Abstract
Background
To assess the importance of the obesity epidemic on cardiovascular disease (CVD) risk, we determined the prevalence of obesity and the relationship of obesity to CVD risk factors and subclinical vascular disease.
Methods
The Multi-Ethnic Study of Atherosclerosis is an observational cohort study involving 6814 persons aged 45 to 84 years who were free of clinical CVD at baseline (2000–2002). The study assessed the association between body size and CVD risk factors, medication use, and subclinical vascular disease (coronary artery calcium, carotid artery intimal medial thickness, and left ventricular mass).
Results
A large proportion of white, African American, and Hispanic participants were overweight (60% to 85%) and obese (30% to 50%), while fewer Chinese American participants were overweight (33%) or obese (5%). Hypertension and diabetes were more prevalent in obese participants despite a much higher use of antihy-pertensive and/or antidiabetic medications. Obesity was associated with a greater risk of coronary artery calcium (17%), internal carotid artery intimal medial thickness greater than 80th percentile (32%), common carotid artery intimal medial thickness greater than 80th percentile (45%), and left ventricular mass greater than 80th percentile (2.7-fold greater) compared with normal body size. These associations persisted after adjustment for traditional CVD risk factors.
Conclusions
These data confirm the epidemic of obesity in most but not all racial and ethnic groups. The observed low prevalence of obesity in Chinese American participants indicates that high rates of obesity should not be considered inevitable. These findings may be viewed as indicators of potential future increases in vascular disease burden and health care costs associated with the obesity epidemic.
The United States and many other countries are experiencing an epidemic of obesity. From 1960 to 2000, the percentage of the US population categorized as obese increased from 11% to 28% in men and 16% to 34% in women, with higher rates observed in certain racial and ethnic groups.1,2 A number of studies have documented the association between obesity and cardiovascular disease (CVD) risk factors,3–5 and some, but not all, with markers of subclinical CVD.6–9 The obesity epidemic has the potential to reduce further gains in the US life expectancy,10 largely through an effect on CVD mortality.11
The Multi-Ethnic Study of Atherosclerosis (MESA) provides an excellent opportunity to evaluate the prevalence and associations with CVD risk factors of obesity and overweight status in a diverse cohort of middle-aged and older adults. Compared with earlier studies conducted in the 1980s and early 1990s, the initial MESA examination began in 2000, coincident with a more recent stage in the obesity epidemic, in a cohort with a multiethnic composition and a greater depth of characterization of CVD risk factors and subclinical CVD. Therefore, this investigation is evaluating the CVD effects of an even greater prevalence of obesity than studies performed only 5 years earlier. As the prevalence of obesity and overweight in the United States further increases, the CVD consequences may also be concurrently increasing. We analyzed data from the MESA baseline examination to (1) document the prevalence of obesity and overweight in middle-aged and older adults by age, race/ethnicity, and sex; (2) assess the associations of obesity with traditional CVD risk factors (eg, blood pressure, lipids and lipoproteins, and diabetes); and (3) evaluate the relationship between obesity and subclinical vascular markers (eg, carotid artery intimal medial thickness [IMT], left ventricular [LV] mass, and coronary artery calcium [CAC] score). These analyses may advance our understanding of the subclinical vascular burden imposed by the ongoing obesity epidemic in an ethnically diverse cross-section of US adults. Furthermore, our goal is to investigate the historical perspective that asserts that the association between CVD and obesity is mediated virtually completely by traditional CVD risk factors.
