Abstract
Lumbar motoneurons can be activated monosynaptically by two glutamatergic synaptic inputs: segmental dorsal root (DR) and descending ventrolateral funiculus (VLF). To determine if their N-methyl-D-aspartate (NMDA) receptors are independent, we used (5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine-hydrogen-maleate (MK-801), known to induce a use-dependent irreversible block of NMDA receptors (NMDAR). In the presence of MK-801 (in bath) and non-NMDA antagonists (in bath, to isolate NMDA receptors pharmacologically) we first stimulated DR. After MK-801 blockade of DR synaptic input, the VLF was stimulated. Its response was found to be not significantly different than its control value suggesting that the DR stimulus activated very few if any receptors also activated by VLF stimulation. Similar findings were made if the stimulation order was reversed. Both inputs also elicited a polysynaptic NMDA receptor- mediated response. Evoking the DR polysynaptic response in the presence of MK-801 eliminated the corresponding VLF response; the reverse did not occur. Surprisingly, when MK-801 was washed from the bath, both DR and VLF responses could recover although the recovery of the DR monosynaptic and polysynaptic responses was reliably greater than those associated with VLF. Recovery was prevented if extrasynaptic receptors were activated by bath applied NMDA in the presence of MK-801 consistent with the possibility that recovery was due to movement of extrasynaptic receptors into parts of the membrane accessible to transmitter released by DR and VLF stimulation. These novel findings suggest that segmental glutamatergic inputs to motoneurons are more susceptible to plastic changes than those from CNS white matter inputs at this developmental stage.
Keywords: MK-801, EPSP, NMDA receptor mobility
Introduction
Developmental studies in the in vitro spinal cord conditions indicate that excitatory glutamatergic transmission to motoneurons undergoes substantial changes during the perinatal period (Arvanian et al., 2004). In evaluating glutamatergic transmission, it is important to recognize that the initial response measured intracellularly from motoneurons consists of 2 components, one mediated by alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) /Kainate receptors and a slightly slower one mediated by NMDA receptors (Ziskind Conhaim,1990; Arvanian and Mendell, 2001a). A third component mediated by metabotropic glutamate receptors appears considerably later, and requires high intensity stimuli activating C-fibers (Arvanian et al., 2005).
Application of pharmacological blockers of AMPA/kainate transmission and the inhibitory transmitters glycine and GABA isolates the NMDA receptor-mediated responses (Arvanian and Mendell, 2001a). This NMDA component declines in amplitude during the initial 2 postnatal weeks (Arvanian et al., 2004) due to its increasing sensitivity to Mg2+ block (Arvanian and Mendell, 2001a) rather than loss of NMDA receptors themselves. During the first postnatal week the VLF NMDA receptor- mediated response resembles that of adults more than the DR response in the same motoneuron in being more sensitive to Mg2+ block (Arvanian and Mendell, 2001a).
The fact that DR and VLF NMDA receptor-mediated responses mature at different rates suggests that the receptors are under the control of their presynaptic inputs rather than solely determined by the motoneuron. This predicts very little cross talk between the monosynaptic responses to these 2 synaptic inputs as might occur, for example, from spillover of transmitter from one input to the receptors normally activated by the other (Kullmann and Asztely, 1998). In order to confirm this, we employed the use-dependent, irreversible NMDA receptor blocker MK-801 (Huettner and Bean, 1988; Foster and Wong, 1987; Kloog et al., 1998; Lipton, 2004) to block the responses to one of these inputs and determined whether it would diminish the initial response to the unstimulated input. If these were independent, stimulation of one input would have little effect on the response to the other (see also Atasoy et al., 2008).
An additional question motivating these experiments was the reversibility of MK-801 blockade of NMDA receptors. MK-801- induced blockade of depolarization elicited by repetitive bath-applied NMDA cannot be reversed (Arvanov et al., 2000). However, recent studies in dissociated hippocampal neurons have shown that blockade of NMDA synaptic responses can be reversed after MK-801 (Tovar and Westbrook, 2002; Zhao et al., 2008). This recovery was attributed to movement of unstimulated functional NMDA receptors from the extrasynaptic regions into the synapse. Here, we observed a similar phenomenon in intact spinal tissue, but surprisingly DR responses displayed more recovery than those made by VLF on the same motoneuron. These findings suggest the possibility that NMDA receptors are mobile in neonatal motoneurons in the intact spinal cord, but more importantly that the sites associated with the different synaptic inputs may vary in their susceptibility to accumulate receptors from extrasynaptic sites.
Some of these studies have been presented in abstract form (Shanthanelson et al., 2005).
Materials and Methods
These studies were performed with the approval of the Institutional Animal Care and Use Committee at SUNY Stony Brook.
Electrophysiology
Electrophysiological experiments were carried out in vitro on neonatal rat spinal cords removed from Sprague Dawley rats ( Taconic, Rensselaer, NY ) aged P1-11 as previously described (Seebach et al., 1998). The rats were anesthetized by placing them on a latex glove lying on a bed of ice (P1/P2), or by halothane (P3-P11). The spinal cord was quickly removed from the animal and the left hemicord was placed in a chamber superfused with ACSF containing (in mM): NaCl (117), KCl (4.7), CaCl2 (2.5), MgSO4 (800 μM), NaHCO3 (25), NaH2PO4 (1.2), dextrose (11), aerated with 95% O2 / 5% CO2 (pH 7.4, 30° C) at 10 ml/min. The VLF was dissected free of the spinal cord at T2 (Pinco and Lev-Tov, 1994). Suction stimulating electrodes were attached to peeled VLF axon bundles for activation of central inputs to motoneurons, to the cut L5 dorsal root for activation of segmental inputs to motoneurons, and to the L5 ventral root for identification of recorded cells as motoneurons by antidromic activation. Intracellular recordings (Axoclamp 2A amplifier, Molecular Devices, Inc. Sunnyvale, CA) were obtained using sharp microelectrodes (resistance 60-80 MΩ, filled with 3 M potassium acetate). Electrical stimulation of the DR and/or VLF (70 μs duration at a rate of 0.025 Hz) was at an intensity sufficient to evoke the just-maximum monosynaptic potential .
Use of pharmacological blockers
The AMPA/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μM), GABAA receptor antagonist bicuculline (5 μM), GABAB receptor antagonist CGP 35348 (10 μM) and glycine receptor antagonist strychnine (5 μM) were added to the perfusion solution to isolate NMDAR-mediated responses pharmacologically (stimulation rate was 0.025 Hz when NMDAR-mediated responses were studied (see Arvanian and Mendell, 2001a for details).
