Abstract
OBJECTIVE: To describe the clinical course and outcome of adults hospitalized with the 2009 H1N1 influenza infection.
PATIENTS AND METHODS: In this retrospective study, we reviewed the electronic medical records of patients with H1N1 influenza infection treated at Mayo Clinic in Rochester, MN, from May 1, 2009, through December 31, 2009.
RESULTS: We identified 1053 patients with H1N1 influenza infection; this study consists of 66 hospitalized adults (6%). Patients' mean ± SD age was 46.9±17.8 years. The 3 most common comorbidities were hypertension in 31 patients (47%), obesity in 29 (44%), and diabetes mellitus in 21 (32%). The most common symptoms were cough in 58 patients (88%), fever or chills in 55 (83%), and dyspnea in 47 (71%). Twenty-nine patients (44%) were admitted to the intensive care unit (ICU). Dyspnea and thrombocytopenia were more common in the ICU patients. The hospital, 28-day, and 90-day mortality rates were 8% (5/66), 11% (7/66), and 14% (9/66), respectively. Among the 29 ICU patients, 23 (79%) received mechanical ventilation, and 16 (55%) developed acute lung injury or acute respiratory distress syndrome. Rescue therapy for refractory respiratory failure was provided for 6 patients (21%). Of the 29 ICU patients, 10 (34%) required vasopressor support, and 4 (14%) required acute renal replacement therapy.
CONCLUSION: Hospitalized adults with H1N1 influenza infection are relatively young, and a significant number require treatment in the ICU. Among the patients who require ICU admission, most develop acute lung injury or acute respiratory distress syndrome and require mechanical ventilator support.
Patients hospitalized with H1N1 influenza infection (n=66) were relatively young, and a significant number required treatment in the intensive care unit (ICU). Most of those admitted to the ICU developed acute lung injury or acute respiratory distress syndrome, necessitating mechanical ventilator support.
APS = Acute Physiology Score; ALI = acute lung injury; APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; BMI = body mass index; CDC = Centers for Disease Control and Prevention; ICU = intensive care unit; IQR = interquartile range; RIFLE = Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease; rRT-PCR = real-time reverse transcriptase polymerase chain reaction; SOFA = Sequential Organ Failure Assessment
An outbreak of 2009 H1N1 influenza infection was identified in Mexico in late March 2009.1 This virus spread rapidly worldwide, prompting an international pandemic alert by the World Health Organization on June 11, 2009.2 As of January 31, 2010, the infection had spread to 211 countries and overseas territories, resulting in 15,174 reported deaths, including 7261 from the Americas and 3605 from Europe.3 These numbers are likely to underestimate the actual numbers because many deaths occurred without testing or were not recognized as influenza-related. The Centers for Disease Control and Prevention (CDC) has estimated that between 41 and 84 million cases of 2009 H1N1 influenza infection occurred in the United States from April 2009 through January 16, 2010, resulting in 183,000 to 378,000 H1N1-related hospitalizations and 8330 to 17,160 H1N1-related deaths.4
Reports from other countries showed that the 2009 H1N1 influenza pandemic had substantial effect on intensive care units (ICUs).5-8 A US President's Council of Advisors on Science and Technology report highlighted a plausible scenario that the epidemic might lead to as many as 1.8 million US hospital admissions, 300,000 requiring ICU care, and 30,000 to 90,000 deaths.9 Anticipating that critical care might be overwhelmed, the European Society of Intensive Care Medicine made recommendations for ICU and hospital preparations for an influenza epidemic.10 The Society of Critical Care Medicine developed a course, Critical Care Cross-Training for Hospital-Based non-ICU Healthcare Providers, designed to provide timely education for health care professionals who would care for critically ill patients during high-volume times.
