Abstract
Objective
To assess the impact of our transcranial doppler ultrasonography (TCD) program on the incidence of first stroke and the rate of transfusion for stroke prevention in children with sickle cell disease.
Study design
In this single-institution, retrospective study, we compared the incidence of stroke and of transfusion for stroke prevention in 475 patients followed in the 8-year period prior to instituting TCD screening to the rate in 530 children in the 8-year period afterwards.
Results
The incidence of overt stroke in the pre-TCD period was 0.67 per 100 patient-years, compared with 0.06 per 100 patient-years in the post-TCD period (p<0.0001). Of the two strokes in the post-TCD period, one occurred in a child too young for the screening protocol and one in a child with high velocities solely in the anterior cerebral arteries. The rate of transfusion therapy for stroke prevention increased from 0.67 per 100 patient-years to 1.12 per 100 patient-years since instituting our program (p=0.008).
Conclusions
Our program has been successful in reducing the rate of first overt stroke, but with increased use of transfusion. Additional modifications to screening might further reduce the risk of first stroke, and studies of alternative treatments may be beneficial.
Keywords: Sickle cell disease, stroke, Transcranial Doppler ultrasonography, blood transfusion, pediatric hematology
Stroke is one of the most devastating complications of sickle cell disease (SCD). The incidence of overt stroke in patients with SCD-SS of all ages in the Cooperative Study of Sickle Cell Disease (CSSCD) was 0.61 events per 100 patient-years.1 Stroke rates were even higher in children with SCD-SS less than 10 years old, with a rate of 1.02 per 100 patient-years in those 2 to 5 years and a rate of 0.79 per 100 patient-years in children ages 6 to 9 years. Over two decades have passed since the collection of CSSCD data, and important advances in stroke prevention in SCD have occurred in that time interval. Transcranial Doppler ultrasonography (TCD) became available to detect children at high risk of stroke and the Stroke Prevention Trial in Sickle Cell Anemia (STOP) showed that regular transfusions reduced the risk of stroke by over 90% in those with abnormal TCD studies.2
The impact of routine TCD screening and primary stroke prevention on the overall incidence of stroke in SCD has not been studied extensively. One study, using a California statewide discharge diagnosis database, found that admissions for first stroke in children with SCD significantly declined following the publication of the STOP results in 1998, from 0.88 per 100 patient-years prior to publication to 0.17 per 100 patient-years, providing indirect evidence that TCD programs reduced stroke risk.3 A more recent report showed a reduction in first stroke incidence from 0.46 to 0.18 per 100 patient-years in children with SCD in the Memphis region following implementation of regular TCD screening, through identification of new strokes seen at their center and the use of population estimates as the denominator.4
The objective of this study was to assess the impact of our TCD screening and prophylactic transfusion program on the incidence of first stroke in children with SCD followed at a large, Comprehensive Sickle Cell Center (CSCC). Secondary aims were to determine the effect of our program on rates of transfusion for stroke prevention and to assess complications of these transfusions and potential limitations to our screening and prophylactic transfusion protocol.
Methods
In this retrospective cohort study of children with homozygous sickle cell disease (SCD-SS) or sickle cell disease-S-β0-thalassemia (SCD-Sβ0-thal) enrolled in the CSCC at the Children's Hospital of Philadelphia (CHOP), the incidence of stroke in the eight-year period before TCD screening was instituted (September 1990 to August 1998) was compared with the rate in the eight-year period after TCD screening began (September 1998 to August 2006). The rates of transfusion therapy for stroke prevention were compared between these groups and complications of transfusion were assessed for children receiving transfusion for abnormal TCD. The Institutional Review Board of CHOP approved this study. Requirement for informed consent was waived.
The study population included individuals under 22 years of age with SCD-SS or SCD-Sβ0-thal enrolled in the CSCC at CHOP – Main Campus. Patients who had had a stroke prior to the study period or prior to enrollment at the CSCC at CHOP were excluded.
