Abstract
Background and purpose:
Recent studies suggest that the effects of cyclooxygenase-2 (COX-2) inhibition are mediated by cannabinoid receptor activation. However, some non-steroidal anti-inflammatory drugs inhibit the enzyme fatty acid amide hydrolase, which regulates levels of some endocannabinoids. Whether COX-2 directly regulates levels of endocannabinoids in vivo is unclear. Here, the effect of the COX-2 inhibitor nimesulide, which does not inhibit fatty acid amide hydrolase, on spinal nociceptive processing was determined. Effects of nimesulide on tissue levels of endocannabinoids and related compounds were measured and the role of cannabinoid 1 (CB1) receptors was determined.
Experimental approach:
Effects of spinal and peripheral administration of nimesulide (1–100 µg per 50 µL) on mechanically evoked responses of rat dorsal horn neurones were measured, and the contribution of the CB1 receptor was determined with the antagonist AM251 (N-(piperidin-1-yl)-5-(-4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide), in anaesthetized rats. Effects of nimesulide on spinal levels of endocannabinoids and related compounds were quantified using liquid chromatography-tandem mass spectrometry.
Key results:
Spinal, but not peripheral, injection of nimesulide (1–100 µg per 50 µL) significantly reduced mechanically evoked responses of dorsal horn neurones. Inhibitory effects of spinal nimesulide were blocked by the CB1 receptor antagonist AM251 (1 µg per 50 µL), but spinal levels of endocannabinoids were not elevated. Indeed, both anandamide and N-oleoylethanolamide (OEA) were significantly decreased by nimesulide.
Conclusions and implications:
Although the inhibitory effects of COX-2 blockade on spinal neuronal responses by nimesulide were dependent on CB1 receptors, we did not detect a concomitant elevation in anandamide or 2-AG. Further understanding of the complexities of endocannabinoid catabolism by multiple enzymes is essential to understand their contribution to COX-2-mediated analgesia.
This article is part of a themed issue on Cannabinoids. To view the editorial for this themed issue visit http://dx.doi.org/10.1111/j.1476-5381.2010.00831.x
Keywords: endocannabinoids, COX-2, nimesulide, LOX, cytochrome P450, CB1, pain
Introduction
The antinociceptive effects of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG) have been well described in animal models of acute and chronic pain (for reviews, see Pertwee, 2001; Iversen and Chapman, 2002; Walker and Huang, 2002). Levels of endocannabinoids are elevated spinally and supraspinally in models of chronic pain (Palazzo et al., 2006; Petrosino et al., 2007).
Fatty acid amide hydrolase (FAAH) and monoacyl glycerol lipase are predominantly responsible for the catabolism of endocannabinoids. AEA and the related fatty acid amides N-oleoylethanolamide (OEA) and N-palmitoylethanolamide are hydrolysed by FAAH (Cravatt et al., 1996; Deutsch et al., 2001), whereas 2-AG is metabolized by monoacyl glycerol lipase (Dinh et al., 2002a,b;). The analgesic potential of preventing the catabolism of endocannabinoids elevated following noxious stimulation has been widely studied. Inhibition of FAAH, either pharmacologically (Lichtman et al., 2004; Kinsey et al., 2009) or by gene deletion (Cravatt et al., 2001), elevates levels of AEA and produces behavioural analgesia in models of acute and chronic pain.
In addition to FAAH, AEA is also a substrate for catabolism by cyclooxygenase-2 (COX-2) (Yu et al., 1997; Kozak et al., 2004), lipoxygenases (LOXs) (Hampson et al., 1995; Ueda et al., 1995; Edgemond et al., 1998) and cytochrome P450 (cP450) (Bornheim et al., 1993; Snider et al., 2008). COX-2 has been shown to contribute to the metabolism of 2-AG to biologically active metabolites (Kozak et al., 2000; Prusakiewicz et al., 2009). The role of COX-2 in the metabolism of these endocannabinoids is of particular relevance to pain processing as this enzyme is constitutively expressed in the spinal cord (Ghilardi et al., 2004) and induced in chronic pain states. COX inhibitors are commonly used in the treatment of chronic pain states, but the role of endocannabinoids in mediating these effects is unclear. Previous studies have demonstrated that ibuprofen can inhibit AEA hydrolysis in rat brain membrane preparations with a potency of the same order of magnitude as required for inhibition of COX-2, and at concentrations comparable to peak plasma concentrations following therapeutic dosing (Fowler et al., 1997; 1999;). The inhibitory effects of non-steroidal anti-inflammatory drugs (NSAIDs) are blocked by cannabinoid 1 (CB1) receptor antagonism, implicating a role of the endocannabinoids in mediating these effects (Guhring et al., 2002; Telleria-Diaz et al., 2010). Nevertheless, the NSAIDs used in previous studies inhibited both COX-2 and FAAH, thus the role of COX-2 in regulating levels of endocannabinoids and the contribution of endocannabinoids to the analgesic effects of NSAIDs in vivo is unclear. In vivo and in vitro studies have shown that nimesulide is a relatively selective COX-2 versus COX-1 inhibitor at therapeutic doses (for review see Famaey, 1997; Shah et al., 2001; Kerola et al., 2009) and does not inhibit FAAH activity (Fowler et al., 2003). The aim of this study was to determine whether the inhibition of spinal COX-2 by nimesulide alters innocuous and/or noxious-evoked responses of spinal neurones, and the contribution of CB1 receptors in mediating these effects. Levels of endocannabinoids and related compounds in the spinal cord following treatment with nimesulide were also determined.