Previous studies have addressed the impact of the 2009 H1N1 influenza infection in the United States. The first cases of 2009 H1N1 influenza infection were described in 2 children from California.11 In April and May 2009, 30 patients with confirmed or probable H1N1 influenza infection were hospitalized in California, 6 (20%) of whom were treated in an ICU.11 Jain et al12 described 272 patients who were hospitalized for the 2009 H1N1 infection from April to mid-June 2009; 25% were admitted to an ICU and 7% died. These reports from the United States had limited information about patients with critical illness and their clinical course in the ICU. A more recent publication from Salt Lake County, Utah, thoroughly described the demographic data and clinical course of 47 patients with the 2009 H1N1 influenza infection treated in the ICU.13 However, the study included only patients from the first wave of the H1N1 influenza epidemic (May-June 2009). The purpose of the current study is to describe the demographics, clinical characteristics, and clinical course of adults hospitalized with H1N1 influenza infection at Mayo Clinic in Rochester, MN, from May 1, 2009 through December 31, 2009.
PATIENTS AND METHODS
This retrospective cohort study was conducted at Mayo Clinic, a tertiary teaching institution with 2 hospitals comprising about 1300 inpatient beds, including 157 adult ICU beds. The study was approved by the Mayo Clinic Institutional Review Board. Patients with laboratory-confirmed 2009 H1N1 influenza infection were identified from the Microbiology Laboratory and the Infection Prevention and Control databases of our institution. We excluded patients younger than 18 years and those who denied use of their medical records for clinical research. All consecutive adult patients (age, ≥18 years) hospitalized with H1N1 infection from May 1, 2009, through December 31, 2009, were included in the study. Laboratory diagnosis of H1N1 influenza was made using a laboratory-developed influenza real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay performed on respiratory specimens.14 Viral nucleic acid was extracted by the MagNA Pure automated instrument (Roche Applied Science, Indianapolis, IN) from respiratory specimens. Influenza virus genomic RNA in the samples was then transcribed to complementary DNA and amplified on the LightCycler 2.0 instrument (Roche Applied Science) using fluorescence resonance hybridization probes. For the purposes of this study, melting curve analysis was used to differentiate the 2009 H1N1 strains from seasonal influenza A strains. This subtyping method was validated through comparison of results with the CDC H1N1 and seasonal influenza rRT-PCR assays and successful completion of a blinded influenza A panel provided by the Minnesota Department of Health.
We abstracted the following demographic and clinical data from the patients' electronic medical records: age, ethnicity, sex, comorbidities, symptoms, vital signs, laboratory values at presentation, infectious complications, antiviral therapy, hospital length of stay, and mortality (hospital, 28-day, and 90-day). For patients admitted to the ICU, we collected admission source (transfer from another hospital, general care ward in our hospital, emergency department); development of acute lung injury (ALI) or acute respiratory distress syndrome (ARDS); vasopressor use; acute renal replacement therapy; ICU length of stay; use, type (invasive or noninvasive), and duration of mechanical ventilation; use of nonconventional ventilation (high-frequency oscillatory ventilation, airway pressure release ventilation); and rescue measures for refractory hypoxemia or hypercapnia (prone positioning, tracheal gas insufflation, inhaled nitric oxide). We abstracted the Acute Physiology Score (APS), Acute Physiology and Chronic Health Evaluation (APACHE) IV score, and predicted mortality from the ICU Data Mart of our institution. The APS, APACHE IV score, and predicted mortality were calculated as described in the literature.15 Organ failure or dysfunction was assessed using the Sequential Organ Failure Assessment (SOFA) scoring system.16 The SOFA score was calculated on days 1, 2, 3, and 7 of ICU stay. In calculation of the SOFA score, the worst values for each parameter in the 24-hour period were used. In case of missing data, normal values were assumed for calculation of individual variables of the SOFA score. For patients who developed acute kidney injury, worst RIFLE (Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease) stage was determined.17