METHODS
The MESA was initiated to investigate the prevalence, correlates, and progression of subclinical CVD. Details about the study design have been published elsewhere.12 In brief, between July 2000 and August 2002, 6814 men and women aged 45 to 84 years old who identified themselves as white, African American, Hispanic, or Chinese American and who were free of clinically apparent CVD were recruited from 6 US communities. Each field site recruited the study participants from locally available sources (lists of residents, dwellings, and telephone exchanges). In the last few months of the recruitment period, supplemental sources (lists of Medicare beneficiaries from the Centers for Medicare and Medicaid Services and referrals by participants) were also used to ensure adequate numbers of minorities and elderly subjects. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
Standardized questionnaires were used to obtain information about level of education, income, smoking history, and medication use. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Body size was classified into 3 categories of BMI (<25.0 [normal], 25.0–29.9 [overweight], and ≥30.0 [obese]). Seated resting blood pressure was measured 3 times with an automated oscillometric sphygmomanometer (Dinamap PRO-100; Critikon, Milwaukee, Wisconsin). The average of the last 2 measurements was used in these analyses. Hypertension was defined as a blood pressure reading greater than or equal to 140/90 mm Hg or current antihypertensive medication use. The levels of lipids, lipoproteins, and glucose were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein (LDL) cholesterol levels were calculated using the Friedewald equation, and LDL particle size was measured using proton nuclear magnetic resonance spectroscopy (LipoScience Inc, Raleigh, North Carolina).13,14 Diabetes status was classified using the American Diabetes Association criteria as normal (fasting glucose, <100 mg/dL [to convert to millimoles per liter, multiply by 0.0555]), impaired (fasting glucose, 100–125 mg/dL), or diabetic (fasting glucose, ≥126 mg/dL, or hypoglycemic medication use).15
CORONARY CALCIUM ASSESSMENT
Computed tomography of the chest was performed either with an electrocardiographic-triggered, electron-beam computed tomography scanner (Imatron C-150; Imatron Inc, South San Francisco, California)16 (at field centers in Chicago, Illinois; Los Angeles, California; and New York, New York) or with prospectively electrocardiographic-triggered scan acquisition at 50% of the RR interval with a multidetector computed tomography system17 that acquired 4 simultaneous 2.5-mm slices for each cardiac cycle in a sequential or axial scan mode. Each participant was scanned twice over phantoms of known calcium concentration. Scans were read centrally at the Harbor-UCLA Research and Education Institute, Los Angeles. The average (phantom-adjusted) Agatston score was used in all analyses.18 The presence of calcification was defined as an average Agatston score of greater than 0.
CAROTID ARTERY IMT
The IMT of the internal carotid artery (ICA) and the common carotid artery (CCA) was assessed using B-mode ultrasound. The maximum IMT was measured and averaged at 12 bilateral locations for the ICAs and at 4 locations for the CCAs. In the analyses, the maximum IMT was dichotomized at the sex-specific 80th percentile of MESA participants who did not have hypertension, diabetes, or impaired fasting glucose levels and who had a BMI of less than 25; for the IMT of the ICA, the cut-points were 0.96 mm for women and 1.14 mm for men, while for the IMT of the CCA, the cutpoints were 0.88 mm for women and 0.92 mm for men.
LV MASS
Magnetic resonance imaging was performed at the 6 MESA field centers with 1.5-T magnets. The protocol and analysis methods have been described elsewhere.19 Briefly, short-axis cine images were acquired from the end diastolic image of the 4-chamber acquisition by prescribing 10 to 12 slices perpendicular to a line from the middle of the mitral valve plane to the cardiac apex. Cine images of the heart were obtained with a temporal resolution of approximately 50 milliseconds or less. The LV mass was determined by the sum of the myocardial area (the difference between endocardial and epicardial borders of the left ventricle) times the slice thickness plus the image gap in the end diastolic phase multiplied by the specific gravity of the myocardium (1.05 g/mL). The LV measures are often indexed by height or body size surface. In MESA, we derived LV mass indices by regressing the log-transformed LV mass on sex and body size variables in the healthy reference sample (a BMI <25 and free of hypertension, diabetes, or impaired fasting glucose levels). The LV mass indices were then given as the ratio of observed LV mass to the predicted value from the regression model. In the present analyses, which assessed body size associations, the sex- and height-adjusted LV mass index was dichotomized at the 80th percentile of the healthy reference sample.
DATA ANALYSIS
In each sex and racial or ethnic group, CVD risk factors were compared across 3-level BMI groups (<25.0, 25.0–29.9, and ≥30.0). Linear regression models were used to assess differences in means. Relative risk (RR) regression models were used to compare proportions for binary outcome variables, and the RR regression coefficients were directly interpreted in terms of prevalence ratios (or risk ratios). In the RR regression, models with subclinical disease markers (CAC, IMT >80th percentile, and LV mass >80th percentile) were adjusted for age, sex, race, and traditional CVD risk factors (systolic blood pressure, hypertension, LDL, high-density lipoprotein [HDL], triglycerides, diabetes, and cigarette smoking), and tested for race-ethnicity interactions. Statistical significance was defined as P<.05.
Commercially available statistical software packages (SPSS Version 13.0; SPSS Inc, Chicago, Illinois; and Intercooled Stata Version 9.2; Stata Corp, College Station, Texas) were used for analyses. The RR regression model was fitted with a generalized linear model in Stata.