Methods
Animals
Experiments were carried out on 70 male (200–220 g) Sprague-Dawley rats (Charles River, Margate, UK), group housed in a temperature controlled (20–22°C) environment with a 12 h light/dark cycle (lights on at 0700 h) with ad libitum access to food and water. All experimental procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and International Association for the Study of Pain (IASP) guidelines.
Surgical procedures
Methods were similar to those previously described (Sokal and Chapman, 2001). Rats were anaesthetized with isoflurane inhalation anaesthetic (3% induction, 2% surgery, 1–1.5% maintenance in 33% O2/67% N2O, Abbott Laboratories Ltd., Maidenhead, UK), and a tracheal cannula was inserted. Rats were then placed in a stereotaxic frame to maintain stability during recordings. A laminectomy was performed, lumbar vertebrae L1–L3 were located, and segments L4–L5 of the spinal cord were exposed using fine rongeurs. The spinal cord was held rigid by clamps rostral and caudal to the exposed section of spinal cord (L4/5), and a small well was formed with the surrounding muscle. Core body temperature was maintained at 36.5–37.5°C throughout the experiment by means of a heating blanket connected to a rectal temperature probe.
In vivo electrophysiology
Extracellular single-unit recordings of deep (500–1000 µm) wide dynamic range (WDR) dorsal horn neurones were made with glass-coated tungsten microelectrodes. Electrodes were descended vertically through the spinal cord with a SCAT-01 microdrive (Digitimer, Welwyn Garden City, UK); depths of recorded neurones from the spinal cord surface were noted. Receptive fields of neurones covering one or two toes were identified using brush, pinch and heat stimuli. Single-unit activity was amplified and filtered (Digitimer). Signals were digitized and analysed using a CED micro1401 interface and Spike 2 data acquisition software (Cambridge Electronic Design, Cambridge, UK). Responses of neurones to a train of 16 transcutaneous electrical stimuli (0.5 Hz, 2 ms pulse-width) applied to the centre of the receptive field were recorded. All neurones selected were WDR, exhibiting a short-latency Aβ-fibre-evoked response (0–20 ms post stimulus) and Aδ-fibre-evoked response (20–90 ms post stimulus). These neurones also exhibited longer-latency C-fibre-evoked responses (90–300 ms post stimulus) and post-discharge responses (300–800 ms post stimulus).
Mechanically evoked responses of neurones to punctate stimuli were characterized using von Frey monofilaments (Semmes-Weinstein monofilaments, North Coast Medical Inc., Morgan Hill, CA, USA, via Linton Instrumentation, Norfolk, UK) applied to the centre of the receptive field on the toes of the hindpaw in ascending (8, 10, 15, 26 and 60 g) bending force order, representing both non-noxious (8 and 10 g) and noxious (15, 26 and 60 g) stimuli (Chaplan et al., 1994). Monofilaments were applied every 10 min for 10 s with 10 s between each monofilament, to the centre of the receptive field. All neurones selected exhibited a graded response to ascending bending force von Frey filaments with <10% variation between stimuli, and quantified by the compilation of stimulus-evoked histograms and analysis of the mean firing rate during application. The effects of drug administration on mechanically evoked responses of WDR neurones were measured as a percentage change of firing rates compared with pre-drug control values. First, the effects of spinal nimesulide (1–100 µg per 50 µL, n= 6 rats per dose, total n= 18 rats) or vehicle (50 µL, n= 6) were studied. Drugs were administered directly onto the exposed spinal cord using a 50 µL Hamilton syringe (Hamilton-Bonaduz, Bonaduz, Switzerland). Each dose was applied for 60 min to the spinal cord, followed by a higher dose, up to a maximum of three doses per rat. In a separate group of rats, the effects of CB1 receptor blockade on nimesulide-mediated effects were determined. The CB1 antagonist AM251 (N-(piperidin-1-yl)-5-(-4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) (1 µg per 50 µL, spinal, n= 6) was given as a 30 min pre-administration prior to nimesulide (25 µg per 50 µL). Finally, the effects of peripherally administered nimesulide (100 µg per 50 µL, n= 6) or vehicle (50 µL, n= 6) were studied for 60 min, injected directly into the hindpaw above the receptive field.
Drugs
A range of doses [1–100 µg (65 µM–6.5 mM)] of the COX-2 inhibitor nimesulide (Tocris Bioscience, Bristol, UK) were dissolved in 100% ethanol, dried and reconstituted in 3% polyethylene glycol sorbitan monooleate (Tween 80; Sigma-Alrdich, Gillingham, UK) in physiological saline (together constituting the vehicle). Because nimesulide has not previously been applied to the spinal cord under similar experimental conditions, a pilot study was conducted to determine the appropriate range of doses, and a wide range of doses were used. Although the actual doses of drug that bathes the surface of the spinal cord are high, the amount of drug that reaches the intracellular targets within neurones is likely to be far lower, especially given the distance of WDR neurones from the surface of the cord. AM251 [1 µg per 50 µL (36 µM), Tocris Bioscience] was dissolved in 3% Tween 80 in physiological saline. The dose, and time point of administration, of AM251 used was based on previous studies (Johanek and Simone, 2004; Jhaveri et al., 2006). Drug/molecular target nomenclature conforms to BJP's guide to Receptors and Channels (Alexander et al., 2008).
