Abstract
Objectives
Effective strategies are needed to manage individuals with chronic non-cancer pain and coexistent opioid addiction. This study compared opioid discontinuation and opioid replacement protocols.
Methods
We planned to enroll 60 individuals into an open-label trial who had been treated with opioids for chronic non-cancer pain, and who also had opioid addiction. Participants were randomly assigned to one of two 6-month treatment protocols of buprenorphine/naloxone sublingual tablets: 1) tapering doses for opioid weaning or “detoxification” (active comparator group) or 2) steady doses for opioid replacement (experimental group). They were followed monthly for the study outcomes: completion of the 6-month treatment protocol and self-reported pain control, physical functioning, alcohol consumption and illicit drug use.
Results
Enrollment was terminated after enrolling 12 participants because none of the 6 assigned to receive tapering doses could successfully complete the protocol (5 were given steady doses and 1 was admitted to an inpatient chemical dependency treatment program); whereas, of the 6 assigned to receive steady doses, 5 completed the protocol (1 withdrew). This difference between the 2 treatment conditions was significant (P = 0.015). Of the 10 participants who completed the 6 month follow-up, 8 reported improved pain control and physical functioning and 5 used alcohol and/or illicit drugs.
Conclusions
We conclude that over 6 months, these participants with chronic pain and co-existent opioid addiction were more likely to adhere to an opioid replacement protocol than an opioid weaning protocol and that opioid replacement therapy with steady doses of buprenorphine/naloxone is associated with improved pain control and physical functioning.
Keywords: Detoxification, addiction, pain, buprenorphine, opioids
BACKGROUND
Chronic non-cancer pain (i.e. pain unrelated to cancer that persists beyond the usual course of disease or injury) is a major problem in the United States.1,2 Opioids are commonly prescribed for chronic pain, but the long-term effectiveness of these medications is not known. For example, the authors of one systematic review of chronic low back pain noted that opioids appear to be effective for pain relief and improving physical functioning in the short-term ( 16 weeks); however, the effectiveness of long-term opioids (> 16 weeks) is less clear.3 Furthermore, some of these patients will misuse their medications.4 It has been estimated that 5% to 24% of patients with chronic back pain who are prescribed long-term opioids exhibit aberrant drug-taking behaviors.3 Other authors have concluded that pharmacologic tolerance, opioid-induced hyperalgesia, and addiction are associated with treatment failures that may limit the value of long-term opioids.5 Patients who have chronic non-cancer pain and co-existent opioid addiction present clinicians with a therapeutic challenge.
In such cases, experts reporting to the National Guideline Clearinghouse suggest these patients “should be referred to an addiction medicine or addiction psychiatric specialist for the management of opioid discontinuation.” However, the scientific evidence to support this recommendation is limited (Quality = level III; expert opinion), and there is “insufficient evidence to recommend for or against it” (Strength = level I; “The clinician will use clinical judgment”).6 Pharmacotherapy for opioid discontinuation is often combined with behavioral counseling, which is considered to be the standard of care.7 The basic rationale for this approach is to provide a “strategic treatment interruption” or “drug holiday,” which is thought to address analgesic failure associated with the use of long-term opioids.4 With this approach, opioids are gradually discontinued (i.e., opioid tapering or “detoxification”). Following this, patients are treated with non-opioid analgesics, other adjuvant medications (e.g., pregabalin for neuropathic pain), physical therapy modalities, and behavioral counseling (e.g., “cognitive behavioral therapy”).