TCD Screening Protocol
TCD screening was instituted at CHOP on September 2, 1998. For the first two years, TCD studies were performed in the Hematology Clinic. Subsequently, studies were performed in the Department of Radiology. All TCD exams were performed by an ultrasound technician, either STOP-certified or trained in the STOP technique by a certified technologist, and all studies were interpreted by an attending radiologist who generated a STOP-quality clinical report.
Screening studies were recommended for all children with SCD-SS and SCD-Sβ0-thal, between the ages of 2 and 18 years and were obtained when children were clinically well. This differs from the STOP protocol, where screening is performed in children from age 2 to 16 years.2 A 2-MHz pulsed Doppler ultrasound system (Nicolet EME TC 2000 through 2005 and subsequently the Pioneer TC 8080; Nicolet, Madison, WI) was utilized for all studies. Using right and left temporal approaches, the highest time-averaged mean velocity was recorded in the middle cerebral artery (MCA), the distal internal carotid artery (ICA) and its bifurcation, the anterior cerebral artery (ACA), and the posterior cerebral artery (PCA). The basilar artery velocity was also recorded using the suboccipital approach. All TCD studies were classified based on the highest time-averaged mean blood flow velocity in the ICA or MCA based on STOP criteria as normal (<170 cm/s), conditional (170-199 cm/s), and abnormal (200 cm/s or higher).2 Studies where readings were unable to be obtained in the ICA and MCA bilaterally were classified as inadequate, unless one side was clearly abnormal.
Our screening protocol includes annual TCD studies for children with normal TCD results, studies every 3 to 6 months for those with conditional results, and a repeat study within 4 weeks for children with abnormal results. Chronic transfusion therapy is recommended for those with confirmed abnormal TCD, defined as two consecutive abnormal TCD studies or a single abnormal TCD with a velocity of at least 220 cm/s. Children with SCD at CHOP who require red cell transfusions receive units from the Blue Tie Tag program primarily.5 This program provides red cell units from self-designated African-American blood donors with phenotype matching for C, E, and Kell.
Data Collection
Each child's sex, SCD genotype, date of initial CSCC visit, and date of transition to adult care or transfer were recorded. The results of all TCD studies were reviewed along with treatment decisions (transfusion therapy, hydroxyurea, no treatment) for those with abnormal studies. All cases of stroke or neurological event were identified from the CSCC comprehensive database. For children receiving transfusions for abnormal TCD, we recorded information on duration of transfusions, method of transfusion, serum ferritin level at the end of the study period, and alloimmunization history.
Determination of Stroke
Medical records, including Emergency Department reports, neurology consultation reports, and admission or event history and physical, radiological studies including images and reports of brain computerized tomography (CT) and magnetic resonance imaging (MRI) for all neurological events were reviewed by the study neurologist (RI). Clinical and radiologic findings were each classified independently as to whether or not a diagnosis of stroke was supported.
Clinical data were first reviewed without prior review of radiology reports or imaging studies and a determination was made as to the classification of the clinical event into one of the following categories: 1) Definite clinical stroke syndrome: neurologic deficit conforming to an arterial ischemic syndrome corresponding to a vascular territory and lasting > 24 hrs, 2) Possible clinical stroke syndrome or transient ischemic attack: neurologic deficit conforming to an arterial ischemic syndrome corresponding to a vascular territory and lasting < 24 hrs, 3) Acute neurologic event not conforming to a stroke syndrome: neurologic sign or symptom not conforming to an arterial ischemic syndrome corresponding to a vascular territory, 4) Indeterminant: documentation of neurologic signs or symptoms insufficient for classification.
Radiologic classification was then carried out and each case classified into one of the following: 1) acute infarct on MRI (region of restricted diffusion) in an arterial territory, 2) MRI negative for acute infarct, or 3) intracerebral hemorrhage consistent with primary intracerebral hemorrhage or hemorrhagic transformation of infarction.