Definitions
We defined shock as recommended by the 2006 International Consensus Conference on Hemodynamic Monitoring in Shock and Implications for Management.18 ALI and ARDS were identified according to the American-European Consensus Conference definition.19,20 Nosocomial infections were defined according to the guidelines from the International Sepsis Forum Consensus Conference.21 Clostridium difficile–associated diarrhea and diarrhea were defined according to the Infectious Diseases Society of America practice guidelines.22 Acute bacterial rhinosinusitis was defined according to the clinical practice guidelines of the American Academy of Otolaryngology.23 Hypertension was identified if the patient had a known diagnosis of hypertension or was taking antihypertensive medication. Diabetes mellitus was identified if the patient's medical record contained an established diagnosis and use of any of the following: oral hypoglycemics, insulin, or exenatide. Asthma, chronic obstructive pulmonary disease, coronary artery disease, and congestive heart failure were identified if the medical record had an established diagnosis. Hematologic malignancy was defined if the patient had a history of leukemia, lymphoma, or clonal plasma cell disorders. Immunosuppression was defined as therapy with immunosuppressants, chemotherapy, radiation therapy, or long-term use of corticosteroids (at least 0.3 mg/kg/day of prednisone or equivalent for ≥1 month). Obesity was defined as body mass index (BMI) equal to or greater than 30 (calculated as weight in kilograms divided by height in meters squared). Partial arterial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio was defined as the ratio of PaO2 (in mm Hg) to the FiO2. The following values were considered normal in our laboratory: leukocyte count, 3.5-10.5 × 109/L; neutrophil count, 1.7-7.0 × 109/L; lymphocyte count, 0.9-2.9 × 109/L; and platelet count, 150-450 × 109/L. (To convert any of these 4 values to × 103/μL, multiply by 1.)
Statistical Analyses
Data were summarized as percentages for categorical variables and as means with standard deviations (SDs) or medians with 25% to 75% interquartile ranges (IQRs) for continuous variables. We used χ2, Fisher exact, Student t, and Mann-Whitney U tests to compare differences between groups. Two-tailed P≤.05 was considered statistically significant. All statistical analyses were performed using SPSS software version 17.0.1 (Chicago, IL).
RESULTS
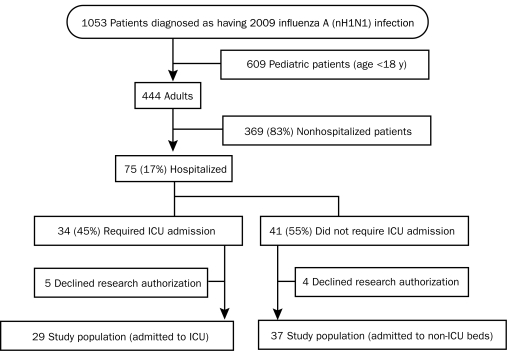
Between May 1, 2009, and December 31, 2009, 1053 patients were diagnosed as having the 2009 H1N1 influenza infection, 444 (42%) of whom were adults (age, ≥18 years) (Figure 1). Seventy-five patients (17%) were hospitalized. After excluding 9 hospitalized adults (5 requiring ICU admission) for lack of research authorization, we had a total of 66 hospitalized adults in the study. The specimens yielding positive H1N1 results were throat swabs in 36 patients, nasal swabs in 18, bronchoalveolar lavage in 8, and tracheal secretions in 1. Three patients were tested at other referring hospitals, and no information was available on the type of diagnostic test. In 3 patients, rRT-PCR tests were negative for influenza on nasal or throat swab specimens, but positive on bronchoalveolar lavage specimens. Antiviral treatment included oseltamivir in 59 patients and zanamivir, peramivir, amantadine, and rimantadine in 1 patient each. Six patients did not receive any antiviral therapy. Of the 66 patients, 29 (44%) required ICU admission.
FIGURE 1.
Flow diagram of patients with the 2009 H1N1 influenza infection diagnosed at Mayo Clinic in Rochester, MN. ICU = intensive care unit; nH1N1 = novel H1N1.