CNQX, MK-801 and NMDA were obtained from Sigma ( St. Louis, MO, US). CGP 46381 and Bicuculline methochloride were from Tocris Bioscience ( Bristol, UK). Strychnine HCl was obtained from Research Biochemical International ( Natick, MA, US)
Protocol
Only cells displaying a stable resting membrane potential greater than −60 mV were included in this study. Membrane potential was monitored throughout the study and corrected by subtracting the value observed after withdrawing the electrode from the motoneuron. Initial AMPA/kainate responses were obtained from both synaptic inputs in the absence of any pharmacological blockers. The AMPA/kainate and inhibitory transmitter antagonist cocktail was then introduced into the bath, and at least 30 min later, control NMDA responses from DR and VLF were obtained at a stimulus intensity sufficient to evoke a maximum monosynaptic response. For both of these control response measures (AMPA/kainate and NMDA) 10 stimuli were delivered at the same intensity with a 40s interval to one input and then the stimulation was switched to the other, and then back, until a total of at least 40 stimuli had been delivered to both DR and VLF. The activity- dependent irreversible NMDAR blocker MK-801 (10 μM) was then added to the bath while continuing administration of the antagonist cocktail. In the presence of MK-801, one input was stimulated exclusively until maximum blockade of the EPSP was achieved, typically after 30- 40 min. with stimulation at 1/ 40s. The other input was then stimulated exclusively until its response was also blocked maximally, i.e., DR and VLF stimulation was not interdigitated as for control measurements.
For experiments where reversibility of MK-801 was studied, the MK-801 was then washed from the bath (still containing the AMPA/kainate and inhibitory transmitter antagonist cocktail) for 45 minutes. The inputs were not stimulated during the 45 minute wash period. Following the wash, the NMDA receptor- mediated response was measured for both inputs, again without alternation. As a final step, all antagonists were washed out for 45 minutes in ACSF. The inputs were not stimulated during this time. DR and VLF were then stimulated in the same order as before to obtain AMPA/kainate responses to verify viability of the cell and its synaptic inputs.
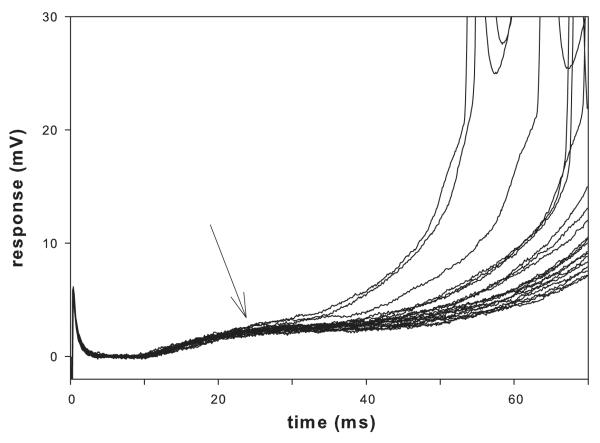
For offline analysis the responses to each stimulus were analyzed using Clampfit 8.2 (Molecular Devices, Inc. Sunnyvale, CA) either in single sweeps, in superimposed sweeps or after averaging. The monosynaptic component of the EPSP from successive stimuli overlaps consistently while the polysynaptic component of the EPSP varies. To obtain the peak of the monosynaptic component, the traces were superimposed and the peak was measured at the latest time where consistent overlap of traces was observed (Fig. 1).
Figure 1.
Several superimposed responses to DR stimulation in a motoneuron. Note that the early monosynaptic responses are superimposed while the later polysynaptic responses are highly variable although they have a similar latency. Stimulation rate was 1/40s.
In most experiments DR was stimulated before VLF for each test (Fig. 2). In several additional experiments the order was reversed and VLF was stimulated before DR (Fig. 3).
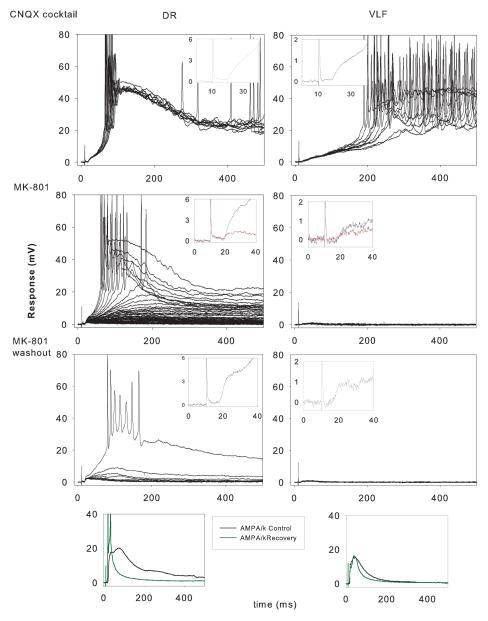
Figure 2.
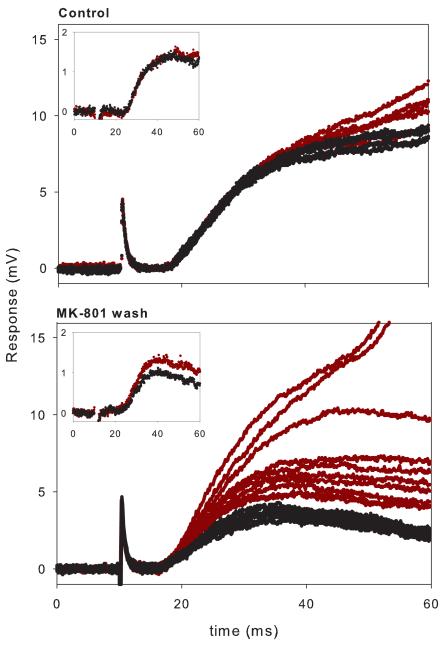
Illustration of the procedures carried out on an individual motoneuron and their inputs. Spinal cord obtained from a P2 rat. Data from a single motoneuron obtained over a 4 hour and 30 min recording period. Vm was about −75 mV throughout. Stimulation rate 1/40s. Control: Blockade of all non NMDA receptors with CNQX, bicuculline, strychnine and CGP 35348 (CNQX Cocktail) administered to the bath. Ten consecutive responses to DR and then to VLF are displayed. Note the reliable responses to DR and the irregular responses to VLF. “Insets” placed at top are average monosynaptic responses at higher gain and faster sweep speed. MK-801. The NMDA receptor- mediated response was then blocked with MK-801, stimulating only DR (60 trials at 0.025 Hz). Trial 1 was obtained immediately after introducing MK-801 (10 μM) into the bath and the decline of the late response took place progressively as stimulation was continued. Stimulation was stopped when steady state was reached. VLF was then stimulated (n=20) until its response declined to steady state. Insets display the initial response (black) and the last response (red) in MK-801. MK-801 was then washed from the bath with ACSF for 45 min. in the absence of stimulation while continuing to apply the non NMDA antagonists. Wash. Superimposed responses (n=10 for both DR and VLF) after MK-801 washout are displayed along with the initial response at high gain (insets). Note the recovery of the DR NMDA response followed by further blockade when repetitively stimulated despite absence of MK-801 in the bath. Note also the lesser recovery of VLF NMDA response- the late response never reappeared in contrast to DR. The bottom graphs are average AMPA/kainate responses before CNQX cocktail (black) and at the very end of the experiment after the CXQX cocktail was washed out for about 45 min. (green). Note the similarity in the responses before and after (except for the briefer time course of the DR response which elicited spikes after the wash phase).
