also mediated via a non-CB1 vascular cannabinoid receptor.
Experimental approach:
Effects of two antagonists (O-1918 and cannabidiol) of the non-CB1 vascular cannabinoid receptor on anandamide-induced changes in mean, systolic and diastolic blood pressure (MBP, SBP, DBP), mesenteric (MBF) and renal (RBF) blood flow and heart rate (HR) in urethane-anaesthetized rats was examined.
Key results:
In anaesthetized rats, anandamide (1.5–3 µmol·kg−1) and its stable analogue methanandamide (0.5 µmol·kg−1) caused a delayed and prolonged decrease in MBP, SBP, DBP, MBF and RBF by about 10–30% of the respective basal values without changing HR. In pithed rats, anandamide (3 µmol·kg−1) decreased blood pressure by about 15–20% of the basal value without affecting HR, MBF and RBF. All vascular changes were reduced by about 30–70% by cannabidiol and O-1918 (3 µmol·kg−1, each).
Conclusions and implications:
Non-CB1 cannabinoid vascular receptors, sensitive to O-1918, contribute to the hypotensive effect of anandamide in anaesthetized rats. Activation of these receptors may be therapeutically important as the endocannabinoid system could be activated as a compensatory mechanism in various forms of hypertension.
Keywords: anandamide, methanandamide, cannabinoid receptors, endothelial cannabinoid receptor, cannabidiol, O-1918, haemodynamic responses, anaesthetized rat, pithed rat
Introduction
Anandamide, originally identified as the first endogenous cannabinoid receptor ligand, is involved in the pathogenesis of hypertension and of hypotension associated with haemorrhagic, endotoxic, and cardiogenic shock and also with advanced liver cirrhosis. The compound induces complex cardiovascular changes via cannabinoid CB1, CB2 and vanilloid TRPV1 receptors (see Malinowska et al., 2008; Pacher et al., 2008: nomenclature follows Alexander et al., 2008). In anaesthetized rats (Varga et al., 1995; Lake et al., 1997; Malinowska et al., 2001a; Kwolek et al., 2005) or mice (Pacher et al., 2004) i.v. injection of anandamine elicits a triphasic response. In the first phase, there is an immediate transient decrease in heart rate (HR) and cardiac contractility that is accompanied by a fall in blood pressure known as the Bezold-Jarisch reflex (phase I), which is mediated via vanilloid TRPV1 receptors located on sensory vagal nerves in the heart (Malinowska et al., 2001a; Pacher et al., 2004). This phase is followed by a brief pressor response and an increase in cardiac contractility (phase II), which involves central and peripheral components, located probably in the medulla oblongata and in blood vessels, respectively (Pacher et al., 2004; Kwolek et al., 2005). Finally, there is a more prolonged decrease in blood pressure accompanied by a marked decrease in cardiac contractility and a slight decrease in total peripheral resistance (phase III) that is mediated via presynaptic cannabinoid CB1 receptors innervating blood vessels and heart (Malinowska et al., 1997; 2001a; Niederhoffer et al., 2003). However, the involvement of TRPV1 receptors in the spinal cord (del Carmen Garcia et al., 2003) and a decrease in cardiac contractility (Bátkai et al., 2004) have also been suggested as additional mechanisms.
On the other hand, in conscious normotensive rats, low doses of anandamide induce a brief pressor response associated with vasoconstriction in renal, mesenteric and hindquarters vascular beds. At higher doses the vasoconstriction in the hindquarters is followed by a β2-adrenoceptor-mediated vasodilatation and an initial bradycardia. A prolonged fall in blood pressure is observed only in hypertensive but not normotensive conscious rats (Lake et al., 1997; Gardiner et al., 2002; 2009; Ho and Gardiner, 2009).
Recently it has been demonstrated that the endocannabinoids anandamide and virodhamine and the synthetic cannabinoid ‘abnormal cannabidiol’ (a neuro-behaviourally inactive cannabinoid that does not bind to CB1 or CB2 receptors) caused a marked relaxation by an, as yet, unidentified non-CB1 vascular cannabinoid receptor sensitive to O-1918 (1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene). This receptor was identified in rat and mouse isolated mesenteric arteries (Járai et al., 1999; Wagner et al., 1999; Ho and Hiley, 2003; 2004; Offertáler et al., 2003), including the mesenteric artery isolated from CB1 receptor−/− knockout mice (Járai et al., 1999). This novel receptor is also involved in the relaxation of isolated human pulmonary arteries elicited by abnormal cannabidiol (Kozłowska et al., 2007) and virodhamine (Kozłowska et al., 2008). There is some speculation as to whether this receptor also participates in the fall in blood pressure elicited by anandamide. This is possible as anandamide decreased blood pressure (DBP) in conscious hypertensive rats, an effect resistant to the CB1 receptor antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM 251) (Ho and Gardiner, 2009). In a study using anaesthetized rats, the hypotensive effect of a high (10 mg·kg−1) but not of lower doses of anandamide was resistant to rimonabant, another CB1 receptor antagonist (Garcia et al., 2001). Furthermore, abnormal cannabidiol has a hypotensive effect in anaesthetized wild-type (Offertáler et al., 2003) or CB1−/− knockout mice (Járai et al., 1999). However, anandamide failed to produce a prolonged decrease in blood pressure in conscious normotensive rats (Gardiner et al., 2002; 2009; Ho and Gardiner, 2009) and in CB1−/− receptor knockout mice (Járai et al., 1999; Ledent et al., 1999).
Therefore, the present study was undertaken in order to examine whether anandamide-induced hypotension in urethane-anaesthetized rats was also mediated via an O-1918-sensitive non-CB1 vascular cannabinoid receptor. We used two antagonists of this receptor, namely O-1918 and cannabidiol. In our study, not only blood pressure but also mesenteric and renal blood flows (MBF and RBF) were examined as anandamide-related and CB1 and CB2 receptor-independent effects were found in the corresponding vascular beds (Járai et al., 1999; Wagner et al., 1999; Ho and Hiley, 2003; 2004; Offertáler et al., 2003; Koura et al., 2004; Ho and Gardiner, 2009). Our results suggest that an O-1918-sensitive non-CB1 vascular cannabinoid receptor did indeed contribute to the delayed hypotension (phase III) induced by anandamide.
