Abstract
Interactions between melanocytes and neighboring cells in the skin are important in regulating skin color in humans. We recently demonstrated that the less pigmented and thicker skin on the palms and soles is regulated by underlying fibroblasts in those areas, specifically via a secreted factor (DKK1) that modulates Wnt signaling. In this study, we tested the hypothesis that dermal fibroblasts regulate the constitutive skin color of individuals ranging from very light to very dark. We used microarray analysis to compare gene expression patterns in fibroblasts derived from lighter skin types compared to darker skin types, with a focus on secreted proteins. We identified a number of genes that differ dramatically in expression and, among the expressed proteins, neuregulin-1, which is secreted by fibroblasts derived from dark skin, effectively increases the pigmentation of melanocytes in tissue culture and in an artificial skin model and regulates their growth, suggesting that it is one of the major factors determining human skin color.
Keywords: Pigmentation, Skin, Melanocyte, Fibroblast, Color
Introduction
The constitutive color of human skin varies widely among different ethnic groups and is frequently classified into one of six distinct skin phototypes (Fitzpatrick, 1988), the most commonly used method to categorize skin color depending on its ability to tan or to burn following exposure to ultraviolet (UV) radiation. UV stimulates the expression and function of many melanocyte-specific proteins, such as tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), tyrosinase-related protein 2 (DCT), and melanoma antigen recognized by T-cells 1 (MART1), and those increases are typically more dramatic in darker skin types than in lighter skin types (Miyamura et al., 2007; Tadokoro et al., 2005). Despite that diversity, the density of melanocytes in all types of skin is virtually identical (Yamaguchi et al., 2006; Yamaguchi and Hearing, 2005) and the differences in visible skin color depend on the amount of melanin produced, the efficiency of melanine transfer from melanocytes to keratinocytes, the ratio of pheomelanin to eumelanin synthesized, etc. (Miyamura et al., 2007; Rees, 2004; Sturm, 2006; Wakamatsu et al., 2006; Yamaguchi et al., 2007a). Levels of constitutive skin pigmentation not only have dramatic social and cosmetic consequences but also are closely and inversely correlated with the risk of skin cancers.
Many studies have shown that interactions between melanocytes and adjacent skin cells, such as keratinocytes in the epidermis and fibroblasts in the dermis, are important in the regulation of melanocyte function and consequent skin pigmentation. Many paracrine factors secreted from keratinocytes and fibroblasts, such as stem cell factor (SCF), hepatocyte growth factor (HGF), interferon-γ (IFN-γ), endothelin-1 (ET-1), basic fibroblast growth factor (bFGF), interleukin-1 (IL-1) and granulocyte-macrophage colony-stimulating factor (GM-CSF), have been identified that are involved in the regulation of skin pigmentation after UV exposure (for reviews, see Imokawa, 2004; Miyamura et al., 2007; Yamaguchi et al., 2007a). It was recently reported that the hypopigmentation of palmoplantar skin (on the palms and soles) compared to nonpalmoplantar (trunk) skin in humans is regulated by dermal fibroblasts in those areas, specifically via a secreted factor (Dickkopf-related protein 1; DKK1) that regulates Wnt signaling (Yamaguchi et al., 2004; Yamaguchi et al., 2007b; Yamaguchi et al., 2008). Furthermore, several other studies have shown the influence of dermal fibroblasts on human epidermal pigmentation (Cario-Andre et al., 2006; Hedley et al., 2002). Therefore, we hypothesized that fibroblasts in the dermis of trunk skin play important functions in regulating the constitutive color of different phototypes of skin and their responses to the environment, particularly via the factors they secrete.
In this study, we used cDNA microarray analysis to identify novel melanogenic factors secreted from fibroblasts derived from human trunk skin of different phototypes to test the hypothesis that they play an important role in regulating constitutive levels of pigmentation in human skin. We demonstrate that one of the secreted factors, neuregulin-1 (NRG-1), which is highly expressed by fibroblasts derived from darker skin, significantly increases pigmentation in a reconstructed skin model and in cultured human melanocytes, suggesting its potential role in regulating constitutive human skin color and perhaps its dysfunction in pigmentary skin diseases.
Results
Microarray analysis of expression patterns of fibroblasts from different skin types
We analyzed the gene expression patterns of 15 different primary fibroblast cell lines derived from skin biopsies from the lower back skin of individuals of three different skin types (five each from skin phototypes I, III and VI) using the Operon V3.0 human whole genome spotted microarray platform. For further analysis, we filtered 11,771 out of 36,288 spots with at least half of the corresponding intensity measures (CIMs) less than the mean of the background signal of the array plus three times the standard deviation of the background. Data were then processed by subtracting background signals, conducting loess within-array and quantile between-array on the common reference channel normalization. However, clustering analysis did not yield strong evidence of distinct gene expression profiles among the three skin types. Supplementary material Fig. S1 shows the CIMs generated using spots with an interquartile range of >1.5.
Expression of known fibroblast-derived melanogenic paracrine factors
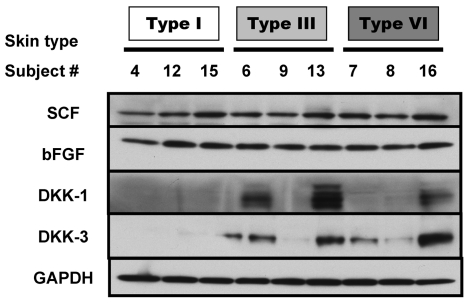
We examined fibroblast-derived melanogenic paracrine factors that were identified in earlier studies to see if any of them were regulated differently depending on the skin phototypes from which the fibroblasts had been derived (Fig. 1). SCF and bFGF are well-known melanogenic paracrine factors, especially with respect to responses to UV exposure (Grichnik et al., 1998; Hachiya et al., 2001), but neither of them showed different expression patterns in the various skin types. DKK1 is a paracrine factor responsible for topographical differences of skin color in palm versus trunk skin (Yamaguchi et al., 2004), but although there was quite a variation in expression patterns of DKK1 and the related DKK3, neither of those showed expression patterns that consistently correlated with skin phototype.
Fig. 1.
Protein expression levels of known fibroblast-derived melanogenic paracrine factors. Proteins were extracted from each of the 15 fibroblast cell lines, and western blotting was used to detect levels of SCF, bFGF, DKK-1, DKK-3, and GAPDH (used as a loading control). Three representative fibroblast lines for each of three skin types are shown here.
Enrichment of secreted genes in differential expression analysis between type I or III and type VI skins
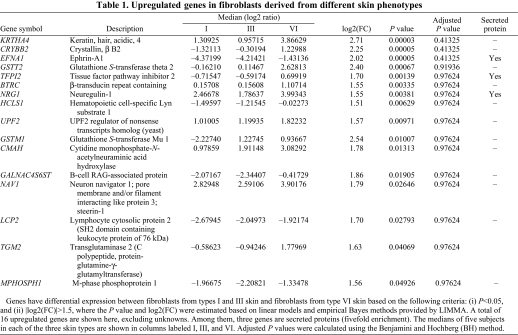
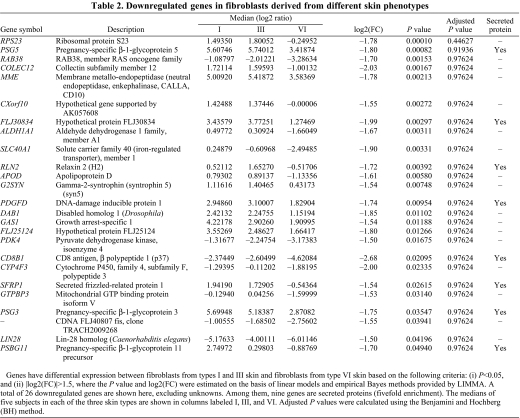
We previously reported that fibroblasts regulate the distinctive pigmented and thick morphology of skin on the palms and soles compared to the remainder of the body via a factor they secrete termed DKK1 (Yamaguchi et al., 2004; Yamaguchi et al., 2007b; Yamaguchi et al., 2008). In a preliminary study, we found that fibroblasts derived from type I or type III skin significantly inhibit the pigmentation of MelanoDerm skin, whereas fibroblasts derived from type VI skin have lesser effects (Choi et al., 2008) (summarized in supplementary material Table S1). Therefore, we hypothesized that fibroblasts might play important roles in regulating the constitutive skin color of different phototypes via their secreted factors. Of the 24,517 genes used for analysis, there are 1178 genes that are secreted (according to the UniProtKB keyword descriptions). Among the total of 50 genes having differential expression between fibroblasts from type I or III skin and fibroblasts from type VI skin, based on the following criteria: (i) P<0.05, and (ii) |log2(FC)|>1.5, 12 of them encoded secreted proteins (fivefold enrichment). Tables 1 and 2 list the detailed expression information on 41 out of these 53 genes (16 upregulated genes and 25 downregulated genes), excluding 12 unknowns. The full microarray database is available at GEO (GSE22022).