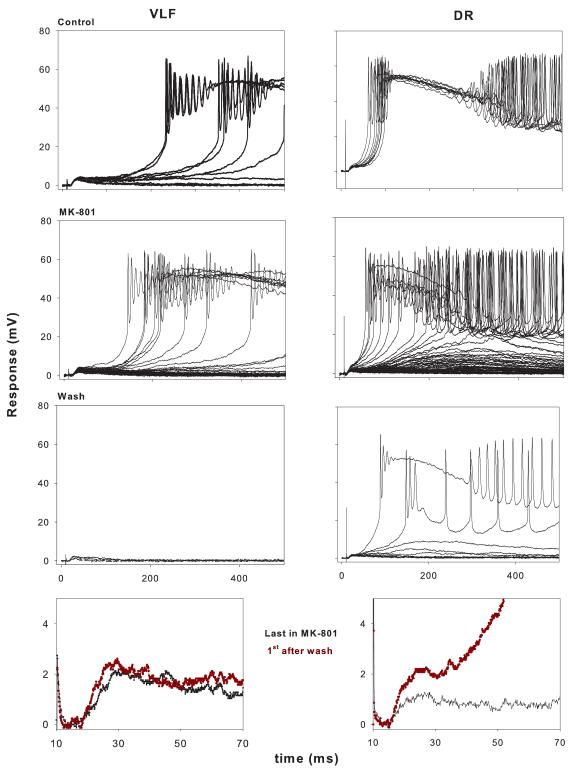
Figure 3.
Organization and protocol similar to figure 2 except for the order of stimulation. P4 rat. Control: NMDA receptor-mediated response (10 consecutive stimuli for DR and VLF); MK-801: response in MK-801 (VLF: 80 stimuli; DR: 60 stimuli); Wash: response after 45 min. washout of MK-801 in the absence of stimulation (20 stimuli for both VLF and DR). Insets in the bottom row display the last monosynaptic response in MK-801 (black) and first response after washout of MK-801 (red). Note recovery of DR response and lack of recovery of VLF response.
Statistics
Only a single cell was studied in each cord, and so the number of observations is the number of cells. The specific tests are described in the Results. Means are reported with standard deviations.
Results
Receptors under the DR and VLF inputs are largely independent
In order to study whether NMDA receptors activated by DR and VLF are independent of each other, we used MK-801’s property of blocking only those NMDA channels that have been activated by neurotransmitter (Huettner and Bean, 1988; Foster and Wong, 1987; Kloog et al., 1998; Lipton, 2004). The first NMDA EPSP elicited after MK-801 administration is not reduced. However, during this initial activation of the NMDA receptor-channel complex, MK-801 inserts into the activated NMDA channel and blocks responses induced by subsequent synaptic activation of the same channel.
After achieving a suitable penetration of a motoneuron, we first recorded DR and VLF-evoked responses in normal ACSF (Fig. 2- bottom graphs). The stimuli to the 2 synaptic inputs were interdigitated, 10 consecutive stimuli to the DR followed by 10 consecutive stimuli to the VLF, with an interval of 40s between each stimulus. Consistent with our previous observations, DR and VLF responses, both largely AMPA receptor- mediated (Arvanian et al., 2004), exhibit a short latency monosynaptic component (Arvanov et al., 2000) of uniform amplitude followed by later highly variable polysynaptic components. We then added non-NMDA antagonists to the perfusing solution to block the AMPA/kainate-, GABA- and glycine-mediated responses and recorded the NMDA receptor-mediated control DR and VLF responses (Arvanian and Mendell, 2001a). Again, these NMDA receptor- mediated control responses were obtained by interdigitating the stimulation of both synaptic inputs. Under these conditions we observed a small monosynaptic response with latency 5-7 ms from the stimulus that was generally similar or slightly longer than that associated with the shortest latency AMPA/kainate response; the rise time, particularly of the VLF response, was longer than the AMPA/kainate mediated response (Fig. 2, control). The amplitude of this presumed monosynaptic response increased as the stimulus intensity was raised. Maximum peak amplitude of the uniform monosynaptic NMDA receptor-mediated EPSPs measured 25-30 ms after the stimulus (Fig. 2, Control) was 1.9 ± 1.4 mV (n=21) and 1.8 ± 2.2 mV (n=25) for DR and VLF responses, respectively. Only cells exhibiting both DR and VLF evoked monosynaptic NMDA receptor- mediated responses were studied. Typical monosynaptic AMPA responses in these cells measured in control ACSF were considerably larger, averaging 5-7 mV (Arvanov et al., 2000).
As reported previously (Arvanian and Mendell, 2001a), stimulation of DR and VLF in the presence CNQX and antagonists of glycine and GABA transmission elicited a second NMDA receptor- dependent depolarization at longer latency that often led to an all-or-none plateau potential with superimposed action potentials (Figs. 2 & 3). The DR- evoked long latency potential has a higher stimulus threshold than the monosynaptic component (Fig. 1A1 in Arvanian and Mendell, 2001a) suggesting that smaller afferent fibers are responsible for eliciting this response, probably via interneurons. The all-or-none behavior of the plateau potential resembles findings with directly applied NMDA in the neonatal spinal cord (MacLean et al., 1997). The late DR- evoked plateau potential occurred reliably at the low frequency of stimulation used here. Although the initial portion of the late VLF response also began reliably soon after the peak of the initial response, the all- or- none plateau potential was initiated at highly irregular intervals due to the variability in the time required to reach its threshold voltage (Fig. 3, Control). We speculate that this may have been the result of a very slow, asynchronous volley arriving from interneurons activated by VLF; this was not studied systematically. The initial component of the late response is not triggered by the monosynaptic EPSP because it often begins on the descending portion of the EPSP, well after the peak of the early response, (see Figs. 1 and 3 of Arvanian and Mendell (2001a)), and in a few cells studied here DR stimulation elicited a late response with no evidence of an early monosynaptic response. As stated above, such cells were not included in the results.
After a stable NMDA receptor- mediated response to DR and VLF was achieved, we introduced the use-dependent NMDA channel blocker MK-801 into the perfusing solution and stimulated DR (but not VLF) repetitively every 40s (Fig. 2, MK-801; see Fig. 3 and below for experiments where VLF was stimulated before DR in MK-801), until maximum blockade of the DR-response was achieved as determined by EPSP amplitude reaching a steady state value. This generally took about 25 minutes. The monosynaptic NMDA-mediated DR-response gradually decreased in amplitude with repeated stimulation and eventually declined to less than half its initial value (average 38 + 21%); only rarely was it completely eliminated (in contrast to results with 2-amino-5-phosphonopentanoic acid (APV), where it was always completely blocked (Arvanian and Mendell, 2001a). The late component also became progressively smaller and slower, i.e., it was no longer all-or-none, and was invariably abolished after repeated stimulation (Fig. 2, MK-801). The finding that the late response declined gradually during MK-801 (Fig. 2, DR in MK-801; Fig. 3, DR during washout) suggests that the channels responsible for the late depolarization were NMDA channels rather than other channels, e.g., Ca2+ channels, triggered by NMDA receptor- mediated depolarization.
