Abstract
Background and purpose:
Cannabis is taken as self-medication by patients with inflammatory bowel disease for symptomatic relief. Cannabinoid receptor agonists decrease inflammation in animal models of colitis, but their effects on the disturbed motility is not known. (-)-Cannabidiol (CBD) has been shown to interact with Δ9-tetrahydrocannabinol (THC) in behavioural studies, but it remains to be established if these cannabinoids interact in vivo in inflammatory disorders. Therefore the effects of CBD and THC alone and in combination were investigated in a model of colitis.
Experimental approach:
The 2,4,6-trinitrobenzene sulphonic acid (TNBS) model of acute colitis in rats was used to assess damage, inflammation (myeloperoxidase activity) and in vitro colonic motility. Sulphasalazine was used as an active control drug.
Key results:
Sulphasalazine, THC and CBD proved beneficial in this model of colitis with the dose–response relationship for the phytocannabinoids showing a bell-shaped pattern on the majority of parameters (optimal THC and CBD dose, 10 mg·kg−1). THC was the most effective drug. The effects of these phytocannabinoids were additive, and CBD increased some effects of an ineffective THC dose to the level of an effective one. THC alone and in combination with CBD protected cholinergic nerves whereas sulphasalazine did not.
Conclusions and implications:
In this model of colitis, THC and CBD not only reduced inflammation but also lowered the occurrence of functional disturbances. Moreover the combination of CBD and THC could be beneficial therapeutically, via additive or potentiating effects.
This article is part of a themed issue on Cannabinoids. To view the editorial for this themed issue visit http://dx.doi.org/10.1111/j.1476-5381.2010.00831.x
Keywords: Δ9-tetrahydrocannabinol, cannabidiol, cannabinoids, sulphasalazine, colitis, inflammation, motility, inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD), which comprises Crohn's disease and ulcerative colitis, is a chronic, relapsing and remitting disorder characterized by excessive inflammatory responses in the gastrointestinal tract leading to damage and occurrence of profound motility and secretory disturbances, which together contribute to bleeding, diarrhoea, abdominal cramping and malnutrition. The disease cannot be cured and the aim of currently accepted treatments is to reduce the symptoms by inducing and maintaining a remission (Baumgart and Sandborn, 2007).
Cannabis is taken as self-medication by patients with IBD in order to relieve symptoms such as abdominal pain, diarrhoea and reduced appetite (Garcia-Planella et al., 2007; Lal et al., 2007). Synthetic cannabinoid (CB) receptor agonists inhibit inflammation and tissue damage in different animal models of colitis, an effect attributed to stimulation of CB1 and CB2 receptors (Massa et al., 2004; Kimball et al., 2006; Storr et al., 2007; Wright et al., 2008; receptor nomenclature follows Alexander et al., 2009). However the effects of the main psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol (THC) have not been evaluated.
It is well known that cannabis possesses immunosuppressive properties and that the main component responsible for this profile of action is THC. In both in vivo and in vitro, THC impairs cell-mediated and humoural immunity, actions that could be beneficial in auto-inflammatory disorders including IBD (Massi et al., 2006). In support of this, THC was effective in attenuating autoimmune responses in an experimental model of diabetes (multiple low-dose streptozotocin injections) (Li et al., 2001) and in experimental autoimmune encephalomyelitis (Lyman et al., 1989).
In addition to THC, cannabis contains a number of non-psychotropic compounds, with (-)-cannabidiol (CBD) being the most abundant and the most extensively studied (Mechoulam, 2005; Pertwee, 2008). Importantly, CBD has been also demonstrated to possess potent anti-inflammatory and immunomodulatory properties which, together with a lack of psychotropic activity and low toxicity, make it a very promising therapeutic candidate for a variety of inflammatory and pain associated disorders, including IBD. CBD is a very potent antioxidant, which results in reduction of the level of reactive oxygen species in the course of inflammation and protection from tissue damage (Malfait et al., 2000; Mechoulam et al., 2002; Weiss et al., 2006). CBD treatment has proven effective in different animal models of inflammation, such as collagen-induced arthritis, non-obese diabetic mice, acute carrageenan-induced inflammation in the rat paw, and it was shown to normalize croton oil-induced hypermotility in mice (Capasso et al., 2008). Notably it was reported that CBD was able to delay and attenuate colitis in interlukin (IL)-10 knockout mice, but the results of the experiment were not published (Malfait et al., 2000). A confirmation of this observation was provided in a recent study, which demonstrated efficacy of CBD pretreatment in dinitrobenzene sulphonic acid (DNBS)-induced colitis in mice (Borrelli et al., 2009).
As the two phytocannabinoids modulate the immune system and differ in their pharmacological profile, their combination could be more beneficial than either drug alone (Russo and Guy, 2006; Pertwee, 2008). Additionally CBD could not only potentiate the therapeutic effects of THC, but also attenuate some of its undesirable effects, such as disorientation, mental clouding, sedation, anxiety, dysphoria and tachycardia (Karniol et al., 1974; Russo and Guy, 2006; Pertwee, 2007; Vann et al., 2008).
Therefore the aim of the present study was to characterize the effects of the two phytocannabinoids, administered alone and in combination, in the 2,4,6-trinitrobenzene sulphonic acid (TNBS)-induced model of colitis in the rat. In addition, the effects of sulphasalazine on macroscopic damage, myeloperoxidase (MPO) activity and in vitro responses of colonic longitudinal muscle strips were evaluated in order to compare the effects of THC and CBD with a standard treatment for IBD (Baumgart and Sandborn, 2007).
Our results demonstrated that treatment with THC and CBD reduced inflammation and motility disturbances associated with colitis. The effects of THC alone and in combination with CBD were similar to and, in some aspects, better than those of sulphasalazine, suggesting potential value of phytocannabinoids for the treatment of IBD.
Methods
All animal care and experimental procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986 and were approved by the University of Hertfordshire Ethics Committee. Male Charles River Wistar rats (200–300 g) were used in the study (n = 85). They were housed in standard cages (2–6 rats per cage) and provided with free access to food and tap water. The animals were maintained at a constant temperature (20–21°C) and a 12 h light/dark cycle.
Induction of colitis
Colitis was induced as described by Boughton-Smith et al., (1988) and Morris et al., (1989) with modifications by Sykes et al., (1999) and minor changes in the procedure. Briefly, the animals were fasted for 20–24 h with free access to water. Colitis was induced under light isoflurane anaesthesia by administering an enema of 6.7 mg of TNBS in 0.25 mL of 25% ethanol (v/v) using an infant feeding tube inserted into the lumen of the colon to a point 8 cm distal from the anus. In a preliminary series of experiments control animals received phosphate-buffered saline (PBS). Rats were matched in their body weights before food withdrawal. After enema administration the animals were kept in a head-down position until they recovered from anaesthesia (1–2 min) to prevent the solution leaking out. After this procedure the animals were placed in separate cages with free access to food and water. The rats were killed 3 days later by carbon dioxide asphyxiation. The abdominal cavity was opened via a midline incision; the colon was removed and immediately placed in fresh Krebs–Henseleit solution with the following composition (in mM): NaCl 118.1, KCl 4.8, NaHCO3 25.0, KH2PO4 1.2, MgSO4 1.2, glucose 11.1 and CaCl2 2.5 (pH 7.4).
Macroscopic damage evaluation
The colon was cleaned of fat and mesentery, cut open longitudinally, gently flushed clean with Krebs solution and scored for visible damage. The degree of inflammation was quantified using a 0–13 scale, according to the criteria presented in Table 1. This wide scale was applied to optimally reflect the differences in the severity of inflammation between animals. The damage assessed visually is referred to as the macroscopic damage score (MDS).
Table 1.