RESULTS
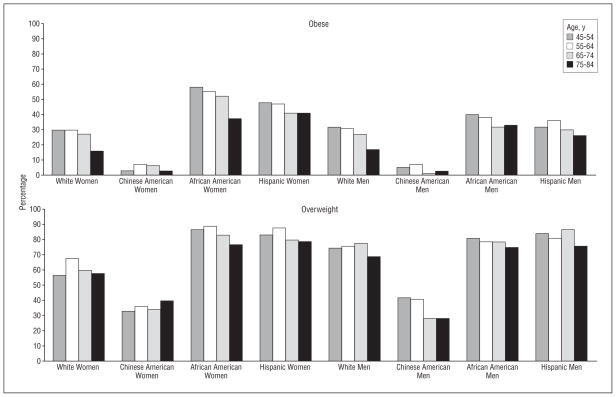
Table 1 presents mean BMI, weight, and waist circumference by race, sex, and age groups. While age and sex differences were observed, the most striking differences were seen across racial/ethnic groups. Chinese American men and women had a lower mean BMI and waist circumference in all age and sex categories (approximately 13% and 10% lower than the next lowest group). White women had a lower BMI and waist circumference than African American or Hispanic women. The prevalence of being obese and overweight by age category are presented in the Figure. A prevalence of 75% or more of being overweight was observed in African American participants, Hispanic participants, and white men, with a more than 60% prevalence observed in white women. In contrast, only one-third of Chinese American participants were classified as overweight. Overall, more than 50% of African American women, 40% of Hispanic women, 30% of African American and Hispanic men, and nearly 30% of white men and women were classified as obese. In contrast, only 5% of Chinese American participants were classified as obese. The pattern of being obese and overweight was similar in middle-aged and older adults.
Table 1.
Body Mass Index (BMI), Weight, and Waist Circumference in the Multi-Ethnic Study of Atherosclerosis Participants by Age, Sex, and Racial/Ethnic Group
| Age, y | Women |
Men |
||||||
|---|---|---|---|---|---|---|---|---|
| White | Chinese American | African American | Hispanic | White | Chinese American | African American | Hispanic | |
| BMIa | ||||||||
| 45–54 | 27.8 | 23.6 | 32.1 | 30.7 | 28.1 | 24.7 | 29.2 | 29.0 |
| 55–64 | 28.1 | 24.1 | 32.0 | 30.4 | 28.3 | 24.5 | 29.1 | 29.0 |
| 65–74 | 27.5 | 23.9 | 31.0 | 29.5 | 28.1 | 23.2 | 28.5 | 28.8 |
| 75–84 | 26.1 | 24.2 | 29.0 | 29.0 | 26.7 | 23.8 | 27.9 | 27.7 |
| Total | 27.5 | 23.9 | 31.3 | 30.0 | 27.9 | 24.1 | 28.8 | 28.8 |
| Weight, lb | ||||||||
| 45–54 | 165 | 130 | 189 | 168 | 196 | 158 | 205 | 186 |
| 55–64 | 165 | 130 | 187 | 162 | 196 | 152 | 199 | 184 |
| 65–74 | 158 | 126 | 178 | 155 | 191 | 143 | 193 | 178 |
| 75–84 | 146 | 122 | 162 | 148 | 177 | 145 | 185 | 171 |
| Total | 160 | 127 | 182 | 160 | 192 | 150 | 197 | 181 |
| Waist, cm | ||||||||
| 45–54 | 92.8 | 82.8 | 100.8 | 98.7 | 99.7 | 88.0 | 100.2 | 99.8 |
| 55–64 | 95.8 | 85.6 | 103.1 | 100.2 | 101.7 | 88.5 | 101.0 | 101.1 |
| 65–74 | 96.7 | 88.5 | 102.1 | 101.8 | 102.2 | 86.2 | 101.2 | 101.5 |
| 75–84 | 95.1 | 90.4 | 98.8 | 102.3 | 99.6 | 89.5 | 99.8 | 101.2 |
| Total | 95.1 | 86.4 | 101.6 | 100.4 | 101.0 | 87.9 | 100.7 | 100.8 |
Conventional conversion factor: To convert pounds to kilograms, multiply by 0.45.
Calculated as weight in kilograms divided by height in meters squared.
Figure.
Obesity (defined as a body mass index [calculated as weight in kilograms divided by height in meters squared] of 25.0–29.9) and overweight (defined as a body mass index of >30.0) prevalence by age and race and ethnic group.
