Abstract
Background and purpose:
N-arachidonoyl glycine (NAGly) is an endogenous lipid that is structurally similar to the endocannabinoid, N-arachidonoyl ethanolamide (anandamide). While NAGly does not activate cannabinoid receptors, it exerts cannabimimetic effects in pain regulation. Here, we have determined if NAGly, like anandamide, modulates vascular tone.
Experimental approach:
In rat isolated small mesenteric arteries, the relaxant responses to NAGly were characterized. Effects of N-arachidonoyl serine and N-arachidonoyl γ-aminobutyric acid were also examined.
Key results:
In endothelium-intact arteries, NAGly-induced relaxation (pEC50%= 5.7 ± 0.2; relaxation at 30 µM = 98 ± 1%) was attenuated by l-NAME (a nitric oxide synthase inhibitor) or iberiotoxin [selective blocker of large conductance Ca2+-activated K+ channels (BKCa)], and abolished by high extracellular K+ concentration. Endothelial removal reduced the potency of NAGly, and the resultant relaxation was inhibited by iberiotoxin, but not l-NAME. NAGly responses were sensitive to the novel cannabinoid receptor antagonist O-1918 independently of endothelial integrity, whereas pertussis toxin, which uncouples Gi/o proteins, attenuated NAGly relaxation only in endothelium-intact arteries. Treatments with antagonists for CB1, CB2 and TRPV1 receptors, or inhibitors of fatty acid amide hydrolase and COX had no effect. The two other arachidonoyl amino acids also induced iberiotoxin- and L-NAME-sensitive relaxations.
Conclusion and implications:
NAGly acts as a vasorelaxant predominantly via activation of BKCa in rat small mesenteric arteries. We suggest that NAGly activates an unknown Gi/o-coupled receptor, stimulating endothelial release of nitric oxide which in turn activates BKCa in the smooth muscle. In addition, NAGly might also activate BKCa through Gi/o- and nitric oxide-independent mechanisms.
Keywords: N-arachidonoyl glycine, potassium channels, nitric oxide, endothelium, cannabinoid receptor, TRPV1 receptor, rat mesenteric artery, N-arachidonoyl serine, N-arachidonoyl γ-aminobutyric acid
Introduction
The endocannabinoid, N-arachidonoyl ethanolamide (anandamide) is known to induce vasorelaxation in a variety of vascular regions in vitro and in vivo, which could manifest as a reduction in mean arterial blood pressure (White et al., 2001; Ho and Gardiner, 2009). The proposed relaxation mechanisms include activation of the Gi/o-coupled cannabinoid CB1 receptors (receptor and ion channel nomenclature follows Alexander et al., 2008), endothelial release of nitric oxide (Deutsch et al., 1997; Mukhopadhyay et al., 2002), activation of Ca2+-activated K+ channels (KCa; White et al., 2001; Romano and Lograno, 2006), inhibition of voltage-gated Ca2+ channels (Gebremedhin et al., 1999), as well as activation of TRPV1 receptors, which are non-selective cation channels expressed on perivascular sensory nerves (Zygmunt et al., 1999). In fact, in rat mesenteric arteries, a large component of anandamide-induced relaxation is mediated by TRPV1 receptors and the subsequent release of the vasorelaxant calcitonin gene-related peptide (Zygmunt et al., 1999; White et al., 2001). Evidence also suggests the existence of a novel cannabinoid (CBx) receptor, which might be activated by anandamide, in the endothelium of some vascular regions especially the mesenteric arteries (Jarai et al., 1999; Ho and Hiley, 2003; Offertaler et al., 2003). While the molecular identity of CBx is yet to be defined, the receptor is thought to activate Gi/o proteins, leading to activation of KCa and perhaps also nitric oxide/cGMP signalling pathways (Begg et al., 2003; Offertaler et al., 2003).
Interestingly, recent studies have indicated that other endogenous lipids which are structurally similar to anandamide might also exert vascular effects. One group of such anandamide analogues comprises N-acyl amino acids, which have been detected in a number of mammalian tissues (Burstein, 1999; Huang et al., 2001). N-arachidonoyl dopamine, which is an agonist for CB1 and TRPV1 receptors, has also been suggested to activate CBx receptors, resulting in mesenteric relaxation (O'Sullivan et al., 2004). Of particular interest, Milman et al. (2006) have shown that N-arachidonoyl serine, which lacks activity at the classical cannabinoid or TRPV1 receptors, is also a vasorelaxant. However, it remains unclear if other N-acyl amino acids also affect vascular reactivity, and thus represent a new group of vasomodulators.
Recently, N-arachidonoyl glycine (NAGly; Figure 1) has attracted much attention because of its analgesic and anti-inflammatory activity (Burstein et al., 2000; Huang et al., 2001) even though NAGly does not activate cannabinoid or TRPV1 receptors (Sheskin et al., 1997; Huang et al., 2001). At present, the cardiovascular effects of NAGly are yet to be explored. Thus, using rat isolated small mesenteric arteries, this study aimed to examine the vasorelaxant effects of NAGly. Mechanisms underlying the relaxations were investigated, with particular focus on the potential involvement of cannabinoid receptors, TRPV1 receptors, nitric oxide and K+ channels. In an effort to examine the involvement of CBx receptors, the vascular actions of the putative CBx antagonist, O-1918, were also examined. In addition, because NAGly has been shown to compete with anandamide for the catabolic enzyme, fatty acid amide hydrolase (FAAH; Burstein et al., 2002), the effect of NAGly metabolism was also investigated. As a comparison, vasorelaxant effects of two other endogenous arachidonoyl amino acids, namely N-arachidonoyl serine and N-arachidonoyl γ-aminobutyric acid (Figure 1) were also examined.
Figure 1.