In each case a final classification was determined based on both clinical and radiologic classification, into one of the following groups: 1) Acute arterial ischemic stroke (AIS), defined as an acute neurological deficit of any duration conforming to an arterial territory with an acute infarct on MRI in a vascular territory corresponding to the clinical deficit; 2) Acute hemorrhagic stroke; 3) Not acute stroke, defined as an acute neurologic deficit or symptom not conforming to an arterial territory or not associated with an acute infarct on MRI corresponding to the clinical deficit; and, 4) Indeterminate, defined as clinical or radiologic data insufficient to adjudicate classification as a stroke. There were no MRI-negative acute infarcts in this study.
Statistical Analysis
Crude incidence rates of stroke were calculated in the pre-TCD (Sept. 1990- Aug 1998) and post-TCD (Sept. 1998 – Aug. 2006) groups as the number of first strokes in each time period divided by the number of person-years of observation in each time period. In each time period, patient follow-up began at the start of the study period or at the time of enrollment at our CSCC, if enrolled after the beginning of the study period. Follow-up time ended at the earliest of occurrence of stroke, date age 22 years was attained (age when transition from our pediatric program usually occurs), date of transfer from CSCC at CHOP, or the end of the study period. Incidence rates were compared by time period using a test of exact binomial proportions.6 In addition, the rate of transfusion for stroke prevention (primary and secondary) was explored by comparing the incidence of stroke in the pre-TCD group to the combined incidence of stroke and initiation of transfusion therapy for TCD findings in the post-TCD group. Descriptive analyses including mean, median, range, and standard deviation were used to describe patient characteristics (number of TCD studies, serum ferritin levels), t-tests were utilized to compare continuous variables between sub-groups, and correlations between continuous variables were calculated using Spearman correlation coefficients.
Results
TCD Screening and Treatment Choices
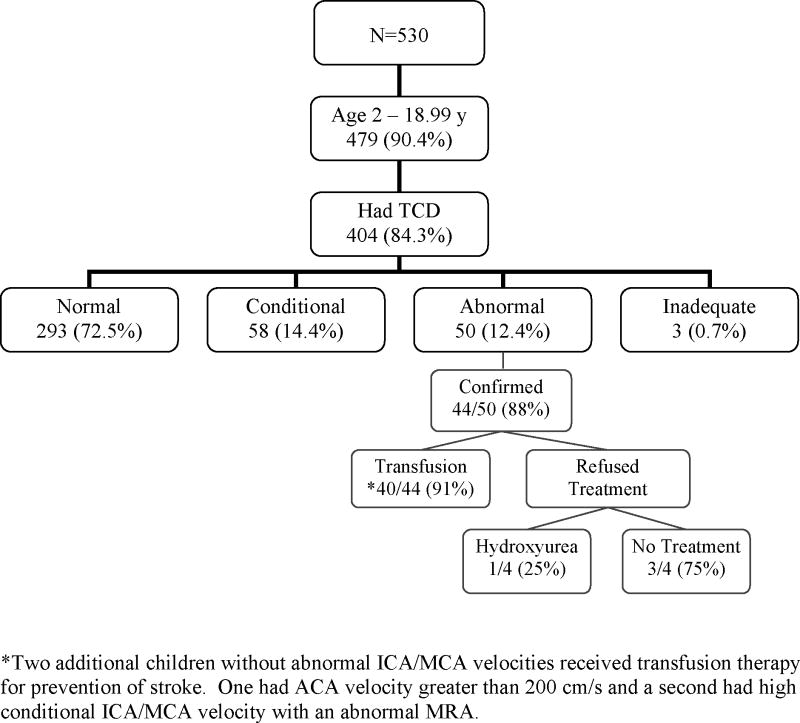
A total of 530 patients with SCD-SS or SCD-Sβ0-thal followed in the post-TCD era were between the ages of 2 and 18 years during the study period and eligible for TCD screening. The distribution of TCD results based on the highest value obtained during the post-TCD study period and treatment choices are shown in the Figure.