Table 1.
Upregulated genes in fibroblasts derived from different skin phenotypes
Table 2.
Downregulated genes in fibroblasts derived from different skin phenotypes
Expression of NRG-1 in normal human skin and in cultured normal human fibroblasts
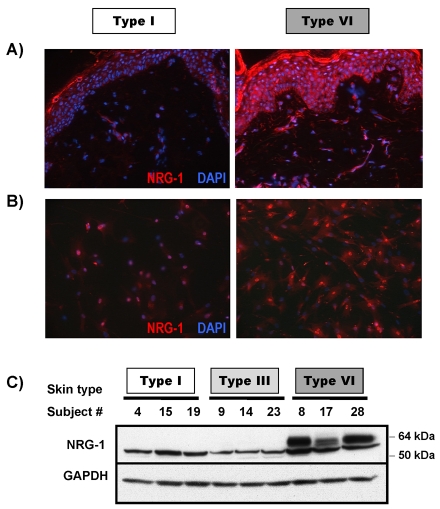
Among the 12 secreted genes that were differentially regulated in fibroblasts from skin type VI compared to skin types I and III (as noted in Tables 1 and 2), NRG-1 was one of the most highly upregulated in fibroblasts derived from type VI skin. Because NRG-1–ErbB signaling has been recently reported to affect melanocyte development and melanoma migration (Gordon-Thomson et al., 2005; Buac et al., 2009), the result was of immediate interest. To validate the expression of NRG-1 by fibroblasts at the protein level, we used immunohistochemistry to examine the expression of NRG-1 in normal human skin samples from types I and VI skin (Fig. 2A). We found that NRG-1 was highly expressed throughout the epidermis as well as in the dermis of type VI skin, but there was no significant expression of NRG-1 in the epidermis and only a very low level of NRG-1 expression in the dermis of type I skin. When fibroblasts derived from skin types I and VI were immunohistochemically stained for NRG-1 (Fig. 2B) using two different antibodies, both of them detected high levels of NRG-1 in most fibroblasts from the type VI skin, both in the cytoplasm and in the nuclei whereas fibroblasts from type I skin showed very low levels of NRG-1 expression and only in a few cells. The localization of NRG-1 in the nuclei has been previously observed in other cell types (Breuleux et al., 2006; Golding et al., 2004; McClell and Gullick, 2009) and whether it serves a function there is currently unknown. Western blotting of NRG-1 in the cell extracts showed that the 60-kDa isoform of NRG-1 is only expressed in fibroblasts derived from type VI skin, but not in fibroblasts derived from type I or III skin (Fig. 2C).
Fig. 2.
Expression of NRG-1 in normal human skin and in cultured normal human fibroblasts. (A) Frozen sections of normal human skin from type I and type VI phototypes were used to examine expression levels of NRG-1. Images are representative of three different subjects of each phototype, which all showed similar results. (B) Fibroblasts were cultured in chamber-slides and stained with NRG-1 antibody. Images are representative of three different fibroblast cell lines of each phototype, which all showed similar results. (C) Proteins were extracted from each of 15 fibroblast cell lines and western blotting was used to detect the level of NRG-1 and GAPDH (used as a loading control). Three representative fibroblast lines for each of three skin types are shown.
We also characterized the expression of three other secreted proteins identified in the microarray analysis (PEDF, TIMP3 and calnexin) but none of them showed any difference in expression that correlated with skin type.
Effect of NRG-1 on the pigmentation of 3D reconstructed skin
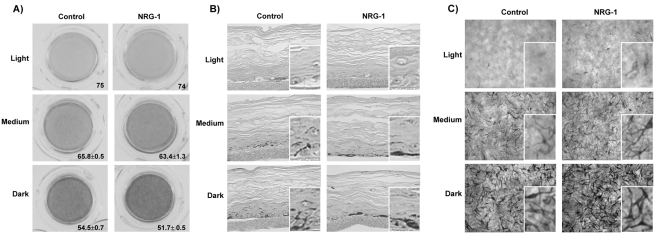
We then used three-dimensional (3D) reconstructed human skin models (termed MelanoDerms) to test the effects of NRG-1 on skin pigmentation. Three types of MelanoDerm, ‘light’ (from Caucasian skin), ‘medium’ (from Asian skin) and ‘dark’ (from African-American skin), were used to compare the effects of NRG-1 on those three types of skin. From preliminary experiments to optimize the concentration of NRG-1 (concentrations of 10–200 ng/ml were tested, supplementary material Fig. S2), 50 ng/ml was chosen, which was consistent with concentrations of NRG-1 used for other cell types by other groups (Carraway et al., 1999; Stove et al., 2005). NRG-1 was added to the MelanoDerm media at 50 ng/ml and the medium was replenished every 2 days. On day 9, we compared the MelanoDerms treated with NRG-1 to controls treated only with vehicle. Pigmentation of the skin was assessed using the L value; higher L values indicating lighter colors, as previously reported (Coelho et al., 2009). The dark MelanoDerms treated with NRG-1 showed increases in pigmentation (ΔL=−3) (Fig. 3A), whereas the effects of NRG-1 on the medium and light MelanoDerms were less, although increases in pigmentation occurred (ΔL=−2 and −1, respectively). It should be noted that relatively small changes in ΔL can represent relatively large changes in visible skin color. For example, the mean L value of White/Caucasian skin is 67, that of Asians and Pacific-Islanders is 65, that of Hispanic/Latinos is 64, and that of American Indians and Alaska Natives is 62 (Beer and Hearing, 2007). Cross-sections of those tissues revealed that most of the melanin pigment in the NRG-1-treated MelanoDerms resided in the basal layer of the epidermis, and had particularly accumulated around the melanocytes (Fig. 3B). In the vehicle-treated control MelanoDerms, melanin pigment was more evenly distributed throughout the entire epidermis and no visible melanin accumulation was observed around melanocytes (Fig. 3B). When melanocyte morphology was examined using bright field microscopy, MelanoDerms treated with NRG-1 showed more abundant cytoplasm with thicker dendrites compared to the vehicle-treated control MelanoDerms (Fig. 3C). Therefore, NRG-1 leads to visible increases in pigmentation in the MelanoDerms, which was greater in skins with more constitutive pigmentation.
Fig. 3.
Effect of NRG-1 on the pigmentation of 3D reconstructed skins. (A) Light (from Caucasian skin, n=1), medium (from Asian skin, n=4) and dark (from African-American skin, n=2) MelanoDerms were treated with or without NRG-1 (50 ng/ml) for 9 days. Images were taken of the inverted MelanoDerms at the end of the protocol. The L value shown for each MelanoDerm was calculated as the average of three spots measured in the center of each MelanoDerm; results are the averages of L values in the number of experiments indicated above ± s.d. (B) Melanin content in human skin equivalents analyzed by Fontana–Masson staining; insets show areas at threefold magnification. (C) Overhead bright field microscopic view of MelanoDerms; insets show areas at threefold magnification.
