Abstract
Background and purpose:
The endocannabinoid-like molecule N-oleoylethanolamine (OEA) is found in the small intestine and regulates food intake and promotes weight loss. The principal aim of the present study was to evaluate the vascular effects of OEA.
Experimental approach:
Perfused isolated mesenteric arterial beds were pre-contracted with methoxamine or high potassium buffers and concentration-response curves to OEA were constructed. Combinations of inhibitors to block nitric oxide production, sensory nerve activity, cyclooxygenase activity, potassium channels, chloride channels and gap junctions, and a cannabinoid CB1 receptor antagonist, were used during these experiments. The effects of OEA on caffeine-induced contractions in calcium-free buffer were also assessed. Isolated thoracic aortic rings were used as a comparison.
Key results:
OEA caused concentration-dependent vasorelaxation in rat isolated mesenteric arterial beds and thoracic aortic rings, with a greater maximal response in mesenteric vessels. This relaxation was sensitive to inhibition of sensory nerve activity and endothelial removal in both preparations. The cyclooxygenase inhibitor indomethacin reversed the effects of capsaicin pre-treatment in perfused mesenteric arterial beds and indomethacin alone enhanced vasorelaxation to OEA. The OEA-induced vasorelaxation was inhibited by a CB1 receptor antagonist only in aortic rings. In mesenteric arteries, OEA suppressed caffeine-induced contractions in calcium-free buffer.
Conclusions and implications:
The vasorelaxant effects of OEA are partly dependent on sensory nerve activity and a functional endothelium in the vasculature. In addition, vasorelaxation to OEA is enhanced following cyclooxygenase inhibition. OEA may also interfere with the release of intracellular calcium in arterial preparations.
This article is part of a themed issue on Cannabinoids. To view the editorial for this themed issue visit http://dx.doi.org/10.1111/j.1476-5381.2010.00831.x
Keywords: N-Oleoylethanolamine, cannabinoid, sensory nerves, endothelium, mesenteric arterial beds, caffeine, cyclooxygenase
Introduction
N-Oleoylethanolamine (OEA) is an endocannabinoid-like molecule (Alexander and Kendall, 2007) that is found in the small intestine and adipose tissue, which acts to reduce food intake via the cannabinoid-like receptor GPR119 and cause weight loss by increased fatty acid metabolism and oxidation (Fu et al., 2003; Guzman et al., 2004; Lo Verme et al., 2005; Overton et al., 2006). Its release in the gastrointestinal tract is promoted by dietary lipids where it acts as a local messenger (Schwartz et al., 2008). In the CNS, OEA acts via peroxisome proliferator-activated receptor (PPAR)-α to act as a neuroprotectant (Sun et al., 2007) and to promote memory consolidation (Campolongo et al., 2009).
OEA is an N-acylethanolamide that is synthesized from an N-acylphosphatidylethanolamine (NAPE), where the acyl group is oleic acid, by a NAPE-specific phospholipase D in phospholipid membranes (Hansen et al., 2000; Okamoto et al., 2004). A second synthetic pathway for OEA has also been determined, whereby NAPE is hydrolysed by phospholipase A2 to lyso-NAPE, and is then further metabolized by lysophospholipase D to OEA (Lo Verme et al., 2005). Like the more commonly studied N-acylethanolamide, anandamide, OEA is synthesized on demand rather than being stored, and can be metabolized into ethanolamine and oleic acid by fatty acid amide hydrolase (FAAH) or N-acylethanolamine-hydrolysing acid amidase (NAAA) (Ueda et al., 2001; Tsuboi et al., 2005). Tissue levels of OEA are consistently higher than those of anandamide in brain, jejunum and liver samples (Richardson et al., 2007; Artmann et al., 2008). In rat mesenteric arteries, tissue levels of OEA are approximately 100 times that of anandamide (Ho et al., 2008), and in rat plasma, the concentration of OEA is approximately 10 nM (Giuffrida et al., 2000).
Cardiovascular responses to endocannabinoids in anaesthetized animals are complex, but usually involve a long-lasting hypotension. The prototypical endocannabinoid anandamide is a vasorelaxant in vitro, yet its mechanism of action is multi-factorial and depends on the species and preparation used. Anandamide can cause vasorelaxation via ‘classical’ CB1 cannabinoid receptors (nomenclature follows Alexander et al., 2009), via a putative endothelial cannabinoid receptor, via sensory nerve activation, or the release of nitric oxide, vasoactive prostanoids or endothelium-derived hyperpolarizing factor (EDHF) (see Randall et al., 2004). There is also evidence that anandamide interferes with intracellular calcium release in vascular smooth muscle preparations (Zygmunt et al., 1997; White and Hiley, 1998). In addition to acute vasorelaxation, some cannabinoids also activate PPARγ, leading to time-dependent vasorelaxation (for a review see O'Sullivan, 2007). Structural similarities to anandamide, its lipophilicity, and signalling via sensory nerves, GPR119 and PPARγ, but no activity at ‘classical’ CB receptors have led to OEA being categorized as an endocannabinoid-like molecule.
Most research into the effects of OEA has focussed on its properties as a satiety factor. However, OEA is also a vasorelaxant of first-, second- and third-order mesenteric arteries, and is reported to be slightly less potent than anandamide (Movahed et al., 2005; Ho et al., 2008). Mesenteric vasorelaxation occurs at least in part via vanilloid (TRPV1) receptors on sensory nerves, an effect which may be due to structural similarities of OEA and anandamide with the TRPV1 agonist capsaicin (Movahed et al., 2005; Ho et al., 2008). Furthermore, in third order mesenteric arteries, vasorelaxation to OEA is partly endothelium-dependent (Ho et al., 2008). There have also been reports of interplay between OEA and anandamide, whereby 1 µM OEA potentiated vasorelaxation to anandamide in small mesenteric arteries via a sensory nerve-dependent, but endothelium-independent, mechanism (Ho et al., 2008).
Given that the tissue levels of OEA are approximately 100 times that of anandamide in rat mesenteric arteries, it is important to understand the effect of this endocannabinoid-like molecule on the vasculature (Ho et al., 2008). These studies aimed to further investigate the mechanism of action of OEA in the mesenteric arterial bed.
Methods
Animals
All animal care and experimental procedures were in accordance with the UK Animal (Scientific Procedures) Act 1986. Male, Wistar rats (200–350 g) (Charles River, Margate, UK) were housed in the University of Nottingham Biomedical Services Unit with access to food and water ad libitum. All rats were stunned by a blow to the head and killed by cervical dislocation prior to isolation of the arterial tissue.
Perfused mesenteric arterial beds
The abdominal cavity was opened by a midline incision, the superior mesenteric artery was cleared of fat and connective tissue and then cannulated in situ. The whole mesenteric vascular bed was then detached from the gastrointestinal tract, removed from the rat, and perfused with warmed (37°C) and gassed (95% O2/5% CO2) modified Krebs'-Henseleit buffer (composition mM: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 d-glucose, 2 CaCl2) at a flow rate of 5 mL·min−1 (McCulloch et al., 1997).