Methods
All animal care, surgical procedures and experimental protocols were approved by the local Animal Ethics Committee in Białystok (Poland). Male Wistar normotensive rats (weighing 200–300 g) with free access to food pellets and water were used.
Anaesthetized rats
Animals were anaesthetized i.p. with urethane (14 mmol·kg−1). The depth of anaesthesia was assessed by monitoring the reflex responses to nociceptive stimuli. The trachea was cannulated. Mean, systolic and diastolic blood pressure (MBP, SBP and DBP, respectively) were measured from the right carotid artery via a transducer (ISOTEC; Hugo Sachs Elektronik, March–Hugstetten, Germany). As DBP reflects changes in vascular resistance, we have mainly concentrated on this cardiovascular parameter (e.g. when selecting the dose of vasopressin and of anandamide). Moreover, for the experiments, in which the influence of cannabinoid receptor antagonists on (-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP 55,940)-induced inhibition of neurogenic tachycardia was examined in pithed rats, only DBP is given (see below). HR was measured by a rate–meter triggered from the pressure record. The left femoral vein was cannulated for i.v. injection of drugs administered in a volume of 0.5 mL·kg−1. Body temperature was kept constant at about 37–38°C using a heating pad (Bio-Sys-Tech, Białystok, Poland) and monitored by a rectal probe (Physitemp BAT10, Clifton, NJ, USA). Middle laparotomy was performed using a longitudinal abdominal incision, and the mesenteric and renal arteries were gently isolated from the surrounding tissues and from the underlying veins for about 0.5 cm. At the end of surgical preparation, ultrasound transit time flow probes (0.5 mm, V-series and 0.7 mm, V-series for mesenteric and renal artery, respectively) were gently placed on the arteries and MBF and RBF continuously measured using directional Ultrasonic Doppler Flowmeters (Transonic Systems Inc., Ithaca, NY, USA). The zero reference level was set at saline solution at the level of heart. Zero flow was determined when the mesenteric and renal artery were completely occluded at the end of the experiment.
Because vasopressor/vasodepressor effects are more marked at a higher level of blood pressure (Malinowska and Schlicker, 1993), in experiments in which changes in blood pressure were studied, vasopressin (0.04–0.4 IU·kg−1·min−1) was infused into the right femoral vein of pithed and some non-pithed animals with a lower basal DBP to raise DBP to a level of about 60–70 mmHg. As in an earlier study (Malinowska et al., 2001b), vasopressin was not used in pithed animals in which electrically induced tachycardia was examined.
Pithed rats
Two types of experiments were performed in pithed rats. (i) The influence of anandamide on basal cardiovascular parameters was examined in rats anaesthetized i.p. with urethane (14 mmol·kg−1); and (ii) the effect of CP 55,940 on the electrically stimulated increase in HR was studied in animals anaesthetized i.p. with pentobarbitone sodium (300 µmol·kg−1). In both cases, atropine (2 µmol·kg−1) was injected i.p. simultaneously with the anaesthetic. After cannulation of the trachea, the animals were pithed by inserting a stainless-steel rod (1.5 mm diameter and 190 mm length) through the right orbit and the foramen magnum and down to the vertebral canal. Artificial respiration (1 mL per 100 g, 60 strokes·min−1) with room air was immediately started using a respirator (7025 Rodent respirator, Hugo Sachs Elektronik, March–Hugstetten, Germany). Both vagal nerves were cut. MBP, SBP, DBP, MBF, RBF, HR and body temperature were measured as described above. In the second type of experiments the pithing rod (enamelled except for a 1 cm section 7 cm from the tip with the uncovered segment situated at vertebra C7-T1) was used as an electrode for electrical stimulation of the preganglionic sympathetic nerve axons leaving the spinal canal; for stimulation an electrical field was generated between the rod and an indifferent electrode placed ventrally by means of a Stimulator T (Hugo Sachs, March–Hugstetten, Germany). After 30 min of equilibration, during which the cardiovascular parameters were allowed to stabilize, experiments were performed.
Experimental protocols
In order to examine the influence of different receptor antagonists on the changes in cardiovascular parameters induced by anandamide or methanandamide (both in anaesthetized and pithed rats) the particular agonist was injected twice (S1 and S2, 20 min apart). Maximal changes in particular cardiovascular parameters (S1 and S2) are given. As individual differences in responses to anandamide were observed, we applied anandamide at doses of 1.5–3 µmol·kg−1. We have chosen a dose of anandamide that decreased DBP during phase III by about 20–30% of the basal value. Methanandamide was administered at 0.5 µmol·kg−1. S1 was applied 5 min after injection of saline solution (anaesthetized rats) or ruthenium red 3 (µmol·kg−1 in pithed rats anaesthetized with urethane). Cannabidiol or O-1918 (3 µmol·kg−1, each) or their solvents were administered 10 min before S2.
Experiments in which the effect of CP 55,940 on the electrically stimulated increase in HR was studied were initiated by the injection of pancuronium (0.8 µmol·kg−1, i.v.) in order to avoid muscle twitches associated with the electrical stimulation. An increase in HR was induced twice (S1 and S2, 10 min apart) by electrical stimulation (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerves. AM 251, cannabidiol or O-1918 (3 µmol·kg−1, each) were administered simultaneously with pancuronium. S1 was applied 5 min later. CP 55,940 (1 µmol·kg−1) was injected 5 min before S2.
Data analysis
Results are given as mean ± SEM (n= number of rats). In order to quantify the effects of antagonists on the anandamide- or methanandamide-induced changes in blood pressure, blood flow and HR, values during S1 and S2 were expressed as percentages of the basal MBP, SBP, DBP, MBF, RBF (Figures 2–4) and HR (data not shown) immediately before injection of that particular agonist. S2 values are expressed as percentages of S1. In order to quantify the effects of drugs on the electrically induced rise in HR, the ratio of the increase in HR induced by S2 over that induced by S1 (S2/S1) was determined. This ratio was expressed as percentage of the corresponding ratio obtained from vehicle-treated animals. For comparison of the mean values, Student's t-test for paired and unpaired data was used. When two or more groups were compared with the same control, one-way analysis of variance (anova) followed by Dunnett's test was used. Differences were considered as significant when P < 0.05.