Measurement of levels of endocannabinoids and related compounds in spinal cord
Rats were anaesthetized and surgically prepared as described above. In two separate experiments, nimesulide (25 µg per 50 µL, n= 8; 100 µg per 50 µL, n= 6), or vehicle (n= 8 and n= 6, respectively), was applied to the spinal cord as described above. The maximal effect of nimesulide on mechanically evoked neuronal responses occurred 30 min post administration and therefore tissue was collected at this time point. Rats were killed by anaesthetic overdose with 5% isoflurane in 33% O2/67% N2O, and the spinal cord was rapidly removed, separated into ipsilateral and contralateral segments, snap-frozen on dry ice and stored at −80°C until analysis. A validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) analytical method was used to measure AEA, OEA and 2-AG as previously described (Richardson et al., 2007), with some alterations as described below. The assay method is in routine use and has been fully validated (Richardson et al., 2007) demonstrating intra- and inter-day precision and accuracy of ≤15% RSD (relative standard deviation). To ensure appropriate control for these studies, which were conducted with an interval of 6 months, time-matched effects of vehicle on levels of endocannabinoids were determined for each of analytical runs to ensure comparability.
Briefly, tissue was weighed, finely minced and homogenized in 5 mL acetonitrile (Fisher Scientific UK., Loughborough, UK) with 15 µL of 28 µM deuterated AEA (AEA-d8) and 100 µL of 10 µM deuterated 2-AG (2-AG-d8) internal standards (Cayman Europe, Tallinn, Estonia). The homogenized mixture was centrifuged, the supernatant collected and the remaining pellet re-homogenized in 2.5 mL acetonitrile before further centrifugation and collection. The solvent was evaporated and the remaining material reconstituted in 200 µL acetonitrile. Analytes were separated chromatographically using a Waters Symmetry C18 column (100 × 2.1 mm id, 3.5 µm particle size; Waters Ltd., Hertfordshire, UK), with a mobile phase from rate of 0.3 mL·min−1, using a gradient elution with mobile phases consisting of A (water, 1 g·L−1 ammonium acetate, 0.1% formic acid) and B (acetonitrile, 1 g·L−1 ammonium acetate, 0.1% formic acid) (both Fisher Scientific). Analytes were injected from a cooled autosampler maintained at 4°C. Analysis was carried out using an Agilent 1100LC system (Agilent Technologies, Böblingen, Germany) coupled to a triple quadropole Quattro Ultima mass spectrometer (Waters Micromass UK Ltd., Manchester, UK) recording in electrospray positive mode. The lower on column limits of detection of AEA and OEA was 5 fmol, while that of 2-AG was 100 fmol.
Statistical analysis
Data from electrophysiology studies are expressed as a percentage of the pre-drug control ± SEM. Statistical analyses comparing effects of nimesulide to vehicle were performed with a one-way anova (Kruskal-Wallis) with post hoc Dunn's test. Statistical analysis comparing effects of 25 µg nimesulide to that of 25 µg nimesulide with CB1 antagonist pretreatment were performed using a non-parametric Mann–Whitney test. Statistical analysis of the effects of nimesulide on levels of endocannabinoids and related compounds were performed using non-parametric Mann–Whitney test.
Results
The mean depths of WDR neurones recorded were similar for each of the treatment groups and were between 500 and 1000 µm from the dorsal surface, corresponding to laminae V–VI (data not shown). Control mechanically evoked responses of WDR neurones used in electrophysiological studies (n= 42 rats) were: 8 g, 22 ± 4; 10 g, 26 ± 4; 15 g, 38 ± 5; 26 g, 60 ± 7; 60 g, 87 ± 7 Hz.
The effects of spinal versus peripheral administration of nimesulide on mechanically evoked responses of dorsal horn neurones
Spinal administration of the COX-2 inhibitor nimesulide (1–100 µg per 50 µL) produced a dose-dependent attenuation of mechanically evoked firing of WDR dorsal horn neurones, both in the non-noxious (8 and 10 g) and noxious (15, 26 and 60 g) range (Figure 1). Nimesulide significantly attenuated evoked responses of WDR dorsal horn neurones, compared with the effects of vehicle, for the majority of the mechanical stimuli applied (Figure 1). The maximal attenuation of mechanically evoked responses was produced by 25 µg of nimesulide, and maximal effects for all doses studied were observed at the following times (min) post drug administration: 8 g, 32 ± 3; 10 g, 37 ± 3; 15 g, 35 ± 3; 26 g, 32 ± 2; 60 g, 33 ± 3; overall 34 ± 1 (n= 18 rats). An example trace of the effect of 25 µg nimesulide on neuronal firing of WDR neurones is shown in Figure 2.
Figure 1.