After maximum blockade of the DR-evoked NMDAR-mediated responses by MK-801 was achieved, we stimulated VLF still in the presence of MK-801 (n=20). In most cases the monosynaptic response to the first VLF stimulus in the presence of MK-801 was similar in amplitude to the mean value obtained before MK-801 administration despite prior stimulation of DR in the presence of MK-801. In other cases this response was diminished to a limited extent. On average, the initial NMDA-mediated monosynaptic VLF-response (Fig. 4) was about 85% of the control amplitude (p> 0.05, rank sign test), and this response was further gradually blocked by MK-801 over the next 40 minutes of VLF stimulation to about 52+ 17% (Fig. 4). Unlike the monosynaptic response, the polysynaptic component, including the plateau potential, in response to VLF was never observed if DR had previously been stimulated in the presence of MK-801 (Fig. 2, MK-801).
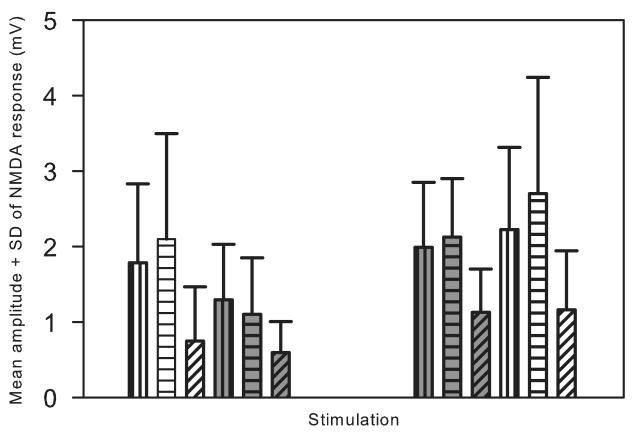
Figure 4.
Bar graphs displaying the mean amplitude of the NMDA receptor- mediated monosynaptic EPSPs elicited in the same motoneuron by DR and VLF stimulation. The left group of bars were experiments (n= 20) where DR (blue) was stimulated before VLF (green) during MK-801. The first blue bar is the mean NMDA control response averaged over all cells. The second blue bar (with horizontal lines) is the mean response at the onset of MK-801 and the third blue bar (with crossed diagonals) is mean final response in MK-801. DR and VLF stimuli in control conditions were interdigitated, but in MK-801 the DR was stimulated until steady state was reached after which VLF was stimulated. Note that the initial VLF response in MK-801 was similar to the control response, i.e., stimulating DR in MK-801 did not affect the response to VLF. The same was observed when the experimental protocol was reversed (n=6), i.e., stimulating VLF first in MK-801 did not affect the response to DR. Note also the depression of all response after stimulation in MK-801. Further details in the text.
In 6 cells the experiment was performed in reverse order (Fig. 3), i.e., we first stimulated VLF in the presence of MK-801, and after VLF-evoked NMDA-mediated responses were blocked we stimulated DR until the response declined to a steady level indicating maximum antagonism by MK-801. The initial monosynaptic DR response (2.7 ± 1.5 mV) after VLF stimulation in the presence of MK-801 was elevated from the control value (2.2 ± 1.1 mV) (Fig. 4), but this difference was not significant (n=6; p> 0.5). Again, the plateau potentials occurred irregularly in response to the initial stimuli of the series, but eventually they ceased. The response to the initial DR stimuli after VLF stimulation always included a late component which gradually decreased in amplitude and increased in rise time until it disappeared. This was in contrast to the VLF response after DR stimulation in which the late component was always abolished from the start (Fig. 2, MK-801—see above).
The finding that the initial monosynaptic response to either synaptic input was virtually unaffected after the other had been stimulated in the presence of MK-801 suggests that relatively few individual NMDA receptors on the motoneuron are activated monosynaptically by both synaptic inputs. A different finding was made with regard to the late response, namely that prior stimulation of DR in the presence of MK-801 almost always abolished the initial VLF late response, but the reverse never occurred.
EPSPs elicited by DR and VLF stimulation recover from “irreversible” MK-801 blockade but the DR EPSP recovers to a greater degree than VLF EPSP
Blockade by MK-801 is described as irreversible, because at −70mv MK-801 unbinds very slowly from NMDA receptors (Huettner and Bean, 1988), Thus we were surprised that the EPSP that had been blocked previously in a use-dependent manner by MK-801 could “recover” from the blockade (Figs. 2 and 3). In this study synaptic NMDA responses were blocked first by repetitive stimulation of one and then the other synaptic input in the presence of MK-801 added to the non-NMDA receptor antagonists cocktail (see above). After achieving maximal block of both DR- and VLF-evoked responses, repetitive stimulation was stopped and MK-801 was washed out by bath application of a solution still containing the non-NMDA antagonists. Following a 45 min period of no-stimulation and wash out of MK-801, DR and VLF inputs were stimulated again.
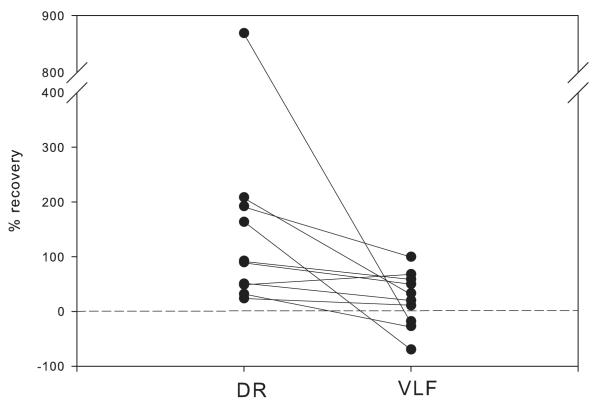
We found several indications that the recovery differs for DR and VLF inputs on the same population of motoneurons. The monosynaptic DR response virtually always exhibited some degree of recovery from the decrease observed in MK-801; VLF often did not recover but could occasionally display a small recovery (Figs 2, 3 and 5). These differences were independent of the order of stimulation (Figs. 2 and 3). In addition, they occurred despite the similarity in the degree of depression by MK-801 (Fig. 4). Recovery of the initial monosynaptic component was calculated as the percent of the initial decline after MK-801 that recovered when MK-801 was washed out. A recovery of 0% indicated that the EPSP amplitude did not change after removal of MK-801, and 100% indicated recovery to the value before MK-801 was added, i.e., control value. Negative values of recovery were indicative of a response that declined further after washout of MK-801. We observed a significantly greater recovery for monosynaptic DR inputs than for those made by VLF (p=0.002; Signed Rank test; n=10, Fig. 5). A further indication of differences between recovery of these inputs during MK-801 wash was the finding in some cases (4 of 10) that the DR response recovery was more than 100% indicating that the response became larger than the original value. This was never observed for VLF responses in the same cells. Similarly, in 3 of 10 cases the VLF responses declined after removal of MK-801 (negative value of % recovery), and this was never observed for DR responses in the same cells. In only 1 case did the VLF response display more recovery than the DR response, and this difference was very small.
Figure 5.
Percent recovery of DR and VLF from MK-801 block when DR was stimulated before VLF. See text for definition of % values. Line connects data from same motoneurons. Note the negative slope for 9 of 10 cells indicating that recovery of response to DR was greater than recovery of response to VLF. Further detail in text.