Figure 2.
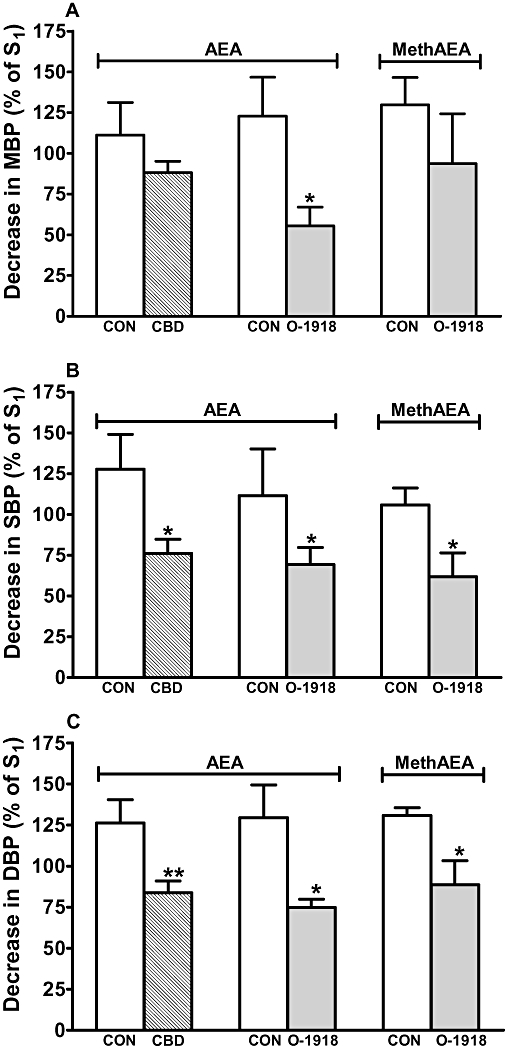
Influence of cannabidiol (CBD, 3 µmol·kg−1) and 1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene (O-1918) (3 µmol·kg−1) on the decreases in mean (A; MBP), systolic (B; SBP) and diastolic (C; DBP) blood pressure (phase III) induced by anandamide (AEA; 1.5–3 µmol·kg−1) and methanandamide (MethAEA; 0.5 µmol·kg−1) in urethane-anaesthetized rats. AEA and MethAEA were given twice (S1 and S2, 20 min apart). S1 and S2 were calculated as percentages of the respective basal values. CBD or O-1918 or their solvents were administered 10 min before S2. All drugs were administered i.v. Means ± SEM of 5–10 rats. *P < 0.05, **P < 0.01 compared with the respective control (CON).
Figure 4.
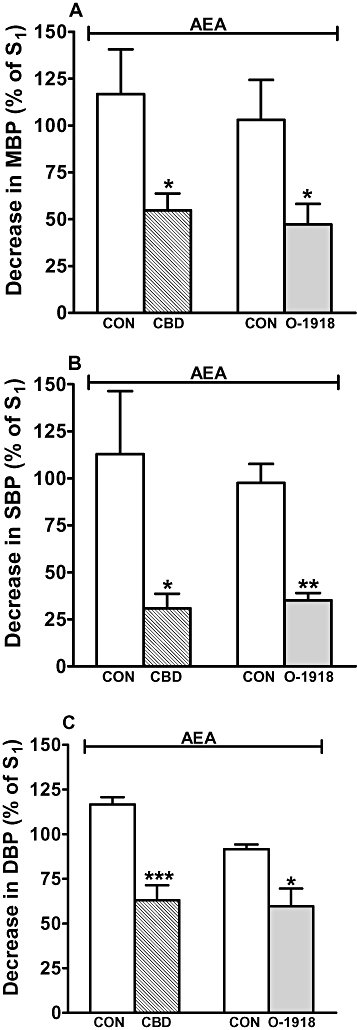
Influence of cannabidiol (CBD, 3 µmol·kg−1) and 1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene (O-1918) (3 µmol·kg−1) on the decreases in mean (A; MBP), systolic (B; SBP) and diastolic (C; DBP) blood pressure induced by anandamide (AEA; 3 µmol·kg−1) in pithed and vagotomized rats anaesthetized with urethane. AEA was given twice (S1 and S2, 20 min apart); ruthenium red (3 µmol·kg−1) was given 5 min before S1. S1 and S2 were calculated as percentages of the respective basal values. CBD or O-1918 or their solvents were administered 10 min before S2. All drugs were administered i.v. Mean ± SEM of 4–6 rats. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the respective control (CON).
Materials
AM 251, anandamide, CP 55,940, (-) cannabidiol, R-(-)-methanandamide (referred to as ‘cannabidiol’ and ‘methanandamide’ in the text; Tocris Cookson, Bristol, UK); atropine sulphate, hydroxypropyl-β-cyclodextrin, pancuronium bromide, ruthenium red, urethane, [Arg8]-vasopressin (Sigma, München, Germany); O-1918 (Cayman Chemicals, Ann Arbor, MI, USA); pentobarbitone sodium (Biowet, Puławy, Poland). Drugs were dissolved in saline with the following exceptions: cannabidiol was dissolved in a mixture of ethanol and saline (1:9), AM 251 in a mixture of ethanol, Cremophor El, dimethyl sulphoxide (DMSO) and saline (1:1:1:9.5) and CP 55,940 in a 19% w·v−1 solution of cyclodextrin. Anandamide was purchased from Tocris Cookson as a 10 mg·mL−1 emulsion in soya water (1:4). Vasopressin was provided by the manufacturer as an aqueous stock solution (100 IU·mL−1), which was diluted (1:74) in isotonic saline before the experiment. The solvents for O-1918 and cannabidiol produced biphasic changes in cardiovascular parameters in anaesthetized rats, lasting for less than 1 min. Thus, saline first decreased and then increased MBP, SBP and DBP by about 3–5 and 12–15 mmHg respectively. Simultaneously, increases in MBF and RBF (by about 1.4 and 0.8 mL·min−1, respectively) were noticed. In anaesthetized rats the solvents for cannabidiol and O-1918 decreased MBP, SBP and DBP by about 3–5 mmHg, which was followed by increases in blood pressure by about 9 mmHg (solvent for CBD) or 13–17 mmHg (solvent for O-1918). Similarly, the solvent for CBD first decreased MBF and RBF by about 0.4 and 0.1 mL·min−1 respectively. Then, an increase in MBF and RBF by about 1.0 and 0.4 mL·min−1 was noticed. Biphasic changes in MBF and RBF were also observed after injection of the solvent for O-1918. Thus, a decrease by about 0.1 was followed by an increase by about 2 mL·min−1 (MBF) and a fall by about 0.05 was followed by an increase by about 0.7 mL·min−1 (RBF). In pithed rats, the solvent for CBD first decreased SBP, DBP and MBP by about 7–10 mmHg and then increased these cardiovascular parameters by about 6–10 mmHg. All biphasic changes lasted for less than 1 min. Cyclodextrin solution caused small and short-lasting decreases in HR and DBP followed by more pronounced increases in both parameters (by about 65 beats·min−1 and 10 mmHg, respectively), which returned to basal values within 1–2 min.