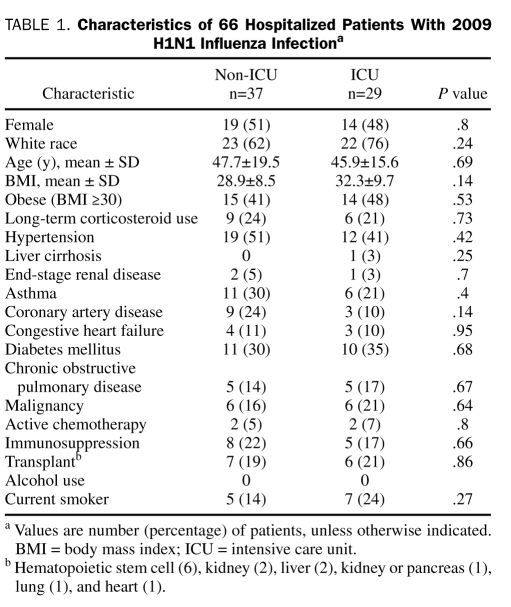
Thirty-three hospitalized patients (50%) were male, and 45 (68%) were white. Their mean ± SD age was 46.9±17.8 years. Twenty-nine (44%) of the patients were obese (Table 1). The 3 most common preexisting comorbidities were hypertension in 31 patients (47%), obesity in 29 (44%), and diabetes mellitus in 21 (32%) (Table 1). There were no statistically significant differences in demographics or preexisting comorbidities between ICU and non-ICU patients (Table 1).
TABLE 1.
Characteristics of 66 Hospitalized Patients With 2009 H1N1 Influenza Infectiona
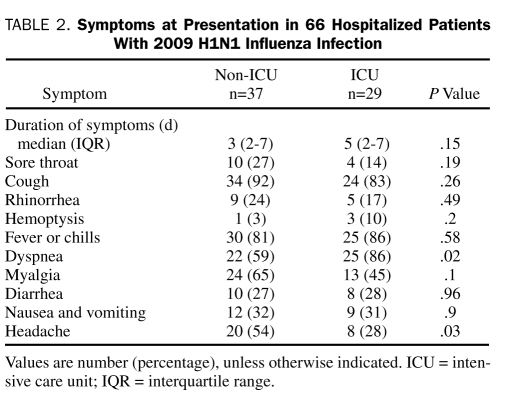
Patients had symptoms for a median (IQR) of 3.0 (2.0-7.0) days before hospital admission. The most common symptoms were cough in 58 patients (88%), fever or chills in 55 (83%), and dyspnea in 47 (71%) (Table 2). There were no statistically significant differences in the duration of symptoms between the ICU and non-ICU groups (Table 2). Dyspnea was more frequent and headache less frequent in ICU patients compared with non-ICU patients (Table 2).
TABLE 2.
Symptoms at Presentation in 66 Hospitalized Patients With 2009 H1N1 Influenza Infection
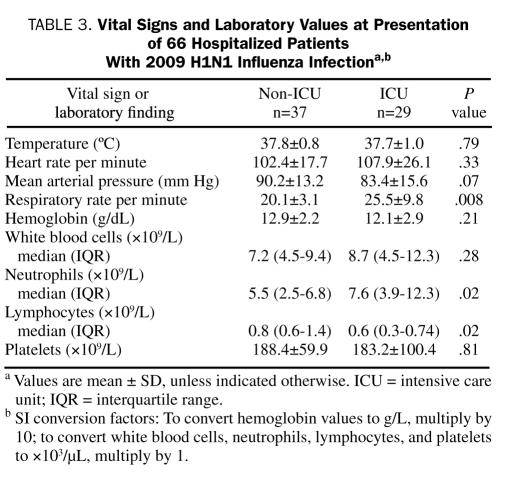
Of the 29 ICU patients, 18 (62%) had received 1 or more antibiotics within 30 days before hospital admission compared with 9 (24%) of the 37 non-ICU patients (P=.002). The respiratory rate and leukocyte count in the ICU group were higher than in the non-ICU group (Table 3). Of the 29 ICU patients, 13 (45%) had thrombocytopenia compared with 7 (19%) of the 37 non-ICU patients (P=.02). There was no statistically significant difference in the presence of leukocytosis and leukopenia between the non-ICU and ICU groups. Neutrophilia and neutropenia were present in 10 (28%) and 4 (11%) of the non-ICU patients compared with 18 (62%) and 8 (12%) of the ICU patients, respectively (P=.007). Lymphocytosis and lymphopenia were present in 4 (11%) and 18 (49%) of the non-ICU patients compared with 2 (7%) and 22 (76%) of the ICU patients, respectively (P=.07).