Opioid replacement therapy is an alternative to opioid discontinuation that places an emphasis on pharmacotherapy for treating patients who have chronic pain and co-existent addiction. With this approach, short-acting opioids (e.g., hydrocodone, oxycodone) are replaced with long-acting opioids (e.g., methadone or buprenorphine). The rationale for this therapeutic approach is that since chronic pain will make it difficult for these individuals to abstain from the short-acting opioid analgesics, which they misuse, replacing those medications with long-acting opioids may result in more effective pain control with a decreased risk for medication misuse.8 Methadone is a full opioid mu-receptor agonist with a half-life of 8–59 hours that can be effective in treating pain and has been recommended for the treatment of chronic pain.9 Two small studies suggest that treating patients who have chronic pain and co-occurring addiction with methadone and adjunctive pain management therapy is superior compared to opioid discontinuation.10, 11 Although methadone can be effective as both an analgesic and for maintenance-oriented treatment of opioid dependence, it has bothersome side effects (e.g., sedation, constipation) and is associated with serious adverse events (e.g., drug overdose, respiratory depression, cardiac rhythm disturbances, and death) that limit its use.12 Buprenorphine, a partial mu-receptor agonist with a half-life of 20–44 hours, is an alternative to methadone for maintenance-oriented treatment. Buprenorphine has good analgesic properties with an excellent safety profile, and pharmacological properties are well documented in the literature.13–16 For outpatient use, buprenorphine is combined with naloxone (hereafter referred to as simply “buprenorphine”) to reduce the potential for intravenous abuse. It has been demonstrated that buprenorphine maintenance leads to better treatment outcomes among those who abuse prescription opioids as compared to those who abuse heroin.17 For example, in one uncontrolled case series of 95 participants, Malinoff and his colleagues concluded that its effectiveness in the treatment of opioid addiction as well as providing analgesia with a low abuse liability make buprenorphine a potentially useful treatment for patients with chronic pain and co-occurring opioid dependency.18 Although buprenorphine is given once daily when used for maintenance therapy, it is typically given in divided doses for pain management; however, there have been no prospective randomized controlled trials to confirm the effectiveness of buprenorphine for the management of chronic non-cancer pain among those with co-existent addiction or to provide dosing guidelines in these situations.
Clinicians need evidence-based guidelines to more effectively manage patients who have both chronic pain and evidence of opioid misuse or addiction. The goal of this study was to compare a buprenorphine tapering protocol (i.e., “detoxification”) for opioid discontinuation with an opioid replacement protocol using steady doses of buprenorphine in a group of patients with chronic non-cancer pain who also had opioid addiction. The hypothesis was that those given steady doses of buprenorphine would be more likely to adhere to the treatment protocol than those given tapering doses.
METHODS
The study protocol was approved by the human studies committee of the sponsoring university and by the Medical Director for Research at the host hospital. It was registered with the Food and Drug Administration (www.ClinicalTrials.gov) and was given the identifier number NCT00552578. Written informed consent was obtained from all participants at the time of enrollment. Participants were free to withdraw from the study at any time without prejudice.
Participant Identification and Randomization
Patients eligible for randomization were men and women aged 18 years or older with well-documented chronic non-cancer pain and a self-identified addiction to prescription opioids. Physicians associated with a multi-disciplinary outpatient pain management program referred patients to the study who were determined to have physical cause for chronic pain that was associated with prescription opioid addiction. Potential participants were screened via a telephone interview by one of the investigators (E.M.F.) who established that the patient wanted help with a self-identified prescription “drug addiction.” A diagnosis of opioid addiction was confirmed using a 7-item checklist based on the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) for “opioid dependency.” 19 We planned to enroll 60 participants into this preliminary study that was designed to test the clinical feasibility of the study treatment protocols. After informed consent was obtained, participants were randomized into one of two groups that were pre-determined by drawing lots (performed by E.M.F.) using a 3:3 ratio, block randomization procedure. The sequence was concealed until interventions were assigned.
Study Interventions
In this open-label trial, potential participants were asked to stop taking all of their opioid medications after the midnight before the morning that they were to be hospitalized for medical stabilization. Following hospital admission, patients were given an initial dose of 4 mg of buprenorphine sublingually after the signs of opioid withdrawal were noted by the nursing staff. Additional doses of 2 mg were given every 2 hours until the symptoms and signs of opioid withdrawal were controlled. The following day, the total amount required to control withdrawal was given in divided doses. The goals were to reduce pain to a level acceptable to the participant and to discharge the participant within 24–48 hours following admission on a stable dose of buprenorphine/naloxone (usually 2/0.5 mg 3 or 4 times daily). Before hospital discharge, the patient was approached by an investigator (L.A. or E.M.F.) who explained the study to the patient and obtained informed consent.
At the time of discharge, participants were assigned to 1 of 2 treatment conditions: the active comparator standard of care protocol for opioid discontinuation (i.e., tapering doses) or opioid replacement treatment (i.e., steady doses). Participants were also advised not to drink any alcoholic beverages, not to obtain prescriptions from other physicians for any controlled substance, not to return to taking any of their previously prescribed opioids, and not to use any illicit drug; however, they were permitted to take non-opioids (e.g., acetaminophen or non-steroidal anti-inflammatory drugs) for breakthrough pain. Prior to discharge, participants were either given or asked to make appointments to follow-up with: 1) a counselor at a chemical dependence treatment program for an “intake assessment,” 2) a pain psychologist to start a 6-week cognitive behavioral treatment, 3) a physical medicine physician for non-opioid pain management, and 4) a primary care physician for pain management with one of the buprenorphine study protocols.