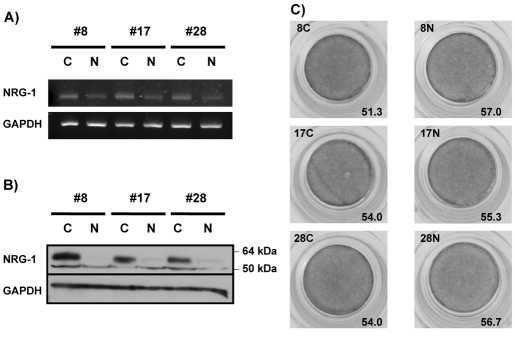
We also examined the effect of NRG-1 knockdown in fibroblasts derived from type VI skin using shRNA transduction. Fig. 4A shows that NRG-1 mRNA was decreased significantly by the NRG-1 shRNA, and Fig. 4B shows that 60-kDa NRG-1 protein, which is only expressed in fibroblasts derived from type VI skin (Fig. 2C), was successfully knocked down as well. When dark MelanoDerms were co-cultured with fibroblasts with knockdown of NRG-1, the pigmentation level was significantly lower than MelanoDerms co-cultured with fibroblasts transduced with control shRNA (Fig. 4C). These results further support the suggestion that NRG-1 secreted from fibroblasts derived from type VI skin regulates the constitutive level of pigmentation in type VI skin.
Fig. 4.
Effect of NRG-1 knockdown by shRNA transduction. (A) mRNA levels and (B) protein levels of NRG-1 in three different fibroblasts (#8, 17, and 28) from type VI skin after control shRNA (C) or NRG-1 shRNA (N) transduction. (C) Change of MelanoDerm pigmentation when fibroblasts were transduced with control shRNA or NRG-1 shRNA. L values shown are the average of three spots measured in the center of each MelanoDerm.
Effect of NRG-1 on the proliferation and pigmentation of human melanocytes
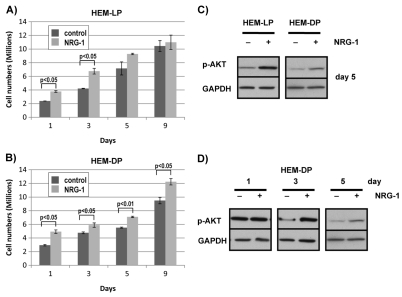
NRG-1 is known to activate intracellular signaling pathways that affect proliferation, apoptosis, and differentiation in many different types of cells (Esper et al., 2006; Falls, 2003). We thus examined the effects of NRG-1 on the proliferation of human epidermal melanocytes (HEM). Melanocytes derived from light skin (HEM-LP) as well as those derived from dark skin (HEM-DP) were used to compare the effects of NRG-1 on different types of melanocytes. When NRG-1 (50 ng/ml) was added to the melanocyte medium, cell numbers were significantly higher as early as day 1, both for HEM-LP and for HEM-DP and that increase continued until they become fully confluent (Fig. 5A,B). The phosphatidylinositol-3-kinase–Akt pathway is one of the pathways known to be involved in NRG-1-mediated effects on proliferation (Esper et al., 2006; Falls, 2003). Therefore, we examined the phosphorylation level of Akt in NRG-1-treated melanocytes compared to controls. In both HEM-LP and HEM-DP, levels of phosphorylated Akt (Akt-P) were significantly higher in NRG-1-treated cells on day 5 (Fig. 5C). We then examined the time course of Akt-P levels after treatment with NRG-1. Levels of Akt-P were equally high in the NRG-1-treated HEM-DP and in the control cells on day 1 when the cells were at a low confluency (~15%). However, the level of Akt-P had significantly decreased in the control by day 3, whereas the NRG-1-treated cells maintained higher levels of Akt-P through day 5 (Fig. 5D).
Fig. 5.
Effect of NRG-1 on the proliferation of human melanocytes in culture. (A) Lightly pigmented (HEM-LP) and (B) darkly pigmented (HEM-DP) melanocytes were treated with or without NRG-1 (50 ng/ml) for 1, 3, 5, or 9 days in 10-cm culture dishes, and the total cell numbers counted. Results are the averages of three experiments ± s.d. (C) Phosphorylated Akt (p-AKT) levels were detected by western blotting for HEM-LP and HEM-DP treated with NRG-1 (50 ng/ml) for 5 days compared to vehicle-treated controls; GAPDH was used as a loading control. (D) HEM-DP were treated with NRG-1 (50 ng/ml) for 1, 3, or 5 days and phosphorylated Akt levels were detected by western blotting compared to vehicle-treated controls; GAPDH was used as a loading control. Results shown are representative of three experiments; statistically significant differences are indicated.
We also examined the effects of NRG-1 on the pigmentation of human melanocytes. In HEM-LP, the level of pigmentation (melanin/protein) was not significantly different between the NRG-1-treated cells and the control cells from days 1 through 5, when the cells were subconfluent and were still actively proliferating. However, on day 9, when the cells had reached confluence, the pigmentation level of NRG-1-treated HEM-LP was about 35% higher than the control (Fig. 6A). In the HEM-DP, the pigmentation level of NRG-1-treated cells was higher than the controls starting at day 5 (Fig. 6B) when the cells were still subconfluent and remained higher at day 9.
Fig. 6.
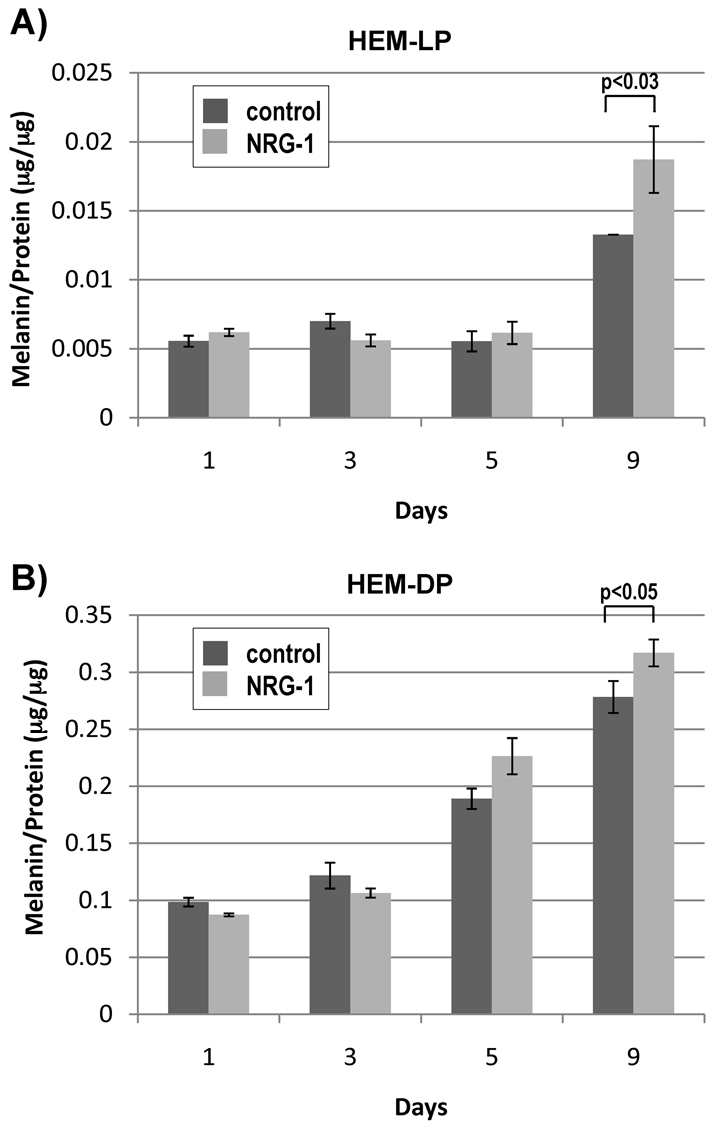
Effect of NRG-1 on the pigmentation of cultured human melanocytes. (A) Lightly pigmented (HEM-LP) and (B) Darkly pigmented (HEM-DP) melanocytes were treated with or without NRG-1 (50 ng/ml) for 1, 3, 5, or 9 days and proteins were extracted from each sample. After centrifugation at 10,000 g for 15 minutes, precipitates were analyzed for melanin content, and the supernatants were analyzed for protein content. Results are the average of three experiments ± s.d.; statistically significant differences are indicated.