We also noted that the late DR response, including plateau potentials, generally recovered in contrast to the late VLF response which never recovered. These differences were observed regardless of the order of stimulation in MK-801 (Figs. 2 and 3). We cannot specify the location of the NMDA receptors responsible for the recovery of these responses, i.e., whether they were restricted to the motoneuron, to the intercalated interneurons, or involved NMDA receptors on both cell types.
Since the experimental protocol required a long period (45 min.) of no stimulation while the MK-801 was being washed out, we considered the possibility that the initial stimulus after the long quiescent period resulted in the release of massive amounts of neurotransmitter which would exaggerate the apparent recovery. This would be true for glutamatergic synapses from DR and not VLF. In control experiments with no blockers, we tested the effect of a 45 min. interval between successive DR stimuli and did not find a large increase in the response after the period of inactivity.
In all of these experiments the state of the motoneuron and its synaptic inputs was determined at the end of the manipulations by perfusing with ACSF for 45 min. to wash all antagonists from the bath Following this 45 min wash with no stimulation, both DR and VLF inputs were stimulated to verify that the AMPA responses had recovered. This further proved that the cell and its inputs had remained viable through out the entire course of the experiment and that there were no substantial changes in excitability of the spinal cord.
The responses to repeated stimuli delivered after the washout of MK-801 were diminished in amplitude, even though MK-801 was no longer in the perfusing solution (Figs. 2 and 3). The percent decrease of the response (49.4 ± 15.1% for DR and 31.6 ± 8.43% for VLF, n=10) was similar to that observed when the inputs were stimulated in the presence of MK-801. This suggests that MK-801 remained in the spinal cord despite being washed from the bath. Further evidence suggesting that MK-801 remained available to block NMDA transmission was obtained from the following experiment (Fig. 6). After blocking the non NMDA receptors with antagonists and demonstrating the stationarity of the monosynaptic NMDA response, MK-801 was applied to the bath. No synaptic pathways were stimulated during MK- 801 application, and 45 minutes later, while maintaining the motoneuron penetration the MK-801 was washed out of the bath for 45 minutes, again with no stimulation. At the end of this period, the DR stimulation was begun and, unlike what had been observed before MK-801 application, the monosynaptic NMDA receptor- mediated EPSP declined with successive stimuli similar to what had been observed in other motoneurons during application of MK- 801. Similar findings were made for the response to VLF stimulation in the same motoneuron. This suggests that MK-801 had not been washed out and remained available to block the NMDA channel upon stimulation (see Discussion).
Figure 6.
Effects of MK-801 in the absence of any stimulation. Control: NMDA receptor mediated EPSPs recorded in CNQX – cocktail in response to 10 consecutive DR stimuli with the initial 5 in red and the final 5 in black. Inset displays average response to the initial 4 VLF stimuli (red) and the last 4 VLF stimuli (black). Stimulation rate 1/40s. Note the uniform amplitude of the monosynaptic component. MK-801 wash: similar records after exposure to MK-801 for 45 min and washout for 45 min., both in the absence of any stimulation throughout this 90 minute period. Red traces are progressively decreasing responses to 1st 10 stimuli; black traces are the last 10 responses (of 50). Note that the monosynaptic EPSP declined progressively in amplitude as if MK-801 was present at the synapse. Inset displays the response to VLF as in the Control records above. Note that unlike controls, the VLF EPSPs exhibited a decline in amplitude with repetitive stimulation.
The recovery of responses from “irreversible” blockade by MK-801 was not dependent on which of these synaptic inputs was stimulated first following wash of MK-801: in 16 spinal cords, DR was stimulated before VLF, and in 6 other spinal cords VLF was stimulated first. In both cases we observed the greater recovery of the response to DR stimulation when MK-801 was washed out.
The EPSP did not recover from “irreversible” MK-801 blockade when NMDA was bath applied
The recovery of the synaptic response after washout of MK-801 can be attributed to several possible mechanisms (see Discussion). One mechanism demonstrated previously in dissociated neurons is the insertion of new NMDA receptors into the synapse from intracellular sources or by lateral diffusion of receptors into the synapse from extrasynaptic regions (Tovar and Westbrook, 2002). To investigate the possible contribution of extrasynaptic receptors to recovery of the response, we carried out a manipulation to inactivate all extrasynaptic NMDA receptors and determined whether recovery from MK-801 blockade would still take place.
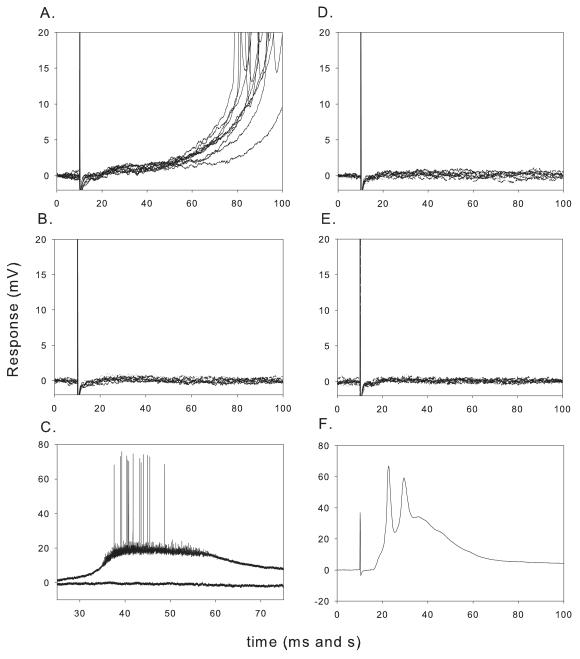
After the DR NMDA receptor- mediated EPSP (Fig. 7A) had declined to a minimum value during repetitive stimulation of DR in the presence of MK-801 (Fig. 7B), a drop of NMDA (10 μl of 10mM NMDA) was applied to the recording chamber from a micropipette. This induced a depolarizing response since receptors extrasynaptic to DR and VLF inputs had not been blocked by MK-801 (Fig. 7C; Arvanov et al., 2000). The response to subsequent NMDA drops was smaller and by the second or third drop the response was completely abolished. Stimulating DR (and VLF) immediately after the NMDA drop applications always elicited no response (Fig. 7D) and there was no recovery after MK-801 and NMDA were washed from the bath for 45 min with a solution containing only the non-NMDA receptor antagonists (Fig. 7E). This was in sharp contrast to the recovery of the response, particularly to DR stimulation, observed in the absence of the NMDA drop (Figs. 2, 3 and 5). Similar findings were made in a total of 6 motoneurons for both DR and VLF synaptic inputs. The DR- evoked late response also never recovered after the NMDA drop in contrast to its recovery in the absence of NMDA (see above). The interpretation of this experiment is that the blockade of the non-DR and non-VLF NMDA receptors by bath applied NMDA in the presence of MK-801 prevented them from contributing to the recovery of the DR- and VLF- responses.
Figure 7.