Those assigned to the active comparator arm were started on tapering doses of buprenorphine that would be gradually decreased over 4 months and then all opioids were to be discontinued for 2 months (i.e., total abstinence from all opioids). During the initial 4 weeks of follow-up, participants were permitted to increase the starting dose up to a maximum total buprenorphine dose of up to 16 mg per day (divided 2 to 4 times per day). They were also permitted to opt out of the tapering protocol and initiate a steady dose schedule for opioid replacement at anytime during the first 4 months of follow-up.
Those assigned to the experimental arm were continued on a steady dose of buprenorphine at the time of hospital discharge, which was scheduled to continue for the entire 6-month follow-up; however, during the first 4 weeks following hospital discharge participants were permitted to increase the stable dose up to a maximum total buprenorphine dose of 16 mg per day (divided 2 to 4 times per day) based on clinical response. The goal of pharmacotherapy was to limit side effects by the use of the lowest dose of buprenorphine that would result in adequate control pain. The buprenorphine dose was divided 2 to 4 times per day as clinically indicated to maximize analgesia. Participants were permitted to opt out of this protocol at any time during follow-up and initiate a tapering schedule.
During the 6-month follow-up period, all participants were requested to continue to receive routine care at their expense that included: routine medical care from their personal physician, non-opioid treatment from a pain medicine specialist (e.g., non-opioid analgesics, physical therapy), and a minimum of 6 therapy sessions from a pain psychologist. They were also requested to obtain an initial evaluation from a chemical dependency counselor and encouraged to attend meetings of mutual-help programs. The pain psychologist developed a program consisting of 6 hour-long sessions once a week designed to help participants cope with chronic pain. The topics included: 1) an overview, 2) theories of pain management, 3) relaxation training, 4) activity and rest cycles, 5) coping choices and 6) attitudes and a personalized chronic pain action plan.
After the 6-month follow-up period, participants were permitted to choose one of the following final treatment plans: 1) continue an abstinence-oriented approach (i.e., non-opioid analgesics only), 2) initiate a tapering schedule that would lead to opioid discontinuation, 3) continue buprenorphine treatment, or 4) return to using their previous opioid medication.
Baseline and Follow-up Evaluations
Baseline data included demographic characteristics, medical history and substance use history. We also attempted to collect opioid use data that could be used to calculate an average daily opioid dose. Every month, participants returned for follow-up data collection that included: adherence to the buprenorphine dosing schedule, number of visits with health care providers (e.g., primary care physicians, pain management physicians, psychiatrists, psychologists, and chemical dependency counselors), attendance at mutual-help group meetings, and their previous 30-day use of alcohol, opioids, and other controlled drugs (e.g., benzodiazepines, marijuana, and cocaine). During each follow-up visit the participants were asked open-ended questions (e.g., “How have you been?”). Adherence to the buprenorphine treatment protocol was judged by tablet counts and/or interviews. For our data set, we used the results of any available urine toxicology obtained by either the treating physician (R.D.B.), the pain management physician (D.M.S.) or by a chemical dependency treatment program. All of these urine drug toxicology tests employed an immunological assay method, and therefore the ability to detect semi-synthetic opiates and synthetic opioids may have been limited.20 At the last follow-up session (6 months after enrollment), all participants were asked 2 open-ended questions: “How would you describe your overall level of pain now as compared to the time right before you started the study?” and “How would you describe your overall level of function now as compared to the time right before you started the study?” Participant comments were recorded with hand-written notes. The clinical records of the pain psychologist were reviewed to determine if the participant: 1) initiated behavioral therapy (i.e., arrived for the initial office consultation), 2) completed the “intake assessment” protocol, and 3) completed the recommended therapy (i.e., all 6 visits).
The main outcome was completion of the tapering dose or steady dose buprenorphine treatment protocols. Other outcomes of interest included: initiation of and engagement in behavioral therapy from a psychiatrist, a psychologist or a certified chemical dependency counselor; the number of drinking days, number of days of licit or illicit drug use, and the number of positive toxicology screening tests. Licit drug use days were considered to be the use of controlled substances (i.e., benzodiazepines) that were prescribed by physicians outside of the study protocol. At he 6-month follow-up, we also noted the level of pain, the level of function with daily activities, and the final treatment plan for medical management for the study participants at the end of the study.