NRG-1 receptors expressed by human melanocytes
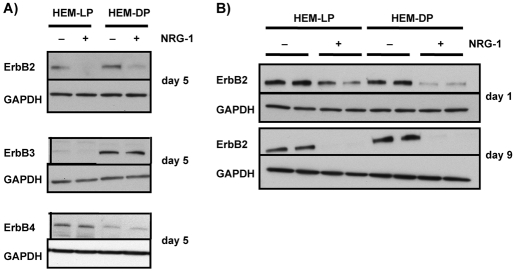
NRG-1 is a ligand for the epidermal growth factor receptor (EGFR) family receptors, ErbB3 and ErbB4 (Esper et al., 2006; Stove et al., 2003). EGFR (ErbB1) is not expressed by human melanocytes (Gordon-Thomson et al., 2001; Gordon-Thomson et al., 2005) (also confirmed in our study), and NRG-1 cannot directly bind to ErbB2 (Carraway et al., 1999; Graus-Porta et al., 1997; Karunagaran et al., 1996). Thus, we examined the levels of ErbB3 and ErbB4 (as well as ErbB2) in HEM-LP and in HEM-DP. ErbB3 is highly expressed by HEM-DP and its expression level was not affected by treatment with NRG-1 (Fig. 7A). By contrast, we were able to detect only trace levels of ErbB3 in HEM-LP and there was no detectable change in that expression elicited by NRG-1. On the other hand, ErbB4 was highly expressed in the HEM-LP, but its expression level was very low in the HEM-DP (Fig. 7A). Levels of ErbB4 expression also did not change after treatment with NRG-1. Interestingly, the expression of ErbB2, a co-receptor for NRG-1 signaling, which was similarly expressed in untreated controls of both types of melanocytes, was almost completely undetectable after 5 days of treatment with NRG-1 both in HEM-LP and in HEM-DP. We thus examined the time course of effects of NRG-1 on ErbB2 expression in more detail. Only 1 day of treatment with NRG-1 was sufficient to significantly decrease the level of ErbB2 in both types of melanocytes (Fig. 7B), and ErbB2 expression had completely disappeared by day 3 (data not shown). This effect lasted until day 9 when the cells were almost 100% confluent.
Fig. 7.
Expression of ErbBs after NRG-1 treatment. (A) HEM-LP and HEM-DP were treated with or without NRG-1 (50 ng/ml) for 5 days, and levels of ErbB2, ErbB3, or ErbB4 were detected by western blotting. Results shown are representative of three experiments. (B) Expression of ErbB2 was examined in HEM-LP and in HEM-DP after 1 or 9 days or treatment with NRG-1 compared to vehicle-treated controls; the results are representative of two independent experiments.
We further investigated whether the decrease in ErbB2 level elicited by NRG-1 is due to transcriptional regulation or to proteasomal degradation. When we measured levels of ErbB2 mRNA after 1 day of treatment with NRG-1, NRG-1-treated cells and controls were similar both for HEM-LP and for HEM-DP (Fig. 8A), despite the marked decrease in ErbB2 protein expression noted above. When the cells were treated with NRG-1 together with MG132 (120 nM), which inhibits proteasomal function (Ando et al., 2004; Ando et al., 2006), the level of ErbB2 protein returned to the control level, which suggests that NRG-1 promotes the proteasomal degradation of ErbB2 (Fig. 8B).
Fig. 8.
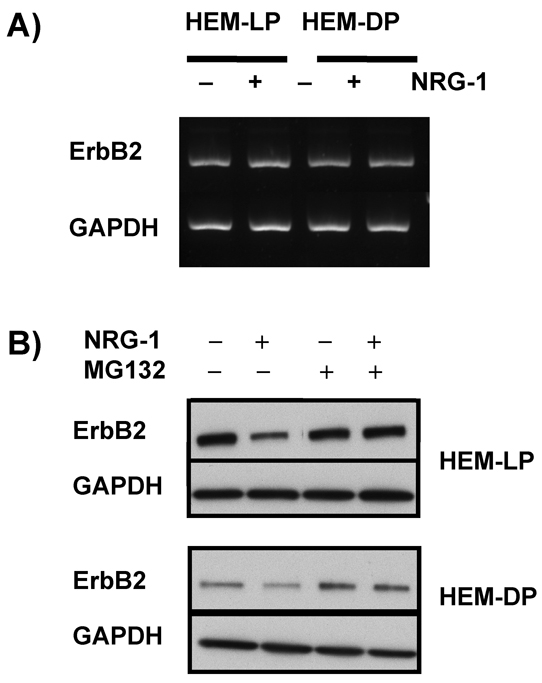
Mechanism of ErbB2 abrogation after NRG-1 treatment. (A) ErbB2 mRNA levels in HEM-LP and in HEM-DP were detected by RT-PCR after treatment with or without NRG-1 (50 ng/ml) for 1 day. (B) MG132 (120 nM) was added to the melanocyte culture medium to inhibit proteasomal degradation during treatment of HEM-LP and HEM-DP with or without NRG-1 (50 ng/ml). Proteins were extracted for each sample, and ErbB2 levels were detected by western blotting. Results are representative of three experiments.
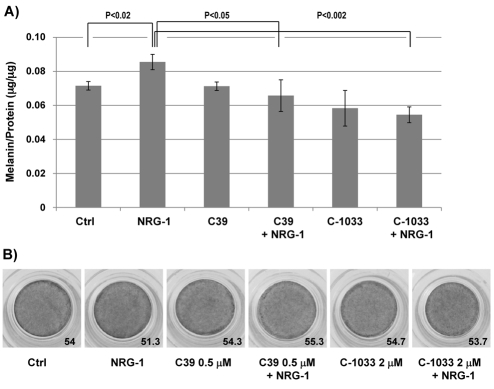
Finally, to confirm the involvement of ErbB receptors in the action of NRG-1, we used two different pan-ErbB inhibitors (C39 and CI-1033) (Ako et al., 2007; Dilworth et al., 2008; Klutchko et al., 2006) to see if they would reverse the effect of NRG-1 in HEM-DP and/or in dark MelanoDerms. C39 (0.5 μM) or CI-1033 (2 μM) alone did not affect the melanin content of HEM-DP, whereas NRG-1 alone stimulated melanin content as expected (Fig. 9). However, when cells were treated with NRG-1 in the presence of either ErbB inhibitor, the melanin content remained at the same level as the control. Similarly, when dark MelanoDerms were treated with either of those ErbB inhibitors, NRG-1 treatment did not increase the pigmentation of MelanoDerms compared to the control. Therefore, ErbB receptors seem to be crucial for the action of NRG-1 on melanocytes.
Fig. 9.
Effect of pan-ErbB inhibitors on NRG-1 action on human melanocytes and MelanoDerms. (A) HEM-DP were treated for 9 days with 50 ng/ml NRG-1 in the presence of C39 (0.5 μM) or CI-1033 (2 μM) or vehicle (DMSO) alone. The results are the average of three independent experiments ± s.d. (B) Dark (from African-American skin) MelanoDerms were treated for 9 days with 50 ng/ml NRG-1 in the presence of C39 (0.5 μM) or CI-1033 (2 μM) or vehicle (DMSO) alone. L values shown are the average of three spots measured in the center of each MelanoDerm.
Discussion
Human skin pigmentation is regulated by complex and intricate interactions between melanocytes and keratinocytes in the epidermis and fibroblasts in the dermis. A number of factors secreted from keratinocytes and/or from fibroblasts have been shown to be involved in regulating skin pigmentation after UV exposure (for reviews, see Imokawa, 2004; Miyamura et al., 2007; Yamaguchi et al., 2007a), and our group previously identified DKK-1 as an important regulator of topological skin color (palmoplantar versus non-palmoplantar skin) (Yamaguchi et al., 2004; Yamaguchi et al., 2007b; Yamaguchi et al., 2008). The gene expression patterns of fibroblasts in different anatomical locations are remarkably distinct and are also stable, even after the cells are put into culture (Chang et al., 2002; Yamaguchi et al., 2000; Yamaguchi et al., 2004). Those characteristics were also found in our study, which shows a remarkable diversity in gene expression patterns in fibroblasts derived from different phototypes of skin (I, III and VI) and there was great consistency in those expression patterns in different individuals.