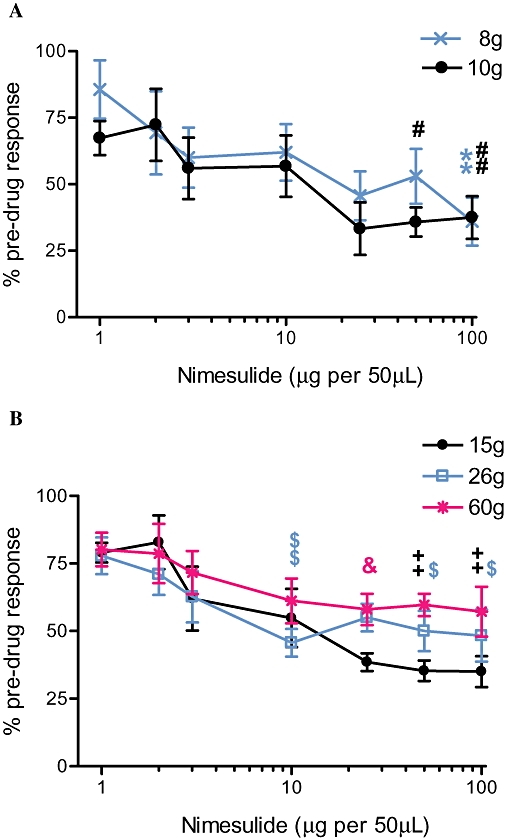
Mean maximal effects of spinal nimesulide on mechanically evoked (8–60 g) (A) non-noxious (8 g and 10 g) and (B) noxious (15 g, 26 g and 60 g) responses of wide dynamic range dorsal horn neurones in naive anaesthetized rats in vivo (n= 6 rats per dose, total n= 18 rats). Nimesulide reduced mechanically evoked responses in a dose-dependent manner. Statistical analyses were performed with one-way anova (Kruskal-Wallis) with post hoc Dunn's test; single symbol (#, $, &)P < 0.05; double symbol (**, ##, ++, $$) P < 0.01 versus vehicle (not shown, no significant difference to pre-drug controls). Data are expressed as a percentage of the pre-drug control ± SEM.
Figure 2.
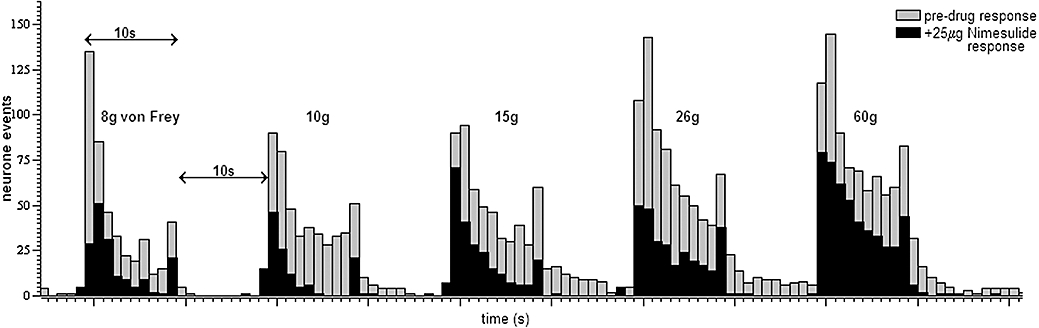
Example trace of mechanically evoked responses of a single wide dynamic range dorsal horn neurone in a naïve anaesthetized rat before (pre-drug response) and after spinal administration of nimesulide (25 µg per 50 µL).
The next series of experiments determined the potential involvement of the cannabinoid receptor system in nimesulide-mediated effects at the level of the spinal cord. The ability of spinal pre-administration of the CB1 receptor antagonist AM251 (1 µg per 50 µL) to modulate nimesulide (25 µg per 50 µL)-mediated inhibition of neuronal firing was determined. AM251 alone did not alter mechanically evoked firing of dorsal horn neurones in the 30 min pre-administration period (Figure 3). AM251 pre-administration blocked the inhibitory effects of nimesulide on mechanically evoked responses of WDR dorsal horn neurones (Figure 4).
Figure 3.
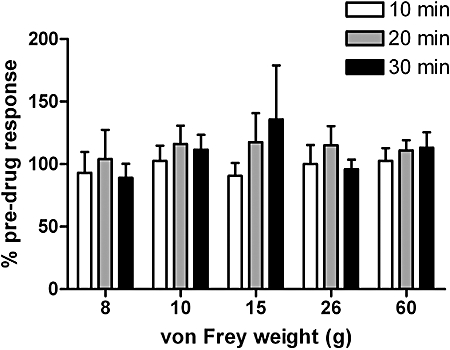
The CB1 antagonist AM251 (N-(piperidin-1-yl)-5-(-4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) (1 µg per 50 µL) alone did not alter mechanically evoked (8–60 g) responses of dorsal horn wide dynamic range neurones in naïve anaesthetized rats (n= 6). Data are expressed as a percentage of the pre-drug control ± SEM.
Figure 4.
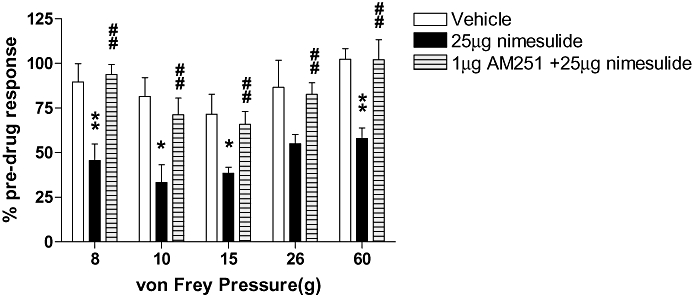
Spinal pretreatment with the CB1 receptor antagonist AM251 (N-(piperidin-1-yl)-5-(-4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) (1 µg per 50 µL) blocked the inhibitory effects of nimesulide (25 µg per 50 µL) on mechanically evoked (8–60 g) responses of wide dynamic range dorsal horn neurones in naïve anaesthetized rats in vivo (n= 6). Statistical analyses were performed with non-parametric Mann–Whitney test; *P < 0.05; **P < 0.01 versus vehicle; ##P < 0.01 versus 25 µg nimesulide. Data are expressed as a percentage of the pre-drug control ± SEM.