In some preparations, the endothelium was removed by perfusion of distilled water for two cycles of 10 min (separated by a gap of approximately 30 min to avoid tissue hypoxia or washing out of the salts and nutrients). Removal of the endothelium was confirmed if 1 µM carbachol failed to reduce methoxamine-induced tone by more than 20%.
Isolated thoracic aortic rings
Thoracic aortae were dissected free of fat and connective tissue, then removed and cut into sections (approximately 4 mm in length) and mounted on metal clips attached by thread to an isometric force displacement transducer (LETICA 210, Barcelona, Spain), then placed in 50 mL organ baths. In one group of rings, the endothelium was removed by gentle rubbing with a matchstick prior to mounting. All vessels were then left to equilibrate at a resting tension of 9.8 mN for 1 h in warmed, gassed modified Krebs'-Henseleit buffer (composition described earlier).
Isolated mesenteric arteries
The superior mesenteric artery was located and removed. Next, the complete mesenteric vascular bed, connected to the intestines, was dissected free from the abdominal cavity, and transferred into Krebs-Henseleit buffer with the composition described above. Segments of first-order branches off the superior mesenteric artery (G1), 2 mm in length, were dissected from the mesenteric arterial bed and cleared of connective and adipose tissue. The superior mesenteric artery (G0) was also finely dissected and segmented at this time. Vessels were then mounted on tungsten wires (40 µm diameter) on a multi channel wire myograph (Model 610M, Danish Myo Technology, Denmark) (Mulvany and Halpern, 1977), and kept at 37°C in Krebs–Henseleit buffer, gassed with 5% CO2/95% O2, with a resting tension of 9.8 mN (O'Sullivan et al., 2004). Tension was measured and recorded on a PowerLab 8/30 recording system (ADInstruments, Chalgrove, Oxfordshire, UK).
Experimental protocols
Preparations were allowed to equilibrate for 1 h, either in the absence or presence of 10 µM capsaicin in order to deplete the sensory nerves of their neurotransmitters (Zygmunt et al., 1999; Harris et al., 2002). Pre-treatment with capsaicin is also thought to cause rapid desensitization of TRPV1 receptors, and may even cause a conformational change in TRPV1 receptor structure, causing the ion channel pore to close (Szallasi and Blumberg, 1999). Indomethacin (a cyclooxygenase inhibitor, 3 µM), flurbiprofen (a cyclooxygenase inhibitor, 10 µM), L-NAME (a nitric oxide synthase inhibitor, 300 µM), carbenoxolone (a gap junction inhibitor, 50 µM) and vapiprost (a thromboxane A2 (TP) receptor antagonist, 30 nM) were present in the buffer throughout some experiments as indicated (Harris et al., 2002).
In some preparations, the involvement of CB1 receptors in OEA-induced vasorelaxation was investigated by inclusion of the CB1 receptor antagonist AM251 (1 µM) in the buffer, before being contracted with the a-adrenoceptor agonist methoxamine. Although 1 µM AM251 is a relatively high concentration, it is still considered a concentration that avoids non-specific actions (White et al., 2001). AM251 is of similar potency to rimonabant at CB1 receptors, with a Ki value of 7.49 nM in rat forebrain, and has greater selectivity for CB1 over CB2 receptors (Ki at CB2 receptors = 2290 nM) (Lan et al., 1999). Therefore, AM251 is routinely used at this concentration. However, at this concentration, binding to CB2 receptors cannot be altogether excluded.
In some experiments, tone was raised by perfusion with high-potassium-containing modified Krebs'-Henseleit buffer (composition mM: 62.5 NaCl, 59.4 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 10 d-glucose, 2 CaCl2) (McCulloch et al., 1997). This was used to depolarize the arterial tissue and investigate the role of potassium channels and/or hyperpolarizing mechanisms.
After a stable tone was achieved using methoxamine or high-potassium buffer, concentration-response curves to OEA were constructed.
Preliminary experiments demonstrated that OEA relaxed the tone induced by methoxamine but not high potassium, suggesting that the relaxation may involve potassium channel activation or the involvement of EDHF. EDHF-mediated responses are non-nitric oxide and non-prostanoid in nature, and are thought to spread from the endothelium to the smooth muscle via myoendothelial gap junctions causing hyperpolarization, and ultimately vasorelaxation, of the smooth muscle (Busse et al., 2002). Therefore, the involvement of EDHF was assessed by removal of the endothelium (as described earlier), and by investigating the effects of the gap junction inhibitor carbenoxolone (50 µM) in the presence of L-NAME and indomethacin (Harris et al., 2002). EDHF-dependent responses and the involvement of potassium channels were investigated by the inclusion of 10 mM tetraethylammonium (TEA) as a non-selective potassium channel inhibitor (McCulloch et al., 1997). The possible involvement of chloride channels in any hyperpolarizing mechanism was investigated by the addition of niflumic acid (10 µM) (Criddle et al., 1997).
Given the sensitivity of vasorelaxation by OEA to methoxamine- but not high-potassium-induced tone, another possibility is that OEA may interfere with the release of intracellular calcium (White and Hiley, 1998). In order to test this possibility, mesenteric arterial beds were perfused with calcium-free buffer and then perfused with a 5 min pulse of 50 mM caffeine, which induced a transient increase in pressure (due to the release of intracellular calcium). Preparations were then perfused with calcium-containing buffer to replenish the intracellular calcium stores, after which they were then perfused with calcium-free buffer, and the response to 50 mM caffeine was repeated in the presence of 10 µM OEA. Preliminary experiments indicated that using this protocol, the caffeine responses in the absence of inhibitors were consistent and reproducible over time (data not shown). These experiments were also conducted following capsaicin pre-treatment to remove any sensory nerve involvement in the response.
In light of the enhancement of responses to anandamide in the presence of low concentrations of OEA (Ho et al., 2008), we examined whether the presence of anandamide (1 µM) in the perfusion fluid affected responses to OEA in the mesenteric arterial bed. In view of the fact that anandamide has vasorelaxant effects alone, this was controlled for by using controls with a matched level of methoxamine-induced pre-contraction.
Statistical analysis
Data are expressed as a mean percentage relaxation of methoxamine- or potassium-induced tone, with error bars representing standard error of the mean (s.e. mean), and n being the number of individual preparations used per group. Sigmoidal concentration-response curves with a variable slope were fitted to these data using a four parameter logistic equation in Prism (Version 5; GraphPad Software, La Jolla, CA, USA). From these curves, pEC50 (negative log of the concentration required to relax the induced tone by half) and Rmax (maximal values of percentage relaxation) values were calculated. When sigmoidal curves failed to reach a plateau, pECX% values (negative log of the concentration required to relax the induced tone by X%) were calculated. Where it was not possible to fit a sigmoidal curve to a dataset (e.g. in Figure 2), a cubic spline curve was fitted instead.
Figure 2.