Macroscopic damage scoring system (0–13 scale)
| Score | Criteria |
|---|---|
| 0 | No damage |
| 1 | Localized hyperaemia or thickening No ulcers |
| 2 | Ulceration without significant inflammation |
| 3 | Ulceration with inflammation at 1 site |
| 4 | ≥2 sites of ulceration and inflammation |
| 5 | Major sites of damage extending >1 cm along length of colon |
| 6–10 | If damage covered >2 cm along the length of the colon, score is increased by 1 for each additional centimetres of involvement |
| Plus | |
| 1 | Minor adhesions (colon could be separated easily from other tissue) |
| 2 | Major adhesions |
| Plus | |
| 1 | Diarrhoea |
Scale adapted from those used previously (Appleyard and Wallace, 1995; Poli et al., 2001).
Tissue preparation for the in vitro studies and the methods employed for measuring motility
The most inflamed segment of the distal colon was cut longitudinally into four strips (whole wall, 2–4 mm × 12–15 mm) one of which was frozen and stored at −70°C for assessment of MPO activity and the remaining three strips were used in motility studies.
Longitudinal muscle strips were placed in 30 mL organ baths and maintained in oxygenated (95% O2/5% CO2) Krebs solution at 37°C. The tissue was suspended in the bathing solution by threads. One end of the tissue was attached to a hook at the bottom of the bath and the other end to an isometric tension transducer (Pioden Controls Ltd., Newport, Isle of Wight, UK and AD Instruments Ltd., Chalgrove, Oxfordshire, UK).
In experiments with electrical field stimulation (EFS), the tissue was attached to a hook between two parallel platinum wire electrodes. Electrical stimuli were delivered by a Harvard Apparatus 6002 stimulator (Harvard Apparatus Ltd., Fircroft Way, Edenbridge, Kent, UK).
An initial load of 1 g was applied. The tissues were left to equilibrate for at least 30 min before experiments were commenced. Wet and dry (after 24 h at room temperature) tissue weights were determined and amplitude of contraction was calculated per gram of dry weight. This normalization procedure was used because of the presence of oedema, as well as possible morphological changes of the smooth muscle layers in the inflamed colon as reported by Wells et al. (2004).
The experiments were recorded with a MacLab data acquisition system (Chart v 3.6., AD Instruments Pty Ltd., Castle Hill, NSW, Australia).
In vitro evaluation of the alterations in motility caused by inflammation
Assessment of the differences in the spontaneous activity
Spontaneous activity was recorded for 10 min. The parameters measured were the amplitude of low-frequency contractions (ALF) and their duration (D).
Responses to carbachol
Cumulative dose–response curves to carbachol were constructed and the final bath concentrations were: 1 × 10−8 M, 1 × 10−7 M, 3 × 10−7 M, 1 × 10−6 M, 3 × 10−6 M, 1 × 10−5 M and 3 × 10−5 M.
Experiments with EFS
These experiments were designed to study neurally mediated responses to electrical stimulation of the enteric nerves. Frequency–response curves were constructed at a voltage supramaximal for 5 Hz, 0.2 ms pulse width in 5 s trains. The frequencies studied were 1, 3, 5, 8, 10 and 15 Hz.
The contractions during the electrical stimulation (‘on’ responses) were cholinergically mediated (completely blocked by 1 × 10−6 M atropine, data not shown). At these stimulation parameters relaxant responses to EFS in the same tissues pre-contracted with carbachol (1 × 10−5 M) were also studied. Carbachol was applied after the contractile response to the last frequency (15 Hz). EFS was started 4–5 min after carbachol application, when the contractile response became stable. Subsequent frequencies were applied after recovery to the baseline.
The relaxant responses are expressed as % relaxation which was calculated as follows: {[responseCON (g) − responseREL (g)] : [responseCON (g) − baseline (g)]} × 100, where responseCON is the contraction to carbachol immediately before application of a given EFS frequency, responseREL is the minimum in response to a given EFS frequency, and the baseline value was assessed directly prior to carbachol administration.
All EFS-evoked responses were tetrodotoxin-sensitive (data not shown).
Myeloperoxidase activity
A MPO assay was used to quantify inflammation. The enzyme activity is considered to be an index of neutrophil infiltration because MPO is predominantly found in these cells (Krawisz et al., 1984).
In the first step of the procedure the enzyme was extracted from the tissue according to the protocol of Bradley et al. (1982) with small modifications from Grisham et al. (1990). Tissue strips were thawed, weighed and homogenized (homogenizer, Kika Labortechnik, T25 basic, Staufen, Germany) in 10 volumes (100 mg tissue·mL−1) of ice-cold 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% (w/v) hexadecyltrimethylammonium bromide (HTAB) and 10 mM Na2EDTA. The homogenate was sonicated on ice (15 s), freeze-thawed, sonicated a second time (15 s) and centrifuged at 4°C and 20 000×g for 20 min. The supernatant was kept on ice and used for the enzyme assay.
Myeloperoxidase activity was measured in 10 µL samples using a microplate procedure according to the method of Venkova et al. (2000) with 3,3′,5,5′-tetramethylbenzidine (TMB) liquid substrate system and horseradish peroxidase (HRP) as the relative standard. The protocol was modified to form an end point assay where the reaction is stopped with acid solution and the absorbance is measured at 450 nm (Microplate reader, Multiskan Ascent, Labsystems Oy, Helsinki, Finland. Software version 1.3.1). MPO activity was expressed as an equivalent to the activity of the standard (nanograms of HRP) converting the same amount of TMB substrate for 3 min at room temperature. Total protein was measured as well (as described below). The MPO data were expressed as nanograms of HRP equivalents per milligram of protein.
Protein determination
Protein content was measured using a BCA™ Protein Assay Kit (PIERCE) in a microplate procedure according to manufacturer's instruction with small modifications. Briefly, bovine serum albumin was used as the standard. Supernatants, as in the MPO assay, were used. After mixing all the reagents, plates were incubated at room temperature for 30 min, and the absorbance was measured at 540 nm on a plate reader.
Experimental design for drug treatment
To assess the effects of various drugs on colonic inflammation the substances were tested using a short-time dosing regimen. The first dose was administered 0.5 h before colitis induction and then 24 and 48 h after induction. Drugs were administered i.p. and the injection volume was 3 mL·kg−1 with the vehicle being a mixture of absolute ethanol, cremophor and saline (1:1:18). To study the effects of CBD, four doses were applied: 5, 10, 15 and 20 mg·kg−1 body weight per day (referred to as CBD5, CBD10, CBD15 and CBD20, n = 4, 6, 5 and 5, respectively, for vehicle group n = 11). Three doses of THC were applied: 5, 10 and 20 mg·kg−1 per day (referred to as THC5, THC10 and THC20, respectively, n = 6 for all apart from the vehicle group –n = 11).
In order to assess the effects of combined treatment with THC and CBD, two mixtures of the two compounds were used (co-administered in one vehicle solution). The dose of CBD (10 mg·kg−1) was chosen on the basis of experiments with CBD alone (the most effective dose), and it was administered with an optimal dose of THC (10 mg·kg−1, T10 + C, 1:1 ratio, n = 6) or with a non-effective dose (5 mg·kg−1, T5 + C, 1:2 ratio, n = 6).
Sulphasalazine (n = 7, for vehicle n = 6) was administered at 300 mg·kg−1 in 3 mL p.o. starting 5 h before the TNBS enema. Three doses were given in total, every 24 h. In order to keep the dosing regimen as close to the i.p. studies as possible the time of first sulphasalazine administration (5 h prior to enema) was chosen on the basis of the duration of gastrointestinal transit in the rat. In a recent study it was demonstrated that about 4–5 h were necessary for sulphasalazine to reach the caecum and to be broken down into 5-aminosalicylic acid (5-ASA), the active moiety and sulphapyridine (detectable in the blood) (Fujioka et al., 2008).
Sulphasalazine was administered in the form of a suspension, prepared in 1% methyl cellulose (for increased viscosity). Control animals received the vehicle solution alone.
The animals were killed 3 days after TNBS instillation and the colonic tissue was taken for the visual assessment of inflammation, MPO assay and the in vitro motility studies.