TABLE 3.
Vital Signs and Laboratory Values at Presentation of 66 Hospitalized Patients With 2009 H1N1 Influenza Infectiona,b
There were no statistically significant differences in serum sodium, creatinine, and bicarbonate levels and anion gap between the ICU and non-ICU groups. The median (IQR) serum urea nitrogen level of the non-ICU group was 14.0 mg/dL (10.0-17.0 mg/dL; to convert to mmol/L, multiply by 0.357) compared with 18.0 mg/dL (11.0-37.0 mg/dL) for the ICU group (P=.03).
Five patients (8%) died before hospital discharge. All hospital deaths occurred in ICU patients. Four more patients died after hospital discharge, 2 ICU and 2 non-ICU patients. The 28- and 90-day mortality rates were 11% (7/66) and 14% (9/66), respectively. The median (IQR) hospital length of stay was 3 days (2-10 days). The median (IQR) hospital length of stay was 11 days (4-17 days) for the ICU patients compared with 2 days (1-3 days) for the non-ICU patients (P<.001).
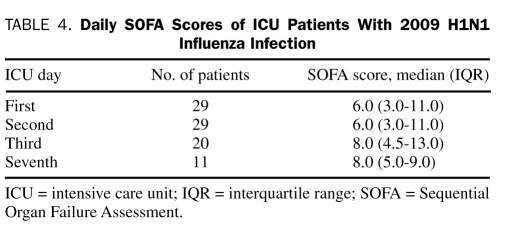
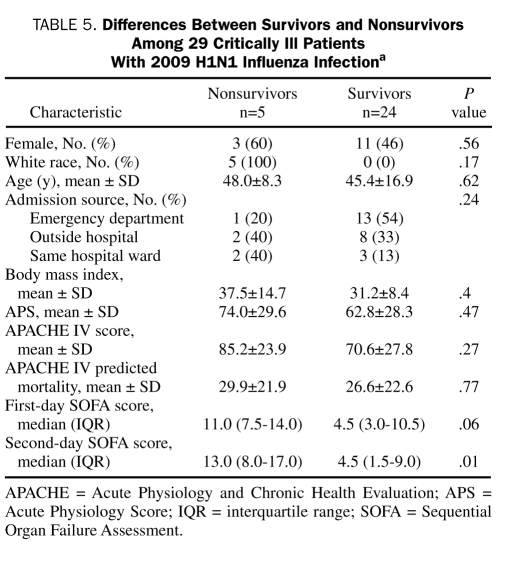
For the 29 patients admitted to the ICU, the mean ± SD APS and APACHE IV scores were 64.7±28.3 and 73.1±27.4, respectively. The mean ± SD and median (IQR) APACHE IV predicted hospitalization rates were 27.2%±22.1% and 21.0% (10.2%-42.6%), respectively. The median (IQR) first-day SOFA score was 6.0 (3.0-11.0) (Table 4). Five (17%) of the 29 patients died in the hospital. There were no statistically significant differences in demographics, severity of illness, and source of ICU admission between the critically ill hospital survivors and nonsurvivors (Table 5). Of the 29 ICU patients, 23 (79%) received mechanical ventilation: 11, invasive mechanical ventilation only; 6, noninvasive mechanical ventilation only; and 6, both invasive and noninvasive mechanical ventilation. The median (IQR) duration of mechanical ventilation was 6.0 days (2.5-15.5 days). Five (22%) of the 23 patients who received mechanical ventilation died compared with none of the 6 who did not receive mechanical ventilation (P=.21). Of the 29 ICU patients, 16 (55%) developed ALI or ARDS. Four (25%) of the 16 patients with ALI or ARDS died compared with 1 (8%) of the 13 without ALI (P=.22). Nonconventional ventilation was used in 8 patients: airway pressure release ventilation in 5 and high-frequency oscillatory ventilation in 3. Rescue therapy for refractory hypoxemia or hypercapnia was provided to 6 patients (21%): inhaled nitric oxide to 4 (14%), tracheal gas insufflation to 3 (10%), and prone ventilation to 2 (7%). Corticosteroids were administered for ARDS in 3 patients (10%).