Results
Anaesthetized rats
In urethane-anaesthetized rats, the basal cardiovascular parameters MBP, SBP and DBP, MBF, RBF and HR, measured immediately before the administration of the first dose of agonist, are given in Table 1. If DBP was lower than 60 mmHg, vasopressin (0.04–0.4 IU·kg−1·min−1) was used to increase DBP to a level of 60–70 mmHg. Cannabidiol (3 µmol·kg−1) decreased HR, blood pressure (MBP, SBP and DBP) and MBF by about 5–9% of the respective basal values, that is, by about 15 beats·min−1, 5–7 mmHg and 0.4 mL·min−1 respectively. It failed to affect RBF. Decreases in HR, blood pressure, MBF and RBF by about 20 beats·min−1, 5–10 mmHg and 0.7 and 0.2 mL·min−1, respectively, were observed after injection of O-1918 (3 µmol·kg−1). The effects of cardiovascular parameters induced by both antagonists were rapid and short-lasting (about 30 s).
Table 1.
Basal cardiovascular parameters and effects of the first injection (S1) of anandamide and methanandamide on basal cardiovascular parameters in anaesthetized and pithed rats
| Group | Agonist | Parameter | Units | Basal |
Changes in cardiovascular parameters noticed in |
||
|---|---|---|---|---|---|---|---|
| Phase I | Phase II | Phase III | |||||
| Anaesthetized rats (anaesthesia with urethane, basal DBP brought to 60–70 mmHg when necessary) | Anandamide (n= 32) | MBP | mmHg | 79.8 ± 1.7 | −4.2 ± 1.9 | 21.5 ± 1.4 | −14.1 ± 1.1 |
| SBP | mmHg | 112.0 ± 2.5 | −2.7 ± 0.9 | 17.3 ± 1.3 | −16.6 ± 1.7 | ||
| DBP | mmHg | 62.8 ± 1.5 | −10.5 ± 2.3 | 24.3 ± 1.0 | −15.8 ± 1.0 | ||
| MBF | mL·min−1 | 4.84 ± 0.23 | −0.11 ± 0.04 | 2.82 ± 0.23 | −0.80 ± 0.14 | ||
| RBF | mL·min−1 | 2.86 ± 0.18 | −0.09 ± 0.06 | 1.00 ± 0.09 | −0.50 ± 0.06 | ||
| HR | beats·min−1 | 367.8 ± 9.2 | −56.8 ± 13.1 | NA | NA | ||
| Methanandamide (n= 13) | MBP | mmHg | 78.6 ± 2.8 | −1.2 ± 0.9 | 14.8 ± 1.0 | −13.5 ± 1.7 | |
| SBP | mmHg | 117.3 ± 5.3 | −1.0 ± 0.7 | 11.8 ± 1.5 | −17.3 ± 2.3 | ||
| DBP | mmHg | 61.7 ± 2.4 | −8.0 ± 1.2 | 17.1 ± 1.6 | −13.0 ± 1.4 | ||
| MBF | mL·min−1 | 4.51 ± 0.36 | −0.06 ± 0.04 | 2.21 ± 0.15 | −0.51 ± 0.06 | ||
| RBF | mL·min−1 | 2.74 ± 0.32 | −0.05 ± 0.05 | 0.88 ± 0.10 | −0.43 ± 0.10 | ||
| HR | beats·min−1 | 354.6 ± 20.6 | −29.2 ± 5.5 | NA | NA | ||
| Pithed rats (anaesthesia with urethane, basal DBP brought to 60–70 mmHg, treatment with ruthenium red 3 µmol·kg−1) | Anandamide (n= 21) | MBP | mmHg | 81.1 ± 1.3 | NA | 15.2 ± 1.0 | −12.5 ± 1.1 |
| SBP | mmHg | 95.6 ± 1.7 | NA | 16.1 ± 5.6 | −13.1 ± 1.0 | ||
| DBP | mmHg | 69.2 ± 1.0 | NA | 15.5 ± 1.0 | −12.1 ± 1.4 | ||
| HR | beats·min−1 | 372.3 ± 5.0 | NA | NA | NA | ||
| Pithed rats (anaesthesia with pentobarbitone; treatment with pancuronium 0.8 µmol·kg−1) | Methanandamide (n= 20) | DBP | mmHg | 51.6 ± 1.4 | ND | ND | ND |
| HR | beats·min−1 | 327.6 ± 3.9 | ND | ND | ND | ||
Basal parameters were determined immediately prior to the first i.v. administration (S1) of anandamide or methanandamide. In pentobarbitone-anaesthetized rats basal cardiovascular parameters immediately before the first electrical stimulation are given. Mean ± SEM of the number of rats given in the second column.
DBP, diastolic blood pressure; HR, heart rate; MBF, mesenteric blood flow; MBP, mean blood pressure; NA, not applicable; ND, not determined; RBF, renal blood flow; SBP, systolic blood pressure.