A higher BMI was associated with more adverse levels of blood pressure, lipoproteins, and fasting glucose despite a higher prevalence of pharmacologic treatment (Table 2). Systolic blood pressure levels were significantly higher in the obese than in the normal BMI group in all racial/ethnic and sex groups (6–20 mm Hg higher, age adjusted), HDL cholesterol levels were significantly lower in all racial/ethnic and sex groups (4–14 mg/dL lower, age adjusted [to convert cholesterol to millimoles per liter, multiply by 0.0259]), fasting glucose levels were significantly higher in white, African American, and Hispanic (8–17 mg/dL higher, age adjusted), while triglyceride levels (18–55 mg/dL higher, age adjusted [to convert triglycerides to millimoles per liter, multiply by 0.0113]) and LDL particle size (109–173 nmol/L higher, age adjusted) were significantly greater in all racial/ethic and sex groups. No consistent differences across body size groups were observed for LDL cholesterol. Compared with the normal body size groups, the overweight (age-adjusted RR, 1.2- to 1.8-fold higher) and obese (age-adjusted RR, 1.5- to 2.3-fold higher) groups had much higher levels of antihypertensive medication use. The use of oral hypoglycemic medication or insulin was also greater in the overweight and obese groups (age-adjusted RR, 2.1- to 9.6-fold higher and 1.2- to 2.8-fold higher, respectively). Less consistent associations between lipid-lowering medication use and body size were observed. For 6459 MESA participants (95%) who were younger than 80 years, the 10-year Framingham coronary heart disease risks were also compared across body size group (Table 2). Greater body size was associated with a significantly higher Framingham coronary heart disease risk in all racial/ethnic and sex groups (except African American and Hispanic women).
Table 2.
Blood Pressure (BP), Glucose, and Lipid Levels and Treatment Across Body Size Group in the Multi-Ethnic Study of Atherosclerosis
| Race/Ethnicity by Sex | BMI | No. of Participants | Mean |
Medication Use, % |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systolic BP, mm Hg | LDL, mg/dL | LDL-P, nmol/L | HDL, mg/dL | Triglycerides, mg/dL | Glucose, mg/dL | For BP Control | For Lipid Lowering | For Diabetes | 10-Year CHD Risk, % | |||
| Women | ||||||||||||
| White | <25.0 | 534 | 119 | 114 | 1181 | 66 | 106 | 91 | 21 | 10 | 1 | 3.1 |
| 25.0–29.9 | 456 | 125 | 119 | 1318 | 56 | 137 | 95 | 35 | 20 | 3 | 4.4 | |
| >30.0 | 373 | 126 | 119 | 1355 | 52 | 161 | 102 | 45 | 23 | 9 | 3.8 | |
| Chinese American | <25.0 | 268 | 123 | 114 | 1231 | 56 | 131 | 102 | 22 | 12 | 9 | 3.5 |
| 25.0–29.9 | 125 | 130 | 113 | 1343 | 48 | 164 | 103 | 43 | 24 | 10 | 4.7 | |
| >30.0 | 20 | 131 | 111 | 1322 | 47 | 180 | 113 | 40 | 20 | 15 | 4.7 | |
| African American | <25.0 | 158 | 128 | 114 | 1142 | 67 | 94 | 96 | 38 | 16 | 8 | 4.7 |
| 25–29.9 | 338 | 132 | 120 | 1254 | 58 | 99 | 103 | 49 | 17 | 10 | 4.7 | |
| >30.0 | 554 | 135 | 119 | 1291 | 53 | 105 | 110 | 60 | 17 | 17 | 4.5 | |
| Hispanic | <25.0 | 130 | 124 | 118 | 1247 | 60 | 129 | 99 | 25 | 12 | 7 | 3.9 |
| 25–29.9 | 298 | 127 | 123 | 1375 | 53 | 154 | 104 | 30 | 15 | 10 | 4.5 | |
| >30.0 | 347 | 129 | 117 | 1380 | 49 | 156 | 115 | 42 | 14 | 18 | 4.6 | |
| Men | ||||||||||||
| White | <25.0 | 314 | 120 | 118 | 1248 | 50 | 106 | 96 | 23 | 14 | 2 | 11.0 |
| 25.0–29.9 | 596 | 124 | 117 | 1339 | 45 | 132 | 101 | 35 | 22 | 5 | 12.6 | |
| >30.0 | 351 | 128 | 117 | 1407 | 41 | 165 | 108 | 42 | 22 | 9 | 13.2 | |
| Chinese American | <25.0 | 252 | 122 | 118 | 1293 | 48 | 128 | 107 | 24 | 10 | 8 | 11.5 |
| 25.0–29.9 | 122 | 127 | 113 | 1304 | 42 | 167 | 112 | 34 | 19 | 14 | 12.4 | |
| >30.0 | 16 | 139 | 120 | 1487 | 37 | 174 | 114 | 50 | 6 | 13 | 14.7 | |
| African American | <25.0 | 175 | 127 | 104 | 1146 | 53 | 90 | 101 | 37 | 13 | 9 | 11.7 |
| 25.0–29.9 | 363 | 130 | 117 | 1311 | 47 | 110 | 108 | 47 | 13 | 13 | 13.2 | |
| >30.0 | 306 | 132 | 114 | 1364 | 43 | 120 | 116 | 53 | 20 | 20 | 13.2 | |
| Hispanic | <25.0 | 122 | 122 | 120 | 1365 | 45 | 137 | 113 | 26 | 8 | 10 | 11.9 |
| 25.0–29.9 | 366 | 125 | 121 | 1428 | 43 | 160 | 109 | 27 | 14 | 14 | 13.2 | |
| >30.0 | 230 | 128 | 116 | 1473 | 41 | 182 | 120 | 37 | 12 | 21 | 13.5 | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CHD, coronary heart disease; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LDL-P, LDL particle count.