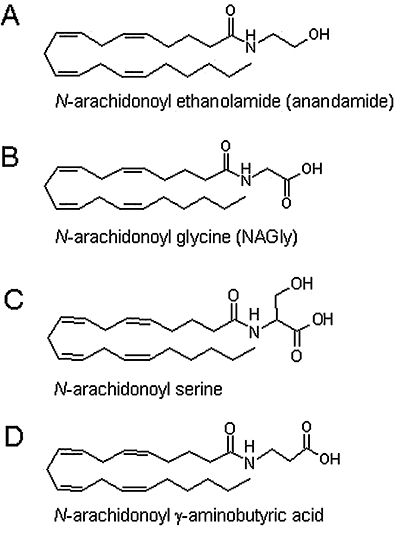
Structures of the endocannabinoid, anandamide (A) and three endogenous conjugates of arachidonic acid and amino acids; N-arachidonoyl glycine (NAGly; B), N-arachidonoyl serine (C), N-arachidonoyl γ-aminobutyric acid (D).
Methods
Myographic studies
All animal care and experimental use were in accordance with the UK Animal (Scientific Procedures) Act 1986. Male Wistar rats (200–350 g; Charles River UK Ltd, Kent, UK) were stunned by a blow to the back of their neck and killed by cervical dislocation. The third-order branches of the superior mesenteric artery, which provides blood supply to the intestine, were removed and cleaned of adherent tissue. Segments (2 mm in length) were mounted in a Mulvany–Halpern-type wire myograph (model 610 M; Danish Myo Technology, Aarhus, Denmark) and maintained at 37°C in gassed (95% O2/5% CO2) Krebs–Henseleit solution of the following composition (mM): NaCl, 118; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3, 25; CaCl2, 2; d-glucose, 10 as previously described (Ho and Randall, 2007). Vessels were equilibrated and set to a basal tension of 2–2.5 mN. The integrity of the endothelium was assessed by precontracting the vessel with 10 µM methoxamine (an α1-adrenoceptor agonist), followed by relaxation with 10 µM carbachol (a muscarinic acetylcholine receptor agonist); vessels showing relaxations of greater than 90% were designated as endothelium intact. When endothelium was not required, it was removed by rubbing the intima with a human hair; carbachol-induced relaxation of less than 10% indicated successful removal.
Experimental protocols
After the test for endothelial integrity, vessels were left for 30 min and then precontracted with 10 µM methoxamine. This was followed by construction of a cumulative concentration–relaxation curve to NAGly, N-arachidonoyl serine, N-arachidonoyl γ-aminobutyric acid or sodium nitroprusside (SNP). The highest concentration used for all N-arachidonoyl amino acids was 30 µM due to limitations in their solubility. It was also observed that NAGly responses showed considerable variations during the course of this study; however, consistent results were obtained in vessels obtained from the same animals. Thus, most experiments were performed in matched vessels; effects of putative modulators or endothelial removal were compared with the control responses obtained in separate vessels of the same rat.
To investigate the relaxation mechanisms of NAGly, cannabinoid receptor antagonists (AM251, JTE907 or O-1918), nitric oxide synthase inhibitor (l-NAME), soluble guanylyl cyclase inhibitor (ODQ) or KCa blockers (apamin, charybdotoxin or iberiotoxin) were used either alone or in combination. These agents were added to the myograph bath 30 min before, and were present during, the construction of the concentration–response curve for NAGly. An incubation time of 20 min was used for the TRPV1 receptor antagonist, SB366791. In some experiments, an FAAH inhibitor (URB597), with or without a COX inhibitor (indomethacin), was incubated with the vessels for 45 min before determination of NAGly responses. In cases where pertussis toxin was used to inhibit activation of Gi/o proteins, it was incubated with the vessels for 2 h.
In a separate series of experiments, treatment with O-1918, iberiotoxin or l-NAME was also performed before determination of relaxant responses to SNP, N-arachidonoyl serine or N-arachidonoyl γ-aminobutyric acid. Furthermore, some vessels were precontracted with high K+ (60 mM) Krebs–Henseleit solution, which was prepared by equimolar substitution of NaCl for KCl in the standard Krebs–Henseleit buffer described above. The mean tension generated by 60 mM KCl (10.2 ± 1.3 mN) was similar to that induced by 10 µM methoxamine in the test for endothelial integrity (9.3 ± 1.2 mN; 18 vessels). It was noted that vessels treated with l-NAME, ODQ, a KCa blocker or O-1918 often displayed enhanced contractile responses to methoxamine. Therefore, a lower concentration of methoxamine (1–3 µM) was used, where required, in order to obtain a similar level of tone to that evoked in the absence of these inhibitors. The basis of such an effect of O-1918 remains unclear, but it is likely related to inhibition of BKCa by O-1918 (see Discussion). The tension generated in the test for endothelial integrity was 10.7 ± 0.4 mN, as compared with 11.7 ± 0.4 mN (113 vessels) in the presence of the inhibitors (either alone or in combination).
Preliminary experiments showed that washing could not fully reverse the effects of NAGly; therefore, only a single concentration–response curve to NAGly (and other arachidonoyl amino acids) was constructed in each preparation. The vehicle of NAGly, which was also the vehicle for N-arachidonoyl serine and N-arachidonoyl γ-aminobutyric acid, had no significant relaxation (up to 0.6% ethanol v/v; data not shown) in methoxamine-precontracted vessels.
Data and statistical analysis
All relaxant responses are expressed as percentage relaxation of the tone induced by methoxamine or KCl. Values are given as mean ± SEM, and n represents the number of animals used. As it was not usually possible to fully define concentration–response curves (solubility limitations prevented use of high-enough concentrations to determine the maximum responses), potency is expressed as pEC50% or pEC20% (the negative logarithm of the concentration of relaxant giving 50 or 20% relaxation of the induced tone, respectively), where appropriate; these values were determined directly from individual log concentration–response curves. Statistical comparisons of concentration-dependent responses were made by two-way anova (Prism 4, GraphPad Software, Inc, San Diego, CA, USA) of the whole data set. Student's t-test was also used where appropriate. P < 0.05 was taken as statistically significant.