The first (S1) i.v. administration of anandamide (1.5–3 µmol·kg−1) induced triphasic changes in cardiovascular parameters in urethane-anaesthetized rats (Figure 1; Table 1). The initial phase I consisted of a rapid, short-lasting fall in MBP, SBP, DBP and a bradycardia, accompanied by very low and, occasionally occurring, decreases in MBF and RBF. The subsequent phase II showed increases in MBP, SBP, DBP, MBF and RBF; the latter alterations lasted for about 30–60 s. Phase III lasted for up to 10 min and encompassed decreases in MBP, SBP, DBP, MBF and RBF and only sporadically a very small fall in HR.
Figure 1.

Typical traces showing the changes in diastolic blood pressure, mesenteric and renal blood flow and heart rate induced by i.v. injection of anandamide (3 µmol·kg−1) in a rat anaesthetized with urethane (A) and in a rat anaesthetized with urethane and pithed (B). Arrows indicate drug application. Pithed rats received an i.v. injection of ruthenium red (3 µmol·kg−1) 5 min before anandamide. Note that the small decrease in heart rate in (B) is related to the injection per se, as it occurred identically when the drug vehicle was administered.
Similar changes were elicited by methanandamide (0.5 µmol·kg−1) (Malinowska et al., 2001a; Table 1). Thus, phase I included rapid decreases in MBP, SBP, DBP and HR; simultaneous decreases in MBF and RBF were very small and occurred only sporadically. In phase II, methanandamide increased MBP, SBP, DBP, MBF and RBF. The long-lasting hypotension (phase III) was accompanied by falls in MBF and RBF but not in HR.
The second administration of anandamide (S2), 20 min after S1 and 10 min after injection of the respective solvents for the antagonists under study, produced comparable triphasic changes in cardiovascular parameters (not shown). During phase I this could be studied best for the decrease in HR, which proved to be most robust among the four cardiovascular components of this phase (Malinowska et al., 2001a). The ratios of the decrease in HR evoked by S2 over that evoked by S1 (S2/S1× 100) were close to 100% when the solvents of the two antagonists were given; these ratios were not affected by cannabidiol or O-1918 (3 µmol·kg−1 each) (n= 5–10, results not shown).
With respect to the increases in MBP, SBP, DBP, MBF and RBF during phase II, virtually identical values occurred after S1 and, following administration of the solvents for the two antagonists, after S2; in other words, the ratios S2/S1× 100 were close to 100%. The ratios were not modified by cannabidiol and O-1918 (n= 8–10, results not shown). O-1918 and its solvent did also not influence the methanandamide-stimulated bradycardia (phase I) and the subsequent increases in MBP, SBP, DBP, MBF and RBF (phase II) (n= 6, data not shown).
The solvents for cannabidiol and O-1918 did not affect or slightly increased the phase III stimulated by anandamide or methanandamide. Thus, the decreases in MBP, SBP, DBP, MBF and RBF obtained by the second administration (S2) were about 90–130% of the respective changes observed during S1 (see respective controls in Figures 2 and 3). By contrast, cannabidiol markedly reduced the anandamide-elicited falls in SBP and DBP by 40 and 34%, respectively, and tended to diminish the decrease in MBP by 21% (Figure 2). It also reduced the fall in MBF and RBF elicited by anandamide by 54 and 50% respectively (Figure 3). O-1918 caused an inhibition of the anandamide-stimulated decreases in MBP, SBP and DBP by 55, 38 and 42% respectively (Figure 2). Similarly, this antagonist reduced the methanandamide-stimulated fall in SBP and DBP by 44 and 32%, respectively, and tended to diminish the decrease in MBP by 28% (Figure 2). It also diminished the fall in MBF and RBF induced by anandamide by 61 and 67% and that induced by methanandamide by 74 and 49% respectively (Figure 3).
Figure 3.

Influence of cannabidiol (CBD, 3 µmol·kg−1) and 1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene (O-1918) (3 µmol·kg−1) on the decrease in (A) mesenteric blood flow (MBF) and (B) renal blood flow (RBF) (phase III) induced by anandamide (AEA; 1.5–3 µmol·kg−1) and methanandamide (MethAEA; 0.5 µmol·kg−1) in urethane-anaesthetized rats. AEA and MethAEA were given twice (S1 and S2, 20 min apart). S1 and S2 were calculated as percentages of the respective basal values. CBD or O-1918 or their solvents were administered 10 min before S2. All drugs were administered i.v. Means ± SEM of 5–10 rats. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the respective control (CON).
Pithed rats
Two experimental series were carried out on pithed rats. In the first series, rats were anaesthetized with urethane, bilaterally vagotomized and treated with ruthenium red (3 µmol·kg−1); the basal cardiovascular parameters are given in Table 1. As DBP was lower than 60 mmHg, infusion of vasopressin (0.04–0.4 IU·kg−1·min−1) was used to increase DBP to a level of 60–70 mmHg. Thus, basal cardiovascular values were similar in anaesthetized and pithed rats. Cannabidiol did not affect basal cardiovascular parameters whereas O-1918 decreased HR by about 15 beats·min−1 and SBP, DBP and MBP by about 15 mmHg. The modifications of cardiovascular parameters were rapid and short-lasting (about 30 s).
As shown in Figure 1B, i.v. injection of anandamide did not produce phase I in pithed rats. As we have shown previously, phase II in these animals is higher and longer than in non-pithed urethane-anaesthetized rats (Kwolek et al., 2005). The pronounced phase II automatically reduces the magnitude of phase III (additionally diminished by the abolition of an influence of presynaptic CB1 receptors on sympathetic nerve endings in pithed rats; e.g. Malinowska et al., 1997). Thus, all experiments in this experimental model were performed in the presence of a blocker of phases I and II, ruthenium red, which was administered 5 min before the first administration of anandamide. Under these conditions anandamide (3 µmol·kg−1) caused a biphasic change in MBP, SBP and DBP (increase followed by decrease; Figure 1B; Table 1). The anandamide-stimulated decrease in DBP in pithed rats was lower (by 27%) than the corresponding change observed in non-pithed animals (Table 1, P < 0.001). The fall in MBP and SBP tended to be lower (by about 11 and 21%, respectively; see Table 1) in pithed than in non-pithed animals. In pithed rats anandamide (3 µmol·kg−1) caused only an increase (without subsequent decrease) in MBF and RBF (Figure 1B). Thus, we did not measure MBF and RBF in further interaction experiments.