Figure 1. TCD screening results and treatment choices.
The distribution of TCD results based on the highest TCD velocity obtained on any study in the post-TCD period is shown. Of the 530 children in the cohort, 479 were eligible for TCD screening based on age and sickle cell diagnosis.
Of the 75 children who were never screened, 22 (29.3%) were older than 16 years of age when screening was instituted (the upper age of screening in the STOP protocol) and 9 (12%) were younger than 3 years of age at the end of the study period and had not yet had their first TCD. The remainder had been followed in our CSCC for a mean duration of 3.4 years (range, 3 weeks to 8 years) during the post-TCD period, and did not undergo TCD screening, mostly related to poor adherence. None of these patients had a stroke.
Among the children who underwent TCD screening, the average number of TCD studies obtained during the study period was 3.3 per child (median 2; range 1 to 18). The number of TCD studies correlated positively with both the maximum TCD velocity (r = 0.58, p<0.001) and duration of follow-up (r = 0.64, p<0.001) and negatively with age (r = -0.24, p<0.001). Seventy-two of 337 children who were eligible for follow-up studies based on initial age ≤ 16 years and follow-up time of at least 18 months did not undergo follow-up TCD. Most of these children had prior normal TCD, but two had conditional studies and one had an abnormal study at the initial screen.
The most recent TCD studies were normal in three of the four patients with abnormal TCD whose families refused transfusion therapy, including one patient treated with hydroxyurea, and conditional (178 cm/s) in the fourth patient; none has had an overt stroke. Four children who initially were placed on transfusion therapy have subsequently discontinued transfusions, two with poor adherence who were switched to hydroxyurea, one who developed a warm autoantibody, and one patient whose parent refused further treatment. All four had normal TCD studies at their last assessment and no history of stroke.
Stroke incidence
The incidence of stroke in the pre and post TCD screening periods is shown in Table I. Of the 19 arterial ischemic strokes in the pre-TCD period, 16 involved the anterior circulation and three involved the posterior circulation. Details of the two overt strokes that occurred in the post-TCD period are shown in Table II.
Table I.
Patient characteristics and stroke/neurological events in the pre- and post-TCD screening periods
| Pre-TCD | Post-TCD | |
|---|---|---|
| Number | 475 | 530 |
| Sex (% male) | 52 | 51.3 |
| Patient years (pt y) of follow-up | 3,137 | 3,578 |
| Overt stroke (number) | 21 | 2 |
| - Ischemic | 19 | 2 |
| - Hemorrhagic | 2 | 0 |
| Other neurological event (number) | 3 | 6 |
| Indeterminate (number) | 2 | 1 |
| Incidence rate of overt stroke (per 100 pt y) - 95% confidence interval | 0.67 (0.41-1.02) | 0.06□ (0.01- 0.20) |
| Rate of transfusion for stroke prevention (per 100 pt y)‡ - 95% confidence interval | 0.67 (0.41-1.02) | 1.12† (0.80-1.52) |
p value < 0.0001.
p value = 0.008
Pre-TCD includes all children transfused for history of overt stroke while Post-TCD includes all children transfused for history of overt stroke or abnormal TCD, as well as one patient transfused for a high conditional TCD with abnormal magnetic resonance angiography and one child transfused for an abnormal ACA velocity without abnormal ICA/MCA velocity.
Table II.