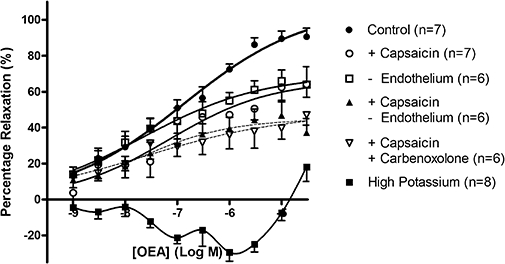
Vasorelaxation to OEA in perfused mesenteric arterial beds. Responses are shown under control conditions, following pre-treatment with 10 µM capsaicin to deplete sensory nerves, or following removal of the endothelium. Combination of both capsaicin pre-treatment and removal of endothelium, or 50 µM carbenoxolone, was also investigated. The effects of pre-contraction with high potassium buffer are also shown. Data points are means ± s.e. mean, with n representing the number of animals per treatment group.
Groups were compared by unpaired Student's t-test, or one-way anova followed by a post hoc test for multiple comparisons. In some situations, two-way anovas were used. In the case of the experiments with caffeine, increases in perfusion pressure were compared in the absence and presence of OEA using Student's paired t-test. P < 0.05 was taken as significant.
Materials
All drugs were supplied by Sigma (Poole, Dorset, UK), with the exception of OEA, which was synthesized in-house by Dr SPH Alexander. Stock solutions (10 mM) of OEA (in dimethylsulphoxide) and anandamide (in ethanol) were further diluted with distilled water. Likewise, stock solutions of capsaicin, indomethacin and flurbiprofen were made in ethanol, whilst niflumic acid, methoxamine and vapiprost (GR 32191) were dissolved in distilled water. Tetraethylammonium and caffeine were added directly to the Krebs-Henseleit buffer.
Results
The effects of AM251 on vasorelaxation to OEA in perfused mesenteric arterial beds
In perfused mesenteric arterial beds, the basal tone (perfusion pressure) was 37.7 ± 4.6 mmHg (n= 6), which was raised to 118.7 ± 8.9 mmHg (n= 6) using methoxamine (26.7 ± 4.9 µM, n= 6). The role of CB1 cannabinoid receptors in the vasorelaxant response to OEA in perfused mesenteric arterial beds was investigated using the CB1 receptor antagonist AM251. Vasorelaxation to OEA was unaffected by the presence of 1 µM AM251 (control pEC50%= 6.12 ± 0.32, n= 6; AM251 pEC50%= 6.26 ± 0.22, n= 6) (Figure 1).
Figure 1.
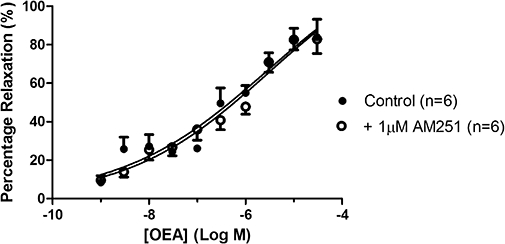
The effects of the cannabinoid CB1 receptor antagonist, AM251, on vasorelaxation to OEA in perfused mesenteric arterial beds. OEA was administered either in the absence or presence of 1 µM AM251. Data points are mean ± s.e. mean with n representing the number of animals used in each group.
The effects of capsaicin pre-treatment on vasorelaxation to OEA in perfused mesenteric arterial beds
In a different set of perfused mesenteric arterial beds, the basal perfusion pressure was 32.0 ± 1.2 mmHg (n= 7), which was raised to 95.6 ± 5.5 mmHg (n= 7) using methoxamine (21.9 ± 5.3 µM, n= 7). In the controls, OEA caused a concentration-dependent fall in methoxamine-induced tone (pEC50= 7.10 ± 0.26, Rmax= 97.8 ± 8.7%, n= 7) (Figure 2). The maximal vasorelaxation to OEA was significantly reduced following pre-treatment with capsaicin (Rmax= 66.5 ± 11.0%, n= 7; P < 0.05, one-way anova plus Bonferroni post hoc test), and to a similar degree following removal of the endothelium (Rmax= 69.4 ± 8.4%, n= 6). Combination of removal of the endothelium with capsaicin pre-treatment further attenuated these responses (capsaicin plus endothelium removal Rmax= 44.9 ± 5.4%, n= 6; P < 0.05, two-way anova vs. capsaicin pre-treatment alone), and the presence of the gap junction inhibitor carbenoxolone following capsaicin pre-treatment had similar effects (capsaicin plus carbenoxolone Rmax= 48.0 ± 12.3%, n= 6) (Figure 2).
The effects on high extracellular potassium on vasorelaxation to OEA
In perfused mesenteric arterial beds contracted using high potassium concentrations (basal pressure = 24.9 ± 1.2 mmHg, induced tone = 80.2 ± 4.0 mmHg, n= 8), OEA failed to evoke a significant vasorelaxation (Figure 2).
The effects of L-NAME and indomethacin on responses to OEA following pre-treatment with capsaicin
The inhibitory effects of capsaicin pre-treatment reported above (see Figure 2) were absent in the presence of L-NAME and indomethacin; in fact, vasorelaxation to OEA was restored to control levels (pEC50= 7.17 ± 0.31, Rmax= 88.1 ± 9.4%, n= 6 (pEC50%= 6.99 ± 0.31, n= 5)) (Figure 3). Furthermore, OEA caused vasorelaxation in the presence of L-NAME and indomethacin and following capsaicin pre-treatment, even when contracted using a high concentration of potassium, albeit with a significantly reduced potency (pEC50%= 4.95 ± 0.09, n= 6). This vasorelaxation was still significantly (P < 0.001, unpaired t-test) different from that observed in methoxamine-contracted preparations. In preparations pre-contracted with methoxamine, following capsaicin pre-treatment and in the presence of L-NAME and indomethacin, the responses to OEA were unaffected by the additional presence of the gap junction/EDHF inhibitor carbenoxolone (pEC50= 7.43 ± 0.44, Rmax= 91.3 ± 12.4%, n= 8) (Figure 3).
Figure 3.
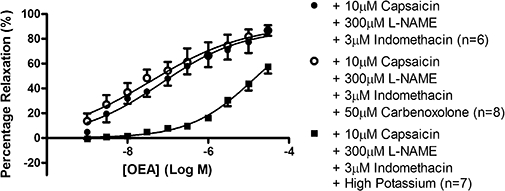
Vasorelaxation to OEA in perfused mesenteric arterial beds in the presence of 10 µM capsaicin pre-treatment, 300 µM L-NAME and 3 µM indomethacin, or those inhibitors and the presence of high potassium or 50 µM carbenoxolone. Data points are mean ± s.e. mean, with n representing the number of animals per group.
The effects of tetraethylammonium on vasorelaxation to OEA
In order to determine whether EDHF or potassium channels contributed towards vasorelaxation to OEA, some experiments were conducted in the presence of 10 mM TEA. TEA significantly enhanced (P < 0.0001, one-way anova plus Bonferroni post hoc test) vasorelaxation to OEA (control pEC50= 7.13 ± 0.32, Rmax= 89.4 ± 9.8%, n= 6; TEA pEC50= 10.5 ± 0.2, Rmax= 69.1 ± 3.6%, n= 6) (Figure 4).
Figure 4.