Statistical Analysis
Baseline and treatment outcome data were collected on paper forms that were specifically designed for use with this study. These data were then entered into an electronic data base using the Statistical Package for Social Sciences (SPSS) version 16 (SPSS Inc., Chicago, IL) by an investigator (C.M.D.) who was not involved with participant enrollment or follow-up data collection. Accuracy of data entry was confirmed by comparing the original paper form with the entered data. Descriptive statistics were used to summarize the baseline characteristics of the study participants. The Fisher exact test or Student’s t-test was used for between group comparisons (2-tailed) as appropriate. The analysis of the main outcome (i.e., completion of the assigned treatment protocol) was made on an intent-to-treat basis.
RESULTS
We screened and enrolled 12 individuals (Figure 1) between December 8, 2007 and April 9, 2008. The baseline characteristics of the participants are summarized in Table 1. There were no statistically significant differences found between the two small samples. A history of substance abuse that preceded the onset of the chronic pain was common among these individuals; 4 admitted to a prior problem with only alcohol, 4 admitted to prior problems with both alcohol and illicit drugs (although none had ever used illicit drugs intravenously), and 5 had previously received treatment for a substance use disorder (See Table 2). Previous criminal problems were also common among the participants; 8 (67%) had been arrested at least once and 3 (25%) had been convicted of a crime. The participants’ estimates of prescription drug use were not considered to be reliable because most had difficulty remembering exactly how much they were taking in the 30 days prior to enrollment, gave a wide range of possible dosages, or even had trouble remembering exactly what drugs they had taken; therefore, these estimates were not included in the data analyses.
Figure 1.
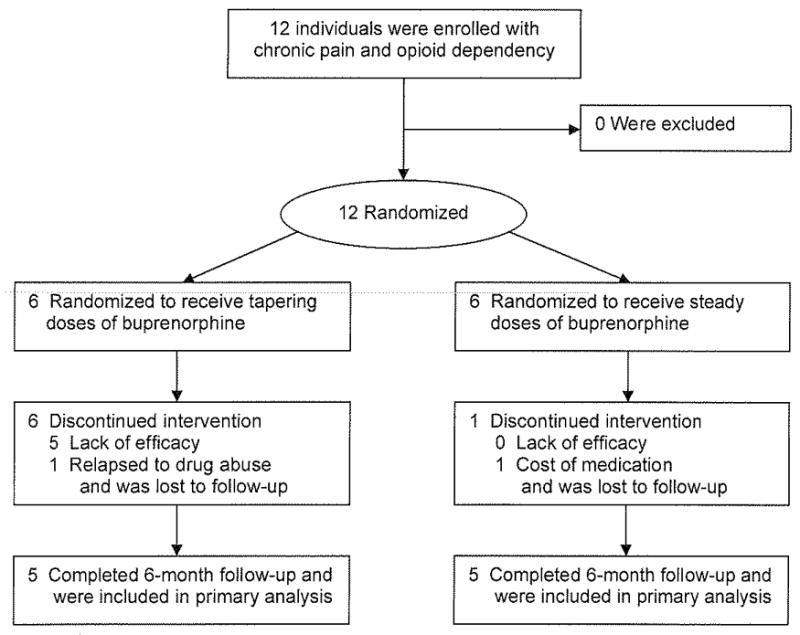
Participant Flow Diagram
Table 1.
Demographic and Other Baseline Characteristics
| Characteristic* | Tapering Doses n=6 | Steady Doses n=6 |
|---|---|---|
| Demographic | ||
| Age (SD) [range], years | 44 (6.4) [37–53] | 46 (14.6) [24–65] |
| Male gender | 2 (33%) | 4 (67%) |
| White race | 5 (83%) | 6 (100%) |
| Married | 4 (67%) | 3 (50%) |
| Some college education | 3 (50%) | 4 (67%) |
| Not working | 5 (83%) | 3 (50%) |
| SUD history | ||
| Family history of SUD | 2 (33%) | 1 (17%) |
| Prior history of alcohol abuse only | 2 (33%) | 2 (33%) |
| Prior history of alcohol and drug abuse | 2 (33%) | 2 (33%) |
| Current opioid useH | ||
| Hydrocodone | 3 (50%) | 2 (33%) |
| Oxycodone | 2 (33%) | 4 (67%) |
| Methadone | 1 (17%) | 0 (0%) |
| Morphine | 1 (17%) | 1 (17%) |
| Fentanyl | 1 (17%) | 2 (33%) |
| Treatment history | ||
| Prior spinal surgery | 2 (33%) | 4 (67%) |
| Prior SUD treatment | 2 (33%) | 3 (50%) |
| Prior mental health treatment | 4 (67%) | 1 (17%) |
| Criminal history | ||
| Any prior arrest | 3 (50%) | 5 (83%) |
| Any prior misdemeanor conviction | 0 (0%) | 0 (0%) |
| Any prior felony conviction | 0 (0%) | 3 (50%) |
| Any prior prison term | 0 (0%) | 1 (17%) |
Participant self-reported data at the time of enrollment. There were no statistically significant differences between the two study groups for any of the characteristics. Abbreviations: SD, standard deviation; SUD, substance use disorder.