Cannabidiol, O-1918 and their solvents did not affect the anandamide-stimulated increases in MBP, SBP and DBP (n= 4–6, results not shown). The anandamide-induced long-lasting fall in MBP, SBP and DBP was reduced by cannabidiol by 40–46% and by O-1918 by 55, 64 and 35, respectively, but not affected by the respective solvents (Figure 4).
In the second experimental series on pithed rats, the animals were anaesthetized with pentobarbitone, bilaterally vagotomized and treated with pancuronium (0.8 µmol·kg−1); basal DBP and HR are given in Table 1. Electrical stimulation (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerve fibres innervating the heart (S1 and S2, 10 min apart) increased HR (increase during S1 by 69.9 ± 3.9 beats·min−1; n= 20). CP 55,940, 1 µmol·kg−1, administered 5 min prior to S2, reduced the neurogenic tachycardia (S2/S1) by about 25%. As shown in Figure 5, the inhibitory effect of CP 55,940 was not modified by cannabidiol and O-1918 but it was strongly attenuated by the cannabinoid CB1 receptor antagonist AM 251 (3 µmol·kg−1 each) (administered 5 min prior to S1). The cannabinoid receptor antagonists did not affect basal HR or the electrically stimulated increase in HR (S1) by themselves.
Figure 5.

Influence of (-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol (CP 55,940) (1 µmol·kg−1) on the electrically stimulated increase in heart rate (HR) in pithed rats anaesthetized with pentobarbitone and its interaction with N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (AM 251) (3 µmol·kg−1), cannabidiol (CBD, 3 µmol·kg−1) and 1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene (O-1918) (3 µmol·kg−1). An increase in HR was induced twice (S1 and S2, 10 min apart) by electrical stimulation (1 Hz, 1 ms, 50 V for 10 s) of the preganglionic sympathetic nerves. AM 251, CBD or O-1918 were administered 5 min before S1. CP 55,940 was injected 5 min before S2. To quantify the effects of CP 55,940, the ratio of S2/S1 was determined. S2/S1 values are expressed as percentages of the corresponding ratios in controls. All drugs were administered i.v. Mean ± SEM of 4–10 rats. **P < 0.01, ***P < 0.001 compared with the respective control (not shown). ΔΔP < 0.001 compared with the group without antagonist.
Discussion
The endocannabinoid anandamide induces triphasic changes in cardiovascular parameters in anaesthetized rats, and the aim of the present study was to investigate whether the putative non-CB1 vascular cannabinoid receptor contributes to the third phase, that is, a delayed and prolonged decrease in blood pressure. Rats were anaesthetized with urethane as, in our previous study (Kwolek et al., 2005), pentobarbitone reduced the cardiovascular effects of anandamide. Also as the vasopressor/vasodepressor effects were more marked at higher levels of blood pressure (Malinowska and Schlicker, 1993), we increased DBP to about 60 mmHg by infusion of vasopressin in anaesthetized animals with low DBP and in pithed rats (series shown in Figure 4). An influence of vasopressin on our results is unlikely as the vasopressin V1a receptor antagonist d(CH2)5[Tyr(Me)2Arg8]-vasopressin failed to affect the cardiovascular responses to anandamide (Kwolek et al., 2005).
Our experiments on anaesthetized rats show that the anandamide-induced prolonged decrease in SBP, DBP and MBP (phase III) was strongly counteracted by two antagonists at the non-CB1 vascular cannabinoid receptor, that is, cannabidiol and O-1918, whereas phases I and II were not affected. These results suggest that the latter receptor did indeed contribute to phase III but not to phases I and II. Admittedly, the two antagonists decreased the cardiovascular parameters under study by themselves. These effects, which were very short-lived and had recovered 9 min prior to the administration of anandamide, may be non-specific in nature and do not suggest a tonic activation of the novel receptors. In this case, one would expect an increase rather than decrease of cardiovascular parameters.
Four series of experiments were carried out in order to support the view that a non-CB1 vascular cannabinoid receptor was involved in the hypotensive effect of anandamide in phase III. First, the possibility had to be considered that anandamide, which is rapidly degraded for example by the fatty acid amide hydrolase (Giuffrida et al., 2001), partially or solely acted via metabolites. In order to exclude this possibility we used methanandamide, a metabolically stable analogue of anandamide. The effect of methanandamide on DBP in phase III was affected by O-1918 in a manner similar to that shown for anandamide. As expected, the hypotensive effect of methanandamide occurred at a somewhat lower dose than that of anandamide (Malinowska et al., 2001a). On the other hand, in conscious rats, the inhibitor of fatty acid hydrolase URB597 augmented the haemodynamic effects of anandamide, but no evidence for an involvement of cyclooxygenase-2 in these actions was found (Gardiner et al., 2009).
Second, cannabidiol and O-1918 have very low affinities for the classical CB1 receptor (Offertáler et al., 2003; Thomas et al., 2007); nonetheless, cannabidiol proved to be a potent non-competitive antagonist against CB1 receptor-mediated effects in vitro (Thomas et al., 2007). The possibility had to be considered that such a mechanism might also occur in vivo (and perhaps also with O-1918); in other words, the effect of both antagonists on the anandamide- and methanandamide-induced phase III might be related to the blockade of CB1 receptors, which play an important role in this phase (Malinowska et al., 1997; 2001a; Niederhoffer et al., 2003). In order to exclude this possibility, experiments were performed on pithed rats in which the inhibition of the neurogenic tachycardia via presynaptic CB1 receptors was considered (Malinowska et al., 2001b). Because urethane strongly attenuates effects mediated via presynaptic CB1 receptors (Kurz et al., 2009), in these particular experiments pentobarbitone was used as anaesthetic; vasopressin was omitted, as in the study by Malinowska et al. (2001b). As expected, the effect of the cannabinoid receptor agonist CP 55,940 was counteracted by the CB1 receptor antagonist AM 251; however, cannabidiol and O-1918 at the doses used in the present study failed to influence the effect of CP 55,940.