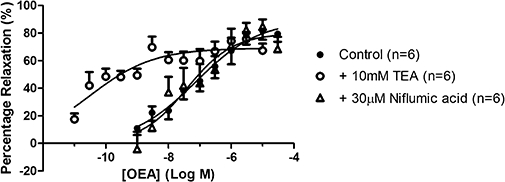
Vasorelaxation to OEA in perfused mesenteric arterial beds in the presence of 10 mM TEA or 10 µM niflumic acid. Data points are mean ± s.e. mean, with n representing the number of animals per group.
The effects of niflumic acid on vasorelaxation to OEA
In order to determine if chloride channel activation contributed towards OEA-induced vasorelaxation, some experiments were carried out in the presence of 10 µM niflumic acid. The presence of niflumic acid did not affect responses to OEA (pEC50= 7.4 ± 0.31, Rmax= 79.9 ± 8.9%, n= 6) (Figure 4).
The effects of cyclooxygenase inhibitors on vasorelaxation to OEA
In light of the reversal of the inhibitory effects of capsaicin on responses to OEA in the presence of L-NAME and indomethacin, we examined the effects of indomethacin alone on responses to OEA in the absence of and after pre-treatment with capsaicin (Figure 5). In these controls, OEA caused a concentration-dependent vasorelaxation in arterial beds (Rmax= 97 ± 11%; pEC50= 7.5 ± 0.4, n= 6) and these responses were similar to those in the presence of indomethacin after pre-treatment with capsaicin (Rmax= 83 ± 13%, n= 5). However, treatment with indomethacin alone significantly (P < 0.05) shifted the concentration-response curve to the left (pEC50 indomethacin = 9.5 ± 0.2, n= 7; indomethacin and capsaicin pEC50= 7.9 ± 0.7, n= 5). Another cyclooxygenase inhibitor, flurbiprofen (10 µM), had effects similar to those of indomethacin (Rmax= 95 ± 11%; pEC50= 9.0 ± 0.5, n= 6; data not shown)
Figure 5.
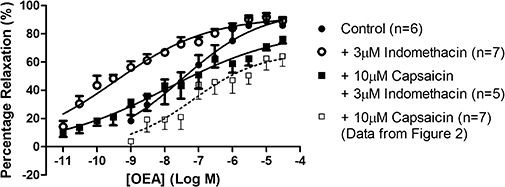
The effects of indomethacin on responses to OEA in the absence and presence of capsaicin pre-treatment in perfused mesenteric arterial beds. OEA was administered either alone, in the presence of 3 µM indomethacin, or a combination of 10 µM capsaicin pre-treatment and 3 µM indomethacin. Data for the vasorelaxation to OEA in the presence of capsaicin alone is repeated here from Figure 2 for ease of comparison to this earlier dataset. Data points are mean ± s.e. mean with n representing the number of animals used in each group.
Following the observations in whole perfused mesenteric arterial beds, the mechanism of enhanced vasorelaxation in the presence of cyclooxygenase inhibition was explored further. In individual vessels of the mesenteric arterial bed, specifically the superior mesenteric artery (G0) and its first-order branches (G1), vasorelaxation to OEA was investigated in the absence and presence of a more specific cyclooxygenase inhibitor, flurbiprofen (10 µM).
OEA caused a concentration-dependent vasorelaxation in isolated mesenteric arteries (G0 pEC50%= 6.79 ± 1.09, n= 5; G1 pEC50= 7.67 ± 0.65, Rmax= 113 ± 14%, n= 10) (Figure 6). This vasorelaxation was not affected by flurbiprofen in G0 arteries, but it was significantly enhanced in the G1 arteries (G1 plus flurbiprofen, pEC50= 8.57 ± 0.22; P < 0.05, comparison of whole datasets by two-way anova vs. control). Incubation of G1 vessels with the thromboxane A2 (TP) receptor antagonist vapiprost (30 nM) also significantly augmented vasorelaxation to OEA (pEC50= 9.25 ± 0.23, n= 6) and this enhancement resulted in responses that were not different from those in the presence of flurbiprofen alone (two-way anova) (Figure 6).
Figure 6.
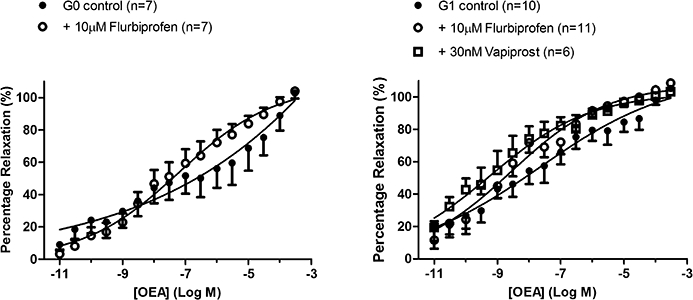
The effect of 10 µM flurbiprofen on responses to OEA in, A) the superior mesenteric artery (G0) and B) its first order branches (G1). Responses in the presence of 30 nM vapiprost are also shown. Data points are mean ± s.e. mean with n representing the number of animals used in each group.
The effects of OEA on caffeine-induced increases in perfusion pressure in calcium-free buffer
In light of the finding that OEA relaxed methoxamine-induced tone but not high-potassium-induced tone, another possibility was that OEA interferes with the release of intracellular calcium. This hypothesis was tested by examining the effects of OEA on caffeine-induced transient increases in perfusion pressure which are likely to be due to the release of intracellular calcium. In calcium-free buffer, 50 mM caffeine led to a transient increase in perfusion pressure (n= 7; Figure 7). However, after the stores were replenished by perfusion with calcium-containing buffer followed by calcium-free buffer, the presence of 10 µM oleoylethanolamide significantly reduced the caffeine-induced increase in perfusion pressure (P < 0.001; Figure 7). OEA alone did not affect the basal perfusion pressure (basal pressure before incubation = 26.4 ± 1.5 mmHg, n= 7; pressure after incubation with OEA = 27.9 ± 1.4 mmHg, n= 7). This experiment was repeated following initial capsaicin pre-treatment in order to determine if the inhibition of caffeine responses was independent of sensory nerve activity. OEA did indeed inhibit caffeine-induced pressor responses following capsaicin pre-treatment (control peak height = 11.3 ± 1.8 mmHg, peak height following incubation with OEA = 2.97 ± 0.51 mmHg, n= 5, P < 0.05).
Figure 7.
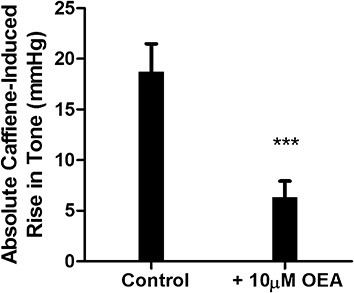
The effects of OEA on caffeine-induced increases in perfusion pressure of mesenteric arterial beds in calcium-free buffer. Data are mean ± s.e. mean, with n= 7 animals per group.
The effects of anandamide on responses to OEA
Following incubation with anandamide, the rise in perfusion pressure induced by methoxamine was much less and it was thus necessary to create a low-tone control (see Table 1) to test subsequent responses to OEA. In the presence of 1 µM anandamide, the relaxant responses to OEA were preserved compared to those in the low-tone matched controls (Figure 8).
Table 1.