SI conversion factors: To convert glucose, LDL and HDL, and trigylcerides to millimoles per liter, multiply by 0.0555, 0.0259, and 0.0113, respectively.
The prevalence and the age-adjusted prevalence ratios of hypertension and diabetes are presented across body size groups in Table 3. Overweight status and obesity were strongly associated with a higher prevalence of hypertension. Compared with the normal body size group, the prevalence ratios of hypertension ranged from 1.05 to 1.37 in the overweight group and 1.29 to 2.31 in the obese group. The prevalence of glucose intolerance (impaired glucose tolerance or diabetes) was higher in the overweight and obese groups in all racial/ethnic and sex groups (except Chinese American women). The glucose intolerance prevalence ratios ranged from a 1.4- to 3.1-fold increase in the obese group compared with the normal body size group and were significantly greater in all groups except Chinese American women.
Table 3.
Hypertension and Glucose Intolerance Across Body Size Group in the Multi-Ethnic Study of Atherosclerosis
| Race/Ethnicity by Sex | BMI | No. of Participants | Hypertension |
Glucose Intolerancea |
||
|---|---|---|---|---|---|---|
| % | Age-Adjusted RR (95% CI) | % | Age-Adjusted RR (95% CI) | |||
| Women | ||||||
| White | <25.0 | 534 | 28 | 1 [Reference] | 13 | 1 [Reference] |
| 25.0–29.9 | 455 | 41 | 1.30 (1.12–1.52) | 26 | 1.96 (1.50–2.56) | |
| >30.0 | 373 | 49 | 1.63 (1.40–1.91) | 41 | 3.15 (2.45–4.05) | |
| Chinese American | <25.0 | 268 | 34 | 1 [Reference] | 37 | 1 [Reference] |
| 25–29.9 | 125 | 51 | 1.37 (1.11–1.69) | 47 | 1.26 (1.00–1.59) | |
| >30.0 | 20 | 50 | 1.39 (0.90–2.13) | 55 | 1.41 (0.90–2.22) | |
| African American | <25.0 | 158 | 47 | 1 [Reference] | 24 | 1 [Reference] |
| 25–29.9 | 338 | 59 | 1.23 (1.04–1.44) | 43 | 1.72 (1.28–2.30) | |
| >30.0 | 554 | 67 | 1.43 (1.23–1.66) | 51 | 2.08 (1.58–2.75) | |
| Hispanic | <25.0 | 130 | 38 | 1 [Reference] | 20 | 1 [Reference] |
| 25–29.9 | 298 | 40 | 1.05 (0.85–1.29) | 41 | 1.96 (1.37–2.80) | |
| >30.0 | 347 | 51 | 1.29 (1.06–1.58) | 53 | 2.64 (1.87–3.74) | |
| Men | ||||||
| White | <25.0 | 314 | 27 | 1 [Reference] | 28 | 1 [Reference] |
| 25–29.9 | 596 | 39 | 1.30 (1.07–1.57) | 40 | 1.38 (1.14–1.68) | |
| >30.0 | 350 | 50 | 1.77 (1.46–2.15) | 53 | 1.90 (1.56–2.31) | |
| Chinese American | <25.0 | 252 | 31 | 1 [Reference] | 51 | 1 [Reference] |
| 25–29.9 | 122 | 39 | 1.32 (1.00–1.73) | 63 | 1.32 (1.11–1.57) | |
| >30.0 | 16 | 69 | 2.31 (1.56–3.41) | 73 | 1.60 (1.22–2.09) | |
| African American | <25.0 | 175 | 49 | 1 [Reference] | 27 | 1 [Reference] |
| 25–29.9 | 363 | 56 | 1.15 (0.98–1.36) | 49 | 1.80 (1.38–2.34) | |
| >30.0 | 305 | 62 | 1.33 (1.13–1.56) | 67 | 2.51 (1.95–3.23) | |
| Hispanic | <25.0 | 122 | 30 | 1 [Reference] | 42 | 1 [Reference] |
| 25–29.9 | 366 | 36 | 1.34 (1.00–1.81) | 51 | 1.23 (0.99–1.54) | |
| >30.0 | 230 | 43 | 1.60 (1.18–2.16) | 71 | 1.70 (1.37–2.11) | |
Abbreviations: CI, confidence interval; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); RR, relative risk.