Third, the question arises as to whether the hypotensive effect caused by anandamide (phase III) and related to a non-CB1 vascular cannabinoid receptor involves the CNS. For this reason, experiments were performed on pithed rats (anaesthetized with urethane), in which the brain and the spinal cord can no longer influence the responses. As expected, phase I of the cardiovascular responses to anandamide, which is related to the Bezold-Jarisch reflex (Malinowska et al., 2001a), was absent under these experimental conditions, whereas phases II and III persisted. The increase in blood pressure in phase II is, however, particularly marked in pithed rats (Kwolek et al., 2005) and it may partially diminish the magnitude of phase III. Thus, in order to have conditions comparable with those in anaesthetized rats, experiments were carried out in the presence of ruthenium red known to attenuate the increase in blood pressure during phase II without affecting its decrease during phase III (Kwolek et al., 2005). The phase III hypotensive effect of anandamide was somewhat less pronounced in pithed when compared with anaesthetized rats but the extent of the antagonistic effect of cannabidiol and O-1918 against anandamide was comparable. These data suggest that the hypotensive effect of anandamide related to the O-1918-sensitive cannabinoid receptor has, at least for the major part, a peripheral site of action. The data on pithed rats support the role of a non-CB1 vascular cannabinoid receptor also indirectly, as they exclude two alternative explanations involving the CNS (Malinowska et al., 1997; 2001b; del Carmen Garcia et al., 2003; Niederhoffer et al., 2003). Thus, CB1 receptors on sympathetic neurones do not function spontaneously in the pithed rat model (and can be studied only if the sympathetic outflow is stimulated electrically) and TRPV1 receptors on sensory vagal nerves cannot have an influence on the CNS
Fourth, as the non-CB1 vascular cannabinoid receptor has been shown to occur in mesenteric and renal vessels (for references see Introduction), it was plausible to assume that the decrease in DBP is associated with an increase in the MBF and RBF. For this reason, MBF and RBF were measured simultaneously with DBP and HR in our experiments but, surprisingly, both parameters were decreased rather than increased in anaesthetized rats (not affected in pithed rats), suggesting that the hypotensive effect of anandamide related to the O-1918-sensitive non-CB1 cannabinoid receptor involves other vascular beds. One candidate is the vasculature of the hindquarters, the conductance of which is strongly increased in response to the hypotensive effect of anandamide in the conscious hypertensive rat; this hypotensive effect is CB1 receptor-independent and possibly involves a non-CB1 vascular cannabinoid receptor (Ho and Gardiner, 2009). Our results related to the MBF and RBF are in line with data obtained in conscious normotensive (Gardiner et al., 2002; 2009;) and hypertensive rats (Ho and Gardiner, 2009), in which anandamide caused vasoconstriction of the mesenteric and renal vascular bed; nonetheless, additional experiments, in which the resistance in the renal and mesenteric vascular bed is determined directly, will be necessary to finally prove the role of either vascular bed in the effect of anandamide in our experimental model.
Although our data suggest that a vascular non-CB1 receptor related to, or identical with, the endothelial cannabinoid receptor (‘abnormal cannabidiol receptor’) originally described by Járai et al. (1999) is implicated in the effect of anandamide and methanandamide, three alternative possibilities had to be considered as well. First, O-1918 has been recently shown to inhibit large-conductance, Ca2+-activated, potassium (BKCa) channels (Godlewski et al., 2009), and anandamide is known to activate these channels (e.g. Kozłowska et al., 2007; 2008;). We cannot exclude the possibility that this mechanism also participates in the hypotensive influence of the endocannabinoid. However, it should be noted that the vasodepressor influence of anandamide was also counteracted by cannabidiol. Second, a decrease in cardiac contractility (Bátkai et al., 2004) has been suggested as an additional mechanism of the hypotensive effect of anandamide in rats. We cannot exclude the possible participation of this mechanism in our study, in which cardiac contractility was not determined directly. Third, the potential involvement of the recently characterized GPR55 receptor in the effect of anandamide had to be considered, as it is an agonist at this receptor (Pertwee, 2007). However, this possibility is very unlikely as recent experiments with a GPR55 receptor knockout mouse clearly showed that this receptor does not lead to a vasodilator response (Johns et al., 2007).
In conclusion, our data based on two agonists, two antagonists and two experimental models show that part of the decrease in blood pressure during the third phase of the cardiovascular effect of anandamide is related to the activation of an O-1918-sensitive non-CB1 cannabinoid receptor. The data show that this receptor, which has been identified in some vascular beds (for references, see Introduction), also plays a role in a very complex phenomenon such as blood pressure regulation. The fact that the non-CB1 vascular cannabinoid receptor is already the fourth mechanism contributing to the anandamide-related delayed decrease in blood pressure in the anaesthetized rat is not so surprising as anandamide is well-known for its pleiotropic effects. Our results are compatible with studies on conscious hypertensive rats (Ho and Gardiner, 2009) and anaesthetized rats (Garcia et al., 2001), in which anandamide caused a CB1 and/or CB2 receptor-independent hypotensive effect although the antagonism by cannabidiol or O-1918 has not been examined. Our data are also compatible with studies on wild-type (Offertáler et al., 2003) and CB1−/− receptor knockout mice (Járai et al., 1999), in which abnormal cannabidiol, an agonist at the endothelial cannabinoid receptor, showed a hypotensive effect that was counteracted by O-1918 and cannabidiol respectively.
It has been suggested that the endocannabinoid system may be activated as a compensatory mechanism in various forms of hypertension (Bátkai et al., 2004). In this context, the non-CB1 vascular cannabinoid receptor, which like CB1 receptors, spinal TRPV1 receptors and a decrease in cardiac contractility contributes to the hypotensive effect of anandamide in the anaesthetized rat, may be of particular interest. This receptor has been identified in vessels of humans (Kozłowska et al., 2007; 2008;) and a hypotensive effect of anandamide, most likely mediated via this receptor, occurred in hypertensive but was absent in normotensive conscious rats (Ho and Gardiner, 2009).