Data for methoxamine-contracted perfused mesenteric arterial beds and vasorelaxation to OEA in the presence of anandamide
| Basal tone (mmHg) | Raised tone (mmHg) | Methoxamine (µM) | OEA potency pEC50 | OEA efficacy Rmax (%) | |
|---|---|---|---|---|---|
| Low tone control | 27.1 ± 2.2 | 52.2 ± 2.5 | 8.29 ± 1.39 | 7.10 ± 0.19 | 88.7 ± 5.9 |
| 1 µM anandamide | 30.3 ± 3.8 | 63.3 ± 4.2 | 100 ± 0 | 6.94 ± 0.83 | 107 ± 23 |
Figure 8.
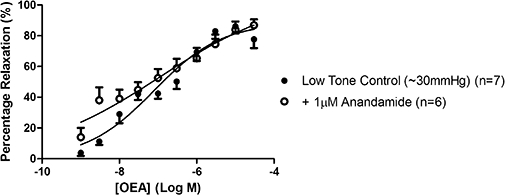
Vasorelaxant responses to OEA in the presence of anandamide in perfused mesenteric arterial beds. Responses to OEA are shown in the presence of 1 µM anandamide and compared with a low-tone matched control. Data points are mean ± s.e. mean with n representing the number of animals used in each group.
Vasorelaxant responses to OEA in rat isolated aorta
OEA caused a concentration-dependent vasorelaxation in rat isolated thoracic aortic rings (pEC50= 7.69 ± 0.47, Rmax= 54.9 ± 8.7%, n= 8). The maximal vasorelaxation was significantly (P < 0.05) reduced by pre-treatment with 10 µM capsaicin (Rmax= 28.9 ± 3.9%, n= 9), or by 1 µM AM251 (Rmax= 31.6 ± 3.9%, n= 9) to a similar degree, but not further by a combination of the two treatments (Rmax= 33.9 ± 3.2%, n= 9) (Figure 9).
Figure 9.
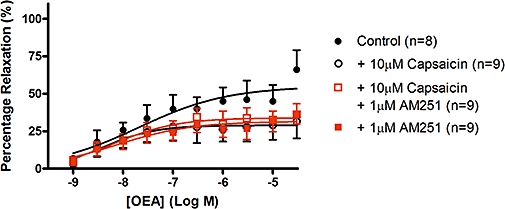
Vasorelaxation to OEA in isolated thoracic aortic rings. Vasorelaxant responses to OEA were obtained in the absence or presence of 1 µM AM251, or a combination of 1 µM AM251 and 10 µM capsaicin pre-treatment. Data points are mean ± s.e. mean, with n representing the number of rings per group.
Removal of the endothelium, or the presence of the nitric oxide synthase inhibitor, L-NAME, significantly reduced the potency of OEA in aortic rings (pEC30% control = 7.41 ± 0.50, n= 6; minus endothelium = 5.86 ± 0.31, n= 10; L-NAME = 5.45 ± 0.33, n= 4) (Figure 10).
Figure 10.
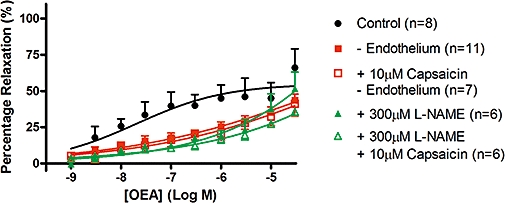
Involvement of the thoracic aortic endothelium in vasorelaxation to OEA. Thoracic aortic rings were relaxed with OEA alone, or in the presence of 10 µM capsaicin pre-treatment, 300 µM L-NAME, or endothelial denudation. Data points are mean ± s.e. mean, with n representing the number of rings per group. The data shown in this graph have been compared using one-way anova of the pEC30% values, as the curves did not reach a plateau and thus calculation of the Rmax values was not possible. These data have been analysed with the inclusion of pEC30% values from Figure 9, as the same control curve was used in both Figures.
The presence of indomethacin did not affect either the potency of, or maximal vasorelaxation to, OEA in aortic rings (control pEC50= 7.23 ± 1.53, Rmax= 81.8 ± 29.7%, n= 5; indomethacin pEC50= 7.86 ± 0.46, Rmax= 81.5 ± 10.0%, n= 6) (Figure 11).
Figure 11.
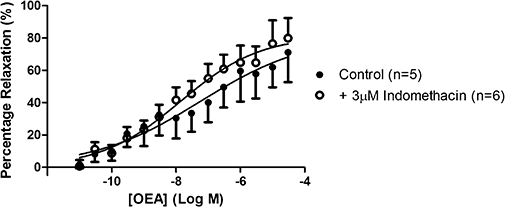
The effects of indomethacin on responses to OEA in isolated thoracic aortic rings. Data points are mean ± s.e. mean, with n representing the number of rings per group.
Contraction of aortic rings using buffer containing a high potassium concentration significantly (P < 0.05) reduced the potency of OEA (control pEC30%= 8.61 ± 0.52, n= 4; high potassium pEC30%= 6.73 ± 0.47, n= 4) (Figure 12).
Figure 12.
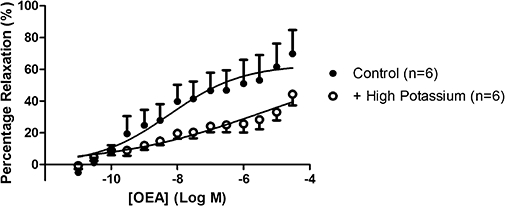
The effects of potassium-induced contraction on responses to OEA in isolated thoracic aortic rings. Data points are mean ± s.e. mean, with n representing the number of rings per group.
Discussion and conclusions
In the present study, we have confirmed previous findings that OEA causes vasorelaxation partly via activation of sensory nerves and partly via an endothelium-dependent mechanism (Movahed et al., 2005; Ho et al., 2008). In addition, we have also identified a proportion of the responses that is insensitive to capsaicin pre-treatment, and appears to be due to interference with release of intracellular calcium. Furthermore, we have found that the responses to OEA are enhanced in the presence of cyclooxygenase inhibition, which suggests a possible modulatory role for prostanoids.
In the current study, OEA was more potent [whole mesenteric arterial bed pEC50−7.1; isolated aortic rings pEC50−7.7) than previously reported in isolated mesenteric resistance arteries (pEC50−6.4 (Movahed et al., 2005); pEC50−5.7 (Ho et al., 2008)]. These responses, in terms of potency and maximal responses, were comparable to those of anandamide in the mesenteric arterial bed (Harris et al., 2002) and rat aorta (O'Sullivan et al., 2005). The potency of OEA in these isolated vessels is much higher than reported at recombinant receptors, with the possible exception of PPARα receptors. At human recombinant CB1 and CB2 cannabinoid receptors the potency (pEC50 values) of OEA is < 4.5 (Ryberg et al., 2007); at GPR55 is 6.4 (Ryberg et al., 2007); at human recombinant GPR119 is 5.5 (Overton et al., 2006); at rat recombinant TRPV1 receptors is 4.8 (Almasi et al., 2008); and in HeLa cells expressing recombinant PPARα, β or γ receptors is 6.9, 6.0 and <5, respectively (Fu et al., 2003).