Either impaired fasting glucose levels or diabetes.
Table 4 presents levels of subclinical CVD markers across body size groups adjusted for race, sex, age, and risk factors. Increased body size was significantly (P<.001) associated with a 1.2-fold greater prevalence of CAC in the obese group compared with the normal body size group. The association with CAC persisted after traditional CVD risk factor adjustment, with no race-ethnicity interaction observed. Using a CAC score cut-point of 100, the association was even stronger. Increased body size was significantly associated with a maximum IMT (>80th percentile). After adjustment for race, sex, and age, there was a 32% greater risk of ICA IMT and a 45% greater risk of CCA IMT exceeding the 80th percentile in the obese group compared with the normal body size group. After further adjustment for traditional CVD risk factors, the association with CCA IMT persisted, but the association with ICA IMT became marginally significant (P=.07). No race-ethnicity interaction was observed. A more than 2-fold greater risk of LV mass exceeding the 80th percentile was observed in the obese group compared with the normal body size group, and this association persisted after traditional CVD risk factor adjustment. For LV mass, we found an interaction between race-ethnicity and body size groups, with a significant association between body size and LV mass observed in all racial/ethnic groups, although with an even stronger association observed in Chinese American participants.
Table 4.
Subclinical Cardiovascular Disease (CVD) by Body Size Group, Adjusted for Age and Risk Factors in the Multi-Ethnic Study of Atherosclerosis
| Subclinical CVD Outcome | BMI | No. (%) of Participants | RR (95% CI) |
|
|---|---|---|---|---|
| Adjusted for Race, Sex, and Age | Adjusted for Race, Sex, Age, and CVD Risk Factorsa | |||
| CAC score >0 | <25.0 | 1953 (48) | 1 [Reference]c | 1 [Reference]b |
| 25–29.9 | 2664 (51) | 1.05 (1.00–1.10) | 0.98 (0.93–1.03) | |
| >30.0 | 2197 (50) | 1.17 (1.11–1.23) | 1.06 (1.00–1.12) | |
| CAC score >100 | <25.0 | 1953 (22) | 1 [Reference]b | 1 [Reference]b |
| 25–29.9 | 2664 (25) | 1.06 (0.96–1.16) | 0.97 (0.88–1.07) | |
| >30.0 | 2197 (24) | 1.28 (1.15–1.41) | 1.12 (1.00–1.25) | |
| Common carotid artery IMT (> sex-specific 80th percentile) | <25.0 | 1938 (29) | 1 [Reference]c | 1 [Reference]c |
| 25–29.9 | 2639 (42) | 1.29 (1.20–1.38) | 1.20 (1.11–1.29) | |
| >30 | 2149 (44) | 1.45 (1.34–1.56) | 1.28 (1.18–1.38) | |
| Internal carotid artery IMT (> sex-specific 80th percentile) | <25.0 | 1931 (29) | 1 [Reference]c | 1 [Reference] |
| 25–29.9 | 2611 (36) | 1.15 (1.06–1.25) | 1.02 (0.94–1.10) | |
| >30.0 | 2087 (40) | 1.32 (1.21–1.43) | 1.09 (1.00–1.19) | |
| LV mass (> 80th percentile, sex and height adjusted) | <25.0 | 1546 (25) | 1 [Reference]c | 1 [Reference]c |
| 25–29.9 | 2034 (49) | 1.82 (1.65–2.02) | 1.65 (1.50–1.82) | |
| >30.0 | 1424 (76) | 2.69 (2.43–2.97) | 2.30 (2.08–2.54) | |
Abbreviations: CAC, coronary artery calcium; CI, confidence interval; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IMT, intimal medial thickness; LV, left ventricular; RR, relative risk
Adjusted for systolic blood pressure, hypertension, low-density lipoprotein, high-density lipoprotein, triglycerides, glycemic status, and cigarette smoking.
P<.01 (between body size groups).
P<.001 (between body size groups).
COMMENT
Findings from this multiethnic cohort of middle-aged and older adults are reflective of the CVD impact of the ongoing obesity epidemic in the United States. These data document the increased burden of CVD risk factors, an exceptionally high prevalence of CVD medication use, and a higher prevalence of subclinical vascular disease (adjusted for traditional CVD risk factors) in participants classified as overweight or obese.
The prevalence of overweight status generally was greater than 60% (with many groups >80%), and the prevalence of obesity generally was greater than 30% (with many groups >50%) in MESA age, racial/ethnicity, and sex groups. Chinese American participants were an exception, with much lower rates of obesity. The high prevalence of obesity occurred even though the selection criteria for MESA (free of clinical CVD and exclusion of the morbidly obese owing to weight limits in the imaging components) would be expected to bias our cohort toward being less obese than their community counterparts. The prevalence of being overweight and obese observed in MESA participants is similar to or even slightly higher than the levels observed in recent national samples.1–3 There is a paucity of national data available for comparison with our results in Chinese Americans. However, international comparisons have documented similarly lower rates of overweight and obesity in China compared with the United States20 as were observed in MESA Chinese American participants.