Some participants were taking more than one opioid at the time of enrollment.
Table 2.
Details of Participant Enrollment Characteristics, Treatment Progress, and Outcomes.*
| Enrollment Characteristics |
Treatment Assignment and Received at the Time of the 4-Month Follow-up |
Clinical Progress during the Course of the 6 Months Follow-up |
Outcomes and Treatment Plans at the Time of 6 Month Follow-up Visit |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Prior Surgery | Prior AODA | Study Group | Protocol Received? Course of Therapy | CD/BT visits | Type of AODA use during follow-up | Toxicology +/Total (%) | PDA | Pain Level | Daily Life | Treatment arrangements made at 6 month follow-up |
| 1 | No | AODA | Taper | No, steady dose at 1 month | 0/0 | CAN, COC, OPI | 4/4 (100%) | 50 | Worse | Worse | Returned to prior opiatesH |
| 3 | No | None | Taper | No, steady dose at 2 months | 1/0 | None | 0/3 (0%) | 100 | Better | Better | Long-term maintenance |
| 5 | No | Alcohol | Taper | No, Withdrew (relapsed) | -- | BNZ, CAN, OPI | 3/3 (100%) | -- | -- | -- | Lost to follow-upI |
| 7 | Yes | Alcohol | Taper | No, steady dose at 1 month | 0/6 | CAN | 1/3 (33%) | 89 | Better | Better | Long-term maintenance |
| 8 | Yes | None | Taper | No, steady dose at 3 months | 17/1 | None | 0/7 (0%) | 100 | Better | Better | Long-term maintenance |
| 10 | No | AODA | Taper | No, steady dose at 4 months | 8/0 | None | 0/3 (0%) | 100 | Better | Better | Long-term maintenance§ |
| 2 | No | None | Steady | Yes, treatment as planned | 4/7 | BNZ | 1/2 (50%) | 96 | Better | Better | Long-term maintenance |
| 4 | Yes | AODA | Steady | Yes, treatment as planned | 0/1 | None | 0/3 (0%) | 100 | Better | Better | Long-term maintenance |
| 6 | Yes | Alcohol | Steady | Yes, treatment as planned | 0/1 | COC, EtOH | 2/3 (67%) | 62 | Worse | Better | Long-term maintenance |
| 9 | Yes | None | Steady | Yes, treatment as planned | 0/0 | CAN, COC, OPI | 2/4 (50%) | 52 | Worse | Worse | Returned to prior opiatesH |
| 11 | Yes | Alcohol | Steady | No, Withdrew (cost) | -- | -- | None | -- | -- | -- | Lost to follow-up¶ |
| 12 | No | AODA | Steady | Yes, treatment as planned | 4/0 | BNZ, EtOH, COC, OPI | 4/4 (100%) | 91 | Worse | Better | Long-term maintenance |
“Prior Surgery” denotes a history of prior spinal surgery for back pain. AODA denotes alcohol and other drugs of abuse: BNZ benzodiazepines, CAN cannabinoids, COC cocaine, EtOH alcohol, and OPI opiates. All self-reported drug used was confirmed by and identical to urine toxicology. Alcohol use was by self-report only. BT denotes a behavioral therapy session with a pain psychologist, CD denotes a chemical dependency therapy session, “Steady” denotes the opioid replacement treatment protocol with steady doses of buprenorphine, PDA denotes percent days abstinent by self-report, “Received Protocol” indicates whether the participant completed the assigned treatment intervention according to the protocol, “Taper” refers to opioid discontinuation (i.e., detoxification) treatment with a 4-month buprenorphine tapering dose protocol, and the “+” sign denotes the number of positive urine toxicology screening tests.
Participant decided that function and pain control was better on prior opiates, however, had clinical evidence of addiction behaviors (e.g., regular use of cocaine).