Materials
Methoxamine, carbachol, SNP, glycine, l-NAME (Nω-nitro-l-arginine methyl ester hydrochloride), apamin, ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one) (Sigma Chemical Co., Poole, UK), pertussis toxin, iberiotoxin and charybdotoxin (Tocris Bioscience, Bristol, UK) were dissolved in deionized water. URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate; Cayman Chemical, Ann Arbor, MI, USA), SB366791 (N-(3-methoxyphenyl)-4-chlorocinnamide), indomethacin (Sigma), AM251 (N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophen yl)-4-methyl-1H-pyrazole-3-carboxamide), JTE907 (N-(1,3-benzodioxol-5-ylmethyl)-1,2-dihydro-7-methoxy-2-oxo-8-(pentyloxy)-3-quinolinecarboxamide), O-1918 (1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methyle thenyl)-2-cyclohexen-1-yl]benzene), N-arachidonoyl glycine, N-arachidonoyl serine and N-arachidonoyl γ-aminobutyric acid (Tocris) were dissolved in 100% ethanol.
Results
Relaxation to NAGly
NAGly induced concentration-dependent relaxation in rat small mesenteric arteries (pEC20%= 5.8 ± 0.2; relaxation at 30 µM = 88 ± 5%; n= 6). Removal of the endothelium caused an apparent rightward displacement (P < 0.01) of the concentration–response curve (pEC20%= 4.9 ± 0.1; relaxation at 30 µM = 54 ± 12%; n= 5). Typical traces of NAGly relaxations are shown in Figure 2.
Figure 2.

Original traces of relaxation to NAGly in endothelium-intact (A) and endothelium-denuded (B) mesenteric arteries. The experiments were conducted in separate vessels obtained from the same rat. The vertical lines indicate addition of drug at the concentrations indicated. MO, methoxamine; NAGly, N-arachidonoyl glycine.
Effects of nitric oxide synthase inhibitor and K+ channel blockers
In endothelium-intact vessels, relaxation to NAGly was significantly (P < 0.01) inhibited by 300 µM l-NAME, an inhibitor of nitric oxide synthase (Table 1; Figure 3A). The combination of l-NAME with 50 nM apamin and 50 nM charybdotoxin, which together block small conductance (SKCa), intermediate conductance (IKCa) and large conductance (BKCa) Ca2+-activated K+ channels, caused further inhibition of NAGly responses (P < 0.01 vs. control or vs. l-NAME alone, Table 1; Figure 3A). In endothelium-denuded vessels, l-NAME had no significant effect on NAGly-induced relaxation (Table 1). Interestingly, additional application of apamin and charybdotoxin resulted in significant rightward displacement (P < 0.05) of the response curve, and revealed contractile responses to NAGly at lower concentrations (Figure 3B; Table 1).
Table 1.
Effects of l-NAME and KCa channel blockers on relaxation to NAGly in small mesenteric arteries precontracted with methoxamine
| With endothelium | pEC50% | Relaxation at 30 µM (%) | n |
|---|---|---|---|
| Control | 5.8 ± 0.4 | 89 ± 4 | 5 |
| +l-NAME | 5.0 ± 0.2 | 77 ± 8 | 5** |
| +l-NAME + apamin + charybdotoxin | – | 54 ± 16 | 6**# |
| Control | 5.7 ± 0.2 | 98 ± 1 | 5 |
| +Iberiotoxin | – | 63 ± 17 | 5** |
| +Iberiotoxin +l-NAME | – | 66 ± 16 | 6** |
| Without endothelium | pEC20% | Relaxation at 30 µM (%) | n |
|---|---|---|---|
| Control | 5.3 ± 0.2 | 68 ± 10 | 6 |
| +l-NAME | 5.2 ± 0.2 | 54 ± 18 | 5 |
| +l-NAME + apamin + charybdotoxin | – | 46 ± 13 | 6* |
| Control | 5.3 ± 0.1 | 82 ± 5 | 7 |
| +Iberiotoxin | 4.7 ± 0.1 | 52 ± 16 | 5** |
Data are expressed as mean ± SEM. Where appropriate, pEC50% and pEC20% values were obtained directly from individual log concentration–response curves; n represents the number of animals.
P < 0.05,
P < 0.01 indicate significant difference from control values (two-way anova of the whole data set).
Significant difference from l-NAME alone (two-way anova of the whole data set; P < 0.01).
Figure 3.
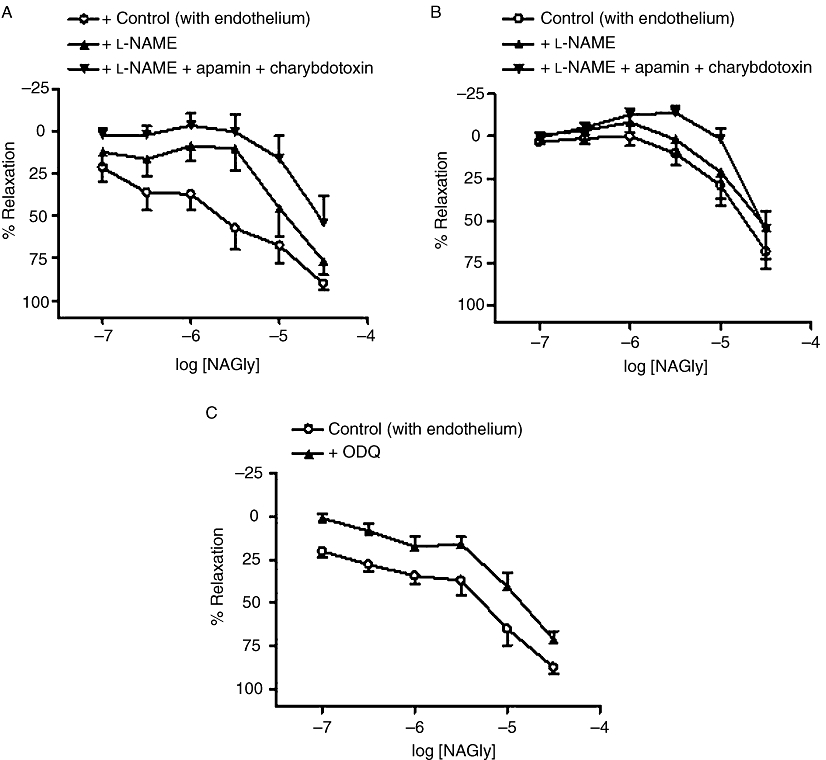
Effects of inhibitors of nitric oxide signalling on relaxation to NAGly in mesenteric arteries. In endothelium-intact (A) and endothelium-denuded (B) vessels, relaxation was elicited by NAGly alone, or after treatment with l-NAME (300 µM) or l-NAME and apamin (50 nM) plus charybdotoxin (50 nM). (C) Relaxation was elicited by NAGly alone, or after treatment with ODQ (10 µM) in endothelium-intact vessels. n= 5–7. Values are shown as means, and vertical bars represent SEM.