Data analysis and statistical procedures
Results are expressed as mean ± SEM. Differences between groups were analysed using unpaired two-tailed t-test (when comparing two groups) or analysis of variance (anova), followed by Dunnett's or Bonferroni post test. Values of P < 0.05 were considered statistically significant. Concentration–response curves for carbachol were fitted using non-linear regression (sigmoidal dose–response, GraphPad Prism). Analysis generated logEC50 (log of the molar concentration of carbachol needed to produce 50% of the maximal response) and Emax (the maximal contractile response to carbachol) values, used to determine differences in potency and efficacy respectively.
Materials
2,4,6-Trinitrobenzene sulphonic acid solution (1 M) and HTAB were obtained from Fluka (Sigma Aldrich, Gillingham, Kent, UK), carbachol, tetrodoxin, atropine, Cremophor® EL, HRP, TMB Liquid Substrate System for elisa, Stop Reagent for TMB substrate, sulphasalazine, methyl cellulose and EDTA disodium salt from Sigma (Sigma Aldrich, Gillingham, Kent, UK); THC [(6aR, 10aR)-6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H -dibenzo[b,d]pyran-1-ol] was obtained from THC Pharm GmbH (Frankfurt am Main, Germany) and CBD (2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol) was kindly supplied by GW Pharmaceuticals (Salisbury, Wiltshire, UK).
Results
In a preliminary series of experiments, rats were given a PBS enema alone and the results are included in all the graphs to represent baseline values in healthy animals and the effects of TNBS administration. TNBS treatment only slightly reduced body weight gain in comparison with control rats (PBS enema), and this effect was not statistically significant.
Treatment of rats with colitis with CBD alone did not affect body weight (Figure 1A). Treatment with THC5, THC20 alone and combined treatment with T10 + C resulted in a significant reduction of body weight gain at 72 h in comparison with the vehicle group (P < 0.05, Figure 1B). Sulphasalazine treatment seemed to increase body weight gain, but this effect was not statistically significant (Figure 1C).
Figure 1.
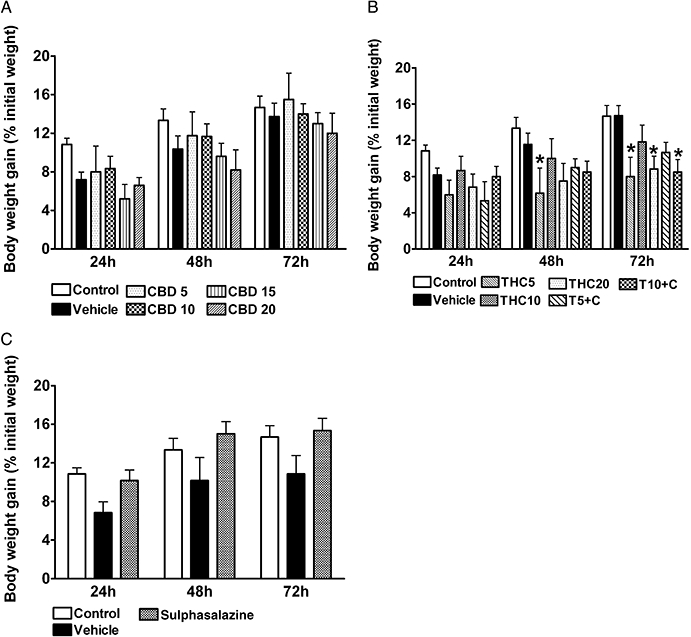
Rat body weight changes over 3 days from administration of 2,4,6-trinitrobenzene sulphonic acid enema. Effects of (A) (-)-cannabidiol (CBD) alone (5, 10, 15 and 20 mg·kg−1 i.p.), (B) Δ9-tetrahydrocannabinol (THC) alone (5, 10 and 20 mg·kg−1 i.p.) and THC in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) and (C) sulphasalazine (300 mg·kg−1 p.o.). Results are expressed as mean ± SEM; n = 4–11; *P < 0.05 compared with respective vehicle group. Vehicle for CBD and THC – mixture of ethanol : cremophor : saline, 1:1:18, vehicle for sulphasalazine – 1% methylcellulose. Control group from preliminary experiments is included – phosphate-buffered saline enema, no treatment.
Effects of drug treatment on macroscopic damage and neutrophil infiltration
2,4,6-Trinitrobenzene sulphonic acid caused colonic injury reflected in increased MDS (Figure 2). The visual assessment revealed the presence of hyperaemia, haemorrhage, mucosal damage, ulceration, necrosis, thickening of the bowel wall and local distension. The damaged area was usually restricted to a small region (about 1–1.5 cm2) in the rat distal colon. Occasionally colonic adhesions to other organs were present. Colitis was also associated with significantly increased neutrophil infiltration (MPO activity) (Figure 3). Treatment with CBD10 seemed to reduce MDS, but this was not statistically significant. Treatment with THC10 and combined treatment with CBD (both T5 + C and T10 + C) as well as treatment with sulphasalazine resulted in significant decrease of MDS (P < 0.05 vs. respective vehicle and vs. THC5 for T5 + C group) (Figure 2). The MPO activity, which was significantly increased in colitis, was dose-dependently reduced by CBD treatment with maximal reduction at CBD20 (P < 0.05 vs. vehicle, Figure 3A). Treatment with THC10 and THC20 also significantly decreased neutrophil infiltration in comparison with the vehicle treated group, while combined treatment T5 + C reduced MPO activity significantly more than THC5 alone (Figure 3B). Sulphasalazine treatment also seemed to decrease MPO activity, but this effect just failed to be statistically significant (P = 0.06, Figure 3C).
Figure 2.
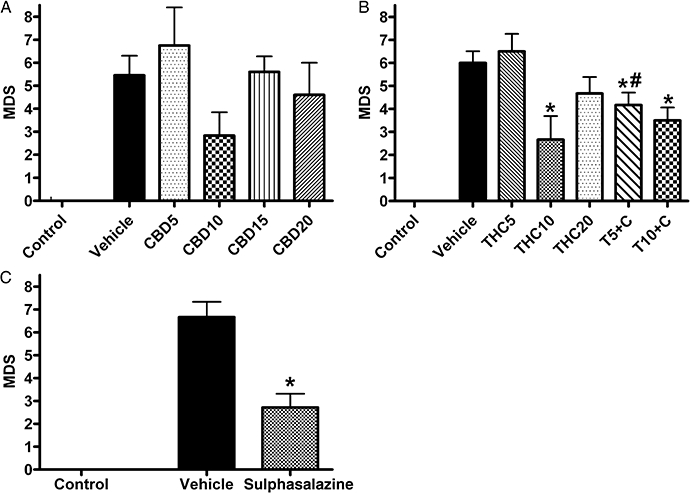
Effects of drug treatment on macroscopic damage score (MDS; 0–13 scale) 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Effects of (A) (-)-cannabidiol (CBD) alone (5, 10, 15 and 20 mg·kg−1 i.p.), (B) Δ9-tetrahydrocannabinol (THC) alone (5, 10 and 20 mg·kg−1 i.p.) and THC in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) and (C) sulphasalazine (300 mg·kg−1 p.o.). Results are expressed as mean ± SEM; n = 4–11; *P < 0.05 compared with respective vehicle group. Vehicle for CBD and THC – mixture of ethanol : cremophor : saline, 1:1:18, vehicle for sulphasalazine – 1% methylcellulose; # P < 0.05 compared with respective THC group. Control group from preliminary experiments is included – phosphate-buffered saline enema, no treatment.
Figure 3.