In agreement with studies by Ho et al. (2008) in isolated third-order mesenteric arteries, OEA caused vasorelaxation of whole perfused mesenteric arterial beds and thoracic aortae, which were sensitive to capsaicin pre-treatment. In the present study, capsaicin pre-treatment reduced the maximal vasorelaxation to OEA, but in the studies by Ho et al. (2008), this treatment reduced the relaxation, and Movahed et al. (2005) even reported abolition of the response to OEA, using this treatment protocol, in first- and second-order mesenteric arteries contracted with phenylephrine. Furthermore, Movahed et al. (2005) reported that the TRPV1 antagonist capsazepine inhibited OEA-induced vasorelaxation of rat mesenteric arteries in a concentration-dependent manner, and reported the near abolition of the OEA response in TRPV1 knockout mice. In vagal sensory nerves, OEA can also activate TRPV1 receptors but this is dependent on protein kinase C activity (Ahern, 2003) and so the effects of OEA may be modulated by other mediators. In our study, we have identified a capsaicin-insensitive component, which suggests a sensory nerve-independent mechanism of action, as previously reported for anandamide (Harris et al., 2002).
The responses to OEA in the whole mesenteric arterial bed were abolished in the presence of high extracellular potassium, and suggest that a significant proportion of the capsaicin-insensitive relaxation could potentially involve potassium channel activation, which may or may not involve the EDHF. Removal of the endothelium produced similar reductions in the maximal vasorelaxation to OEA as those observed in capsaicin pre-treated preparations. Therefore, it is possible that the location of the potassium channels involved in this response could be on the vascular smooth muscle as removal of the endothelium only attenuated maximal relaxations, instead of abolishing the response in whole mesenteric beds, as in those preparations contracted using high potassium. Furthermore, a major role for EDHF can be ruled out as removal of the endothelium or treatment with the gap junction inhibitor (therefore, inhibitor of the diffusion of EDHF), carbenoxolone, only had a small effect on capsaicin-independent responses to OEA. This is comparable to the modest effects reported by Ho et al. (2008) and would suggest that EDHF plays a minor role in the capsaicin-insensitive relaxation.
Somewhat interestingly, vasorelaxation to OEA was not affected by inhibition of nitric oxide synthase and cyclooxygenase. Indeed, this treatment reversed the inhibitory effects of capsaicin pre-treatment. This prompted us to carry out further experiments that demonstrated that the cyclooxygenase inhibitor, indomethacin, was responsible for this reversal of inhibition. Indeed, we found that indomethacin alone potentiated responses to OEA.
The ability of indomethacin to increase the potency of OEA in naïve tissues and to reverse, or prevent, the inhibitory effects of capsaicin pre-treatment is a novel observation. We also demonstrated that the structurally different cyclooxygenase inhibitor, flurbiprofen, had comparable effects. This would suggest that it is a property of cyclooxygenase inhibitors in general, as opposed to a unique property of indomethacin, which has resulted in the increased potency of OEA. Furthermore, various cyclooxygenase inhibitors have been shown to augment vasorelaxation to other endocannabinoids (anandamide and 2-arachidonoylglycerol) in mesenteric arterial segments (Ho and Randall, 2007). Anandamide and 2-arachidonoylglycerol can be metabolized to arachidonic acid, which may be metabolized via endothelial cyclooxygenase to prostanoids, or they themselves may be metabolized to vasoactive prostamides via cyclooxygenases (Fowler, 2007a). By contrast, vasorelaxation of human pulmonary arteries via virodhamine is reduced by cyclooxygenase inhibitors and is thought to occur via the release of vasodilator prostanoids derived from arachidonic acid (Kozlowska et al., 2008). Such differences could, of course, reflect differences in the balance between the production of vasoconstrictor and vasodilator prostanoids.
Our study does not provide evidence for the mechanism of the enhancement of vasorelaxation to OEA by cyclooxygenase inhibitors in whole mesenteric arterial beds. Possible explanations include that OEA, or products of its hydrolysis, are metabolized by cyclooxygenase, thus blocking cyclooxygenase would increase the concentration of vasoactive OEA. However, it is unlikely that these are substrates for cyclooxygenase, due to the mono-unsaturated nature of OEA and oleic acid. There is evidence, however, that unsaturated fatty acids, which are not substrates for cyclooxygenase, may modulate cyclooxygenase activity in an allosteric manner (Yuan et al., 2009). A second suggestion is that OEA activates a cyclooxygenase pathway causing release of vasoconstrictor prostanoids, which, if inhibited, would enhance the vasorelaxant response. Thirdly, there is evidence that NSAIDs, including ibuprofen and flurbiprofen, interfere with other enzymes capable of transforming OEA, including a dual inhibition of cyclooxygenase and FAAH, and it is blockade of the latter enzyme that is increasing the concentration of available OEA. However, the concentrations employed here are below those effective at inhibiting FAAH (Fowler, 2007b). Combined with evidence from Ho et al. (2008), neither the tissue concentration nor the vasoactivity of OEA is affected by blockade of FAAH, in contrast to observations with anandamide. However, we cannot rule out the possibility that indomethacin could be acting on one of the other metabolic enzymes, such as NAAA (Ueda et al., 2001; Tsuboi et al., 2005).
The modulation of vasorelaxation to endocannabinoids and related compounds, including OEA, by prostanoids, is not unique, as we have previously shown that inclusion of cyclooxygenase inhibitors uncovers or enhances vasorelaxation to the sex steroids testosterone (Tep-Areenan et al., 2003b) and 17β-oestradiol (Tep-Areenan et al., 2003a). Whether there is a common mechanism that underlies this effect is unclear. However, there are implications for pharmacological studies that routinely include endocannabinoids and cyclooxygenase inhibitors in their protocols, and potential cardiovascular effects of cyclooxygenase inhibitors when used therapeutically. Therefore, further investigations into the mechanism of the augmented vasorelaxation to OEA in the presence of the cyclooxygenase inhibitor flurbiprofen were conducted in isolated arteries taken from the mesenteric arterial bed. There appears to be some heterogeneity of these enhanced responses, which were only observed in first-order branches (G1) of the superior mesenteric artery, and not the superior mesenteric artery itself (G0). Furthermore, we have shown that a similar augmentation of responses to OEA was also observed in the presence of the thromboxane A2 receptor antagonist vapiprost. This would suggest that there is a release of vasoconstrictor prostanoids (most likely thromboxane A2) which modulates the dilator response of OEA. Therefore on inhibition of cyclooxygenase, or in the presence of a thromboxane receptor antagonist, the underlying vasodilator response is enhanced.
The presence of the capsaicin-insensitive relaxation prompted us to investigate further the mechanisms of relaxation. As mentioned above, we were able to exclude a major role for EDHF. The inability of OEA to relax tone induced by high potassium could suggest that the mechanisms involve potassium channel activation, independent of EDHF. To explore this possibility we used the non-selective potassium channel inhibitor TEA, which significantly enhanced responses to OEA. This observation did not mimic the results obtained using high potassium-induced tone, and thus excludes a role for potassium channels. However, the results may be a reflection of a non-specific action of TEA due to the high concentration used, such as causing the release of acetylcholine (Drukarch et al., 1989).