In this study, we used the cDNA microarray technique to analyze the gene expression patterns of 15 different primary dermal fibroblast populations derived from the dorsal trunk skin of three different skin phototypes (I, III and VI). Previously, we showed that the level of pigmentation of MelanoDerms co-cultured with fibroblasts derived from skin type VI was much higher than MelanoDerms co-cultured with fibroblasts derived from skin types I or III (Choi et al., 2008) (summarized in supplementary material Table S1). In this study, we were able to identify 12 differentially regulated secreted factors in fibroblasts derived from type VI skin, compared to fibroblasts derived from types I or III skin, that might play a role in regulating the constitutive skin colors of different skin phototypes.
Among them, we investigated NRG-1 for its potential to regulate pigmentation in the MelanoDerm skin model and in cultured human melanocytes. NRG-1 is widely expressed in brain and in nervous tissues and is required for the differentiation, migration, and development of neurons and for dendritic development (Corfas et al., 1995; Krivosheya et al., 2008). Neuregulins (NRGs) are a diverse group of secreted peptide growth factors that mediate cell–cell interactions in the nervous system, heart, breast, and other organ systems, signaling through tyrosine kinase receptors of the ErbB family (Esper et al., 2006; Falls, 2003). NRG-1 is the term for a family of proteins, derived by alternative splicing from a single gene, that function as ligands for the receptors ErbB3 and ErbB4. The calcium-binding EGF-like domain is sufficient for the activation of ErbB receptor-tyrosine kinases (Yarden and Sliwkowski, 2001). Many isoforms of NRG-1 protein of various sizes have been reported, e.g. 140, 110, 95, 60, 55 and 45 kDa (Law et al., 2004; Tan et al., 2007). In this study, we showed that exogenous recombinant human NRG-1 (rh-NRG-1) was able to increase pigmentation both in melanocyte culture and in the MelanoDerm skin model. Furthermore, we found that only fibroblasts derived from type VI skin expressed the 60-kDa isoform of NRG-1, and that when the 60-kDa isoform of NRG-1 was knocked down in fibroblasts, the darkening effect of fibroblasts derived from type VI skin was significantly decreased. Overall, these results strongly support the idea that NRG-1 is an important melanogenic factor regulating the constitutive pigmentation of darker human skin.
Once NRG-1 binds to ErbB3 or ErbB4, it can produce ErbB3/ErbB2 or ErbB4/ErbB2 heterodimers or ErbB4 homodimers to initiate the downstream signaling (Esper et al., 2006). Receptor dimerization leads to the activation of intracellular signaling pathways that include phosphatidylinositol-3-kinase and the mitogen-activated protein kinase (MAPK) pathways (Esper et al., 2006). Given the developmental origin of melanocytes (from neural crest cells), it is no surprise that many nervous-system-specific proteins might also be important in regulating melanocyte function. As an example, it has already been reported that nerve growth factor (NGF), a neurotropic polypeptide, affects the survival and proliferation of melanocytes and melanoma cells (Bothwell, 1997; Marconi et al., 2006; Truzzi et al., 2008). The role of NRG-1 during melanocyte development was noted some time ago, including the fact that it plays a crucial role as a survival factor for postmigratory neural crest cells in a Sox10-dependent manner (Paratore et al., 2001) and in determining the targeted differentiation of neural crest cells (Sieber-Blum et al., 2004). The expression of ErbB receptors (ErbB2, ErbB3 and ErbB4) and their role in regulating migration and proliferation of human melanocytes (and even melanoma cells) was previously reported (Gordon-Thomson et al., 2005). Very recently, Buac and colleagues (Buac et al., 2009) reported the role of NRG-1–ErbB3 signaling in melanocyte development in mice and showed that signaling inhibited differentiation but stimulated proliferation. Therefore, it seems possible that the functions differ between the murine and human pigmentary systems or according to other interacting factors present.
Our results show that NRG-1 increases the proliferation of human melanocytes, possibly via the phosphorylation of Akt, and that it increases pigmentation levels in the MelanoDerm skin model and in cultured human melanocytes. One interesting aspect of our results is that NRG-1 is much more efficient in increasing melanin content as the melanocytes became confluent. Therefore, it seems that the initial effect of NRG-1 is to increase proliferation, and then to increase pigmentation when proliferation becomes limiting. In the MelanoDerm skin model, NRG-1 did not increase melanocyte growth, but it did increase the size of melanocytes, accompanied by an accumulation of melanin pigments, and also increased the thickness of dendrites and/or degree of dendricity of melanocytes in the basal layer. This might be closer to the physiological situation, in which the number of melanocytes in lighter and darker skin is comparable and melanocytes do not usually proliferate unless there is a strong stimulatory signal such as UV. Therefore, we hypothesize that NRG-1 affects the dendricity and pigment synthesis of melanocytes and the distribution of melanin in human skin. Further investigation will characterize the mechanism of action of NRG-1 to visibly increase pigmentation in darker skin.
NRG-1 binds to ErbB3 or ErbB4, which in turn can form heterodimers with ErbB2 or can form homodimers by themselves (i.e. ErbB4–ErbB4) to activate downstream signaling (Esper et al., 2006; Falls, 2003). There have been several studies on the role of ErbB receptors on the proliferation of melanocytes and melanoma cells (Carraway et al., 1999; Gordon-Thomson et al., 2005; Stove et al., 2003; Stove et al., 2005). In fact, when we treated melanocyte cultures or MelanoDerms with pan-ErbB inhibitors, they were able to prevent the effects of NRG-1. Interestingly, we found that under normal conditions, ErbB3 is highly expressed only in HEM-DP, whereas ErbB4 is highly expressed only in HEM-LP. Therefore, the different levels of ErbB3 and ErbB4 receptors in the HEM-LP and HEM-DP might explain why the effect of NRG-1 was more evident in dark MelanoDerms and in HEM-DP than in light MelanoDerms and in HEM-LP. Other mechanisms and/or factors unique to melanocytes from dark skin might function together with NRG-1 for synergistic effects on melanogenesis, and further investigation will be needed to clarify this issue.
ErbB2 is known as an oncogene, which is constitutively activated in melanoma cells and in some other types of cancer cells and is a well-characterized cancer drug target (Carraway et al., 1999; Falls, 2003; Gschwind et al., 2004; Maatta et al., 2006; Stove et al., 2003). NRG-1 cannot bind to ErbB2 directly, but it has been suggested that ErbB2 might augment ErbB receptor signaling by acting as an auxiliary co-receptor (Carraway et al., 1999; Graus-Porta et al., 1997; Karunagaran et al., 1996). One of the most interesting findings of our study is that NRG-1 ligand binding leads to the complete abrogation of ErbB2 receptor levels due to increased proteasomal degradation both in HEM-LP and in HEM-DP. The implications of this need to be further studied, especially regarding whether targeting ErbB2 would be an effective strategy for melanoma prevention or intervention.
Overall, this study provides a database of potential novel melanogenic factors that could regulate the constitutive pigmentation of different phototypes of human skin. Further investigation of those melanogenic paracrine factors should clarify the regulation of melanocyte function in different skin phototypes and might explain different susceptibilities to skin cancers. Ultimately, this could lead to developing approaches to prevent and/or target melanomas.
Materials and Methods
Human fibroblast and melanocyte cultures
Adult human fibroblasts derived from the dermis of 4 mm punch biopsies were taken from the lower backs of 15 healthy subjects of three different phototypes (types I, III and VI). This study was approved by the Human Research Ethics Committee of Hamburg. Subjects with type I skin were #4, 12, 15, 19 and 26; subjects with type III skin were # 6, 9, 13, 14 and 23; and subjects with type VI skin were # 7, 8, 16, 17 and 28. The fibroblasts were cultured from the biopsies and were grown in monolayer culture in high-glucose Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and 1% L-glutamine plus 1% penicillin and streptomycin at 37°C in a humidified 5% CO2 atmosphere. Fibroblasts were subcultured using routine methods and were used at passages 3 to 6.