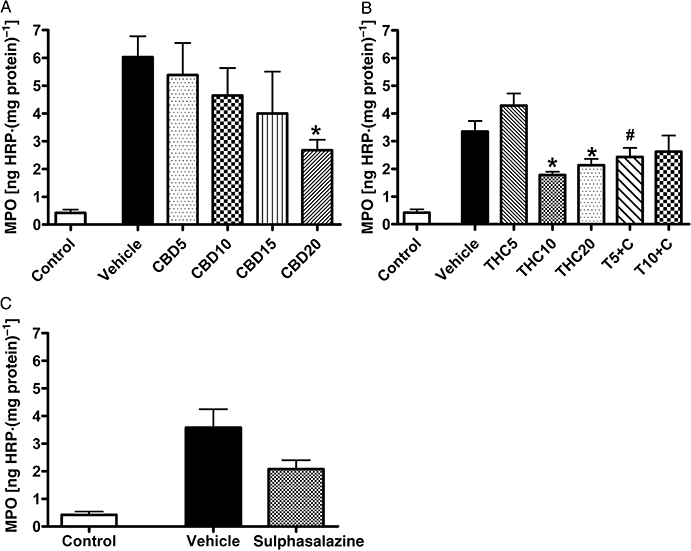
Effects of drug treatment on myeloperoxidase (MPO) activity in rat colon 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Effects of (A) (-)-cannabidiol (CBD) alone (5, 10, 15 and 20 mg·kg−1 i.p.), (B) Δ9-tetrahydrocannabinol (THC) alone (5, 10 and 20 mg·kg−1 i.p.) and THC in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) and (C) sulphasalazine (300 mg·kg−1 p.o.). Results are expressed as mean ± SEM; n = 4–11; *P < 0.05 compared with respective vehicle group, #P < 0.05 compared with respective THC group. Vehicle for CBD and THC – mixture of ethanol : cremophor : saline, 1:1:18, vehicle for sulphasalazine – 1% methylcellulose. Control group from preliminary experiments is included – phosphate-buffered saline enema, no treatment.
Effects of drug treatment on motility disturbances
Spontaneous activity
Colitis resulted in significant reduction of the amplitude and prolonged duration of spontaneous low-frequency contractions in colonic muscle strips. Treatment with CBD10, THC10, T10 + C and sulphasalazine resulted in significant increase of ALF (Tables 2–4). Treatment with CBD10, CBD20, THC10, THC20 and both combined treatments significantly reduced the duration of spontaneous contractions (Table 3). The duration also appeared to be decreased by sulphasalazine, but this effect was not statistically significant due to the large variability in the vehicle group (Table 4).
Table 2.
Effects of (-)-cannabidiol (CBD) (5, 10, 15 and 20 mg·kg−1 i.p.) on the amplitude (ALF) and duration (D) of low-frequency spontaneous contractions
| Control | Vehicle | CBD5 | CBD10 | CBD15 | CBD20 | |
|---|---|---|---|---|---|---|
| ALF (g·g−1) | 195 ± 19 | 37 ± 5 | 41 ± 13 | 67 ± 11* | 47 ± 8 | 55 ± 10 |
| D (s) | 26.9 ± 1.7 | 62.8 ± 4.5 | 57.0 ± 7.8 | 46.2 ± 6.5* | 52.6 ± 2.8 | 38.7 ± 2.8* |
Longitudinal muscle strips from rat colon 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema.
Control values (preliminary experiments, phosphate-buffered saline enema, no treatment) are shown. Contractions expressed in grams per gram of dry tissue weight. Results are expressed as mean ± SEM; n = 4–11.
P < 0.05 compared with the vehicle (ethanol : cremophor : saline, 1:1:18).
Table 4.
Effects of sulphasalazine (300 mg·kg−1 p.o.) on the amplitude (ALF) and duration (D) of low-frequency spontaneous contractions
| Vehicle | Sulphasalazine | |
|---|---|---|
| ALF (g·g−1) | 36 ± 8 | 133 ± 24* |
| D (s) | 89.0 ± 32.1 | 36.3 ± 3.0 |
Longitudinal muscle strips from rat colon 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Contractions expressed in grams per gram of dry tissues weight. Results are expressed as mean ± SEM; n = 6–7.
P < 0.05 compared with the vehicle (1% methylcellulose).
Table 3.
Effects of THC alone (5, 10 and 20 mg·kg−1 i.p.) and in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) on the amplitude (ALF) and duration (D) of low-frequency spontaneous contractions
| Vehicle | THC5 | THC10 | THC20 | T5 + C | T10 + C | |
|---|---|---|---|---|---|---|
| ALF (g·g−1) | 41 ± 6 | 49 ± 10 | 92 ± 13* | 62 ± 14 | 66 ± 10 | 89 ± 9* |
| D (s) | 77.7 ± 11.0 | 61.6 ± 6.5 | 50.7 ± 7.5* | 40.6 ± 3.3* | 46.3 ± 3.4* | 44.5 ± 5.6* |
Longitudinal muscle strips from rat colon 3 days after administration of TNBS enema. Contractions expressed in grams per gram of dry tissue weight. Results are expressed as mean ± SEM; n = 6–11.
P< 0.05 compared with the vehicle (ethanol : cremophor : saline, 1:1:18).
CBD, (-)-cannabidiol; THC, Δ9-tetrahydrocannabinol; TNBS, 2,4,6-trinitrobenzene sulphonic acid.
Responses to carbachol
The contractile responses to carbachol application were markedly reduced in inflamed tissues (see concentration–response curves Figure 4 and Emax values Table 5), but there was no significant change in the potency of carbachol between treatment groups (see logEC50 values, Tables 5–7). Treatment with CBD10 resulted in a significant increase in the contractions to carbachol (P < 0.05 vs. vehicle at concentrations 1 × 10−6 M to 3 × 10−5 M, Figure 4A and P < 0.05 for Emax, Table 5). THC10 treatment alone improved the responses (P < 0.05 for Emax, Table 6). The effect was statistically significant in the groups that received combined treatment with CBD (T10 + C, P < 0.05 vs. vehicle at concentrations 3 × 10−7 M to 3 × 10−5 M, Figure 4B and T5 + 10 and T10 + C, P < 0.05 for Emax, Table 6), suggesting additive effects of CBD and THC. The T5 + C group reached the level of contractility of the THC10 group. Treatment with sulphasalazine also significantly enhanced the responses at carbachol concentrations 3 × 10−7 M to 3 × 10−5 M (Figure 4C, P < 0.05 for Emax, Table 7).
Figure 4.
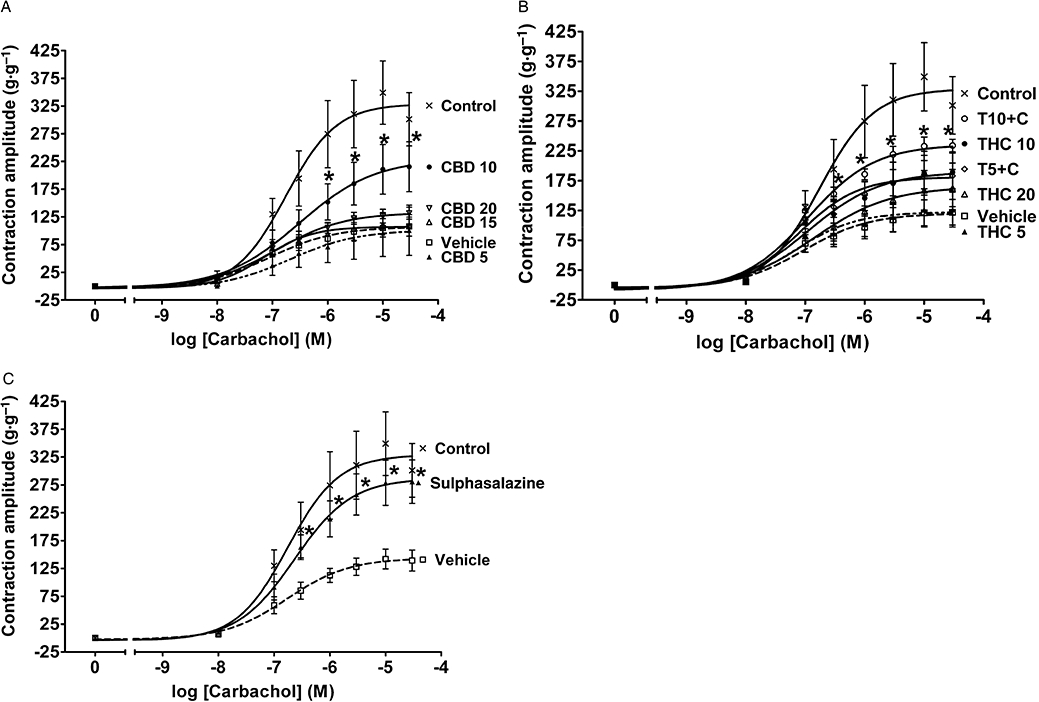
Effects of drug treatment on contractile responses of longitudinal muscle strips from rat colon to carbachol 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Effects of (A) (-)-cannabidiol (CBD) alone (5, 10, 15 and 20 mg·kg−1 i.p.), (B) Δ9-tetrahydrocannabinol (THC) alone (5, 10 and 20 mg·kg−1 i.p.) and THC in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) and (C) sulphasalazine (300 mg·kg−1 p.o.). Contractions expressed in grams per gram of dry tissue weight. Results are expressed as mean ± SEM; n = 4–11; *P < 0.05 compared with respective vehicle group. Vehicle for CBD and THC – mixture of ethanol : cremophor : saline, 1:1:18, vehicle for sulphasalazine – 1% methylcellulose. Control group from preliminary experiments is included – phosphate-buffered saline enema, no treatment.