In addition, treatment with the selective blocker of BKCa, iberiotoxin (50 nM) alone greatly inhibited (P < 0.01) the relaxation to NAGly (Table 1; Figure 4A), but the combined treatment of iberiotoxin and l-NAME did not cause significantly larger inhibition (P < 0.01 vs. control, P > 0.05 vs. iberiotoxin alone, Table 1; Figure 4A). In endothelium-denuded vessels, iberiotoxin also induced rightward displacement (P < 0.01) of NAGly response curve, which showed notable contractions to lower concentrations of NAGly (Table 1; Figure 4B). Moreover, NAGly responses were abolished by precontracted vessels with high extracellular [K+] (60 mM KCl; n= 4; P < 0.01; Figure 4A).
Figure 4.
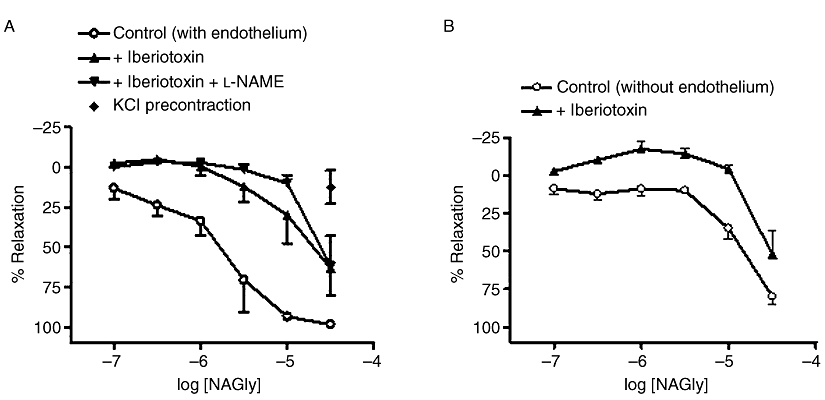
Effects of K+ channel blockade on relaxation to NAGly in mesenteric arteries. (A) Relaxation was elicited by NAGly alone, or after treatment with iberiotoxin (50 nM), or iberiotoxin (50 nM) plus l-NAME (300 µM) in endothelium-intact vessels. Relaxation was also elicited by NAGly alone in vessels precontracted with 60 mM KCl, instead of 10 µM methoxamine. (B) Relaxation was elicited by NAGly alone, or after treatment with iberiotoxin (50 nM) in endothelium-denuded vessels. n= 4–6. Values are shown as means, and vertical bars represent SEM.
Effects of a soluble guanylyl cyclase inhibitor
Inhibition of soluble guanylyl cyclase by 10 µM ODQ significantly attenuated NAGly responses in endothelium-intact (control, pEC50%= 5.3 ± 0.2; relaxation at 30 µM = 89 ± 4%; n= 7; +ODQ, pEC50%= 4.9 ± 0.1; relaxation at 30 µM = 73 ± 5%; n= 7; P < 0.01; Figure 3C), but not endothelium-denuded vessels (control, pEC50%= 4.9 ± 0.1; relaxation at 30 µM = 91 ± 1%; n= 4; +ODQ, pEC50%= 5.0 ± 0.1; relaxation at 30 µM = 85 ± 8%; n= 5).
Effects of a TRPV1 receptor antagonist
The TRPV1 receptor antagonist, SB366791 (2 µM) had no significant effect on relaxation to NAGly (with endothelium: control, pEC50%= 5.1 ± 0.2; relaxation at 30 µM = 87 ± 5%; n= 5; +SB366791, pEC50%= 5.3 ± 0.2; relaxation at 30 µM = 81 ± 7%; n= 5; without endothelium: control, pEC20%= 5.0 ± 0.1; relaxation at 30 µM = 63 ± 11%; n= 4; +SB366791, pEC20%= 5.1 ± 0.2; relaxation at 30 µM = 58 ± 11%; n= 4).
Effects of CB1 and CB2 receptor antagonists
The presence of 1 µM AM251 (selective CB1 receptor antagonist) and 1 µM JTE907 (selective CB2 receptor antagonist) had no significant effect on NAGly-induced relaxation (Table 2).
Table 2.
Effects of cannabinoid receptor antagonists, O-1918 and pertussis toxin, on relaxation to NAGly in small mesenteric arteries precontracted with methoxamine
| With endothelium | pEC50% | Relaxation at 30 µM (%) | n |
|---|---|---|---|
| Control | 5.7 ± 0.2 | 98 ± 1 | 5 |
| +AM251 + JTE907 | 5.5 ± 0.2 | 93 ± 2 | 6 |
| Control | 5.3 ± 0.2 | 85 ± 5 | 6 |
| +O-1918 | – | 38 ± 17 | 6** |
| Control | 5.5 ± 0.2 | 91 ± 3 | 5 |
| +Pertussis toxin | 5.2 ± 0.2 | 87 ± 4 | 5* |
| Without endothelium | pEC20% | Relaxation at 30 µM (%) | n |
|---|---|---|---|
| Control | 5.0 ± 0.1 | 63 ± 11 | 4 |
| +O-1918 | – | 54 ± 18 | 4** |
| Control | 5.3 ± 0.1 | 83 ± 7 | 5 |
| +Pertussis toxin | 5.4 ± 0.1 | 89 ± 3 | 5 |
Data are expressed as mean ± SEM. Where appropriate, pEC50% and pEC20% values were obtained directly from individual log concentration–response curves; n represents the number of animals.