The effects of peripheral administration of nimesulide were also studied. Intra-plantar injection of 100 µg per 50 µL nimesulide did not alter mechanically evoked responses of dorsal horn neurones, compared with either pre-drug controls or vehicle (Table 1).
Table 1.
Effects of peripherally administered nimesulide (100 µg per 50 µL) or vehicle (50 µL) on mechanically evoked responses of dorsal horn wide dynamic range neurones in naïve anaesthetized rats
| von Frey stimulus |
Vehicle (3% Tween 80 in saline) (percentage of pre-drug firing rate) |
Nimesulide 100 µg (percentage of pre-drug firing rate) |
||||
|---|---|---|---|---|---|---|
| 10 min | 30 min | 50 min | 10 min | 30 min | 50 min | |
| 8 g | 112.9 ± 41.7 | 103.5 ± 7.8 | 97.8 ± 8.8 | 141.3 ± 53.9 | 138.0 ± 19.1 | 163.6 ± 35.2 |
| 10 g | 143.5 ± 38.8 | 112.4 ± 15.3 | 106.3 ± 20.5 | 168.8 ± 18.9 | 118.2 ± 16.9 | 87.8 ± 15.3 |
| 15 g | 128.2 ± 26.6 | 104.1 ± 13.0 | 138.8 ± 39.6 | 117.5 ± 19.9 | 104.9 ± 16.8 | 77.4 ± 9.8 |
| 26 g | 97.9 ± 20.6 | 102.7 ± 17.9 | 123.2 ± 21.6 | 107.4 ± 16.7 | 111.8 ± 15.4 | 103.8 ± 12.8 |
| 60 g | 99.2 ± 17.1 | 95.7 ± 9.5 | 101.9 ± 15.3 | 95.4 ± 8.9 | 95.4 ± 9.2 | 100.5 ± 9.7 |
Data are expressed as a percentage of the pre-drug control ± SEM (n= 6).
Spinal administration of nimesulide decreased spinal levels of endocannabinoids and related molecules
To determine the potential contribution of changes in levels of endocannabinoids to the CB1 receptor-mediated effects of nimesulide, the effects of nimesulide (25 µg; n= 8, and 100 µg; n= 6) versus vehicle (n= 14) on spinal levels of endocannabinoids and related molecules in vivo were determined. Nimesulide significantly decreased levels of AEA (25 µg P < 0.005, 100 µg P < 0.01) and OEA (100 µg P < 0.01), without altering levels of 2-AG in the spinal cord of rats (Figure 5).
Figure 5.
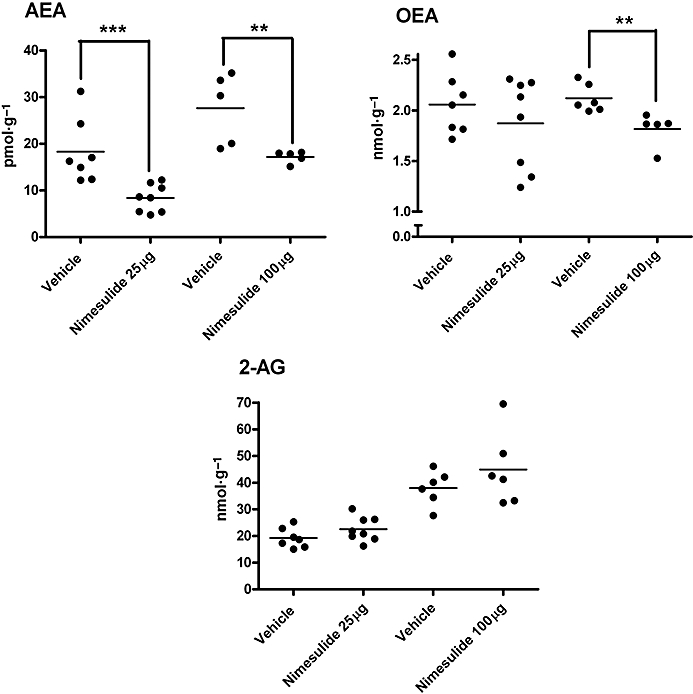
Effects of spinal nimesulide on levels of anandamide (AEA), N-oleoylethanolamine (OEA) and 2-arachidonoylglycerol (2-AG) in spinal cord of naïve anaesthetized rats. Two analytical runs were conducted; effects of nimesulide 25 µg per 50 µL versus 50 µL vehicle (n= 8 samples per group) were analysed in one LC/MS run, followed by nimesulide 100 µg per 50 µL versus 50 µL vehicle (n= 6 samples per group) in a second analytical run. Statistical analyses were performed with non-parametric Mann–Whitney test; **P < 0.01, ***P < 0.005 nimesulide versus vehicle. Data are expressed as individual values and median value is depicted by the line.
Discussion and conclusions
In the present study, spinal, but not peripheral, administration of the COX-2 inhibitor nimesulide reduced mechanically evoked firing of WDR dorsal horn neurones in the anaesthetized rat. The effects of nimesulide were blocked by a CB1 receptor antagonist, and accompanied by a decrease in spinal levels of AEA and OEA; levels of 2-AG were unaltered compared with matched vehicle controls. Our pharmacological data support the proposal that COX-2-mediated analgesia is mediated at least in part by CB1 receptors. The association of the inhibitory effect of nimesulide with a decrease in the levels of AEA and OEA suggests that there are complex interactions between the effects of metabolizing enzymes and levels of endocannabinoids and related compounds in vivo.