Table 5.
Effects of (-)-cannabidiol (CBD) (5, 10, 15 and 20 mg·kg−1 i.p.) on the potency and efficacy of carbachol
| Control | Vehicle | CBD5 | CBD10 | CBD15 | CBD20 | |
|---|---|---|---|---|---|---|
| logEC50 | −6.76 ± 0.24 | −7.06 ± 0.30 | −6.68 ± 0.65 | −6.52 ± 0.39 | −7.16 ± 0.29 | −6.94 ± 0.17 |
| Emax (g·g−1) | 329 ± 35 | 107 ± 11 | 102 ± 31 | 230 ± 45* | 107 ± 10 | 133 ± 9 |
Longitudinal muscle strips from rat colon 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Control values (preliminary experiments, phosphate-buffered saline enema, no treatment) are shown. Contractions expressed in grams per gram of dry tissue weight. Results are expressed as mean ± SEM; n = 4–11.
P < 0.05 compared with the vehicle (ethanol : cremophor : saline, 1:1:18).
Table 7.
Effects of sulphasalazine (300 mg·kg−1 p.o.) on the potency and efficacy of carbachol
| Vehicle | Sulphasalazine | |
|---|---|---|
| logEC50 | −6.76 ± 0.20 | −6.64 ± 0.19 |
| Emax (g·g−1) | 144 ± 13 | 289 ± 27* |
Longitudinal muscle strips from rat colon 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Contractions expressed in grams per gram of dry tissues weight. Results are expressed as mean ± SEM; n = 6–7.
P < 0.05 compared with the vehicle (1% methylcellulose).
Table 6.
Effects of THC alone (5, 10 and 20 mg·kg−1 i.p.) and in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) on the potency and efficacy of carbachol
| Vehicle | THC5 | THC10 | THC20 | T5 + C | T10 + C | |
|---|---|---|---|---|---|---|
| logEC50 | −7.04 ± 0.31 | −7.17 ± 0.36 | −6.95 ± 0.31 | −6.85 ± 0.37 | −7.10 ± 0.20 | −6.95 ± 0.10 |
| Emax (g·g−1) | 120 ± 14 | 123 ± 14 | 190 ± 23* | 165 ± 26 | 181 ± 128 | 235 ± 98 |
Longitudinal muscle strips from rat colon 3 days after administration of TNBS enema. Contractions expressed in grams per gram of dry tissue weight. Results are expressed as mean ± SEM; n = 6–11.
P < 0.05 compared with the vehicle (ethanol : cremophor : saline, 1:1:18).
CBD, (-)-cannabidiol; THC, Δ9-tetrahydrocannabinol; TNBS, 2,4,6-trinitrobenzene sulphonic acid.
EFS experiments
Both contractile and relaxant responses to EFS were dramatically decreased in inflamed colonic strips (Figures 5 and 6). CBD treatment did not have any significant effects on EFS-evoked responses (Figures 5A and 6A), while treatment with THC10 alone and in combination with CBD (T10 + C) significantly increased both the contractile cholinergic (Figure 5B) and relaxant (Figure 6B) responses. As shown in Figure 6B the relaxant responses were also improved to a similar extent in THC20 and T5 + C groups (P < 0.05 for T5 + C vs. vehicle at 15 Hz).
Figure 5.
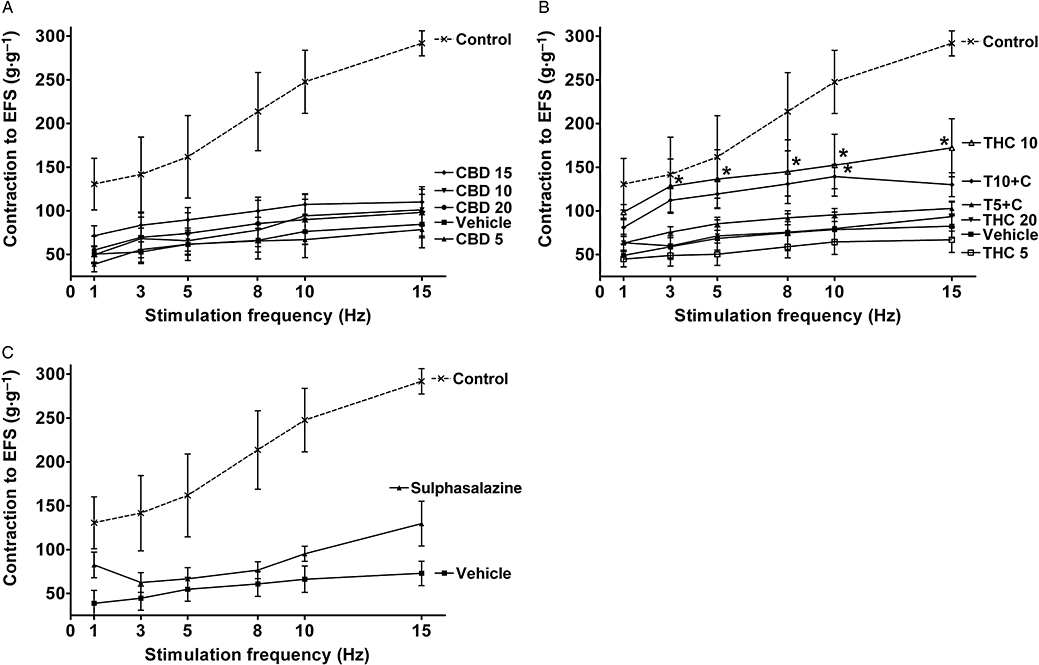
Effects of drug treatment on contractile responses of longitudinal muscle strips from rat colon to electrical field stimulation (EFS) 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Effects of (A) (-)-cannabidiol (CBD) alone (5, 10, 15 and 20 mg·kg−1 i.p.), (B) Δ9-tetrahydrocannabinol (THC) alone (5, 10 and 20 mg·kg−1 i.p.) and THC in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) and (C) sulphasalazine (300 mg·kg−1 p.o.). EFS parameters: 5 s stimulation, 0.2 ms pulse width, voltage (V) supramaximal for 5 Hz. Cholinergic ‘on’ responses are shown. Contractions expressed in grams per gram of dry tissue weight. Results are expressed as mean ± SEM; n = 4–11; *P < 0.05 compared with respective vehicle group. Vehicle for CBD and THC – mixture of ethanol : cremophor : saline, 1:1:18, vehicle for sulphasalazine – 1% methylcellulose. Control group from preliminary experiments is included – phosphate-buffered saline enema, no treatment.
Figure 6.