P < 0.05,
P < 0.01 indicate significant difference from control values (two-way anova of the whole data set).
Effects of a novel endothelial receptor antagonist
The presence of 3 µM O-1918, which is thought to be a selective antagonist for a novel endothelial receptor, induced rightward displacements (P < 0.01) of NAGly concentration–response curves in the presence and absence of a functional endothelium (Table 2; Figure 5A,B). It can also be seen that lower concentrations of NAGly caused small contractions in O-1918-treated vessels (Figure 5A,B). In contrast, 0.3 µM O-1918 had no significant effect on NAGly responses (with endothelium: pEC50%= 5.2 ± 0.1; relaxation at 30 µM = 89 ± 6%; n= 6; +O-1918, pEC50%= 5.3 ± 0.2; relaxation at 30 µM = 90 ± 5%; n= 6).
Figure 5.
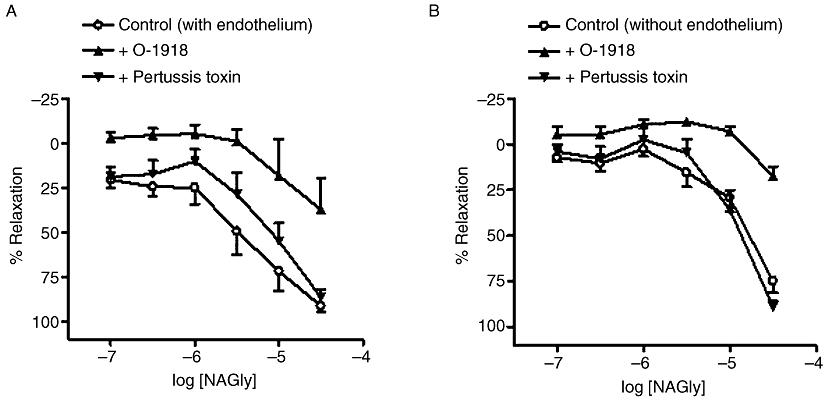
Effects of O-1918 (3 µM) or pertussis toxin (400 ng·mL−1) on relaxation to NAGly in endothelium-intact (A) and endothelium-denuded (B) mesenteric arteries. Control NAGly responses have been pooled for clarity in these graphs. n= 4–7. Values are shown as means, and vertical bars represent SEM.
It was noted that the combination of O-1918 (3 µM) and iberiotoxin (50 nM) had similar effect compared with iberiotoxin alone (with endothelium, +O-1918 + iberiotoxin, pEC20%= 5.0 ± 0.1; relaxation at 30 µM = 78 ± 13%; n= 4; without endothelium, +O-1918 + iberiotoxin, pEC20%= 4.9 ± 0.1; relaxation at 30 µM = 61 ± 10%; n= 5; in both cases, P < 0.01 vs. control, P > 0.05 vs. iberiotoxin alone).
Effects of an inhibitor of Gi/o signalling
Treatment with 400 ng·mL−1 pertussis toxin, which uncouples Gi/o from its receptors, significantly (P < 0.05) attenuated relaxation to NAGly in endothelium-intact vessels (Table 2; Figure 5A). However, pertussis toxin had no significant effect in endothelium-denuded vessels (Table 2; Figure 5B).
Effects of FAAH and COX inhibitors
The selective FAAH inhibitor, URB597 (1 µM) applied either alone, or in combination with the COX inhibitor, indomethacin (10 µM) had no significant effect on relaxation to NAGly (with endothelium: control, pEC50%= 5.5 ± 0.2; relaxation at 30 µM = 95 ± 1%; n= 6; +URB597, pEC50%= 5.2 ± 0.1; relaxation at 30 µM = 87 ± 5%; n= 4; +URB597 + indomethacin, pEC50%= 5.3 ± 0.3; relaxation at 30 µM = 90 ± 2%; n= 5; Figure 6).
Figure 6.
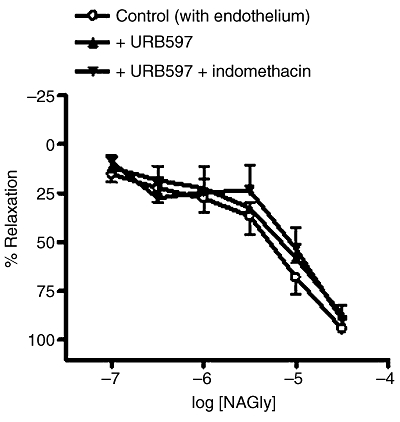
Effects of URB597 (1 µM) and indomethacin (10 µM) on relaxation to NAGly in endothelium-intact mesenteric arteries. n= 4–6. Values are shown as means, and vertical bars represent SEM.
The amino acid, glycine, which can be formed from hydrolysis of NAGly, induced small relaxations at 30 µM (31 ± 7%; n= 4).
Relaxation to SNP
Additional experiments revealed that the novel endothelial receptor antagonist, O-1918 (at 3 µM) also significantly attenuated the relaxant effects of SNP, a nitric oxide donor (without endothelium: control, pEC50%= 6.9 ± 0.3; relaxation at 300 µM = 90 ± 3%; n= 8; +O-1918, pEC50%= 6.0 ± 0.3; relaxation at 300 µM = 82 ± 6%; n= 7, P < 0.01; Figure 7). However, a lower concentration of O-1918 (0.3 µM) had no significant effect on SNP responses (without endothelium: pEC50%= 6.7 ± 0.4; relaxation at 300 µM = 98 ± 1%; n= 5; +O-1918, pEC50%= 7.1 ± 0.2; relaxation at 300 µM = 101 ± 4%; n= 5).
Figure 7.