Effects of NMDA drop on recovery of synaptic response after MK-801. P2 rat. DR stimulation. A. NMDA receptor- mediated response to DR stimulation. Note the monosynaptic response followed by the late polysynaptic response. B. NMDA response after DR stimulation for 1 h with MK-801 in the bath illustrating the blockade of the synaptic response. C. Response to bath applied 2 NMDA drops in the presence of MK-801. Note the initial depolarizing response (black) despite the presence of MK-801 beginning about 1 h previously. The second drop elicited no response (red). D. Response to DR after immediately after NMDA administration. E. Response to DR after 1 hour MK-801 washout without any stimulation. Note the lack of the usual recovery of the early or late DR- mediated NMDA receptor- mediated response after MK-801 washout when NMDA drop is delivered (compare to recovery in Figs 2 and 3). F. Despite lack of recovery of NMDA response, AMPA response recovered after washout of all non NMDA antagonists. Thus the failure for NMDA recovery was not due to loss of synaptic input.
In all of these experiments, at the end of all manipulations, all the antagonists were washed out from the bath for 45 min using ACSF without any stimulation. Following the 45 min wash period, AMPA responses were obtained by first stimulating the DR and then the VLF. The recovery of the AMPA response indicated that the motoneuron and its synaptic inputs were still viable (Fig. 7F).
Discussion
These experiments were undertaken because previous work had shown that DR and VLF NMDA receptor- mediated synapses on the same motoneuron exhibit different properties during early postnatal development. (Arvanian and Mendell, 2001a; Arvanian et al., 2004). The expectation from these findings was that there would be little or no overlap between the receptors activated by these different inputs. We began by taking advantage of the use-dependence of MK-801- elicited blockade to determine whether stimulating one input thereby blocking the receptors associated with that input would have any effect on the response to the other input. We found that blocking the monosynaptic response to either input first had virtually no significant effect on the monosynaptic response to the other input. The long latency responses displayed a different pattern; stimulation of DR during MK-801 administration always resulted in elimination of the late VLF response (Fig. 2), but never vice versa (Fig. 3).
One implication of these findings is convergence between pathways activated by VLF and DR. These experiments do not permit us to specify the location of such interactions except to say that they can occur on interneurons, on motoneurons or both. Petruska et al. (2007) demonstrated in adult rats that individual ascending axons in the VLF have cell bodies in the vicinity of L5 motoneurons and send collaterals to terminate on these motoneurons. Thus at the level of the motoneuron, some of the interaction may take place between inputs from muscle spindle afferents in the dorsal root and ascending axons in the VLF activated antidromically. The present findings suggest that there are also interneurons projecting to motoneurons that receive inputs from spinal white matter axons and segmental inputs. The general concept of common interneurons activated by descending and segmental projections and projecting to motoneurons has been described (rev. by Jankowska, 2008) and interneurons influencing motoneuron activity that are activated by NMDA have been described (Kiehn et al., 1996; Hochman et al., 1994). Thus neural elements and circuits that could mediate the interactions described here appear to exist although at present we cannot specify their identity and location.
The fact that the interaction occurs from DR to VLF but not in the opposite direction suggests that these 2 inputs normally do not activate common NMDA receptors and that the interaction may occur by a process of “spillover” (Diamond, 2002) whereby transmitter spreads to activate receptors beyond its normal postsynaptic zone of influence (Huang and Bordey, 2004). Spillover is normally limited by transmitter uptake mechanisms (Diamond and Jahr, 1997; Diamond, 2001), particularly into glia (Bergles and Jahr, 1997). Evidence for such a mechanism has been obtained recently by decreasing transmitter uptake using inhibitors of glutamate transporters (Harney et al., 2008; Hires et al., 2008). We speculate that the much larger, faster polysynaptic EPSPs we observed in the same motoneuron from electrical stimulation of DR inputs compared to VLF inputs might be associated with a higher local density of synapses which would result in a higher concentration of glutamate in the synaptic cleft. This might overwhelm mechanisms for transmitter reuptake and result in activation of non DR receptors. However, under normal physiological conditions, the asynchronous discharge of inputs in the DR pathway may cause very little spillover because of lower concentrations of transmitter in the synaptic cleft although a physiological role for spillover has been suggested (Jahr, 2003).
Recovery from MK-801 blockade occurred for both the short latency monosynaptic and longer latency, presumed polysynaptic NMDA receptor- mediated responses. The recovery was uniformly greater for responses elicited by DR than VLF. This was ascertained quantitatively for the monosynaptic response and qualitatively for the late polysynaptic response. The fact that the differences were also observed for polysynaptic responses suggests that NMDA receptors on interneurons (Hochman et al., 1994) associated with DR and VLF inputs may differ in the same way as those on motoneurons, i.e., NMDA receptor properties may differ according to their synaptic input (see below).
The recovery from MK-801 blockade was unexpected in view of the literature suggesting that this agent is a non- competitive, irreversible inhibitor of NMDA receptor- mediated transmission (Wong et al., 1986). The possibility that MK-801 becomes unbound from the receptors that it had previously blocked during the wash period seems very unlikely since it unbinds very slowly from NMDA receptors at −70 mV (Huettner and Bean, 1988) which is very close to the membrane potential of cells recorded in these preparations. Furthermore, our finding that the MK-801 appears to remain associated with the NMDA receptor after an extended washout period, even in the absence of stimulation throughout its time of application, argues that unbinding did not occur. Another possible explanation is that a population of initially desensitized receptors at the activated synapses was not blocked by MK-801 (Dzubay and Jahr, 1996) and became functional during the washout of MK-801 from the bath when synaptic inputs to the motoneuron were not activated. According to this hypothesis, one would have also expected these receptors to also become active during washout in experiments where NMDA and MK-801 were co-applied because no stimulation was given during this period. The lack of recovery under these conditions suggests that inability to block a population of desensitized receptors is not the explanation.
Other possible explanations for the apparent recovery of NMDA transmission include increased presynaptic release probability due to the long period of inactivity during washout. Although we cannot completely rule out this possibility, the similarity of the monosynaptic AMPA component after recovery to that observed at the onset of the pharmacological manipulations (Fig. 2) suggest that large changes in transmitter release probability did not occur. Motoneuron membrane potential was monitored throughout and remained relatively constant (typically < 5 mV variation) and so fluctuations in Mg2+ block known to affect NMDA transmission (Nowak et al., 1984) were very unlikely to account for our findings.
Tovar and Westbrook (2002) observed similar recovery of synaptic responses after MK-801 blockade in autapses made by hippocampal neurons studied in culture. These findings were interpreted as NMDA receptor trafficking from extrasynaptic regions based on the abolition of the recovery by application of NMDA in the presence of MK-801, a finding also made in the present experiments. The similarity of our results to those observed in the earlier experiments, suggests that similar mechanisms are operating in both situations. We speculate that the greater recovery of DR responses compared to VLF reflects greater ability of trafficking receptors to be incorporated stably at those synapses. This might reflect differences in factors such as scaffolding proteins or the composition of the NR2 regulatory subunits (El Husseini et al., 2000; Wenthold et al., 2003) associated with these synaptic inputs. The similarity in recovery from MK-801 of polysynaptic responses from DR and VLF might reflect differences in properties of DR and VLF synapses on interneurons similar to those on motoneurons although this would require direct recording from these cells for verification.