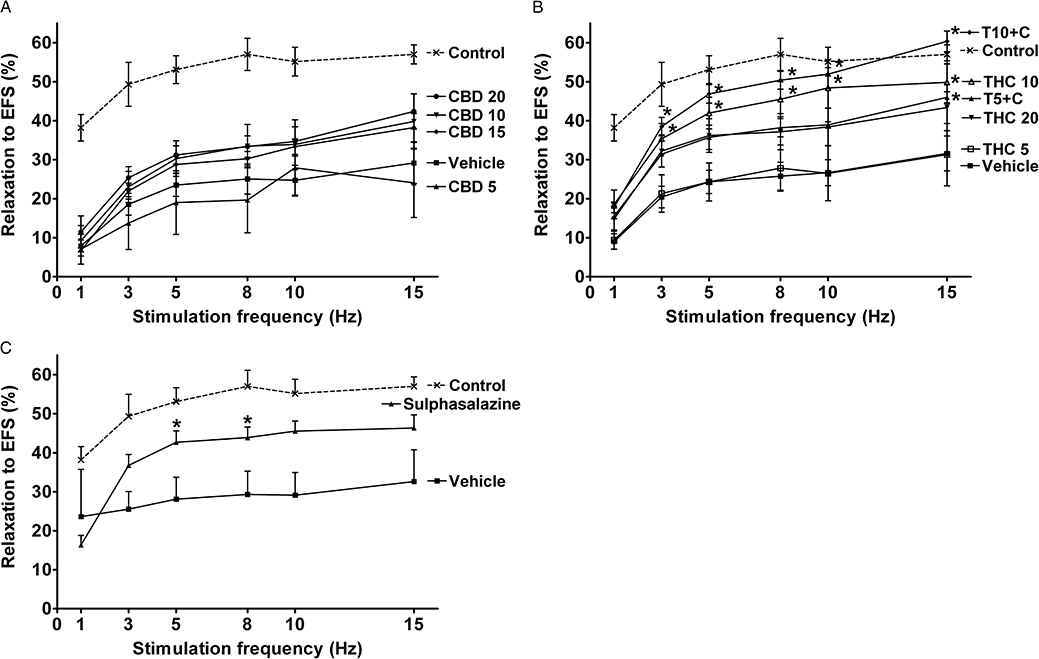
Effects of drug treatment on relaxant responses of longitudinal muscle strips from rat colon to electrical field stimulation (EFS) 3 days after administration of 2,4,6-trinitrobenzene sulphonic acid enema. Effects of (A) (-)-cannabidiol (CBD) alone (5, 10, 15 and 20 mg·kg−1 i.p.), (B) Δ9-tetrahydrocannabinol (THC) alone (5, 10 and 20 mg·kg−1 i.p.) and THC in combination with 10 mg·kg−1 CBD (5 and 10 mg·kg−1 THC, T5 + C and T10 + C, respectively) and (C) sulphasalazine (300 mg·kg−1 p.o.). Strips pre-contracted with 1 × 10−5 M carbachol. EFS parameters: 5 s stimulation, 0.2 ms pulse width, voltage (V) supramaximal for 5 Hz. Responses expressed as % relaxation. Results are expressed as mean ± SEM; n = 4–11; *P < 0.05 compared with respective vehicle group. Vehicle for CBD and THC – mixture of ethanol : cremophor : saline, 1:1:18, vehicle for sulphasalazine – 1% methylcellulose. Control group from preliminary experiments is included – phosphate-buffered saline enema, no treatment.
Treatment with sulphasalazine did not have significant effect on EFS-evoked contractile responses (Figure 5C), but it increased the relaxant responses (Figure 6C), although to a smaller extent than THC10 and T10 + C.
Discussion
2,4,6-Trinitrobenzene sulphonic acid-induced colitis in rats is a well-established animal model of colitis (Boughton-Smith et al., 1988; Morris et al., 1989; Sykes et al., 1999; Whittle et al., 2008; Arribas et al., 2009). It has been reported that colitis alters spontaneous activity as well as smooth muscle contractility in response to pharmacological and nerve stimulation (Poli et al., 2001). In the present study, TNBS administration caused a mild, acute colitis associated with damage, neutrophil infiltration and impairment of in vitro motility parameters. Sulphasalazine, a standard treatment for IBD, not only reduced injury and inflammation, as reported previously (Daddaoua et al., 2007), but also improved motility. Similar to the effects of sulphasalazine, treatment with the phytocannabinoids, CBD and THC, resulted in a reduction of damage, neutrophil infiltration and in vitro motility disturbances.
Inhibitory effects of phytocannabinoids on MPO activity are an essential feature of their actions as demonstrated in other in vivo studies (Hayakawa et al., 2007; Napimoga et al., 2009). Interestingly, evaluation of the mechanism of action of CBD in a study on the inhibition of human neutrophil chemotaxis revealed that a new target, distinct from CB1 and CB2 receptors, may be involved. THC, unlike CBD, does not affect neutrophil function directly (Mchugh et al., 2008). Therefore, the reduction of MPO activity seen in the present study was most likely to be mediated by indirect effects of THC, such as a decrease in levels of cytokines, such as tumour necrosis factor-α and IL-8, involved in neutrophil recruitment. Additional studies are necessary to clarify this. In addition, the dissociation of the positive effects of phytocannabinoids upon damage, functional parameters and neutrophil infiltration suggests that other inflammatory cell types involved in tissue injury and motility disturbances (e.g. macrophages, mast cells) were affected by CBD and THC in the present study.
In relation to the reduction of motility disturbances, both phytocannabinoids have been demonstrated to reduce the release of pro-inflammatory mediators, such as tumour necrosis factor-α, interferon-γ, IL-1β and nitric oxide, both in vivo and in vitro (Watzl et al., 1991; Srivastava et al., 1998; Malfait et al., 2000; Esposito et al., 2007; Kaplan et al., 2008; Borrelli et al., 2009). These pro-inflammatory mediators were shown to impair smooth muscle contractility and/or decrease neurotransmitter release from the myenteric plexus (Collins, 1996; Jacobson et al., 1997; Kinoshita et al., 2006). Importantly, the profile of phytocannabinoid action on motility parameters in the present study was similar to that of the reference drug, sulphasalazine: that is, a significant improvement of responses. The effectiveness of THC (10 mg·kg−1) alone and in combination with CBD was even greater than that of sulphasalazine in the EFS-evoked responses because, unlike sulphasalazine, the former treatments significantly increased cholinergic contractions and increased relaxant responses to EFS to a greater extent. Therefore, THC alone and in combination with CBD was more protective of the myenteric plexus than the active control drug, sulphasalazine, as it improved the function of both the excitatory and inhibitory nerves.
Notably, in the groups treated with CBD or sulphasalazine alone, the cholinergic motor function (responses to EFS) remained abnormal despite improvement of smooth muscle contractility (responses to carbachol). The reason for this remains unknown. A possible explanation could be a direct inhibition of the function of cholinergic motor neurons; however, such an effect of CBD or sulphasalazine has not been reported. Another possibility is that these two drugs, unlike THC, do not protect the excitatory neurons from damage caused by TNBS enema and/or do not reduce inflammatory mediators involved in neuronal dysfunction in the course of colitis
In the present study, the effects of in vivo treatment with phytocannabinoids on the in vitro motility parameters are most likely to reflect anti-inflammatory actions of THC and CBD and to result from reduced damage. This is supported by the observation that the MDS correlates with the amplitude of spontaneous contractions and with the responses to carbachol. The dominance of anti-inflammatory effects of phytocannabinoids over any direct effects on motility explains why there is an apparent discrepancy between the effects of THC and CBD observed in the present study (increased motility parameters at 10 mg·kg–1) and known effects of cannabinoids (reduced gastrointestinal contractility to EFS and acetylcholine) (Duncan et al., 2005; Capasso et al., 2008). We also found that both CBD and THC displayed bell-shaped dose–response curves (Tables 2 and 3, Figures 2, 4–6) that is a characteristic feature of cannabinoid pharmacology.