Spinal administration of the COX-2 inhibitor nimesulide dose-dependently reduced mechanically evoked responses of dorsal horn neurones to both non-noxious and noxious stimuli, compared with both pre-drug responses and effects of vehicle. Maximal inhibitory effects of nimesulide were observed with a dose of 25 µg, and occurred at approximately 30 min post administration for all of the evoked responses studied. These data are consistent with the established constitutive expression of COX-2 by the spinal cord (Ghilardi et al., 2004) and indicate that COX-2 has a role in modulating the responses of spinal neurones in naïve rats. Previously, both systemic and intrathecal administration of a COX-2 inhibitor has been shown to suppress acute thermal hyperalgesia and reduce the spinal release of prostaglandin E2 (Yaksh et al., 2001). Further work has indicated a role of spinal prostaglandins in the development, but not the maintenance of spinal hyperexcitability produced by joint inflammation (Vasquez et al., 2001). Thus, the effects of nimesulide on mechanically evoked responses of spinal neurones may arise, at least in part, from decreased levels of prostaglandin E2.
In the present study, the inhibitory effects of spinal nimesulide on mechanically evoked responses of dorsal horn neurons were blocked by pre-administration of a CB1 receptor antagonist, AM251, implicating a role of the endocannabinoid receptor system. Despite this pharmacological evidence the two higher doses of nimesulide, both of which inhibited neuronal responses, decreased levels of AEA and OEA in the spinal cord, while levels of 2-AG remained unchanged. Our electrophysiological data are consistent with the report that the inhibitory effects of spinal administration of indomethacin are also blocked by AM251 (Guhring et al., 2002). It is important to note, however, that unlike indomethacin (Fowler et al., 2003; Holt et al., 2007), nimesulide does not inhibit FAAH (Fowler et al., 2003) and therefore our data implicate a direct role of COX-2 in the regulation of endocannabinoid function in vivo. Recently, the effects of two COX-2 inhibitors (NS398 and L-745 337) on evoked neuronal responses in naïve rats and in a model of joint inflammation have been reported (Telleria-Diaz et al., 2010). In this study, spinal administration of the COX-2 inhibitor L-745 337 did not alter evoked neuronal responses in naïve rats, but attenuated responses in the model of joint inflammation. Although this previous study only investigated the effects of the COX-2 inhibitor NS398 on the spinal release of 2-AG, it is of interest to note that spinal NS398 did not alter spinal release of 2-AG compared with the pre-drug application period (Telleria-Diaz et al., 2010). These data are consistent with our observation that spinal nimesulide, at doses that attenuate evoked neuronal responses in a CB1 receptor-dependent manner, does not alter levels of 2-AG in the spinal cord.
In contrast to the effects of spinally administered nimesulide, intra-plantar injection of nimesulide did not alter mechanically evoked responses of WDR dorsal horn neurones in naïve rats, consistent with the established literature that COX-2 is not constitutively expressed in peripheral tissue (Vane et al., 1998). Under conditions of peripheral inflammation, hindpaw injection of the COX-2 inhibitor rofecoxib enhances the inhibitory effects of AEA on pain behaviour and elevates levels of AEA, OEA and N-palmitoylethanolamide in the hindpaw, indicating a role of peripheral COX-2 in the modulation of endocannabinoid function under these conditions (Guindon et al., 2006).
It is established from in vitro studies that COX-2 can metabolize AEA and 2-AG (Yu et al., 1997; Kozak et al., 2004). Furthermore, the physiological relevance of COX-2 regulation of endocannabinoids has been demonstrated in work using the hippocampal slice preparation, although levels of endocannabinoids were not measured (Slanina and Schweitzer, 2005). Evidence for a role of COX-2 metabolism of endocannabinoids is further supported by the detection of COX-2 metabolites of AEA in FAAH knockout mice dosed with AEA (Weber et al., 2004) and the presence of COX-2 metabolites of 2-AG in the rat (Hu et al., 2008). Our data demonstrating a mismatch between the pharmacological effects of nimesulide mediated by the CB1 receptor and levels of endocannabinoids suggest that, at least at the level of the spinal cord, the COX-2 regulation of endocannabinoids is complex.
The mechanism by which inhibition of COX-2 by nimesulide can produce CB1 receptor-dependent effects in the absence of overt increases in levels of AEA or 2-AG is unclear, but may involve catabolism via other pathways. Indeed, it has been established that LOXs (Hampson et al., 1995; Ueda et al., 1995) and cP450 enzymes (Snider et al., 2007; 2009; Awumey et al., 2008; Stark et al., 2008) metabolize AEA and 2-AG. Importantly, a number of the LOX and cP450 metabolites of the endocannabinoids and their precursor, arachidonic acid, have activity at the CB1 receptor. For example, the LOX-12 metabolite of AEA, 12-(S)-hydroxyarachidonoylethanolamide (12-hydroxyanandamide, 12-HAEA) binds the CB1 receptor with affinity comparable to, or greater than, that of AEA (Hampson et al., 1995; Edgemond et al., 1998). cP450 metabolism of AEA and its precursor arachidonic acid results in the formation of eicosatrienoic acids (EETs), in particular the 2-epoxyeicosatrienol glycerols (2-EG), 2-(11,12-epoxyeicosatrienol)glycerol (2-11,12-EG), 2-(14,15-epoxyeicosatrienol)glycerol (2-14,15-EG) from arachidonic acid (Chen et al., 2008) and 2-(5,6-epoxyeicosatrienol)ethanolamide (5,6-EET-EA) from AEA (Stark et al., 2008; Snider et al., 2009), which are agonists at CB1 receptors with comparable or greater binding affinity than 2-AG (Chen et al., 2008).