Participant relapsed to illicit drug use during the first follow-up month, was referred for inpatient detoxification and completed a 28-day inpatient program.
Participant delayed the onset of tapering for 2 months, but then could not tolerate a lower dose and requested opioid replacement treatment at month 4.
Participant was unable to afford buprenorphine, returned to using short-acting opioids during the second follow-up month, but considered buprenorphine to be better.
Of the 12 participants, 1 dropped out of the study and 1 relapsed to illicit drug use and was lost to follow-up. See Table 2. Of the remaining 10 participants, only 2 completed the 6-session cognitive behavioral therapy program that was recommended to help them cope with chronic pain during follow-up, 3 went to the initial evaluation sessions only and 5 did not attend any of the sessions. Similarly, most participants did not engage in other behavioral therapy for chemical dependency. Of the 10 who completed the 6-month follow-up, 1 completed 12 weeks of outpatient therapy for chemical dependency and attended meetings of a 12-Step mutual-help program regularly, 3 attended some of the initial sessions then dropped out after 1, 2 and 4 sessions respectively (one of these attended some 12-Step meetings), and 4 did not initiate any chemical dependency counseling or attend any 12-Step meetings. The other participant completed 2 weeks of inpatient chemical dependency treatment and subsequently attended 12-Step meetings regularly, completed the 6-session pain coping skills program, but did not attend any outpatient behavioral therapy sessions at a chemical dependency treatment program.
Among the 12 participants, the mean stable dose of buprenorphine at the time of hospital discharge was 7.5 mg per day (range: 6–16 mg per day) that was taken in divided doses 2 to 4 times per day; and at the end of the first 4 weeks of follow-up, the mean final dose of buprenorphine for the 11 participants still in the study was 9.8 mg per day (range: 4–16 mg per day) that was taken in divided doses 2 to 3 times per day. Anecdotally, participants indicated that divided doses of buprenorphine provided superior pain control as compared to once-a-day dosing.
Participant recruitment and enrollment was terminated early before the 60-participant target was achieved because none of the 6 participants in the tapering dose arm (the “active comparator” arm) could successfully complete the 6-month protocol (i.e., 4 months of buprenorphine tapering followed by 2 months of opioid abstinence). Of these 6 participants, 5 could not complete the 4-month taper and requested to be given buprenorphine replacement therapy for pain control during the first month (2 participants), second month (1 participant), third month (1 participant) or forth month (1 participant); and 1 was admitted to a “28-day” inpatient chemical dependency treatment program following a relapse to illicit drug use during the second month and then was lost to follow-up. Of the 6 assigned to the 6-month opioid replacement protocol, 5 completed the 6-month protocol and 1 withdrew during the second month due to the cost of buprenorphine. This difference between the 2 treatment conditions (0/6 vs. 5/6) in terms of the primary outcome (i.e., completion of the 6-month treatment protocol) was significant (P = 0.015). In addition to this statistical finding, the physicians involved in the study observed that those assigned to receive steady doses of buprenorphine seemed to do extremely well clinically, whereas those assigned to the tapering protocol appeared to be “miserable” (in the words of one participant). Thus, the physicians involved in clinical aspects of the study determined that they could no longer ethically recruit patients into a study that involved a tapering dose (i.e., opioid discontinuation) protocol. Since all the participants who completed the study eventually received steady doses of buprenorphine, an intent-to-treat analysis of the other 6-month outcomes was not appropriate.
Of the 12 participants who enrolled in the study, 4 returned to using their prior opioid. Of these 4, 1 participant did so illicitly, and 2 did so by licit prescriptions due to inadequate pain control by buprenorphine; although the remaining participant was doing well on buprenorphine, this participant requested to return to prior opioids due to the cost of buprenorphine (i.e., lack of insurance coverage), but at doses less than prior to study enrollment. Of the 10 participants who completed the 6-month follow-up, 8 were receiving opioid replacement treatment, and 6 reported improved pain control and physical functioning. One of the 4 participants who reported worsening pain sustained a work-related back injury during follow-up. Although 5 participants initiated counseling specifically for their addiction disorder and 5 initiated pain management behavioral therapy, only 4 (40%) participants (2 for each type) continued with therapy beyond the initial intake evaluation procedure. Details are provided in Table 2.