(-)-Cannabidiol has been shown to interact with THC in behavioural and physiological studies, but there have been no reports indicating whether these cannabinoids do or do not interact in vivo in inflammatory disorders. Therefore, the current study aimed to evaluate the possible interaction of an ineffective (5 mg·kg−1) and an optimal dose of THC (10 mg·kg−1) with an optimal dose of CBD (10 mg·kg−1). The choice of the doses of the two drugs used in the present study was not only based on a literature review but also on the ratio used in Sativex® (Russo and Guy, 2006). The ineffective dose of THC was used to see if CBD could potentiate its effects, as demonstrated in other studies (Karniol and Carlini, 1973; Anderson et al., 1974; Fernandes et al., 1974; Murphy et al., 1990).
The analysis of CBD and THC interaction in the present study is complicated, because both phytocannabinoids proved beneficial in TNBS-induced colitis in the rat. The effects of combined treatment with CBD and THC were greater than additive for one parameter (MPO activity) and additive for three others (MDS, spontaneous activity and contractility to carbachol). There were also statistically non-significant signs of a potentiation for two additional parameters (body weight gain and increased EFS-evoked relaxations) but no evidence of an interaction for EFS-evoked contractions.
In the present study CBD did not affect body weight gain when administered alone. A similar observation for 10 mg·kg−1 CBD was recently made in healthy mice although there was a trend towards a reduction in food intake (Riedel et al., 2009). Analysis of combined treatment with THC in the present study leads to the interesting observation that CBD reinforced the effects of THC and the effect of combined treatment was the same as a higher dose of THC administered alone. This observation is in agreement with a previous study that demonstrated that CBD potentiates the inhibitory effects of THC on food intake (Fernandes et al., 1974).
A similar pattern of changes was observed when analysing MPO activity, that is, combined treatment with T5 + C reduced neutrophil infiltration by about 50% when compared with 5 mg·kg−1 THC alone, which suggests that CBD augmented the effect of THC. However analysis of the interaction in the T10 + C group was inconclusive because of the larger variability in responses. Like the effects on MPO activity, an enhancement in the T5 + C group versus T5 alone was observed in the EFS-evoked relaxations. As suggested by other studies, potentiation of the effects of THC could be explained by inhibition of its metabolism by concomitant administration of CBD (Fernandes et al., 1974; Zuardi et al., 1984). In addition, it was demonstrated recently that the CBD-induced potentiation of such pharmacological effects of THC as hypoactivity, hypothermia and impairment of spatial memory was accompanied by increased expression of CB1 receptors in hippocampus and hypothalamus (Hayakawa et al., 2008) and a similar mechanism could contribute to interactions observed in the current study. However in the study by Hayakawa et al. the effects of CBD were observed only at a very high dose (50 mg·kg−1), and the measurements were done 1 h after i.p. injection. Therefore, additional studies with repeated dosing of CBD will be necessary to clarify if lower doses would induce changes in CB1 receptor expression and it also needs to be established if such changes can occur outside the CNS. Because CBD reduced MDS in the present study, albeit not significantly, the lowering of this parameter in T5 + C group could be attributed to additive effects of the drugs. Importantly a similar interaction was observed in the improvement of contractile responses to carbachol and the spontaneous activity parameters.
In conclusion, treatment with THC, CBD and sulphasalazine reduced signs of damage, inflammation and functional disturbances in a rat model of Crohn's disease. THC was the most effective drug, because it significantly improved all parameters and importantly, unlike the reference drug sulphasalazine, it improved the function of cholinergic motoneurons. CBD on its own also displayed beneficial actions, such as improved spontaneous activity and contractility to carbachol, which extends previous findings (Malfait et al., 2000; Borrelli et al., 2009) and further suggests that this phytocannabinoid, which is devoid of psychoactive properties, could help alleviate symptoms in human IBD. Combined treatment with CBD and THC proved beneficial in TNBS-induced colitis in the rat, as it resulted in additive effects on some functional parameters and as CBD caused an ineffective dose of THC (5 mg·kg−1) to produce beneficial effects of the same magnitude as those produced by a higher dose of THC (10 mg·kg−1) in the absence of CBD. It is possible therefore that the benefit-to-risk ratio may well be greater when CBD and THC are co-administered to ameliorate colitis than when THC is administered alone. Further studies will be necessary to identify the mechanisms responsible for the observed effects.
Acknowledgments
This article is dedicated to the memory of Professor Mike Parsons, both our friend and a co-author, who died on 26 February 2010. We would like to thank GW Pharmaceuticals for kindly providing CBD for the present study.
Glossary
Abbreviations:
- ALF
amplitude of low-frequency spontaneous contractions
- CBD
(-)-cannabidiol
- EFS
electrical field stimulation
- HRP
horseradish peroxidase
- MDS
macroscopic damage score
- MPO
myeloperoxidase
- PBS
phosphate-buffered saline
- THC
Δ9-tetrahydrocannabinol
- TNBS
2,4,6-trinitrobenzene sulphonic acid
Conflict of interests
One of the authors (Roger Pertwee) has formal links with and funding from GW Pharmaceuticals.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edition. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PF, Jackson DM, Chesher GB. Interaction of delta9-tetrahydrocannabinol and cannabidiol on intestinal motility in mice. J Pharm Pharmacol. 1974;26:136–137. doi: 10.1111/j.2042-7158.1974.tb09240.x. [DOI] [PubMed] [Google Scholar]
- Appleyard CB, Wallace JL. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am J Physiol. 1995;269:G119–G125. doi: 10.1152/ajpgi.1995.269.1.G119. [DOI] [PubMed] [Google Scholar]
- Arribas B, Rodriguez-Cabezas ME, Camuesco D, Comalada M, Bailon E, Utrilla P, et al. A probiotic strain of Escherichia coli, Nissle 1917, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br J Pharmacol. 2009;157:1024–1033. doi: 10.1111/j.1476-5381.2009.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- Borrelli F, Aviello G, Romano B, Orlando P, Capasso R, Maiello F, et al. Cannabidiol, a safe and non-psychotropic ingredient of the marijuana plant Cannabis sativa, is protective in a murine model of colitis. J Mol Med. 2009;87:1111–1121. doi: 10.1007/s00109-009-0512-x. [DOI] [PubMed] [Google Scholar]
- Boughton-Smith NK, Wallace JL, Morris GP, Whittle BJ. The effect of anti-inflammatory drugs on eicosanoid formation in a chronic model of inflammatory bowel disease in the rat. Br J Pharmacol. 1988;94:65–72. doi: 10.1111/j.1476-5381.1988.tb11500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F, et al. Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. Br J Pharmacol. 2008;154:1001–1008. doi: 10.1038/bjp.2008.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996;111:1683–1699. doi: 10.1016/s0016-5085(96)70034-3. [DOI] [PubMed] [Google Scholar]
- Daddaoua A, Martinez-Plata E, Lopez-Posadas R, Vieites JM, Gonzalez M, Requena P, et al. Active hexose correlated compound acts as a prebiotic and is antiinflammatory in rats with hapten-induced colitis. J Nutr. 2007;137:1222–1228. doi: 10.1093/jn/137.5.1222. [DOI] [PubMed] [Google Scholar]
- Duncan M, Davison JS, Sharkey KA. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- Esposito G, Scuderi C, Savani C, Steardo L, Jr, De Filippis D, Cottone P, et al. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br J Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M, Schabarek A, Coper H, Hill R. Modification of delta9-THC-actions by cannabinol and cannabidiol in the rat. Psychopharmacologia. 1974;38:329–338. doi: 10.1007/BF00429130. [DOI] [PubMed] [Google Scholar]