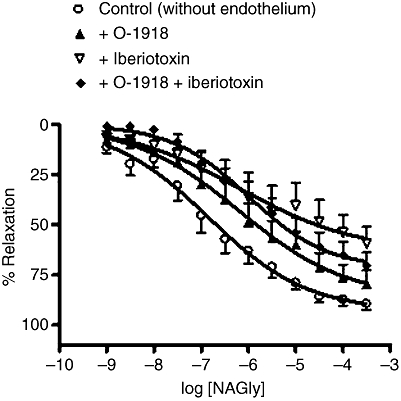
Effects of iberiotoxin (50 nM) and O-1918 (3 µM) on relaxation to SNP in endothelium-denuded mesenteric arteries. n= 5–8. Values are shown as means, and vertical bars represent SEM.
Similar to the case for NAGly, SNP responses were sensitive to iberiotoxin (50 nM), and the combination of iberiotoxin and O-1918 did not cause further inhibition (Figure 7; +50 nM iberiotoxin, relaxation at 300 µM = 63 ± 7%; n= 5, P < 0.01; +iberiotoxin + O-1918, relaxation at 300 µM = 71 ± 7%; n= 6, P < 0.01 vs. control, P > 0.05 vs. iberiotoxin alone). Precontracting vessels with 60 mM KCl, instead of methoxamine, significantly reduced SNP-induced relaxation, to a similar extent compared with iberiotoxin alone or the combination of iberiotoxin and O-1918 (relaxation at 300 µM = 72 ± 6%; n= 5).
Relaxation to two other N-arachidonoyl amino acids
N-Arachidonoyl serine (Figure 1) induced concentration-dependent relaxation in mesenteric arteries (with endothelium: control, pEC50%= 4.9 ± 0.1; relaxation at 30 µM = 84 ± 6%; n= 6). Interestingly, the presence of l-NAME or iberiotoxin significantly reduced N-arachidonoyl serine responses (with endothelium: +300 µM l-NAME, pEC50%= 4.7 ± 0.1; relaxation at 30 µM = 74 ± 7%; n= 6; P < 0.01; +50 nM iberiotoxin, relaxation at 30 µM = 40 ± 19%; n= 6; P < 0.01; Figure 8A). It was noted that N-arachidonoyl serine induced relaxation was abolished by precontracting the vessels with 60 mM KCl (n= 5).
Figure 8.

Effects of iberiotoxin (50 nM) or l-NAME (300 µM) on relaxation to N-arachidonoyl serine (A) and N-arachidonoyl γ-aminobutyric acid (B) in endothelium-intact mesenteric arteries. n= 5–7. Values are shown as means, and vertical bars represent SEM.
Another N-arachidonoyl amino acid, N-arachidonoyl γ-aminobutyric acid (cf. Figure 1) also induced mesenteric relaxation (with endothelium: control, pEC50%= 6.0 ± 0.1; relaxation at 30 µM = 99 ± 1%; n= 5; Figure 8B). l-NAME and iberiotoxin significantly reduced the potency, but not the maximal effect, of N-arachidonoyl γ-aminobutyric acid (with endothelium: +300 µM l-NAME, pEC50%= 5.7 ± 0.1; relaxation at 30 µM = 100 ± 1%; n= 5; P < 0.01; +50 nM iberiotoxin, pEC50%= 5.8 ± 0.1; relaxation at 30 µM = 99 ± 1%; n= 5; P < 0.01; Figure 8B). Relaxation to N-arachidonoyl γ-aminobutyric acid was also abolished by precontraction with 60 mM KCl (n= 4).
Discussion and conclusions
NAGly was originally synthesized as an analogue of the endocannabinoid, anandamide (Sheskin et al., 1997). More recently, endogenous NAGly has been detected, particularly in the gut, spinal cord and brain (Huang et al., 2001). This study shows, for the first time, that NAGly is a vasorelaxant in mesenteric arteries. Unlike anandamide, NAGly does not activate cannabinoid receptors (Sheskin et al., 1997). Consistent with this result, we found that mesenteric relaxation to NAGly was insensitive to the selective CB1 and CB2 receptor antagonists, AM251 and JTE907, respectively, which were used in combination (Lan et al., 1999; Iwamura et al., 2001). In addition to cannabinoid receptors, anandamide is also known to activate TRPV1 receptors in perivascular sensory nerves (Zygmunt et al., 1999). Mesenteric relaxation to anandamide (Ho et al., 2008), but not NAGly (this study) was reduced by SB366791, an antagonist with high affinity for TRPV1 receptors (Gunthorpe et al., 2004). These data argue against the involvement of TRPV1 receptors in NAGly responses, and corroborate with the finding that NAGly has no activity (<5% at 10 µM) at human TRPV1 receptors (Huang et al., 2001).
Anandamide is primarily degraded by the FAAH into arachidonic acid and ethanolamide (Cravatt et al., 1996). In a recent study, we have found that inhibition of anandamide hydrolysis via FAAH potentiates anandamide-induced relaxation of rat mesenteric arteries (Ho and Randall, 2007). NAGly might also serve as a substrate for FAAH, resulting in arachidonic acid and glycine (Huang et al., 2001; Burstein et al., 2002). However, URB597, a selective FAAH inhibitor, had no significant effect on responses to NAGly, suggesting that FAAH plays little role in either regulating or mediating the relaxant effects of NAGly. Moreover, because the combined treatment of URB597 and the COX inhibitor, indomethacin also had no effect on relaxation to NAGly, COX-mediated metabolism of the hydrolysis product, arachidonic acid or NAGly itself (Prusakiewicz et al., 2002) was not involved in relaxation to NAGly.