Description of overt stroke and other neurological events in post-TCD period
| Age (y) | TCD results | Time from TCD to neurological event (days) | Clinical presentation | Brain MRI/A |
|---|---|---|---|---|
| Overt Stroke | ||||
| 1.2 | Not Done | NA | R hemiparesis during admission for acute chest syndrome/splenic sequestration | L MCA distribution infarction – DWI+; Occlusion of L ICA/MCA |
| 3.4 | Nomal “Abnormal” ACA velocities | 12 | R arm weakness | L MCA distribution infarction-DWI+; Mod-severe stenosis bilateral ICA |
| Other Neurological Events | ||||
| 1.1 | Not Done | NA | Febrile seizure; non-focal neurological exam | Punctate R caudate infarct- DWI+; scattered old foci of increased T2/FLAIR |
| 5.5 | Abnormal | 96 | Headache for 1 day, 30 min episode L arm and bilateral leg weakness 3 mo after starting transfusions; non-focal neurological exam | Possible acute punctate L caudate infarct; scattered old foci of increased T2/FLAIR |
| 9.5 | Normal | 72 | Aplastic episode (Hb 2.7); Dizzy. Non-focal neurological exam | Punctate L centrum semiovale infarct- DWI+; scattered old foci of increased T2/FLAIR |
| 10.6 | Normal | 28 | Acute infarct on screening MRI. Next day complaints of fatigue, dizziness. Non-focal neurological exam | Punctate L periventricular infarct-DWI+; scattered old foci of increased T2/FLAIR |
| 11.6 | Conditional | 145 | Dizzy for 2 days. Non-focal neurological exam | Punctate R frontal deep white matter infarct- DWI+; scattered old foci of increased T2/FLAIR |
| 4.9 | Not Done | NA | Viral syndrome; Multiorgan failure; Encephalopathy | Symmetrical bilateral caudate, putamen, globus palladi and L anterior-medial temporal lobe infarcts |
NA, not applicable; L, Left; R, Right; MCA, middle cerebral artery; ICA; internal carotid artery; ACA anterior cerebral artery; MRI, magnetic resonance imaging; DWI, diffusion weighted imaging; and Hb, hemoglobin.
Other Neurological Events
There were three neurological events not classified as acute stroke in the pre-TCD period. One patient had acute encephalopathy and behavioral changes. The second had multiple seizures, and the third had one episode of syncope with a suspected transient ischemic attack. Details of the six neurological events not classified as acute stroke in the post-TCD period are shown in Table II.
Transfusion Therapy for Stroke Prevention – Rates and complications
The rate of initiation of transfusion therapy for stroke prevention significantly increased in the post-TCD period (Table I). Most children (40/42, 95%) initially received simple transfusions, but 20 of these 40 (50%) subsequently switched to automated exchange transfusion after a mean of 2.5 years (range, 0.1 to 5.3 years) in order to reduce the rate if iron accumulation. Two children received only automated exchange transfusions. Only two of the forty-two children required central venous line placement.
The median serum ferritin level was 1,064 ng/mL (mean 1,566 ng/mL and range 38 to 7,240 ng/mL) and six children had ferritin levels of 3,000 ng/mL or above, consistent with severe iron overload.7 Serum ferritin level correlated positively with duration of simple transfusion (r = 0.63, p<0.001) and negatively with duration of exchange transfusion (r = -0.47, p=0.002). Serum ferritin level was significantly lower in children receiving exchange transfusion (n=22, mean = 1,002 ng/ml) compared with children receiving simple transfusion (n=20, mean = 2,187 ng/ml, p= 0.019). Six children were receiving chelation therapy and their mean ferritin level was significantly higher than those not receiving chelation (3,516 vs 1,241 ng/ml, p=0.001).
Eleven children (26.2%) developed red cell antibodies while receiving chronic red cell transfusions for primary stroke prevention. Six of these had prior alloimmunization. Twenty-seven new antibodies developed in this cohort; most (78%) were directed against the Rh group. The remaining antibodies were anti-Jkb, anti-LeA, anti-Jsa and anti-Wra.
Discussion
Within this single, large pediatric sickle cell center population, TCD screening and treatment of children with abnormal TCD studies have been successful, resulting in a more than 10-fold reduction in stroke incidence. Most (84%) eligible children were screened with TCD and the acceptance rate for transfusion therapy was very high. Implementation of a TCD screening and treatment protocol within a single institution is feasible and effective. Our work further adds to the evidence that the use of TCD screening and prophylactic transfusions has had a major impact on the incidence of overt stroke in children with SCD, but at the expense of an increased number of children receiving transfusion therapy.