We observed a strong relationship between obesity and traditional CVD risk factors. The relationship between risk factors for CVD and obesity was similar in all racial/ethnic and sex groups. An interesting finding was that despite fewer Chinese American participants being classified as overweight or obese, a similar relationship between greater body size and CVD consequences was observed in Chinese Americans as in other groups.
A higher prevalence of hypertension, a higher prevalence of diabetes, and lower levels of HDL cholesterol were observed in participants classified as obese when compared with those in the normal body size group, confirming previously documented associations between increased body size and hypertension, dyslipidemia, and diabetes.4,5 These differences occurred despite the large number of obese participants who reported current pharmacologic treatment for hypertension, dyslipidemia, or diabetes. It should be noted that the large number of individuals receiving pharmacologic treatment for CVD risk factors likely represents appropriate medical care. While obesity was associated with subclinical CVD after adjustment for traditional CVD risk factors, it should be noted that aggressive treatment of risk factors would be expected to blunt the impact of obesity on future CVD events. That said, our data point to potentially large societal costs associated with the obesity epidemic, with a high proportion of US adults requiring lifelong treatment for chronic disease risk factors. The fiscal importance of a continued obesity epidemic is great, with the direct economic costs of obesity estimated to exceed $90 billion annually in US health care costs, including increased Medicare health care costs among retirees.21,22
We observed a higher prevalence of CAC, a greater carotid artery IMT, and a greater LV mass in the obese group compared with the normal body size group. Importantly, these relationships between body size and subclinical CVD markers persisted even after adjustment for traditional CVD risk factors. To our knowledge, previous studies have not consistently observed a relationship between body size and IMT. Obesity has been shown to be associated with CAC7 and LV mass6,9 but less consistently with carotid artery IMT.8,23–25 The higher prevalence of CAC, thickening of the walls of the carotid artery, and greater LV mass with greater body size represents a probable precursor to increased future CVD events.
The observed association between obesity and CVD risk is likely mediated through a variety of different pathways. Obesity has been shown to adversely affect blood pressure, dyslipidemia, and glycemic state, which in turn likely have an impact on atherosclerosis progression and LV mass over an extended period (ie, a lifetime). Obesity may also mediate its effects through inflammatory mechanisms such as the increased production of adipokines, cytokines, and inflammatory markers by adipocytes through increases in insulin resistance and the alteration of endothelial function.26,27 Strategies to further ameliorate the obesity epidemic need to consider these important pathways.
There are limitations to our study: MESA selected participants who were free of clinical CVD and excluded those who were morbidly obese (owing to imaging component weight restrictions). Given that our sample would be expected to represent a somewhat lower risk group when compared with the entire community, this selection strategy likely underestimates the true burden of obesity and CVD in the population. We used standard BMI nationally recognized cutpoints to define obesity28 that may be less valid across the heterogeneous racial/ethnic, sex, and age groups in MESA. The data presented herein are cross-sectional and cannot incorporate the known time lag between the development of CVD risk factors and their impact on subclinical atherosclerosis. The continued association between obesity and subclinical CVD after CVD risk factor adjustment may be misleading because adjusting for current levels does not fully consider past or lifelong history of a more adverse CVD risk factor profile.
In summary, these data from MESA document the widespread epidemic of overweight and obesity across racial/ethnic, sex, and age groups. Findings from our Chinese American participants suggest that high rates of population obesity should not be viewed as being inevitable. An important component of our analyses was the association of obesity with subclinical CVD that persisted after adjustment for traditional CVD risk factors. Previous studies have shown that increased prevalence of traditional CVD risk factors (hypertension, diabetes, and dyslipidemia) and a greater burden of subclinical vascular disease are strong predictors of CVD risk.29 Prospective data from MESA will allow us to assess the effects of body size on CVD morbidity and mortality and to document the mediating effects of obesity-related risk factors. Our findings support the imperative to redouble our efforts to assist in increasing healthy behaviors and to remove environmental barriers to maintaining a healthy weight.