The possibility that the receptors came from intracellular rather than membrane sources (Groc and Choquet, 2006) cannot be discounted, but seems unlikely because bath application of NMDA, which should not affect intracellular sources of NMDA receptors, prevented the recovery from MK-801 blockade. However, if receptors cycle rapidly through the cytoplasm and the plasma membrane as suggested by Washbourne et al. (2004), the outcome of NMDA drop experiment would be more difficult to predict, particularly in the present experiment where it was applied to an intact spinal cord.
A surprising finding was that MK-801 generally did not completely abolish the synaptic response. This raises the possibility that transmitters other than glutamate were released by the presynaptic fibers or that MK-801 failed to penetrate to all the NMDA receptors. The former seems very unlikely because the reversible antagonist APV completely blocks the response remaining after the non NMDA antagonist cocktail under identical conditions to those in the present experiments (Arvanian and Mendell, 2001a). The latter is contradicted by our finding that MK-801 completely blocked the response produced by the second or third bath applied NMDA. The suggestion that the synaptic response remaining after exposure to MK-801 for 45 minutes and of the order of 50 DR stimuli was mediated by another transmitter also seems unlikely because the response was permanently abolished when a drop of NMDA was added in the presence of MK-801. This would not have been expected to affect transmission at another receptor unless the NMDA drop elicited presynaptic inhibition of Ia transmission to the motoneuron thereby blocking all transmitter release. NMDA receptors are present on the presynaptic terminals of afferent fibers, and NMDA does elicit presynaptic inhibition of transmitter release from the terminals of dorsal root afferent fibers as monitored via the AMPA/kainate receptor response (Arvanian and Mendell, 2001a, Bardoni et al., 2004). However, it only partially depressed transmitter release, and so this could not account for the uniform total loss of NMDA receptor responsiveness observed after the NMDA drop. Furthermore, VLF transmission was not inhibited by exogenous NMDA (Arvanian and Mendell, 2001a) in contrast to the strong depression observed in the current experiments (not illustrated). Together these comparisons indicate that the NMDA effects on the recovery from MK-801 were not the exclusive result of presynaptic inhibition of glutamate release.
The failure of bath applied MK-801 to completely block the NMDA receptor- mediated synaptic response might also be explained by a constant replacement of NMDA receptors at the synapse by independently functioning unblocked extrasynaptic receptors (Clark et al., 1997; Moniyama, 2000; Thomas et al., 2005). In agreement with this hypothesis, when all NMDA receptors were inactivated by exogenous NMDA in the presence of MK-801, replacement by functional NMDA receptors was not possible and the synaptic response disappeared. NMDA receptor transport within neurons is fast enough to be consistent with such a mechanism (Groc et al., 2004; Guillaud et al., 2003: Washbourne et al., 2002). This replacement hypothesis might also help to explain the variable recovery of synaptic transmission to levels above or below the original response after synapse specific MK-801 block in the absence of NMDA.
These experiments were based on the use dependence of MK-801 blockade, but the results also revealed that this antagonist can be bound in the absence of synaptic activity. MK-801 blocks transmission when administered (45 min) and washed out (45 min.) in the absence of stimulation. Previous biochemical work has indicated that MK-801 binds to NMDA receptors in cortical membrane preparations via 2 distinct processes: a kinetically fast (half time 10 min.) binding requiring the presence of a ligand for the NMDA receptor, e.g., glutamate, and a much slower effect not requiring the presence of any agonist (half time 2-3 hours) (Javitt and Zukin, 1989). It remains to be determined precisely where the MK-801 is located before inserting into the pore as a result of the initial stimulus. The biochemical experiments suggested that the slow accumulation of MK-801 is hydrophobic raising the possibility that it is lipophilic and remains associated with the membrane.
Although the in vitro experiments provide important guidance for interpreting the current experiments mechanistically, the present experiments in a largely intact hemicord preparation permit an additional important functional implication to be drawn, namely, that different classes of NMDA receptor- mediated connections on the same motoneuron, i.e., spindle afferent fibers in the dorsal root and fibers in the VLF, differ in their properties. Whether these differences are related to the increased susceptibility of VLF NMDAR- mediated synapses to Mg2+ block in neonatal rat motoneurons (Arvanian et al., 2004) is not presently known. Another unknown is whether the reduced ability of NMDA receptors to be stabilized at VLF synapses contributes to the relative lack of sensitivity of these synapses to the sensitizing effects of neurotrophins (Arvanov et al., 2000; Arvanian and Mendell, 2001b).
Still other interesting questions remain. It will be important to determine whether adult rats exhibit similar evidence for membrane trafficking, whether it is synapse specific, and whether it is related to the susceptibility to synaptic plasticity. The finding that recovery of responses at VLF synapses was less than that associated with DR synapses suggests that maturation might be a factor since we have previously shown that VLF synapses on motoneurons display more signs of maturation than those from DR in neonatal rats (Arvanian et al., 2004). It will be important to understand at what factors underlie the apparent difference in ability of synapses under different presynaptic inputs to stabilize trafficking NMDA receptors. There is evidence that the molecular composition of the postsynaptic density as well as the composition of the NMDA receptor itself may be important contributors to the stabilization of the postsynaptic receptors. In view of the well documented role of NMDA receptors in strengthening excitatory connections during development (Aamodt and Constatine Paton, 1999), the solution to these problems at the molecular and cellular level could improve the ability to regulate the formation of new synaptic connections in the adult nervous system which could help improve the outcome after injury and degenerative diseases.