Acknowledgments
This work was supported by Medical University of Białystok (Poland; grant No. 3-13433F; 3-13453F). B Malinowska and E Schlicker received the Copernicus Award from the Foundation for Polish Science (FNP; Warsaw, Poland) and the German Research Foundation (DFG; Bonn, Germany). The authors are also indebted to the Alexander von Humboldt-Stiftung (Bonn, Germany) for generously providing some of the equipment.
Glossary
Abbreviations:
- AM 251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CBD
cannabidiol
- CP 55,940
(-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol
- DBP
diastolic blood pressure
- DMSO
dimethyl sulphoxide
- HR
heart rate
- MBF
mesenteric blood flow
- MBP
mean blood pressure
- O-1918
1,3-dimethoxy-5-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-benzene
- RBF
renal blood flow
- SBP
systolic blood pressure
Conflicts of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, et al. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Carmen Garcia M, Adler-Graschinsky E, Celuch SM. Hypotensive effect of anandamide through the activation of CB1 and VR1 spinal receptors in urethane-anesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:270–276. doi: 10.1007/s00210-003-0800-x. [DOI] [PubMed] [Google Scholar]
- Garcia N, Járai Z, Mirshahi F, Kunos G, Sanyal AJ. Systemic and portal hemodynamic effects of anandamide. Am J Physiol Gastrointest Liver Physiol. 2001;280:G14–G20. doi: 10.1152/ajpgi.2001.280.1.G14. [DOI] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennet T. Complex regional haemodynamic effects of anandamide in conscious rats. Br J Pharmacol. 2002;135:1889–1896. doi: 10.1038/sj.bjp.0704649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner SM, March JE, Kemp PA, Bennet T. Factors influencing the regional haemodynamic responses to methanandamide and anandamide in conscious rats. Br J Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00363.x. on line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Beltramo M, Piomelli D. Mechanisms of endocannabinoid inactivation: biochemistry and pharmacology. J Pharmacol Exp Ther. 2001;298:7–14. [PubMed] [Google Scholar]
- Godlewski G, Offertáler L, Osei-Hyiaman Mo FM, Harvey-White J, Liu J, et al. The endogenous brain constituent N-arachidonoyl L-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther. 2009;328:351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Gardiner SM. Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br J Pharmacol. 2009;156:94–104. doi: 10.1111/j.1476-5381.2008.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasorelaxant activities of the putative endocannabinoid virodhamine in rat isolated small mesenteric artery. J Pharm Pharmacol. 2004;56:869–875. doi: 10.1211/0022357023682. [DOI] [PubMed] [Google Scholar]
- Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, et al. Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol. 2004;15:1488–1494. doi: 10.1097/01.asn.0000130561.82631.bc. [DOI] [PubMed] [Google Scholar]
- Kozłowska H, Baranowska M, Schlicker E, Kozłowski M, Laudański J, Malinowska B. Identification of the vasodilatory endothelial cannabinoid receptor in the human pulmonary artery. J Hypertens. 2007;25:2240–2248. doi: 10.1097/HJH.0b013e3282ef7a0a. [DOI] [PubMed] [Google Scholar]
- Kozłowska H, Baranowska M, Schlicker E, Kozłowski M, Laudański J, Malinowska B. Virodhamine relaxes the human pulmonary through the endothelial cannabinoid receptor and indirectly through a COX product. Br J Pharmacol. 2008;155:1034–1042. doi: 10.1038/bjp.2008.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz C, Baranowska U, Łupiński S, Göthert M, Malinowska B, Schlicker E. Urethane, but not pentobarbitone, attenuates presynaptic receptor function in rats: a contribution to the choice of anaesthetic. Br J Pharmacol. 2009;157:1474–1482. doi: 10.1111/j.1476-5381.2009.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwolek G, Zakrzeska A, Schlicker E, Göthert M, Godlewski G, Malinowska B. Central and peripheral components of the pressor effect of anandamide in urethane-anaesthetized rats. Br J Pharmacol. 2005;145:567–575. doi: 10.1038/sj.bjp.0706195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake KD, Martin BR, Kunos G, Varga K. Cardiovascular effects of anandamide in anesthetized and conscious normotensive and hypertensive rats. Hypertension. 1997;29:1204–1210. doi: 10.1161/01.hyp.29.5.1204. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Schlicker E. Identification of endothelial H1, vascular H2 and cardiac presynaptic H3 receptors in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:55–60. doi: 10.1007/BF00168772. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Godlewski G, Bucher B, Schlicker E. Cannabinoid CB1 receptor-mediated inhibition of the neurogenic vasopressor response in the pithed rat. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:197–202. doi: 10.1007/pl00005041. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Kwolek G, Göthert M. Anandamide and methanandamide induce both vanilloid VR1- and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001a;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Piszcz J, Koneczny B, Hryniewicz A, Schlicker E. Modulation of the cardiac autonomic transmission of pithed rats by presynaptic opioid OP4 and cannabinoid CB1 receptors. Naunyn Schmiedebergs Arch Pharmacol. 2001b;364:233–241. doi: 10.1007/s002100100450. [DOI] [PubMed] [Google Scholar]
- Malinowska B, Łupiński S, Godlewski G, Baranowska U, Schlicker E. Role of endocannabinoids in cardiovascular shock. J Physiol Pharmacol. 2008;59(Suppl. 8):91–107. [PubMed] [Google Scholar]
- Niederhoffer N, Schmidt K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Pacher P, Bátkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mukhopadhyay P, Mohanraj R, Godlewski G, Bátkai S, Kunos G. Modulation of the endocannabinoid system in cardiovascular disease: therapeutic potential and limitations. Hypertension. 2008;52:601–607. doi: 10.1161/HYPERTENSIONAHA.105.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. GPR55: a new member of the cannabinoid receptor clan? Br J Pharmacol. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Philips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150:613–623. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Lake K, Martin BR, Kunos G. Novel antagonist implicates the CB1 cannabinoid receptor in the hypotensive action of anandamide. Eur J Pharmacol. 1995;278:279–283. doi: 10.1016/0014-2999(95)00181-j. [DOI] [PubMed] [Google Scholar]
- Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]