Human neonatal epidermal melanocytes, lightly and darkly pigmented (HEM-LP and HEM-DP, respectively) were obtained from Cascade Biologics (Portland, OR) and were cultured in melanocyte growth medium (MGM) consisting of Medium 254 and human melanocyte growth supplement (both from Cascade Biologics) at 37°C under 5% CO2. Melanocytes from the third to ninth passage were used in these experiments.
Microarray procedures
Oligo-cDNA microarray hybridization was performed according to the National Cancer Institute in-house protocol, as detailed previously (Yamaguchi et al., 2008). Briefly, total RNAs were prepared from cultured fibroblasts using an RNeasy mini kit (Qiagen, Valencia, CA). The quality (purity and integrity) and quantity of each total RNA preparation was measured using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE). A universal human reference RNA (Stratagene, La Jolla, CA) was used as a control. cDNA samples generated from RNA samples and purified before the coupling reaction were labeled with Cy3 (for fibroblast samples) or Cy5 (for reference RNA) monoreactive dyes (GE Healthcare, Piscataway, NJ), and were hybridized simultaneously on an oligo-DNA chip (Hs-OperonV3.0-v1p24, p27, p31) overnight at 42°C. Two fluorescent intensities of the oligo-DNA chip were scanned using a microarray scanner (GenePix 4000B; Axon Instruments, Molecular Devices, Sunnyvale, CA). Differential gene expression was profiled using Genepix Pro 5.0 software and was analyzed by Miltenyi Biotec (Bergisch Gladbach, Germany). The full microarray database is available at GEO (GSE22022).
shRNA transduction
Control shRNA lentiviral particles (sc-108080) and NRG-1 shRNA (sc-37210-V) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and were transduced to fibroblasts derived from type VI skin (#8, 17 and 28) according to the manufacturer's instructions. Briefly, cells were plated at 50,000 cells per well in six-well plates 24 hours prior to viral infection. The next day, cells were transduced with lentiviral particles (MOI=4) in the presence of 2 μg/ml polybrene (Santa Cruz Biotechnology). Selection with puromycin (2 μg/ml) was performed for 10 days in normal fibroblast growth medium; normal fibroblasts without shRNA transduction did not survive under this selection condition. Stably transduced puromycin-resistant cells were subcultured and expanded for the confirmation of NRG-1 knockdown and also for further experiments.
3D skin reconstructs
Light (from Caucasian skin), medium (from Asian skin), and dark (from African-American skin) human epidermal equivalents (MelanoDerm) were purchased from MatTek Corp. (Ashland, MA). MelanoDerms were grown at the air–liquid interface of the maintenance medium MEL-LLMM (MatTek Corp.), and the culture medium was renewed every 2 days. Where noted, MelanoDerms were treated for 9 days with 50 ng/ml NRG-1 (rhNRG-1-β1; R&D Systems, Minneapolis, MN) prepared in phosphate-buffered saline (PBS) with 0.1% bovine serum albumin (BSA) and MelanoDerm maintenance medium. The same concentrations of PBS and BSA were used for mock-treated controls.
For the experiments where the MelanoDerms were co-cultured with shRNA-transduced fibroblasts, the NRG-1 shRNA-transduced fibroblasts or control shRNA-transduced fibroblasts from type VI skin were grown in normal fibroblast growth medium, and the dark MelanoDerms were placed on top of the confluent monolayer culture in the MelanoDerm maintenance medium. Every 2 days, MelanoDerms were transferred to freshly prepared confluent fibroblast culture in the fresh MelanoDerm maintenance medium, and MelanoDerms were harvested after 9 days of treatment.
For the experiments where the MelanoDerms were treated with NRG-1 and/or various pan-ErbB inhibitors, the dark MelanoDerms were first incubated with a pan-ErbB inhibitor (0.5 μM C39, EMD Chemicals, Gibbistown, NJ or 2 μM CI-1033, Selleck Chemicals LLC, US) for 30 minutes or with vehicle (DMSO) only before treatment with 50 ng/ml NRG-1; identical amounts of DMSO (0.02%) were added to all samples. Treatment was renewed every 2 days in fresh medium and was continued for 9 days.
Western blotting analysis
Fibroblast cultures in 100-mm dishes were solubilized in appropriate volumes of mammalian protein extraction reagent (M-PER; Pierce Biotechnology, Rockford, IL) containing a protease inhibitor mixture (Roche, Mannheim, Germany) and phosphatase inhibitors. The protein concentration of each extract was measured using a BCA protein assay kit (Pierce, Rockford, IL). Cell extracts (20 μg) were separated on 8–16% gradient SDS polyacrylamide gels (Invitrogen, Carlsbad, CA), after which proteins were transferred to PVDF membranes (Invitrogen). Membranes were blocked for 1 hour in PBS or Tris-buffered saline (TBS) containing 0.1% Tween 20 and 5% (w/v) nonfat dry milk powder and were incubated overnight at 4°C with the primary antibody in PBS containing 0.1% Tween 20 and 2.5% (w/v) nonfat dry milk powder or in 0.1% Tween–TBS with 5% BSA, depending on the antibody. The following primary antibodies were used: anti-human DKK-1 (0.2 μg/ml), anti-human DKK-3 (0.1 μg/ml), anti-human bFGF (0.1 μg/ml), anti-human SCF (0.1 μg/ml), anti-human mouse monoclonal ErbB3 (1 μg/ml), and anti-human mouse monoclonal ErbB4 (2 μg/ml) antibodies from R&D Systems; neuregulin-1α/β1/2 (C-20) (1:500) and GAPDH (FL-335) (1:10,000) antibodies from Santa Cruz Biotechnology; ErbB2 rabbit monoclonal (1:1000) and phosphorylated Akt (Ser473) (1:1000) antibodies from Cell Signaling Technology. Membranes were then incubated with an HRP-linked anti-rabbit antibody (GE Healthcare) at 1:10,000 dilution or with an anti-goat antibody (DAKO) at 1:2,000 dilution at room temperature for 1 hour. Antigen–antibody complexes were detected using an ECL-plus western blotting detection system (GE Healthcare). Each experiment was performed at least in triplicate.
Immunohistochemical staining
Fibroblasts were cultured in two-well Lab-Tek chamber slides (Nalge Nunc International, Naperville, IL) and were processed for indirect fluorescence to detect the expression of proteins using two different primary antibodies to NRG-1 (rabbit polyclonal, 20 μg/ml, Abcam; and neuregulin-1α/β1/2 (C-20), 2 μg/ml, Santa Cruz Biotechnology). Bound antibodies were visualized with Alexa Fluor 594 goat anti-rabbit IgG (H+L) (Molecular Probes, Eugene, OR), at 25°C for 1 hour at a 1:400 dilution with 5% goat serum. Nuclei were counterstained with DAPI (Vector Laboratories). The red fluorescence produced by Alexa Fluor 594 and blue fluorescence by DAPI were observed and captured using a Leica DMR B/D MLD fluorescence microscope (Leica, Wetzlar, Germany) and a Dage-MTI 3CCD three-chip color video camera (Dage-MTI, Michigan City, IN).
Immunohistochemistry and melanin staining
The expression of NRG-1 in frozen sections of skin specimens from the same volunteers from whom the fibroblasts were derived was detected by indirect immunofluorescence with the primary antibody to NRG-1 (rabbit polyclonal, 20 μg/ml, Abcam). Bound antibodies were visualized with Alexa Fluor 594 goat anti-rabbit IgG (H+L) at 25°C for 1 hour at a 1:400 dilution with 5% normal goat serum. Fluorescence was observed and analyzed with a fluorescence microscope as detailed above. Paraffin-embedded tissues were also processed for the Fontana-Masson silver stain (Tadokoro et al., 2003) to observe the melanin distribution in skin specimens.