TABLE 4.
Daily SOFA Scores of ICU Patients With 2009 H1N1 Influenza Infection
TABLE 5.
Differences Between Survivors and Nonsurvivors Among 29 Critically Ill Patients With 2009 H1N1 Influenza Infectiona
The SOFA score on the second ICU day was lower for hospital survivors than for nonsurvivors (Table 5). Vasopressors for shock were administered in 10 (34%) of the ICU patients (6 with septic shock, 3 with cardiogenic shock due to right ventricular failure, and 1 with obstructive shock from cardiac tamponade). Of the 10 patients receiving vasopressors, 4 (40%) died compared with 1 (5%) of the 19 who did not receive vasopressors (P=.02). One patient had end-stage renal disease before ICU admission. The worst RIFLE stage was normal in 14 (48%), Risk in 6 (21%), Injury in 2 (7%), and Failure in 6 (21%). There was no statistically significant association between RIFLE stage and mortality (P=.29). Acute renal replacement therapy was provided to 4 ICU patients (14%). Two (50%) of the 4 patients who received renal replacement therapy died compared with 3 (12%) of the 25 patients who did not receive renal replacement (P=.06).
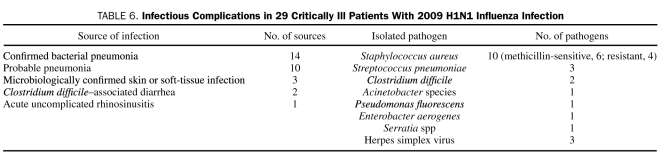
The most common infectious complication noted in the 29 ICU patients was pneumonia, and the most common pathogen was Staphylococcus aureus (Table 6). The pathogens isolated from patients with microbiologically confirmed bacterial pneumonia were S aureus in 10, Streptococcus pneumoniae in 3, Pseudomonas fluorescens in 1, Enterobacter aerogenes in 1, and Serratia species in 1. Herpes simplex virus type 1 was identified in the 3 skin infections. C difficile–associated diarrhea developed in 2 patients.
TABLE 6.
Infectious Complications in 29 Critically Ill Patients With 2009 H1N1 Influenza Infection
Two of the patients died after life support was withdrawn per their wishes. Seventeen patients (59%) were discharged to a general hospital ward from the ICU, and 7 patients (24%) were transferred to a respiratory care unit for weaning from mechanical ventilation. All 24 patients transferred out of the ICU survived the rest of the hospitalization. Nineteen patients were discharged home, 4 patients were sent to a skilled nursing facility, and 1 patient was sent to a long-term acute care facility for continued weaning from mechanical ventilation.