Although speculative, the metabolism of endocannabinoids via some, or all of the pathways described above, may be increased when COX-2 is inhibited by nimesulide. Nevertheless, a rigorous investigation of this hypothesis, including the in vivo measurement of alternative metabolites of AEA and 2-AG alongside behavioural or neuronal measurements of ant-nociceptive responses, is required to substantiate this proposal. At the present time it is not possible to confirm this hypothesis as, to the best of our knowledge, deuterated standards for these novel CB ligands are not available. The generation of these standards is crucial for further elucidation, via LC-MS/MS techniques, of the role of these pathways in the regulation of endocannabinoid levels in vivo.
In conclusion, we have demonstrated a major contribution of the CB1 receptor in mediating nimesulide-induced inhibition of the spinal processing of innocuous and noxious inputs; however, these effects occur alongside a decrease in spinal levels of AEA and no change in levels of 2-AG.
Acknowledgments
This work was funded by GSK, the MRC and the University of Nottingham.
Glossary
Abbreviations:
- 2-AG
2-arachidonoylglycerol
- 2-AG-d8
deuterated 2-arachidonoylglycerol
- 5,6-EET-EA
2-(5,6-epoxyeicosatrienol)ethanolamide
- 2-EG
2-epoxyeicosatrienol glycerol
- 2-11,12-EG
2-(11,12-epoxyeicosatrienol)glycerol
- 2-14,15-EG
2-(14,15-epoxyeicosatrienol)glycerol
- 12-HAEA
12-(S)-hydroxyarachidonoylethanolamide
- AEA
anandamide
- AEA-d8
dueterated anandamide
- AM251
N-(piperidin-1-yl)-5-(-4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- CB1
cannabinoid receptor 1
- COX
cyclooxygenase
- cP450
cytochrome p450
- EET
eicosatrienoic acid
- FAAH
fatty acid amide hydrolase
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LOX
lipoxygenase
- NSAID
non-steroidal anti-inflammatory drug
- OEA
N-oleoylethanolamide
- WDR
wide dynamic range
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153:S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awumey EM, Hill SK, Diz DI, Bukoski RD. Cytochrome P-450 metabolites of 2-arachidonoylglycerol play a role in Ca2+-induced relaxation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2008;294:H2363–H2370. doi: 10.1152/ajpheart.01042.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornheim LM, Kim KY, Chen B, Correia MA. The effect of cannabidiol on mouse hepatic microsomal cytochrome P450-dependent anandamide metabolism. Biochem Biophys Res Commun. 1993;197:740–746. doi: 10.1006/bbrc.1993.2541. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen JK, Chen J, Imig JD, Wei S, Hachey DL, Guthi JS, et al. Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283:24514–24524. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, Glaser ST, Howell JM, Kunz JS, Puffenbarger RA, Hillard CJ, et al. The cellular uptake of anandamide is coupled to its breakdown by fatty-acid amide hydrolase. J Biol Chem. 2001;276:6967–6973. doi: 10.1074/jbc.M003161200. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002a;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chem Phys Lipids. 2002b;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Human platelets and polymorphonuclear leukocytes synthesize oxygenated derivatives of arachidonylethanolamide (anandamide): their affinities for cannabinoid receptors and pathways of inactivation. Mol Pharmacol. 1998;54:180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- Famaey JP. In vitro and in vivo pharmacological evidence of selective cyclooxygenase-2 inhibition by nimesulide: an overview. Inflamm Res. 1997;46:437–446. doi: 10.1007/s000110050221. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Stenstrom A, Tiger G. Ibuprofen inhibits the metabolism of the endogenous cannabimimetic agent anandamide. Pharmacol Toxicol. 1997;80:103–107. doi: 10.1111/j.1600-0773.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Janson U, Johnson RM, Wahlstrom G, Stenstrom A, Norstrom K, et al. Inhibition of anandamide hydrolysis by the enantiomers of ibuprofen, ketorolac, and flurbiprofen. Arch Biochem Biophys. 1999;362:191–196. doi: 10.1006/abbi.1998.1025. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Holt S, Tiger G. Acidic nonsteroidal anti-inflammatory drugs inhibit rat brain fatty acid amide hydrolase in a pH-dependent manner. J Enzyme Inhib Med Chem. 2003;18:55–58. doi: 10.1080/1475636021000049726. [DOI] [PubMed] [Google Scholar]
- Ghilardi JR, Svensson CI, Rogers SD, Yaksh TL, Mantyh PW. Constitutive spinal cyclooxygenase-2 participates in the initiation of tissue injury-induced hyperalgesia. J Neurosci. 2004;24:2727–2732. doi: 10.1523/JNEUROSCI.5054-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhring H, Hamza M, Sergejeva M, Ates M, Kotalla CE, Ledent C, et al. A role for endocannabinoids in indomethacin-induced spinal antinociception. Eur J Pharmacol. 2002;454:153–163. doi: 10.1016/s0014-2999(02)02485-8. [DOI] [PubMed] [Google Scholar]
- Guindon J, Lo Verme J, De Lean A, Piomelli D, Beaulieu P. Synergistic antinociceptive effects of anandamide, an endocannabinoid, and nonsteroidal anti-inflammatory drugs in peripheral tissue: a role for endogenous fatty-acid ethanolamides. Eur J Pharmacol. 2006;550:68–77. doi: 10.1016/j.ejphar.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Hill WA, Zan-Phillips M, Makriyannis A, Leung E, Eglen RM, et al. Anandamide hydroxylation by brain lipoxygenase:metabolite structures and potencies at the cannabinoid receptor. Biochim Biophys Acta. 1995;1259:173–179. doi: 10.1016/0005-2760(95)00157-8. [DOI] [PubMed] [Google Scholar]