We then examined the effects of chloride channel inhibition with niflumic acid (10 µM), showing no effect on OEA-induced relaxation of methoxamine-induced tone, which excludes a role for chloride channels in this response. Interestingly, niflumic acid at the concentration used is also a cyclooxygenase inhibitor (Johnson et al., 1995). Niflumic acid is 100 times more selective for COX-2 than COX-1; thus, at the concentration used in these experiments, it is likely that COX-2 activity is fully inhibited, whilst COX-1 activity is only partially inhibited (Johnson et al., 1995). Therefore, the lack of inhibition of responses to OEA in the presence of niflumic acid, compared to the enhanced vasorelaxation to OEA seen in the presence of indomethacin, may suggest that the enhanced OEA responses are due to a COX-1-mediated mechanism.
The lack of effect of chloride channel inhibitors and EDHF inhibition led us to consider whether OEA altered the release of calcium from intracellular stores to cause vasorelaxation. High extracellular potassium causes vasoconstriction via depolarization and the entry of extracellular calcium, while methoxamine involves both calcium influx and release of intracellular calcium. Furthermore, we have also shown that, in isolated aortae taken from spontaneously hypertensive rats, OEA relaxes methoxamine-induced tone but not tone established via the thromboxane mimetic, U46619 (Wheal et al., 2009), which can act via thromboxane receptors (Coleman et al., 1981), but may also act via allowing calcium influx via L-type calcium channels and increasing the calcium sensitivity of smooth muscle contractile proteins (Yamagishi et al., 1992), or by inhibiting the activation of calcium-activated potassium channels (KCa) to cause a prolonged depolarization and ultimately a constriction of the vessels (Scornik and Toro, 1992). Zygmunt et al. (1997) and White and Hiley (1998) have both previously shown that anandamide interferes with the release or activity of intracellular calcium in vascular smooth muscle. We repeated the protocol used by White and Hiley (1998) and found that OEA substantially reduced caffeine-induced increases in perfusion pressure in the absence of extracellular calcium, an effect which was independent of sensory nerve activity. This would suggest that OEA causes vasorelaxation via interfering with the release of intracellular calcium. In this regard, Gamberucci et al. (1997) reported that unsaturated fatty acids, including oleic acid (a product of OEA metabolism), inhibited store-dependent capacitative calcium influx in a range of haematological and tumour cell lines. These effects, which could include increased calcium extrusion, occurred in the micromolar range and appeared to be independent of G-protein coupling or metabolism (Gamberucci et al., 1997). It is possible that that OEA also acts in this way, or acts as a donor of oleic acid, although in mesenteric vessels oleic acid does not cause vasorelaxation at 10 µM or below (Sudhahar et al., 2009). It is also of note that Gamberucci et al. (1997) found that these responses to unsaturated fatty acids were reversed by the presence of albumin. Such an effect of albumin could potentially explain why some of the vascular effects of endocannabinoids are not seen in vivo (see Randall et al., 2004). Unsaturated fatty acids have also been shown to deplete intracellular calcium from the endoplasmic reticulum in the Jurkat leukaemic cell line (Chow and Jondal, 1990). The metabolically stable analogue of anandamide, methanandamide, has also been shown to deplete calcium stores in astrocytes (Venance et al., 1997). However, this effect was not mimicked by arachidonic acid and was Pertussis toxin sensitive. In conclusion, our results are consistent with OEA, or oleic acid as a metabolite, causing vasorelaxation via interfering with intracellular calcium, possibly via depletion of stores or increased extrusion of intracellular calcium. These findings are comparable to those reported for anandamide by Zygmunt et al. (1997) and White and Hiley (1998) and so we have identified an important mechanism of vasorelaxation for OEA.
Ho et al. (2008) reported that OEA enhanced the activity of anandamide in isolated mesenteric arterial segments. We therefore examined the effects of anandamide on responses to OEA. Vasorelaxation to OEA was not affected by 1 µM anandamide which would seem to rule out a significant interaction.
In thoracic aortae, the mechanism of action of OEA also appears to be partly endothelium-dependent, as demonstrated in the present study, and by Ho et al. (2008) in isolated small mesenteric arteries by a reduction in the potency of OEA following mechanical removal of the endothelium. However, this mechanism of action was not evident in perfused mesenteric arterial beds, as removal of the endothelium did not significantly inhibit OEA-induced vasorelaxation in addition to capsaicin pre-treatment, thus highlighting differential mechanisms of OEA action in the vasculature.
A further difference between mesenteric beds and aortae is the involvement of CB1 receptors. It is widely accepted that OEA does not act via ‘classical’ cannabinoid receptors such as CB1 or CB2 (Lambert et al., 1999; Alexander and Kendall, 2007). However, whilst this was evident in mesenteric beds, the presence of 1 µM AM251 reduced the maximal vasorelaxation to OEA in aortic rings, indicative of a role for CB1 receptors. The magnitude of inhibition by AM251 was similar to that of capsaicin pre-treatment, and was not additive when the two treatments were combined, thus suggesting a linked signalling pathway. This may be possible as CB1 receptors and TRPV1 have been reported to be co-localized on cultured sensory dorsal root ganglion neurones (Ahluwalia et al., 2000).
In conclusion, we have shown that the potent vasorelaxant effects of OEA are partly dependent on sensory nerve activity and the endothelium in the vasculature, and that it also interferes with the intracellular release of calcium, which may lead to vasorelaxation. We have also shown that cyclooxygenase inhibitors increase the potency of OEA as a vasorelaxant and this may suggest that cyclooxygenase activity may modulate the vascular activity of OEA.
Acknowledgments
We are most grateful to the British Heart Foundation for funding this research.
Glossary
Abbreviations:
- EDHF
endothelium-derived hyperpolarizing factor
- FAAH
fatty acid amide hydrolase
- KCa
calcium-activated potassium channels
- NAAA
N-acylethanolamine-hydrolysing acid amidase
- NAPE
N-acylphosphatidylethanolamine
- OEA
N-oleoylethanolamine
- PPAR
peroxisome proliferator-activated receptor
- TEA
tetraethylammonium
- TP
thromboxane A2 receptor
- TRPV1
transient receptor potential vanilloid receptor 1
Conflicts of interest
The authors state no conflict of interest.