DISCUSSION
In this retrospective study, we describe the clinical pictures and outcomes of 66 hospitalized adults with 2009 H1N1 influenza infection. Twenty-nine patients (44%) were treated in the ICU. The hospital, 28-day, and 90-day mortality rates were 8%, 11%, and 14%, respectively. Sixty-eight percent of patients were white, and 44% were obese. The 3 most common preexisting comorbidities were hypertension, obesity, and diabetes mellitus. Patients had symptoms for a median of 3.0 days before hospital admission. The most common symptoms at presentation were cough, fever or chills, and dyspnea. Dyspnea was more frequent in ICU patients than in non-ICU patients. More ICU patients than non-ICU patients had thrombocytopenia. Most of the ICU patients received mechanical ventilation, and more than half developed ALI or ARDS. Nonconventional ventilation was used in 8, and rescue therapy for refractory respiratory failure was provided to 6 patients. Microbiologically confirmed bacterial pneumonia developed in 14 patients. The most commonly identified pathogen was S aureus.
At our institution, 17% (75/444) of the adults diagnosed as having the 2009 H1N1 influenza infection required hospitalization between May 1, 2009, and December 31, 2009. Our microbiology laboratory did not detect any H1N1 infection in January and February 2010, suggesting that the 2 waves of the 2009 H1N1 influenza epidemic may have ended in our region. Several studies have described the clinical course of hospitalized patients with the 2009 H1N1 influenza infection from different countries. Most of these studies may have reported only the first wave of the epidemic. Studies from the southern hemisphere, which were conducted in Australia and New Zealand, were limited to 3 winter months.8,24 Rello et al7 reported the ICU support of patients with H1N1 infection in Spain. Their study was limited to June and July 2009. The Canadian multicenter study that addressed the critical care support of patients with the 2009 H1N1 influenza infection spanned from April to June 2009.6 Similarly, most of the reported studies from the United States and Mexico deal with the early period of the epidemic.13,25-28 Our study differs from these studies by the likelihood of including both waves of the epidemic. Similar to our study, the more recent publications from France and Canada may have included all the 2009 H1N1 influenza infections.29-31
Previous studies have shown racial disparity associated with the 2009 H1N1 influenza infection.32 In studies from Australia and Canada, there was a relatively higher representation of nonwhite racial groups among patients with H1N1 infection.6,8,31 A similar overrepresentation of Hispanics was noted in the United States.12,25 Our inpatient hospital population is about 90% white. However, only 68% of the current study population was white, suggesting a similar trend in our patients.
Pregnancy and obesity have been cited as risk factors for severe H1N1 influenza infection.8,33-37 A recent case-cohort study analyzing data from the first wave of the H1N1 pandemic in the United States suggested that morbid obesity (defined as BMI >40) was statistically associated with hospitalization among adults (age, ≥20 years), independent of the presence of the chronic medical conditions recognized by the CDC Advisory Committee on Immunization Practices (ACIP) to increase the risk of influenza-related complications.38 In studies from Canada and the United States, 33% and 55% of hospitalized patients with H1N1 influenza infection were obese.6,12,35 In our study, 44% of the patients were obese. We did not collect data on non-hospitalized patients with H1N1 infection; hence, we are unable to comment on the association between the risk of hospitalization or death and the BMI status or presence of chronic medical conditions. No pregnant patients were in our study cohort. Pregnant women were given high priority by ACIP for H1N1 vaccination.39 In accordance with this recommendation, pregnant women were among the first to receive vaccine as soon as vaccine was made available to us. This could be a likely reason why no pregnant women were hospitalized because of severe illness from H1N1 infection. However, we did not have sufficient data to measure the effectiveness of the H1N1 vaccine. Only 1 hospitalized (to non-ICU bed) patient had received vaccination against H1N1 before hospital admission. The common presenting symptoms in our patients did not differ from those reported in other studies.7,12,13,24,25,40 Dyspnea was more frequent and headache less frequent in our ICU patients.