We demonstrated that the potency of NAGly relaxations is partly dependent on an intact endothelium, in that the endothelium contributes to, but is not obligatory for, the relaxant effects of NAGly. Importantly, in endothelium-intact, but not -denuded, vessels, relaxation to NAGly was attenuated by the nitric oxide synthase inhibitor, l-NAME, and the soluble guanylyl cyclase inhibitor, ODQ, suggesting the involvement of nitric oxide production and subsequent increase in intracellular cGMP levels. We therefore hypothesize that NAGly stimulates endothelial release of nitric oxide, which underlies the endothelium-dependent component of NAGly-induced relaxation. Another downstream signalling effector of nitric oxide is large conductance Ca2+-activated K+ channels (BKCa) in vascular smooth muscle cells. We found that NAGly responses were greatly inhibited by the selective BKCa blocker, iberiotoxin (Galvez et al., 1990), indicating a major role of this K+ channel in the vascular action of NAGly. Interestingly, adding l-NAME to iberiotoxin did not cause further inhibition of NAGly relaxations in endothelium-intact vessels. This would suggest that BKCa is downstream of endothelial-derived nitric oxide. Increases in BKCa activity would repolarize vascular smooth muscle cells, reduce Ca2+ entry via voltage-gated Ca2+ channels and thus lead to vasorelaxation. Given that BKCa can be activated directly by nitric oxide (Mistry and Garland, 1998) or indirectly via generation of cGMP and subsequent activation of protein kinase K (Peng et al., 1996), a combination of these two mechanisms might be involved in the NAGly–nitric oxide–BKCa pathway.
Further experimentation is required to elucidate exactly how NAGly activates nitric oxide production. Nonetheless, we have explored the possibility that NAGly acts through the novel endothelial cannabinoid receptor, referred to as CBx receptor, and is thought to be coupled to Gi/o proteins as are the classical cannabinoid receptors (Jarai et al., 1999; Begg et al., 2003). Mesenteric relaxation to NAGly was slightly attenuated by the Gi/o protein uncoupler, pertussis toxin in endothelium-intact, but not endothelium-denuded, vessels. The effects of pertussis toxin were small compared to those of l-NAME and iberiotoxin. Nonetheless, this result, combined with the lack of effect of CB1 and CB2 antagonists, suggests that an unknown, endothelial Gi/o protein-coupled receptor also contributes to NAGly relaxations. The CBx receptor might be involved because NAGly responses were also reduced by 3 µM O-1918, a putative CBx receptor antagonist that is frequently used at concentrations ≥3 µM (Offertaler et al., 2003), although 0.3 µM O-1918 was ineffective. CBx receptors have been reported to activate phosphatidylinositol 3-kinase and Akt kinase via Gi/o proteins, resulting in phosphorylation and activation of the endothelial nitric oxide synthase (Begg et al., 2003; McCollum et al., 2007). This provides a mechanism by which NAGly could stimulate the release of nitric oxide. It should be noted that the orphan receptor GPR55 has recently been proposed as the CBx receptor, but this remains a contentious issue, especially as GPR55 neither couples to Gi/o proteins nor mediates mesenteric relaxation attributed to CBx receptors (Johns et al., 2007; Ryberg et al., 2007). It also remains unclear if O-1918 is an antagonist, and NAGly an agonist, for GPR55.
On the other hand, it is clear that K+ channels are fundamental to the relaxant effects of NAGly, which were abolished in the presence of depolarizing, high extracellular K+ solution. As mentioned before, BKCa plays an important role in NAGly relaxations in endothelium-intact vessels. Our data suggest that, in the absence of endothelium, NAGly also activates BKCa, albeit by nitric oxide- and Gi/o-independent mechanisms. This possibility is likely to explain the observation that endothelial removal tended to reduce the potency, but not the maximal relaxant effect, of NAGly. Very recently, N-arachidonoyl serine has been reported to directly activate BKCa in cultured cells, which is likely to contribute to the mesenteric relaxation induced by N-arachidonoyl serine (Godlewski et al., 2009). It is therefore possible that NAGly also activates BKCa by direct binding. Future electrophysiological experiments measuring BKCa activity in mesenteric smooth muscle cells would be required to test this hypothesis.
Of note, the inhibitory effects of O-1918 on relaxation to NAGly resembled those of iberiotoxin (Figures 4 and 5); in particular, O-1918 also inhibited NAGly responses in endothelium-denuded vessels. This could be accounted for by O-1918-induced inhibition of BKCa activity because we observed that O-1918 (at 3 µM, but not 0.3 µM) also significantly attenuated BKCa-dependent relaxation induced by nitric oxide (released by SNP). Indeed, during the course of this study, O-1918 has been found to inhibit BKCa-mediated currents in cultured human embryonic kidney cells expressing BKCa (Godlewski et al., 2009). These results therefore call for cautious interpretation of reports of sensitivity to O-1918, especially if nitric oxide and/or BKCa activation is involved.
In addition to BKCa, the involvement of other subtypes of K+ channels, namely SKCa and IKCa, cannot be excluded because the combination of apamin (selective inhibitor of SKCa) and charybdotoxin (blocker of IKCa and BKCa; Ledoux et al., 2006) reduced relaxation to NAGly. Nonetheless, it is noteworthy that present evidence points to the predominant expression of SKCa and IKCa in the endothelium, as opposed to the predominant expression of BKCa in the smooth muscle (Ledoux et al., 2006). Because the channel blockers also attenuated NAGly responses in endothelium-denuded vessels, we would suggest that the effects of apamin plus charybdotoxin were likely to be due to inhibition by charybdotoxin of BKCa.
In this study, lower concentrations (between 0.1 and 3 µM) of NAGly induced small contractions, notably in the absence of the endothelium and after treatment with KCa blockers or O-1918. This perhaps indicates that NAGly responses are biphasic, with initial vasocontraction followed by vasorelaxation, in isolated mesenteric arteries. While the predominant vascular action of NAGly is relaxation, mechanisms underlying NAGly-induced vasocontraction warrant further investigation.