The optimal timing and frequency of re-screening with TCD has not been well-established. Our stroke rate was quite low, even with incomplete adherence to our screening regimen, suggesting that such rigorous re-screening may not be necessary for all children. In our cohort, follow-up was more complete for children with prior conditional studies and for younger children, groups known to have a higher risk of conversion to abnormal TCD,8 supporting the importance of close follow-up in these groups.
Our TCD screening and prophylactic transfusion protocol did not prevent stroke in two children. The first involved a child who had abnormally high blood flow velocity in the ACA, but failed to meet protocol criteria for transfusion because velocities in the ICA/MCA were not elevated. In addition to this stroke, we had also previously reported stroke that occurred in a child with SCD-SS and isolated elevated ACA velocities followed in a CHOP satellite site.9 Sub-analysis of STOP data showed that ACA velocities of 170 cm/s or higher were associated with an increased risk of stroke, even after adjusting for ICA/MCA TCD classification10 and further study is needed to determine the optimal treatment for these patients.
The second stroke not prevented by our TCD protocol involved a 14 month-old child who had a stroke prior to the age of 2 years when TCD screening began, suggesting a need to begin TCD screening at an earlier age. In the BABY HUG study, baseline TCD performed in 192 infants and toddlers with SCD at a mean age of 12.6 months showed a TAMMvel of 115 cm/s,11 substantially lower than approximately 140 cm/s reported for older children with SCD.12 Furthermore, none of the infants in the BABY HUG study had abnormal studies as defined for older children (TAMMvel ≥ 200 cm/s).11 Thus, cutoff velocities associated with high risk of stroke in children younger than two years need to be established. Lastly, this 14 month-old child's stroke occurred during an episode of acute chest syndrome and splenic sequestration. There may be differences in the underlying pathophysiology of strokes that occur in temporal relationship to an acute medical event compared with stroke not associated with such events given the lower stroke recurrence rate in the former group.13 It is unclear whether TCD screening and treatment protocols are as effective in preventing event-related strokes.
In the post-TCD group, five children were found to have acute silent infarcts with punctate areas of T2 hyperintensity with restricted diffusion in a location that did not correspond to neurological symptoms. The preceding TCD results in these children were variable, ranging from normal to abnormal (Table II). Given that diffusion-weighted imaging was not universally used at our center in the pre-TCD period, and that our threshold for obtaining imaging studies in children with SCD is lower in more recent years, it is not possible to compare the rate of such events in the pre- and post-TCD groups. Nonetheless, this finding suggests that TCD screening protocols, alone, are not an optimal approach to prevent silent infarcts in children with SCD. In support of this observation, Wang et al did not find any correlation of TCD velocities with silent infarcts.14 It is possible that silent infarcts are caused by occlusion of smaller diameter vessels, which TCD cannot detect. Of note, we discovered acute silent infarcts in the context of a febrile illness in one patient and with severe anemia in another, suggesting that these additional stressors may precipitate silent infarcts in children who are at risk. In support of this theory, in a recent report of seven children with SCD and acute silent cerebral infarcts, four had severe anemia at presentation and one had a recent acute illness.15
Although the rate of first stroke has declined at our Center, there has been a significant increase in the number of children who are being transfused to prevent stroke. Regular transfusions may have the added benefit of preventing other complications of SCD such as acute chest syndrome and pain,16 but carry the risks of infection, alloimmunization, and iron overload. Too few liver biopsies were performed during the study period for meaningful analysis, but the majority of children receiving transfusions for abnormal TCD at our Center had serum ferritin levels below 1,500 ng/ml, a level at which significant liver iron overload in SCD is uncommon.7 The use of exchange transfusion limits iron loading and may obviate the need for chelation therapy in SCD.17 In the present study, children receiving exchange transfusion had lower serum ferritin levels than children managed solely with simple transfusions. This supports a role for utilizing exchange transfusion as early as feasible in this patient population. Exchange transfusion involves greater blood donor exposure than simple transfusion, which may increase the risk of alloimmunization and transfusion-transmitted infection.17 This potential downside as well as the need for large intravenous access and the local availability of outpatient apheresis services should be considered in making the decision about transfusion method. Deferasirox, an oral chelator, only became commercially available in early 2006, towards the end of our study period, and thus its impact on transfusional iron overload in our patient population could not be evaluated. It is possible that expanded chelation options also may improve the management of iron overload in these patients.