Acknowledgments
We thank Dr. Indira Raman for the helpful suggestion concerning the use of MK-801 in these experiments. The research was supported by a grant from the NIH to LMM (5 RO1 NS16996) as well as the Christopher and Dana Reeve Foundation (LMM), the New York State Spinal Cord Injury Research Board (VLA) and the Veterans Administration (VLA).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- APV
2-amino-5-phosphovaleric acid
- CGP 35348
Ciba Geigy Product 35348
- CNS
Central Nervous system
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DR
dorsal root
- EPSP
excitatory postsynaptic potential
- GABA
γ-amino butyric acid
- MK-801
(5R,10S)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine-hydrogen-maleate
- NMDA
N-methyl-d-aspartate
- NMDAR
N-methyl-d-aspartate receptor
- NR2
Subunit 2 of the NMDA receptor
- VLF
ventrolateral funiculus
Literature Cited
- Aamodt SM, Constantine-Paton M. The role of neural activity in synaptic development and its implications for adult brain function. Adv. Neurol. 1999;79:133–144. [PubMed] [Google Scholar]
- Arvanian VL, Bowers WJ, Petruska JC, Manuzon H, Narrow WC, Motin V, Federoff HJ, Mendell LM. Viral delivery of NR2D subunits reduces Mg2+ block of NMDA receptor and restores NT-3-induced potentiation of AMPA/kainate responses in maturing rat motoneurons. J. Neurophysiol. 2004;92:2394–2404. doi: 10.1152/jn.00278.2004. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur. J. Neurosci. 2001a;14:1800–1808. doi: 10.1046/j.0953-816x.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Mendell LM. Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. Eur. J. Neurosci. 2001b;14:1800–1808. doi: 10.1046/j.0953-816x.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Motin V, Mendell LM. Comparison of metabotropic glutamate receptor responses at segmental and descending inputs to motoneurons in neonatal rat spinal cord. J. Pharm. Exp. Ther. 2005;312:669–677. doi: 10.1124/jpet.104.075077. [DOI] [PubMed] [Google Scholar]
- Arvanian VL, Mendell LM. Removal of NMDA receptor Mg2+ block extends the action of neurotrophin-3 on synaptic transmission in neonatal rat motoneurons. J. Neurophysiol. 2001;86:123–129. doi: 10.1152/jn.2001.86.1.123. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Seebach BS, Mendell LM. NT-3 evokes an LTP- like facilitation of AMPA/Kainate- mediated synaptic transmission in the neonatal rat spinal cord. J. Neurophysiol. 2000;84:752–758. doi: 10.1152/jn.2000.84.2.752. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Ertunc M, Moulder KL, Blackwell J, Chung C, Su J, Kavalali ET. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J. Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J. Neurosci. 2004;24:2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Clark BA, Farrant M, Cull-Candy SG. A direct comparison of the single-channel properties of synaptic and extrasynaptic NMDA receptors. J. Neurosci. 1997;17:107–116. doi: 10.1523/JNEUROSCI.17-01-00107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS. A broad view of glutamate spillover. Nat. Neurosci. 2002;5:291–292. doi: 10.1038/nn0402-291. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J. Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS. Neuronal glutamate transporters limit activation of NMDA receptors by neurotransmitter spillover on CA1 pyramidal cells. J. Neurosci. 2001;21:8328–8338. doi: 10.1523/JNEUROSCI.21-21-08328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzubay JA, Jahr CE. Kinetics of NMDA channel opening. J. Neurosci. 1996;16:4129–4134. doi: 10.1523/JNEUROSCI.16-13-04129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. [PubMed] [Google Scholar]
- Foster AC, Wong EH. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-D-aspartate receptor in rat brain. Br. J. Pharmacol. 1987;91:403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groc L, Choquet D. AMPA and NMDA glutamate receptor trafficking, multiple roads for reaching and leaving the synapse. Cell Tissue Res. 2006;326:423–438. doi: 10.1007/s00441-006-0254-9. [DOI] [PubMed] [Google Scholar]
- Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent regulation of the lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J. Neurosci. 2003;23:131–140. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney SC, Jane DE, Anwyl R. Extrasynaptic NR2D-containing NMDARs are recruited to the synapse during LTP of NMDAR-EPSCs. J. Neurosci. 2008;28:11685–11694. doi: 10.1523/JNEUROSCI.3035-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hires SA, Zhu Y, Tsien TY. Optical measurement of synaptic gluatamate spillover and reuptake by linker optimized glutamate-sensitive fluorescent reporters. Porc Natl Acad Sci. 2008;105(11):4411–6. doi: 10.1073/pnas.0712008105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman S, Jordan LM, MacDonald JF. N-methyl-D-aspartate receptor-mediated voltage oscillations in neurons surrounding the central canal in slices of rat spinal cord. J. Neurophysiol. 1994;72:565–577. doi: 10.1152/jn.1994.72.2.565. [DOI] [PubMed] [Google Scholar]
- Huang H, Bordey A. Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J. Neurosci. 2004;24:5659–5669. doi: 10.1523/JNEUROSCI.1338-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801, selective binding to open channels. Proc. Natl. Acad. Sci. USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE. Drooling and stuttering, or do synapses whisper? Trends Neurosci. 2003;26:7–9. doi: 10.1016/s0166-2236(02)00003-6. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal networks in the cat, elementary components. Brain Res. Rev. 2008;57:46–55. doi: 10.1016/j.brainresrev.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Biexponential kinetics of [3H]MK-801 binding, evidence for access to closed and open N-methyl-D-aspartate receptor channels. Mol. Pharmacol. 1989;35:387–393. [PubMed] [Google Scholar]
- Kiehn O, Johnson BR, Raastad M. Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience. 1996;75:263–273. doi: 10.1016/0306-4522(96)00250-3. [DOI] [PubMed] [Google Scholar]
- Kloog Y, Nadler V, Sokolovsky M. Mode of binding of [3H]dibenzocycloalkenimine (MK-801) to the N-methyl-D-aspartate (NMDA) receptor and its therapeutic implication. FEBS Lett. 1988;230:167–170. doi: 10.1016/0014-5793(88)80664-1. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus, evidence and implications. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Failures and successes of NMDA receptor antagonists, molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004;1:101–110. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Schmidt BJ, Hochman S. NMDA receptor activation triggers voltage oscillations, plateau potentials and bursting in neonatal rat lumbar motoneurons in vitro. Eur. J. Neurosci. 1997;9:2702–2711. doi: 10.1111/j.1460-9568.1997.tb01699.x. [DOI] [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. J. Physiol. 2000;523:621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Ichiyama RM, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training following spinal cord transection in rats. J. Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinco M, Lev-Tov A. Synaptic transmission between ventrolateral funiculus axons and lumbar motoneurons in the isolated spinal cord of the neonatal rat. J. Neurophysiol. 1994;72:2406–2419. doi: 10.1152/jn.1994.72.5.2406. [DOI] [PubMed] [Google Scholar]
- Seebach BS, Arvanov VL, Mendell LM. Neurotrophin influence on the development of segmental reflexes in the rat. J. Neurophysiol. 1999;81:2398–2405. doi: 10.1152/jn.1999.81.5.2398. [DOI] [PubMed] [Google Scholar]
- Shanthanelson M, Arvanian VL, Bowers WJ, Federoff HJ, Mendell LM. Independence and interdependence of NMDA receptor mediated synaptic inputs on motoneurons in the developing spinal cord, Role of NMDA-2D subunits in receptor trafficking. Society for Neuroscience Abstracts. 2005 #175.13. [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J. Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Mobile NMDA receptors at hippocampal synapses. Neuron. 2002;34:255–264. doi: 10.1016/s0896-6273(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat. Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Liu XB, Jones EG, McAllister AK. Cycling of NMDA receptors during trafficking in neurons before synapse formation. J. Neurosci. 2004;24:8253–8264. doi: 10.1523/JNEUROSCI.2555-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu. Rev. Pharmacol. Toxicol. 2003;43:335–358. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc. Natl. Acad. Sci. USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Peng Y, Xu Z, Chen RQ, Gu QH, Chen Z, Lu W. Synaptic metaplasticity through NMDA receptor lateral diffusion. J. Neurosci. 2008;28:3060–3070. doi: 10.1523/JNEUROSCI.5450-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ziskind-Conhaim L. NMDA receptors mediate poly- and monosynaptic potentials in motoneurons of rat embryos. J. Neurosci. 1990;10:125–135. doi: 10.1523/JNEUROSCI.10-01-00125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]