Here, we also report that N-arachidonoyl serine and N-arachidonoyl γ-aminobutyric acid induced mesenteric relaxation; in descending order of potency, N-arachidonoyl γ-aminobutyric acid > NAGly > N-arachidonoyl serine. Furthermore, like NAGly, relaxation to these two endogenous conjugates of arachidonic acid and amino acid was sensitive to l-NAME and iberiotoxin, suggesting a role for nitric oxide and BKCa. Interestingly, N-arachidonoyl serine, but not N-arachidonoyl γ-aminobutyric acid, also induced small contractions (at concentrations <10 µM) in the presence of l-NAME or iberiotoxin. Based on data from the current and previous studies, we hypothesize that N-arachidonoyl amino acids represent a new group of vasomodulators. The physiological relevance of NAGly and other N-arachidonoyl amino acids in vascular control warrants further investigation. For instance, the vascular content of these compounds is yet to be examined. Nonetheless, Huang et al. (2001) reported that the gut has relatively high levels of NAGly (at about 130 pmol·g−1 dry tissue), which could be released in close proximity to the mesenteric arteries, and thereby induce vasorelaxation, as observed in this study.
In conclusion, our present results suggested that NAGly caused mesenteric arterial relaxation predominantly via activation of BKCa. It is hypothesized that NAGly activated an unknown Gi/o-coupled receptor, stimulating endothelial release of nitric oxide which in turn activated BKCa. In addition, NAGly also activated BKCa through Gi/o- and nitric oxide-independent mechanisms, resulting in relaxation of endothelium-denuded vessels.
Acknowledgments
We are grateful for the financial support from St George's University of London, and for the technical help from Thomas Spelman.
Glossary
Abbreviations:
- AM251
N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CBx
novel cannabinoid receptor
- FAAH
fatty acid amide hydrolase
- JTE907
N-(1,3-benzodioxol-5-ylmethyl)-1,2-dihydro-7-methoxy-2- oxo-8-(pentyloxy)-3-quinolinecarboxamide
- KCa
Ca2+-activated K+ channels
- l-NAME
Nω-nitro-l-arginine methyl ester hydrochloride
- NAGly
N-arachidonoyl glycine
- O-1918
1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methyle thenyl)-2-cyclohexen-1-yl]benzene
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one
- SB366791
N-(3-methoxyphenyl)-4-chlorocinnamide
- TRPV1
transient receptor potential vanilloid type 1
- URB597
3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Mo FM, Offertaler L, Batkai S, Pacher P, Razdan RK, et al. G protein-coupled endothelial receptor for atypical cannabinoid ligands modulates a Ca2+-dependent K+ current. J Biol Chem. 2003;278:46188–46194. doi: 10.1074/jbc.M307258200. [DOI] [PubMed] [Google Scholar]
- Burstein SH. The cannabinoid acids: nonpsychoactive derivatives with therapeutic potential. Pharmacol Ther. 1999;82:87–96. doi: 10.1016/s0163-7258(98)00069-2. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Rossetti RG, Yagen B, Zurier RB. Oxidative metabolism of anandamide. Prostaglandins Other Lipid Mediat. 2000;61:29–41. doi: 10.1016/s0090-6980(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Burstein SH, Huang SM, Petros TJ, Rossetti RG, Walker JM, Zurier RB. Regulation of anandamide tissue levels by N-arachidonylglycine. Biochem Pharmacol. 2002;64:1147–1150. doi: 10.1016/s0006-2952(02)01301-1. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Deutsch D, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK, et al. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, Kaczorowski GJ, et al. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Campbell WB, Hillard CJ, Harder DR. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit l-type Ca2+ channel current. Am J Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Offertaler L, Osei-Hyiaman D, Mo FM, Harvey-White J, Liu J, et al. The endogenous brain constituent N-arachidonoyl l-serine is an activator of large conductance Ca2+-activated K+ channels. J Pharmacol Exp Ther. 2009;328:351–361. doi: 10.1124/jpet.108.144717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Ho WS, Gardiner SM. Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br J Pharmacol. 2009;156:94–104. doi: 10.1111/j.1476-5381.2008.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Vasodilator actions of abnormal-cannabidiol in rat isolated small mesenteric artery. Br J Pharmacol. 2003;138:1320–1332. doi: 10.1038/sj.bjp.0705160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, et al. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- Iwamura H, Suzuki H, Ueda Y, Kaya T, Inaba T. In vitro and in vivo pharmacological characterization of JTE-907, a novel selective ligand for cannabinoid CB2 receptor. J Pharmacol Exp Ther. 2001;296:420–425. [PubMed] [Google Scholar]
- Jarai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, et al. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci USA. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, et al. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Fernando SR, McCallion D, Pertwee R, et al. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;25:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 2006;21:69–78. doi: 10.1152/physiol.00040.2005. [DOI] [PubMed] [Google Scholar]
- McCollum L, Howlett AC, Mukhopadhyay S. Anandamide-mediated CB1/CB2 cannabinoid receptor – independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther. 2007;321:930–937. doi: 10.1124/jpet.106.117549. [DOI] [PubMed] [Google Scholar]
- Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Batkai S, et al. N-arachidonoyl l-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci USA. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Chapnick BM, Howlett AC. Anandamide-induced vasorelaxation in rabbit aortic rings has two components: G protein dependent and independent. Am J Physiol Heart Circ Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- Offertaler L, Mo FM, Batkai S, Liu J, Begg M, Razdan RK, et al. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br J Pharmacol. 2004;141:803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Hoidal JR, Farrukh IS. Regulation of Ca(2+)-activated K+ channels in pulmonary vascular smooth muscle cells: role of nitric oxide. J Appl Physiol. 1996;81:1264–1272. doi: 10.1152/jappl.1996.81.3.1264. [DOI] [PubMed] [Google Scholar]
- Prusakiewicz JJ, Kingsley PJ, Kozak KR, Marnett LJ. Selective oxygenation of N-arachidonylglycine by cyclooxygenase-2. Biochem Biophys Res Commun. 2002;296:612–617. doi: 10.1016/s0006-291x(02)00915-4. [DOI] [PubMed] [Google Scholar]
- Romano MR, Lograno MD. Cannabinoid agonists induce relaxation in the bovine ophthalmic artery: evidences for CB1 receptors, nitric oxide and potassium channels. Br J Pharmacol. 2006;147:917–925. doi: 10.1038/sj.bjp.0706687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheskin T, Hanus L, Slager J, Vogel Z, Mechoulam R. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J Med Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