The prevalence of red cell alloimmunization in our study was high, although only one child discontinued transfusion for this reason. Alloimmunization was primarily related to the Rh group despite prospective C, D, and E matching. This finding may be due to the increased African-American donor representation in our blood donor pool, given that Rh variants are more common in this donor group.18, 19 Further modifications to transfusion protocols, such as the use of red blood cell genotyping, may limit this complication.
Thus, our TCD screening and prophylactic transfusion program has been successful in reducing the incidence of overt stroke. Modifications such as the addition of ACA velocity to treatment criteria, earlier screening, or the addition of other neuroimaging studies might further reduce the risk of first stroke. With the reduction in overt stroke has come an increased use of transfusions. Transfusional iron overload can be managed well with exchange transfusions, and although alloimmmunization occurred, use of antigen negative blood allowed for continued transfusion. Further optimization of transfusion protocols and chelation regimens may limit complications of treatment. Finally, studies of alternative treatments, such as hydroxyurea, are needed.
Acknowledgments
The authors thank Kwaku Ohene-Frempong, MD, and Alan Cohen, MD for their thoughtful review of this manuscript.
Supported in part by grant U54 HL70596 from the National Heart, Lung, and Blood Institute (NHLBI). In addition, some of the TCD studies were performed as part of the Optimizing Stroke Prevention in Sickle Cell Anemia (STOP 2) study supported by grants (U01 HL 052193 and U01 HL 052016) from the NHLBI. The authors declare no conflicts of interest.
- ACA
Anterior Cerebral Artery
- AIS
Arterial Ischemic Stroke
- CHOP
Children's Hospital of Philadelphia
- CSCC
Comprehensive Sickle Cell Center
- CSSCD
Cooperative Study of Sickle Cell Disease
- CT
Computerized Tomography
- ICA
Internal Carotid Artery
- MCA
Middle Cerebral Artery
- MRA
Magnetic Resonance Angiography
- MRI
Magnetic Resonance Imaging
- PCA
Posterior Cerebral Artery
- SCD
Sickle Cell Disease
- STOP
Stroke Prevention Trial in Sickle Cell Anemia
- TCD
Transcranial Doppler ultrasonography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91:288–94. [PubMed] [Google Scholar]
- 2.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 3.Fullerton HJ, Adams RJ, Zhao S, Johnston SC. Declining stroke rates in Californian children with sickle cell disease. Blood. 2004;104:336–9. doi: 10.1182/blood-2004-02-0636. [DOI] [PubMed] [Google Scholar]
- 4.McCarville MB, Goodin GS, Fortner G, Li CS, Smeltzer MP, Adams R, et al. Evaluation of a comprehensive transcranial doppler screening program for children with sickle cell anemia. Pediatr Blood Cancer. 2008;50:818–21. doi: 10.1002/pbc.21430. [DOI] [PubMed] [Google Scholar]
- 5.Sesok-Pizzini DA, Friedman DF, Smith-Whitley K, Nance SJ. Transfusion support of patients with sickle cell disease at the Children's Hospital of Philadelphia. Immunohematology. 2006;22:121–5. [PubMed] [Google Scholar]
- 6.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. New York, NY: John Wiley & Sons; 1982. [Google Scholar]