Melanin content assay
Melanin contents were determined as described previously (Virador et al., 1999). In brief, cell pellets were dissolved overnight at room temperature in 200 μl of 1 N NaOH, and melanin concentrations were quantitated by absorbance at 405 nm in a SpectraMax 250 ELISA reader (Molecular Devices) using a standard curve generated from synthetic melanin (Sigma-Aldrich). Melanin content is expressed as micrograms melanin per microgram protein. Each experiment was repeated at least three times.
RT-PCR
Total RNAs were isolated from cells using an RNeasy mini kit (Qiagen), and 500 ng RNA of each sample was used for reverse transcription. The following primers were used for PCR: human ErbB2 sense primer 5′-ACAGTGGCATCTGTGAGCTG-3′; ErbB2 antisense primer 5′-AGCAGAGGTGGGTGTTATGG-3′; NRG-1 sense primer 5′-CTGTGTGAATGGAGGGGAGT-3′; NRG-1 antisense primer 5′-GCTTTTTCCGCTGTTTCTTG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sense primer 5′-ACCACAGTCCATGCCATCAC-3′; GAPDH antisense primer 5′-TCCACCACCCTGTTGCTGTA-3′. After denaturation at 94°C for 3 minutes, PCR was performed for 32 cycles for ErbB2, 29 cycles for NRG-1, and 28 cycles for GAPDH (30 seconds at 94°C, 30 seconds at 60°C, and 45 seconds at 68°C). Control reactions were performed in the absence of reverse transcriptase and were negative. Each experiment was repeated in duplicate independently.
Statistical analysis
We used the LIMMA (Linear Models for Microarray Data) package in R BioConductor for microarray analysis (Smyth, 2004). Each array was subjected to two types of normalization, a nonlinear locally weighted scatterplot smoothing (LOWESS) normalization, which corrects intensity-dependent variation in dye bias, and a quantile normalization to ensure all reference channels have the same empirical distribution across arrays, leaving the M values (log ratios) unchanged. Differential expression between groups was assessed using linear models and empirical Bayes methods provided by LIMMA. Spots with |log2(FC)|>1.5 and a t-statistics-based P value of <0.05 were retained for further exploration.
Supplementary Material
Acknowledgments
The authors thank Yuji Yamaguchi (Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan) for his helpful discussions and critical review of the manuscript. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/18/3102/DC1
References
- Ako E., Yamashita Y., Ohira M., Yamazaki M., Hori T., Kubo N., Sawada T., Hirakawa K. (2007). The pan-erbB tyrosine kinase inhibitor CI-1033 inhibits human esophageal cancer cells in vitro and in vivo. Oncol. Rep. 17, 887-893 [DOI] [PubMed] [Google Scholar]
- Ando H., Watabe H., Valencia J. C., Yasumoto K., Furumura M., Funasaka Y., Oka M., Ichihashi M., Hearing V. J. (2004). Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase-a new aspect of ubiquitin-proteasome function. J. Biol. Chem. 279, 15427-15433 [DOI] [PubMed] [Google Scholar]
- Ando H., Wen Z.-M., Kim H.-Y., Salem N., Valencia J. C., Costin G. E., Watabe H., Yasumoto K., Niki Y., Kondoh H., et al. (2006). Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem. J. 394, 43-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer J. Z., Hearing V. J. (2007). Skin color, melanin, race/ethnicity and UV-induced DNA damage. In Biophysical and Physiological Effects of Solar Radiation on Human Skin (ed. Giacomoni P. U.), pp. 99-126 Cambridge: Royal Society of Chemistry; [Google Scholar]
- Bothwell M. (1997). Neurotrophin function in skin. J. Investig. Dermatol. Symp. Proc. 2, 27-30 [DOI] [PubMed] [Google Scholar]
- Breuleux M., Schoumacher F., Rehn D., Kung W., Mueller H., Eppenberger U. (2006). Heregulins implicated in cellular functions other than receptor activation. Mol. Cancer Res. 4, 27-37 [DOI] [PubMed] [Google Scholar]
- Buac K., Xu M., Cronin J., Weeraratna A. T., Hewitt S. M., Pavan W. J. (2009). NRG1 / ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation. Pigment Cell Melanoma Res. 22, 773-784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario-Andre M., Pain C., Gauthier Y., Casoli V., Taieb A. (2006). In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 19, 434-442 [DOI] [PubMed] [Google Scholar]
- Carraway K. L., III, Rossi E. A., Komatsu M., Price-Schiavi S. A., Huang D., Guy P. M., Carvajal M. E., Fregien N., Carraway C. A., Carraway K. L. (1999). An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J. Biol. Chem. 274, 5263-5266 [DOI] [PubMed] [Google Scholar]
- Chang H. Y., Chi J. T., Dudoit S., Bondre C., van de Rijn M., Botstein D., Brown P. O. (2002). Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA 99, 12877-12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W., Wolber R., Gerwat W., Mann T., Hearing V. J. (2008). Characterization of the influence of fibroblasts on melanocyte function and pigmentation. In Proceedings of the 20th International Pigment Cell Conference (ed. Jimbow K.), pp. 79-82 Bologna, Italy: Medimond; [Google Scholar]
- Coelho S. G., Koo E., Hearing V. J. (2009). Standardization of in vitro macrophotography for assessment of cutaneous responses. Photochem. Photobiol. 85, 1032-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G., Rosen K. M., Aratake H., Krauss R., Fischbach G. D. (1995). Differential expression of ARIA isoforms in the rat brain. Neuron 14, 103-115 [DOI] [PubMed] [Google Scholar]
- Dilworth J. T., Wojtkowiak J. W., Mathieu P., Tainsky M. A., Reiners J. J., Jr, Mattingly R. R., Hancock C. N. (2008). Suppression of proliferation of two independent NF1 malignant peripheral nerve sheath tumor cell lines by the pan-ErbB inhibitor CI-1033. Cancer Biol. Ther. 7, 1938-1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper R. M., Pankonin M. S., Loeb J. A. (2006). Neuregulins: versatile growth and differentiation factors in nervous system development and human disease. Brain Res. Rev. 51, 161-175 [DOI] [PubMed] [Google Scholar]
- Falls D. L. (2003). Neuregulins: functions, forms, and signaling strategies. Exp. Cell Res. 284, 14-30 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick T. B. (1988). The validity and practicability of sun-reactive skin types I through VI. Arch. Dermatol. 124, 869-871 [DOI] [PubMed] [Google Scholar]
- Golding M., Ruhrberg C., Sandle J., Gullick W. J. (2004). Mapping nucleolar and spliceosome localization sequences of neuregulin1-beta3. Exp. Cell Res. 299, 110-118 [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C., Mason R. S., Moore G. P. (2001). Regulation of epidermal growth factor receptor expression in human melanocytes. Exp. Dermatol. 10, 321-328 [DOI] [PubMed] [Google Scholar]
- Gordon-Thomson C., Jones J., Mason R. S., Moore G. P. (2005). ErbB receptors mediate both migratory and proliferative activities in human melanocytes and melanoma cells. Melanoma Res. 15, 21-28 [DOI] [PubMed] [Google Scholar]
- Graus-Porta D., Beerli R. R., Daly J. M., Hynes N. E. (1997). ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 16, 1647-1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grichnik J. M., Burch J. A., Burchette J., Shea C. R. (1998). The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J. Invest. Dermatol. 111, 233-238 [DOI] [PubMed] [Google Scholar]
- Gschwind A., Fischer O. M., Ullrich A. (2004). The discovery of receptor tyrosine kinases: targets for cancer therapy. Nat. Rev. Cancer 4, 361-370 [DOI] [PubMed] [Google Scholar]