References
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Kendall DA. The complications of promiscuity: endocannabinoid action and metabolism. Br J Pharmacol. 2007;152:602–623. doi: 10.1038/sj.bjp.0707456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158:S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasi R, Szoke E, Bolcskei K, Varga A, Riedl Z, Sandor Z, et al. Actions of 3-methyl-N-oleoyldopamine, 4-methyl-N-oleoyldopamine and N-oleoylethanolamide on the rat TRPV1 receptor in vitro and in vivo. Life Sci. 2008;82:644–651. doi: 10.1016/j.lfs.2007.12.022. [DOI] [PubMed] [Google Scholar]
- Artmann A, Petersen G, Hellgren LI, Boberg J, Skonberg C, Nellemann C, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, Fu J, et al. Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci U S A. 2009;106:8027–8031. doi: 10.1073/pnas.0903038106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow SC, Jondal M. Polyunsaturated free fatty acids stimulate an increase in cytosolic Ca2+ by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in T cells through a mechanism independent of phosphoinositide turnover. J Biol Chem. 1990;265:902–907. [PubMed] [Google Scholar]
- Coleman RA, Humphrey PP, Kennedy I, Levy GP, Lumley P. Comparison of the actions of U-46619, a prostaglandin H2-analogue, with those of prostaglandin H2 and thromboxane A2 on some isolated smooth muscle preparations. Br J Pharmacol. 1981;73:773–778. doi: 10.1111/j.1476-5381.1981.tb16814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle DN, de Moura RS, Greenwood IA, Large WA. Inhibitory action of niflumic acid on noradrenaline- and 5-hydroxytryptamine-induced pressor responses in the isolated mesenteric vascular bed of the rat. Br J Pharmacol. 1997;120:813–818. doi: 10.1038/sj.bjp.0700981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drukarch B, Kits KS, Leysen JE, Schepens E, Stoof JC. Restricted usefulness of tetraethylammonium and 4-aminopyridine for the characterization of receptor-operated K+-channels. Br J Pharmacol. 1989;98:113–118. doi: 10.1111/j.1476-5381.1989.tb16870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol. 2007a;152:594–601. doi: 10.1038/sj.bjp.0707379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CJ. The pharmacology of the cannabinoid system – a question of efficacy and selectivity. Mol Neurobiol. 2007b;36:15–25. doi: 10.1007/s12035-007-0001-6. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gamberucci A, Fulceri R, Benedetti A. Inhibition of store-dependent capacitative Ca2+ influx by unsaturated fatty acids. Cell Calcium. 1997;21:375–385. doi: 10.1016/s0143-4160(97)90031-2. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulie P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. Eur J Pharmacol. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- Guzman M, Lo Verme J, Fu J, Oveisi F, Blazquez C, Piomelli D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha) J Biol Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Hansen SH, Schousboe A, Hansen HS. Determination of the phospholipid precursor of anandamide and other N-acylethanolamine phospholipids before and after sodium azide-induced toxicity in cultured neocortical neurons. J Neurochem. 2000;75:861–871. doi: 10.1046/j.1471-4159.2000.0750861.x. [DOI] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155:837–846. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Wimsatt J, Buckel SD, Dyer RD, Maddipati KR. Purification and characterization of prostaglandin H synthase-2 from sheep placental cotyledons. Arch Biochem Biophys. 1995;324:26–34. doi: 10.1006/abbi.1995.9934. [DOI] [PubMed] [Google Scholar]
- Kozlowska H, Baranowska M, Schlicker E, Kozlowski M, Laudanski J, Malinowska B. Virodhamine relaxes the human pulmonary artery through the endothelial cannabinoid receptor and indirectly through a COX product. Br J Pharmacol. 2008;155:1034–1042. doi: 10.1038/bjp.2008.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert DM, DiPaolo FG, Sonveaux P, Kanyonyo M, Govaerts SJ, Hermans E, et al. Analogues and homologues of N-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors. Biochim Biophys Acta. 1999;1440:266–274. doi: 10.1016/s1388-1981(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Lan R, Liu Q, Fan P, Lin S, Fernando SR, McCallion D, et al. Structure-activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J Med Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci. 2005;62:708–716. doi: 10.1007/s00018-004-4494-0. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Bottrill FE, Randall MD, Hiley CR. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br J Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahed P, Jonsson BA, Birnir B, Wingstrand JA, Jorgensen TD, Ermund A, et al. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem. 2005;280:38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- Mulvany MJ, Halpern W. Contractile properties of small resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582. doi: 10.1038/sj.bjp.0707423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br J Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan SE, Kendall DA, Randall MD. Vascular effects of delta 9-tetrahydrocannabinol (THC), anandamide and N-arachidonoyldopamine (NADA) in the rat isolated aorta. Eur J Pharmacol. 2005;507:211–221. doi: 10.1016/j.ejphar.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, et al. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Randall MD, Kendall DA, O'Sullivan SE. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D, Ortori CA, Chapman V, Kendall DA, Barrett DA. Quantitative profiling of endocannabinoids and related compounds in rat brain using liquid chromatography-tandem electrospray ionization mass spectrometry. Anal Biochem. 2007;360:216–226. doi: 10.1016/j.ab.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, et al. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992;262:C708–C713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- Sudhahar V, Shaw S, Imig JD. Mechanisms involved in oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Eur J Pharmacol. 2009;607:143–150. doi: 10.1016/j.ejphar.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, et al. Cannabinoid activation of PPAR alpha; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tep-Areenan P, Kendall DA, Randall MD. Mechanisms of vasorelaxation to 17beta-oestradiol in rat arteries. Eur J Pharmacol. 2003a;476:139–149. doi: 10.1016/s0014-2999(03)02152-6. [DOI] [PubMed] [Google Scholar]
- Tep-Areenan P, Kendall DA, Randall MD. Mechanisms of vasorelaxation to testosterone in the rat aorta. Eur J Pharmacol. 2003b;465:125–132. doi: 10.1016/s0014-2999(03)01453-5. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Ueda N. Molecular characterization of N-acylethanolamine-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. J Biol Chem. 2005;280:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamanaka K, Yamamoto S. Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J Biol Chem. 2001;276:35552–35557. doi: 10.1074/jbc.M106261200. [DOI] [PubMed] [Google Scholar]
- Venance L, Sagan S, Giaume C. R)-methanandamide inhibits receptor-induced calcium responses by depleting internal calcium stores in cultured astrocytes. Pflugers Arch. 1997;434:147–149. doi: 10.1007/s004240050376. [DOI] [PubMed] [Google Scholar]
- Wheal AJ, Alexander SPH, Randall MD. Effect of contractile agents on vasorelaxation to oleoylethanolamide in isolated aortic rings from spontaneously hypertensive rats. 2009. Abstract presented at the British Pharmacological Society's summer meeting: http://www.pA2online.org/abstracts/Vol7Issue2abst022P.pdf.
- White R, Hiley CR. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Yanagisawa T, Taira N. Ca2+ influx induced by the agonist U46619 is inhibited by hyperpolarization induced by the K+ channel opener cromakalim in canine coronary artery. Jpn J Pharmacol. 1992;59:291–299. doi: 10.1254/jjp.59.291. [DOI] [PubMed] [Google Scholar]
- Yuan C, Sidhu RS, Kuklev DV, Kado Y, Wada M, Song I, et al. Cyclooxygenase Allosterism, Fatty Acid-mediated Cross-talk between Monomers of Cyclooxygenase Homodimers. J Biol Chem. 2009;284:10046–10055. doi: 10.1074/jbc.M808634200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Hogestatt ED, Waldeck K, Edwards G, Kirkup AJ, Weston AH. Studies on the effects of anandamide in rat hepatic artery. Br J Pharmacol. 1997;122:1679–1686. doi: 10.1038/sj.bjp.0701601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]