As shown in Table 2, alcohol and drug use by the participants was not uncommon during the 6 months of follow-up; only 4 were able to abstain completely from alcohol and drugs. Those who used cocaine during the follow-up period did not do as well as those who did not use cocaine; all 4 who used cocaine indicated that their pain was worse at the end of the study compared to none of the 6 who did not use cocaine, which was significant (P = 0.005). Interestingly, 2 of these 4 elected to return to the use of their previous opioid medications, because they claimed that those medications controlled their pain better than buprenorphine.
We also noted subjective improvements in clinical outcomes that were not adequately reflected in the numerical outcome data that we collected. Responses to the open-ended questions indicated that those assigned to opioid replacement treatment appeared well and frequently noted dramatic improvements in function.
DISCUSSION
These study participants with chronic non-cancer pain and co-existent opioid addiction were more likely to adhere to steady doses of buprenorphine for opioid replacement therapy than tapering doses of buprenorphine for opioid discontinuation therapy. None of the 6 participants in the tapering arm could successfully complete the 6-month protocol (i.e., 4 months of tapering doses and 2 months of non-opioid treatment); and of these, 5 were switched to steady doses of sublingual buprenorphine, and 1 was admitted to an inpatient chemical dependency treatment program. Of the 6 assigned to the opioid replacement, 5 completed the 6-month protocol although 1 withdrew from the study due to financial considerations. Because this difference in treatment retention between the 2 treatment conditions was significant, study enrollment was terminated early (i.e., lack of efficacy of the tapering dose arm).
The mean final dose of buprenorphine for the participants was 9.8 mg per day (range: 4–16 mg per day) that was taken in divided doses 2 to 3 times per day. This suggests that as compared to the doses of buprenorphine that are used for the maintenance therapy of illicit opioid dependence (e.g., due to heroin), lower doses of buprenorphine can be used for this population with chronic pain if a “split dosing” strategy is used.
Most of the participants did not engage in behavioral therapy for their addiction disorder; although 8 initiated behavioral therapy, only 3 continued with therapy beyond 3 sessions. Finding ways to engage these patients in treatment with behavioral health professionals will be a challenge for physicians. This is important because the authors of a systematic review concluded that there is “moderate evidence of the positive effectiveness” of multidisciplinary teams for subacute low back pain.21 In particular, psychiatric treatment is “modestly effective” for reducing chronic back pain.22
There are several limitations to this preliminary study. First, the participants of this study may not be representative of other types of chronic pain patients. For example, prior treatment for a substance use disorder and at least 1 prior arrest were common among the participants of this study. It is not clear if the findings of this study would apply to patients without a prior history of substance abuse or legal problems. Second, the small sample size limited our ability to identify sub-groups of patients (e.g., those with no prior treatment for substance abuse or with no prior arrests) who might do well with non-opioid pain management. Third, this was an open label trial without a placebo. The outcomes might have been different if a placebo had been used and if the treatment conditions were masked to the participants. Fourth, we were not able to collect urine for toxicology every month on every participant. We might have observed higher rates of relapse if sampling was more complete. Fifth, it is not clear whether outcomes would have been better in the opioid discontinuation group if they had engaged in behavioral therapy or if the buprenorphine dosing schedule were different. Sixth, the follow-up interval may be too narrow. It is not clear if steady doses of buprenorphine would continue to be beneficial beyond 6 months. Finally, the study could have been improved if we were able to collect better baseline psychometric data, more detailed information about pre-enrollment doses of prescription and illicit drug use and more objective data about the quality of life and level of pain outcomes during follow-up.
Contrary to the current practice guidelines that recommend opioid discontinuation, we found that it was quite difficult to wean opioids among those with chronic non-cancer pain and co-existent opioid addiction. Despite the limitations of the study, opioid replacement pharmacotherapy using steady doses of buprenorphine may have an important role to play in the management of patients with chronic non-cancer pain and coexistent opioid addiction.
Acknowledgments
This study was supported, in part, by a grant (K23 AA 015616) from the National Institute on Alcohol Abuse and Alcoholism (R.D.B. and L.A.), by a grant (No. 29926) from the Donald W. Reynolds Foundation (A.L.Z.), and by a grant (No. 1060512-1-35905) from the University of Buffalo Interdisciplinary Research Fund (E.M.F.) The authors are grateful for the help of Lisa A. Keenan, PhD and Andy Danzo for their assistance in the preparation of this manuscript.