- Holt S, Paylor B, Boldrup L, Alajakku K, Vandevoorde S, Sundstrom A, et al. Inhibition of fatty acid amide hydrolase, a key endocannabinoid metabolizing enzyme, by analogues of ibuprofen and indomethacin. Eur J Pharmacol. 2007;565:26–36. doi: 10.1016/j.ejphar.2007.02.051. [DOI] [PubMed] [Google Scholar]
- Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol. 2008;153:1538–1549. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L, Chapman V. Cannabinoids: a real prospect for pain relief? Curr Opin Pharmacol. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Richardson D, Kendall DA, Barrett DA, Chapman V. Analgesic effects of fatty acid amide hydrolase inhibition in a rat model of neuropathic pain. J Neurosci. 2006;26:13318–13327. doi: 10.1523/JNEUROSCI.3326-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. Pain. 2004;109:432–442. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- Kerola M, Vuolteenaho K, Kosonen O, Kankaanranta H, Sarna S, Moilanen E. Effects of nimesulide, acetylsalicylic acid, ibuprofen and nabumetone on cyclooxygenase-1- and cyclooxygenase-2-mediated prostanoid production in healthy volunteers ex vivo. Basic Clin Pharmacol Toxicol. 2009;104:17–21. doi: 10.1111/j.1742-7843.2008.00332.x. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JJ, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des. 2004;10:659–667. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, et al. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Petrosino S, Marabese I, Vita D, Giordano C, et al. Neuropathic pain and the endocannabinoid system in the dorsal raphe: pharmacological treatment and interactions with the serotonergic system. Eur J Neurosci. 2006;24:2011–2020. doi: 10.1111/j.1460-9568.2006.05086.x. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. doi: 10.1016/s0301-0082(00)00031-9. [DOI] [PubMed] [Google Scholar]
- Petrosino S, Palazzo E, de Novellis V, Bisogno T, Rossi F, Maione S, et al. Changes in spinal and supraspinal endocannabinoid levels in neuropathic rats. Neuropharmacology. 2007;52:415–422. doi: 10.1016/j.neuropharm.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Prusakiewicz JJ, Duggan KC, Rouzer CA, Marnett LJ. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochem. 2009;48:7353–7355. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Shah AA, Thjodleifsson B, Murray FE, Kay E, Barry M, Sigthorsson G, et al. Selective inhibition of COX-2 in humans is associated with less gastrointestinal injury: a comparison of nimesulide and naproxen. Gut. 2001;48:339–346. doi: 10.1136/gut.48.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina KA, Schweitzer P. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology. 2005;49:653–659. doi: 10.1016/j.neuropharm.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Snider NT, Kornilov AM, Kent UM, Hollenberg PF. Anandamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Ther. 2007;321:590–597. doi: 10.1124/jpet.107.119321. [DOI] [PubMed] [Google Scholar]
- Snider NT, Sikora MJ, Sridar C, Feuerstein TJ, Rae JM, Hollenberg PF. The endocannabinoid anandamide is a substrate for the human polymorphic cytochrome P450 2D6. J Pharmacol Exp Ther. 2008;327:538–545. doi: 10.1124/jpet.108.141796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Nast JA, Tesmer LA, Hollenberg PF. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol Pharmacol. 2009;75:965–972. doi: 10.1124/mol.108.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal DM, Chapman V. Spinal GABA(B)-receptor antagonism increases nociceptive transmission in vivo. Neuroreport. 2001;12:3247–3250. doi: 10.1097/00001756-200110290-00021. [DOI] [PubMed] [Google Scholar]
- Stark K, Dostalek M, Guengerich FP. Expression and purification of orphan cytochrome P450 4X1 and oxidation of anandamide. FEBS J. 2008;275:3706–3717. doi: 10.1111/j.1742-4658.2008.06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria-Diaz A, Schmidt M, Kreusch S, Neubert AK, Schache F, Vazquez E, et al. Spinal antinociceptive effects of cyclooxygenase inhibition during inflammation: Involvement of prostaglandins and endocannabinoids. Pain. 2010;148:26–35. doi: 10.1016/j.pain.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, et al. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochim Biophys Acta. 1995;1254:127–134. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vasquez E, Bar KJ, Ebersberger A, Klein B, Vanegas H, Schaible HG. Spinal prostaglandins are involved in the development but not the maintenance of inflammation-induced spinal hyperexcitability. J Neurosci. 2001;21:9001–9008. doi: 10.1523/JNEUROSCI.21-22-09001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol Ther. 2002;95:127–135. doi: 10.1016/s0163-7258(02)00252-8. [DOI] [PubMed] [Google Scholar]
- Weber A, Ni J, Ling KH, Acheampong A, Tang-Liu DD, Burk R, et al. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J Lipid Res. 2004;45:757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Dirig DM, Conway CM, Svensson C, Luo ZD, Isakson PC. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J Neurosci. 2001;21:5847–5853. doi: 10.1523/JNEUROSCI.21-16-05847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]