- Hachiya A., Kobayashi A., Ohuchi A., Takema Y., Imokawa G. (2001). The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J. Invest Dermatol. 116, 578-586 [DOI] [PubMed] [Google Scholar]
- Hedley S., Layton C., Heaton M., Chakrabarty K. H., Dawson R. A., Gawkrodger D. J., Mac Neil S. (2002). Fibroblasts play a regulatory role in the control of pigmentation in reconstructed human skin from skin types I and II. Pigment Cell Res. 15, 49-56 [DOI] [PubMed] [Google Scholar]
- Imokawa G. (2004). Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 17, 96-110 [DOI] [PubMed] [Google Scholar]
- Karunagaran D., Tzahar E., Beerli R. R., Chen X., Graus-Porta D., Ratzkin B. J., Seger R., Hynes N. E., Yarden Y. (1996). ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 15, 254-264 [PMC free article] [PubMed] [Google Scholar]
- Klutchko S. R., Zhou H., Winters R. T., Tran T. P., Bridges A. J., Althaus I. W., Amato D. M., Elliott W. L., Ellis P. A., Meade M. A., et al. (2006). Tyrosine kinase inhibitors. 19. 6-Alkynamides of 4-anilinoquinazolines and 4-anilinopyrido[3,4-d]pyrimidines as irreversible inhibitors of the erbB family of tyrosine kinase receptors. J. Med. Chem. 49, 1475-1485 [DOI] [PubMed] [Google Scholar]
- Krivosheya D., Tapia L., Levinson J. N., Huang K., Kang Y., Hines R., Ting A. K., Craig A. M., Mei L., Bamji S. X., et al. (2008). ErbB4-neuregulin signaling modulates synapse development and dendritic arborization through distinct mechanisms. J. Biol. Chem. 283, 32944-32956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law A. J., Shannon W. C., Hyde T. M., Kleinman J. E., Harrison P. J. (2004). Neuregulin-1 (NRG-1) mRNA and protein in the adult human brain. Neuroscience 127, 125-136 [DOI] [PubMed] [Google Scholar]
- Maatta J. A., Sundvall M., Junttila T. T., Peri L., Laine V. J., Isola J., Egeblad M., Elenius K. (2006). Proteolytic cleavage and phosphorylation of a tumor-associated ErbB4 isoform promote ligand-independent survival and cancer cell growth. Mol. Biol. Cell 17, 67-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi A., Panza M. C., Bonnet-Duquennoy M., Lazou K., Kurfurst R., Truzzi F., Lotti R., De Santis G., Dumas M., Bonte F., et al. (2006). Expression and function of neurotrophins and their receptors in human melanocytes. Int. J. Cosmet. Sci. 28, 255-261 [DOI] [PubMed] [Google Scholar]
- McClell C. M., Gullick W. J. (2009). Neuregulins in the Nucleus. In Breast Cancer in the Post-Genomic Era (ed. Giordano A., Normanno N.). New York: Humana Press; [Google Scholar]
- Miyamura Y., Coelho S. G., Wolber R., Miller S. A., Wakamatsu K., Zmudzka B. Z., Ito S., Smuda C., Passeron T., Choi W., et al. (2007). Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 20, 2-13 [DOI] [PubMed] [Google Scholar]
- Paratore C., Goerich D. E., Suter U., Wegner M., Sommer L. (2001). Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128, 3949-3961 [DOI] [PubMed] [Google Scholar]
- Rees J. L. (2004). The genetics of sun sensitivity in humans. Am. J. Hum. Gen. 75, 739-751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber-Blum M., Grim M., Hu Y. F., Szeder V. (2004). Pluripotent neural crest stem cells in the adult hair follicle. Dev. Dyn. 231, 258-269 [DOI] [PubMed] [Google Scholar]
- Smyth G. K. (2004). Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3 [DOI] [PubMed] [Google Scholar]
- Stove C., Stove V., Derycke L., Van Marck V., Mareel M., Bracke M. (2003). The heregulin/human epidermal growth factor receptor as a new growth factor system in melanoma with multiple ways of deregulation. J. Invest. Dermatol. 121, 802-812 [DOI] [PubMed] [Google Scholar]
- Stove C., Boterberg T., Van Marck V., Mareel M., Bracke M. (2005). Bowes melanoma cells secrete heregulin, which can promote aggregation and counteract invasion of human mammary cancer cells. Int. J. Cancer 114, 572-578 [DOI] [PubMed] [Google Scholar]
- Sturm R. A. (2006). A golden age of human pigmentation genetics. Trends Genet. 22, 464-468 [DOI] [PubMed] [Google Scholar]
- Tadokoro T., Kobayashi N., Zmudzka B. Z., Ito S., Wakamatsu K., Yamaguchi Y., Korossy K. S., Miller S. A., Beer J. Z., Hearing V. J. (2003). UV-induced DNA damage and melanin content in human skin differing in racial/ethnic origin and photosensitivity. FASEB J. 17, 1177-1179 [DOI] [PubMed] [Google Scholar]
- Tadokoro T., Yamaguchi Y., Batzer J., Coelho S. G., Zmudzka B. Z., Miller S. A., Wolber R., Beer J. Z., Hearing V. J. (2005). Mechanisms of skin tanning in different racial/ethnic groups in response to ultraviolet radiation. J. Invest. Dermatol. 124, 1326-1332 [DOI] [PubMed] [Google Scholar]
- Tan W., Wang Y., Gold B., Chen J., Dean M., Harrison P. J., Weinberger D. R., Law A. J. (2007). Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J. Biol. Chem. 282, 24343-24351 [DOI] [PubMed] [Google Scholar]
- Truzzi F., Marconi A., Lotti R., Dallaglio K., French L. E., Hempstead B. L., Pincelli C. (2008). Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J. Invest Dermatol. 128, 2031-2040 [DOI] [PubMed] [Google Scholar]
- Virador V., Kobayashi N., Matsunaga J., Hearing V. J. (1999). A standardized protocol for assessing regulators of pigmentation. Anal. Biochem. 270, 207-219 [DOI] [PubMed] [Google Scholar]
- Wakamatsu K., Kavanagh R., Kadekaro A. L., Terzieva S., Sturm R. A., Leachman S., Abdel-Malek Z., Ito S. (2006). Diversity of pigmentation in cultured human melanocytes is due to differences in the type as well as quantity of melanin. Pigment Cell Res. 19, 154-162 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Hearing V. J. (2005). Melanocyte distribution and function in human skin: effects of UV radiation. In From Melanocytes to Malignant Melanoma (ed. Hearing V. J., Leong S. P. L.), pp. 101-116 Totowa: Humana Press; [Google Scholar]
- Yamaguchi Y., Crane S., Zhou L., Ochoa S. M., Falanga V. (2000). Lack of co-ordinate expression of the alpha1(I) and alpha1(III) procollagen genes in fibroblast clonal cultures. Brit. J. Dermatol. 143, 1149-1153 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Itami S., Watabe H., Yasumoto K., Abdel-Malek Z. A., Kubo T., Rouzaud F., Tanemura A., Yoshikawa K., Hearing V. J. (2004). Mesenchymal-epithelial interactions in the skin: Increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J. Cell Biol. 165, 275-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Takahashi K., Zmudzka B. Z., Kornhauser A., Miller S. A., Tadokoro T., Berens W., Beer J. Z., Hearing V. J. (2006). Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 20, 1486-1488 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Brenner M., Hearing V. J. (2007a). The regulation of skin pigmentation. J. Biol. Chem. 282, 27557-27561 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Passeron T., Watabe H., Yasumoto K., Rouzaud F., Hoashi T., Hearing V. J. (2007b). The effects of dickkopf 1 on gene expression and Wnt signaling by melanocytes: mechanisms underlying its suppression of melanocyte function and proliferation. J. Invest. Dermatol. 127, 1217-1225 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Passeron T., Hoashi T., Watabe H., Rouzaud F., Yasumoto K., Hara T., Tohyama C., Katayama I., Miki T., et al. (2008). Dickkopf 1 (DKK1) regulates skin pigmentation and thickness by affecting Wnt/β-catenin signaling in keratinocytes. FASEB J. 22, 1009-1020 [DOI] [PubMed] [Google Scholar]
- Yarden Y., Sliwkowski M. X. (2001). Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127-137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.