Footnotes
Trial Registration: clinicaltrials.gov Identifier, NCT00552578
References
- 1.Joranson DE, Leitman R. The McNeil National Pain Study. New York: Louis Harris and Associates; 1994. [Google Scholar]
- 2.Turk DC, Okifuji A, Kaluaokalani D. Clinical Outcome and Economic Evaluation of Multidisciplinary Pain Centers. In: Block AR, Kremer EF, Fernandez E, editors. Handbook of Pain Syndromes: Biopsychosocial Perspectives. Mahwah, NJ: Erlbaum; 1999. [Google Scholar]
- 3.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146:116–127. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Brown KR, Singh V. National All Schedules Prescription Electronic Reporting Act (NASPER): balancing substance abuse and medical necessity. Pain Physician. 2002;5:294–319. [PubMed] [Google Scholar]
- 5.Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain. 2008;24:469–478. doi: 10.1097/AJP.0b013e31816b2f26. [DOI] [PubMed] [Google Scholar]
- 6.Veterans Health Administration, Department of Defense. Washington (DC): Veterans Health Administration, Department of Defense; 2003. [Accessed April 24, 2009]. VA/DoD clinical practice guideline for the management of opioid therapy for chronic pain. Available at: http://www.guideline.gov/summary/summary.aspx?ss=15&doc_id=4812&nbr=003474&string=chronic+AND+pain. [Google Scholar]
- 7.Fingerhood AI. Illicit and therapeutic use of drugs with abuse liability. In: Barker LR, Burton JR, Zieve PD, editors. Principles of Ambulatory Medicine. 5. Philadelphia: Lippincott, Williams & Wilkins; 1999. pp. 264–286. [Google Scholar]
- 8.Savage SR. Opioid medications in the management of pain. In: Graham AW, Schultz TK, Mayo-Smith MF, Ries RK, Wilford BB, editors. Principles of Addiction Medicine. 3. Chevy Chase, MD: American Society of Addiction Medicine; 2003. pp. 1451–1463. [Google Scholar]
- 9.Altier N, Dion D, Boulanger A, Choiniere M. Management of chronic neuropathic pain with methadone: a review of 13 cases. Clin J Pain. 2005;21:364–369. doi: 10.1097/01.ajp.0000125247.95213.53. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy JA, Crowley TJ. Chronic pain and substance abuse: a pilot study of opioid maintenance. J Subst Abuse Treat. 1990;7:233–238. doi: 10.1016/0740-5472(90)90046-s. [DOI] [PubMed] [Google Scholar]
- 11.Tennant FS, Jr, Rawson RA. Outpatient treatment of prescription opioid dependence: comparison of two methods. Arch Intern Med. 1982;142:1845–1847. [PubMed] [Google Scholar]
- 12.Rhodin A, Gronbladh L, Nilsson LH, Gordh T. Methadone treatment of chronic non-malignant pain and opioid dependence--a long-term follow-up. Eur J Pain. 2006;10:271–278. doi: 10.1016/j.ejpain.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Heel RC, Brogden RN, Speight TM, Avery GS. Buprenorphine: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1979;17:81–110. doi: 10.2165/00003495-197917020-00001. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29:297–326. doi: 10.1016/j.jpainsymman.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Kakko J, Svanborg KD, Kreek MJ, Heilig M. 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: a randomised, placebo-controlled trial. Lancet. 2003;361(9358):662–668. doi: 10.1016/S0140-6736(03)12600-1. [DOI] [PubMed] [Google Scholar]
- 16.Bridge TP, Fudala PJ, Herbert S, Leiderman DB. Safety and health policy considerations related to the use of buprenorphine/naloxone as an office-based treatment for opiate dependence. Drug Alcohol Depend. 2003;70(2 Suppl):S79–85. doi: 10.1016/s0376-8716(03)00061-9. [DOI] [PubMed] [Google Scholar]
- 17.Torrington M, Domier CP, Hillhouse M, Ling W. Buprenorphine 101: treating opioid dependence with buprenorphine in an office-based setting. J Addict Dis. 2007;26(3):93–99. doi: 10.1300/J069v26n03_10. [DOI] [PubMed] [Google Scholar]
- 18.Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–384. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- 19.Diagnosis and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 20.Heit HA, Gourlay DL. Urine drug testing in pain medicine. J Pain Symptom Manage. 2004;27:260–267. doi: 10.1016/j.jpainsymman.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Karjalainen KA, Malmivaara A, van Tulder M, et al. Multidisciplinary biopsychosocial rehabilitation for subacute low-back pain among working age adults. The Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD002193. [DOI] [PubMed] [Google Scholar]
- 22.Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80(1–2):1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]