Acknowledgments
Funding/Support: This study was supported by contracts N01-HC-95159 through N01-HC-95166 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by the Wake Forest University General Clinical Research Center (GCRC) M01-RR07122.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Drs Burke and Chung had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Burke, Shea, and Tracy. Acquisition of data: Burke, Shea, Tracy, and Watson. Analysis and interpretation of data: Burke, Bertoni, Watson, Blumenthal, Chung, and Carnethon. Drafting of the manuscript: Burke, Watson, and Chung. Critical revision of the manuscript for important intellectual content: Burke, Bertoni, Shea, Tracy, Watson, Blumenthal, Chung, and Carnethon. Statistical analysis: Chung. Obtained funding: Burke, Shea, and Tracy. Administrative, technical, and material support: Burke, Shea, Tracy, and Watson. Study supervision: Shea and Watson.
Additional Contributions: We thank the other investigators, the staff, and especially the participants of MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesanhlbi.org.
References
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Truong KD, Sturm R. Weight gain trends across sociodemographic groups in the United States. Am J Public Health. 2005;95(9):1602–1606. doi: 10.2105/AJPH.2004.043935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman DS, Khan LK, Serdula MK, Galuska DA, Dietz WH. Trends and correlates of class 3 obesity in the United States from 1990 through 2000. JAMA. 2002;288(14):1758–1761. doi: 10.1001/jama.288.14.1758. [DOI] [PubMed] [Google Scholar]
- 4.Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev. 2002;18(suppl 3):S21–S26. doi: 10.1002/dmrr.289. [DOI] [PubMed] [Google Scholar]
- 5.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293(15):1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 6.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry: the Framingham Heart Study. JAMA. 1991;266 (2):231–236. [PubMed] [Google Scholar]
- 7.Snell-Bergeon JK, Hokanson JE, Kinney GL, et al. Measurement of abdominal fat by CT compared to waist circumference and BMI in explaining the presence of coronary calcium. Int J Obes Relat Metab Disord. 2004;28(12):1594–1599. doi: 10.1038/sj.ijo.0802796. [DOI] [PubMed] [Google Scholar]
- 8.Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE, Bots ML. Change in body mass index from adolescence to young adulthood and increased carotid intima-media thickness at 28 years of age: the Atherosclerosis Risk in Young Adults study. Int J Obes Relat Metab Disord. 2003;27(11):1383–1390. doi: 10.1038/sj.ijo.0802404. [DOI] [PubMed] [Google Scholar]
- 9.Schunkert H. Obesity and target organ damage: the heart. Int J Obes Relat Metab Disord. 2002;26(suppl 4):S15–S20. doi: 10.1038/sj.ijo.0802214. [DOI] [PubMed] [Google Scholar]
- 10.Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352(11):1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 11.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293(15):1861–1867. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 14.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48(3-4):171–180. [PubMed] [Google Scholar]
- 15.Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 16.Breen JF, Sheedy PF, Schwartz RS, et al. Coronary artery calcification detected with ultrafast CT as an indication of coronary artery disease. Radiology. 1992;185(2):435–439. doi: 10.1148/radiology.185.2.1410350. [DOI] [PubMed] [Google Scholar]
- 17.Carr JJ, Crouse JR, III, Goff DC, Jr, D’Agostino RB, Jr, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174(4):915–921. doi: 10.2214/ajr.174.4.1740915. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 suppl 2):S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 20.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35(1):93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- 21.Daviglus ML, Liu K, Yan LL, et al. Relation of body mass index in young adulthood and middle age to Medicare expenditures in older age. JAMA. 2004;292 (22):2743–2749. doi: 10.1001/jama.292.22.2743. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, McDonald T, Reffitt B, Edington DW. BMI, physical activity, and health care utilization/costs among Medicare retirees. Obes Res. 2005;13(8):1450–1457. doi: 10.1038/oby.2005.175. [DOI] [PubMed] [Google Scholar]
- 23.Juonala M, Raitakari M, Viikari JS, Raitakari OT. Obesity in youth is not an independent predictor of carotid IMT in adulthood: the Cardiovascular Risk in Young Finns Study. Atherosclerosis. 2006;185(2):388–393. doi: 10.1016/j.atherosclerosis.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Davis PH, Dawson JD, Riley WA, Lauer RM. Carotid intimal-medial thickness is related to cardiovascular risk factors measured from childhood through middle age: the Muscatine Study. Circulation. 2001;104(23):2815–2819. doi: 10.1161/hc4601.099486. [DOI] [PubMed] [Google Scholar]
- 25.Chambless LE, Folsom AR, Davis V, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987–1998. Am J Epidemiol. 2002;155(1):38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 26.Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288 (5):H2031–H2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 27.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: World Health Organization; 1998. [PubMed] [Google Scholar]
- 29.Kuller LH, Arnold AM, Psaty BM, et al. 10-Year follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the Cardiovascular Health Study. Arch Intern Med. 2006;166(1):71–78. doi: 10.1001/archinte.166.1.71. [DOI] [PubMed] [Google Scholar]