- Fujioka Y, Metsugi Y, Ogawara K, Higaki K, Kimura T. Evaluation of in vivo dissolution behavior and GI transit of griseofulvin, a BCS class II drug. Int J Pharm. 2008;352:36–43. doi: 10.1016/j.ijpharm.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Planella E, Marin L, Domenech E, Bernal I, Manosa M, Zabana Y, et al. Use of complementary and alternative medicine and drug abuse in patients with inflammatory bowel disease. Med Clin (Barc) 2007;128:45–48. doi: 10.1157/13097468. [DOI] [PubMed] [Google Scholar]
- Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–742. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Nozako M, Hazekawa M, Irie K, Fujioka M, et al. Delayed treatment with cannabidiol has a cerebroprotective action via a cannabinoid receptor-independent myeloperoxidase-inhibiting mechanism. J Neurochem. 2007;102:1488–1496. doi: 10.1111/j.1471-4159.2007.04565.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Mishima K, Hazekawa M, Sano K, Irie K, Orito K, et al. Cannabidiol potentiates pharmacological effects of Delta(9)-tetrahydrocannabinol via CB(1) receptor-dependent mechanism. Brain Res. 2008;1188:157–164. doi: 10.1016/j.brainres.2007.09.090. [DOI] [PubMed] [Google Scholar]
- Jacobson K, McHugh K, Collins SM. The mechanism of altered neural function in a rat model of acute colitis. Gastroenterology. 1997;112:156–162. doi: 10.1016/s0016-5085(97)70230-0. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Springs AE, Kaminski NE. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T cells (NFAT) Biochem Pharmacol. 2008;76:726–737. doi: 10.1016/j.bcp.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniol IG, Carlini EA. Pharmacological interaction between cannabidiol and delta 9-tetrahydrocannabinol. Psychopharmacologia. 1973;33:53–70. doi: 10.1007/BF00428793. [DOI] [PubMed] [Google Scholar]
- Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172–177. doi: 10.1016/0014-2999(74)90129-0. [DOI] [PubMed] [Google Scholar]
- Kimball ES, Schneider CR, Wallace NH, Hornby PJ. Agonists of cannabinoid receptor 1 and 2 inhibit experimental colitis induced by oil of mustard and by dextran sulfate sodium. Am J Physiol Gastrointest Liver Physiol. 2006;291:G364–G371. doi: 10.1152/ajpgi.00407.2005. [DOI] [PubMed] [Google Scholar]
- Kinoshita K, Hori M, Fujisawa M, Sato K, Ohama T, Momotani E, et al. Role of TNF-alpha in muscularis inflammation and motility disorder in a TNBS-induced colitis model: clues from TNF-alpha-deficient mice. Neurogastroenterol Motil. 2006;18:578–588. doi: 10.1111/j.1365-2982.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- Lal C, Ryan M, Tangri S, Silverberg MS, Gordon A, Steinhart AH. Cannabis use by patients with inflammatory bowel disease. Gastroenterology. 2007;132:A183. doi: 10.1097/MEG.0b013e328349bb4c. [DOI] [PubMed] [Google Scholar]
- Li X, Kaminski NE, Fischer LJ. Examination of the immunosuppressive effect of delta9-tetrahydrocannabinol in streptozotocin-induced autoimmune diabetes. Int Immunopharmacol. 2001;1:699–712. doi: 10.1016/s1567-5769(01)00003-0. [DOI] [PubMed] [Google Scholar]
- Lyman WD, Sonett JR, Brosnan CF, Elkin R, Bornstein MB. Delta 9-tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol. 1989;23:73–81. doi: 10.1016/0165-5728(89)90075-1. [DOI] [PubMed] [Google Scholar]
- McHugh D, Tanner C, Mechoulam R, Pertwee RG, Ross RA. Inhibition of human neutrophil chemotaxis by endogenous cannabinoids and phytocannabinods: evidence for a site distinct from CB1 and CB2. Mol Pharmacol. 2008;73:441–450. doi: 10.1124/mol.107.041863. [DOI] [PubMed] [Google Scholar]
- Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, et al. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Marsicano G, Hermann H, Cannich A, Monory K, Cravatt BF, et al. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Parolaro D. Cannabinoids, immune system and cytokine network. Curr Pharm Des. 2006;12:3135–3146. doi: 10.2174/138161206777947425. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Plant cannabinoids: a neglected pharmacological treasure trove. Br J Pharmacol. 2005;146:913–915. doi: 10.1038/sj.bjp.0706415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42:11S–19S. doi: 10.1002/j.1552-4604.2002.tb05998.x. [DOI] [PubMed] [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Murphy LL, Steger RW, Smith MS, Bartke A. Effects of delta-9-tetrahydrocannabinol, cannabinol and cannabidiol, alone and in combinations, on luteinizing hormone and prolactin release and on hypothalamic neurotransmitters in the male rat. Neuroendocrinology. 1990;52:316–321. doi: 10.1159/000125604. [DOI] [PubMed] [Google Scholar]
- Napimoga MH, Benatti BB, Lima FO, Alves PM, Campos AC, Pena-Dos-Santos DR, et al. Cannabidiol decreases bone resorption by inhibiting RANK/RANKL expression and pro-inflammatory cytokines during experimental periodontitis in rats. Int Immunopharmacol. 2009;9:216–222. doi: 10.1016/j.intimp.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Cannabinoids and multiple sclerosis. Mol Neurobiol. 2007;36:45–59. doi: 10.1007/s12035-007-0005-2. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli E, Lazzaretti M, Grandi D, Pozzoli C, Coruzzi G. Morphological and functional alterations of the myenteric plexus in rats with TNBS-induced colitis. Neurochem Res. 2001;26:1085–1093. doi: 10.1023/a:1012313424144. [DOI] [PubMed] [Google Scholar]
- Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol. 2009;156:1154–1166. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. doi: 10.1016/j.mehy.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Srivastava MD, Srivastava BI, Brouhard B. Delta9 tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology. 1998;40:179–185. doi: 10.1016/s0162-3109(98)00041-1. [DOI] [PubMed] [Google Scholar]
- Storr M, Keenan CM, Patel KD, Sharkey KA. The cannabinoid-2 (Cb2) receptor mediates protection against TNBS colitis in mice. Gastroenterology. 2007;132(Suppl 2):A231. [Google Scholar]
- Sykes AP, Bhogal R, Brampton C, Chander C, Whelan C, Parsons ME, et al. The effect of an inhibitor of matrix metalloproteinases on colonic inflammation in a trinitrobenzenesulphonic acid rat model of inflammatory bowel disease. Aliment Pharmacol Ther. 1999;13:1535–1542. doi: 10.1046/j.1365-2036.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- Vann RE, Gamage TF, Warner JA, Marshall EM, Taylor NL, Martin BR, et al. Divergent effects of cannabidiol on the discriminative stimulus and place conditioning effects of Delta(9)-tetrahydrocannabinol. Drug Alcohol Depend. 2008;94:191–198. doi: 10.1016/j.drugalcdep.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkova K, Dunn ST, Adesina AM, Meerveld BG, Greenwood-Van Meerveld B. Neuromuscular dysfunction in the jejunum and colon of human leukocyte antigen B27 transgenic rats. J Pharmacol Exp Ther. 2000;293:60–66. [PubMed] [Google Scholar]
- Watzl B, Scuderi P, Watson RR. Influence of marijuana components (THC and CBD) on human mononuclear cell cytokine secretion in vitro. Adv Exp Med Biol. 1991;288:63–70. doi: 10.1007/978-1-4684-5925-8_7. [DOI] [PubMed] [Google Scholar]
- Weiss L, Zeira M, Reich S, Har-Noy M, Mechoulam R, Slavin S, et al. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity. 2006;39:143–151. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- Wells RW, Blennerhassett MG. Persistent and selective effects of inflammation on smooth muscle cell contractility in rat colitis. Pflugers Arch. 2004;448:515–524. doi: 10.1007/s00424-004-1286-1. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Varga C, Berko A, Horvath K, Posa A, Riley JP, et al. Attenuation of inflammation and cytokine production in rat colitis by a novel selective inhibitor of leukotriene A4 hydrolase. Br J Pharmacol. 2008;153:983–991. doi: 10.1038/sj.bjp.0707645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Teixeira NA, Karniol IC. Pharmacological interaction of the effects of delta 9-trans-tetrahydrocannabinol and cannabidiol on serum corticosterone levels in rats. Arch Int Pharmacodyn Ther. 1984;269:12–19. [PubMed] [Google Scholar]