- 7.Adamkiewicz TV, Abboud MR, Paley C, Olivieri N, Kirby-Allen M, Vichinsky E, et al. Serum ferritin level changes in children with sickle cell disease on chronic blood transfusion are non-linear, and are associated with iron load and liver injury. Blood. 2009;114:4632–8. doi: 10.1182/blood-2009-02-203323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams RJ, Brambilla DJ, Granger S, Gallagher D, Vichinsky E, Abboud MR, et al. Stroke and conversion to high risk in children screened with transcranial Doppler ultrasound during the STOP study. Blood. 2004;103:3689–94. doi: 10.1182/blood-2003-08-2733. [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowski JL, Zimmerman R, Greenbaum B, Ohene-Frempong K. Stroke and elevated blood flow velocity in the anterior cerebral artery in sickle cell disease. J Pediatr Hematol Oncol. 2004;26:323–6. doi: 10.1097/00043426-200405000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Kwiatkowski JL, Granger S, Brambilla DJ, Brown RC, Miller ST, Adams RJ. Elevated blood flow velocity in the anterior cerebral artery and stroke risk in sickle cell disease: extended analysis from the STOP trial. Br J Haematol. 2006;134:333–9. doi: 10.1111/j.1365-2141.2006.06193.x. [DOI] [PubMed] [Google Scholar]
- 11.Pavlakis SG, Rees RC, Huang X, Brown RC, Casella JF, Iyer RV, et al. Transcranial doppler ultrasonography (TCD) in infants with sickle cell anemia: Baseline data from the BABY HUG trial. Pediatr Blood Cancer. 2010;54:256–9. doi: 10.1002/pbc.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams RJ, McKie VC, Carl EM, Nichols FT, Perry R, Brock K, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42:699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- 13.Scothorn DJ, Price C, Schwartz D, Terrill C, Buchanan GR, Shurney W, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140:348–54. doi: 10.1067/mpd.2002.122498. [DOI] [PubMed] [Google Scholar]
- 14.Wang WC, Gallagher DM, Pegelow CH, Wright EC, Vichinsky EP, Abboud MR, et al. Multicenter comparison of magnetic resonance imaging and transcranial Doppler ultrasonography in the evaluation of the central nervous system in children with sickle cell disease. J Pediatr Hematol Oncol. 2000;22:335–9. doi: 10.1097/00043426-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Dowling MM, Quinn CT, Rogers ZR, Buchanan GR. Acute silent cerebral infarction in children with sickle cell anemia. Pediatr Blood Cancer. 2010;54:461–4. doi: 10.1002/pbc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller ST, Wright E, Abboud M, Berman B, Files B, Scher CD, et al. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the Stroke Prevention Trial (STOP) in sickle-cell anemia. J Pediatr. 2001;139:785–9. doi: 10.1067/mpd.2001.119593. [DOI] [PubMed] [Google Scholar]
- 17.Kim HC, Dugan NP, Silber JH, Martin MB, Schwartz E, Ohene-Frempong K, et al. Erythrocytapheresis therapy to reduce iron overload in chronically transfused patients with sickle cell disease. Blood. 1994;83:1136–42. [PubMed] [Google Scholar]
- 18.Faas BH, Beckers EA, Wildoer P, Ligthart PC, Overbeeke MA, Zondervan HA, et al. Molecular background of VS and weak C expression in blacks. Transfusion. 1997;37:38–44. doi: 10.1046/j.1537-2995.1997.37197176949.x. [DOI] [PubMed] [Google Scholar]
- 19.Singleton BK, Green CA, Avent ND, Martin PG, Smart E, Daka A, et al. The presence of an RHD pseudogene containing a 37 base pair duplication and a nonsense mutation in africans with the Rh D-negative blood group phenotype. Blood. 2000;95:12–8. [PubMed] [Google Scholar]