In our study, 44% of the hospitalized patients were treated in the ICU, and all hospital deaths occurred in the ICU patients. Studies from the United States have shown that 20% to 36% of hospitalized patients with H1N1 infection require ICU admission.12,25,27 Similar proportions of hospitalized patients requiring critical care support have been reported from other countries as well.28,29,30 The hospital mortality rate of our ICU patients was 17.2%. This is comparable to the hospital mortality figures reported from other countries.7,8,29 Limited data are available addressing mortality rates after hospital discharge. In a Canadian study, the 28-day and 90-day mortality rates of critically ill patients with H1N1 infection were 14.3% and 17.3%, respectively. In our study, the 28-day and 90-day mortality rates of hospitalized patients with H1N1 infection were 11% and 14%, respectively.
Respiratory failure is common in critically ill patients with H1N1 influenza infection. In our critically ill patients with H1N1 infection, 79% received mechanical ventilation, and 55% developed ALI or ARDS. Nonconventional and rescue therapies were instituted in 28% and 21% of the ICU patients, respectively, for refractory respiratory failure. None of our patients needed extracorporeal membrane oxygenation. Previous studies have also shown that most critically ill patients with H1N1 infection receive mechanical ventilator support6-8,11,29 and 25% to 50% develop ALI or ARDS.8,12,29
Multiple organ failure requiring end-organ support often complicates critical illness. Among the 29 patients admitted to the ICU in our study, 34% received vasopressor support, and 14% received acute renal replacement therapy. The median SOFA score on the first ICU day was 6, and the mean APACHE IV score was 73.1. In a recent study by Miller et al,13 the median SOFA score was 7. Previous studies of critically ill patients with H1N1 influenza infection report mean APACHE II scores of 13 to 20.6,13 Because we used the APACHE IV prognostic model to assess severity of illness, we cannot compare our findings with others.
Our study has several limitations. It is a single-center study, so the sample size is small. Because of the small sample size and relatively low number of deaths, our analyses are not adequately powered to determine statistically significant differences between groups.
CONCLUSION
In this study of 66 adults hospitalized with H1N1 influenza infection, 29 (44%) required ICU admission, and the 90-day mortality was 14%. Among the patients admitted to the ICU, most developed ALI or ARDS, and most received mechanical ventilation. Secondary infections associated with health care are common complications in these patients.
Supplementary Material
Acknowledgments
We thank Bobbi S. Pritt, MD, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, for providing us information on the laboratory diagnostic methodology of the 2009 H1N1 influenza infection.
Footnotes
An earlier version of this article appeared Online First.
REFERENCES
- 1.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, et al. INER Working Group on Influenza Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680-689 [DOI] [PubMed] [Google Scholar]
- 2.Zarocostas J. World Health Organization declares A (H1N1) influenza pandemic. BMJ. 2009;338:b2425 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Pandemic (H1N1) 2009–update 86. WHO Web site. http://www.who.int/csr/don/2010_02_5/en/index.html Accessed June 10, 2010
- 4.Centers for Disease Control and Prevention (CDC) CDC Estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States, April 2009 – January 16, 2010. http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm Accessed June 10, 2010
- 5.Dominguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880-1887 [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872-1879 [DOI] [PubMed] [Google Scholar]
- 7.Rello J, Rodriguez A, Ibanez P, et al. H1N1 SEMICYUC working group Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13(5):R148 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2784367/?tool=pubmed Accessed June 10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb SA, Pettila V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925-1934 [DOI] [PubMed] [Google Scholar]
- 9.White House Report. Report to the president on the US preparations for 2009-H1N1influenza. White House Web site. http://www.whitehouse.gov/administration/eop/ostp/pcast/docsreports Accessed July 26, 2010
- 10.Sprung CL, Zimmerman JL, Christian MD, et al. Recommendations for intensive care unit and hospital preparations for an influenza epidemic or mass disaster: summary report of the European Society of Intensive Care Medicine's Task Force for intensive care unit triage during an influenza epidemic or mass disaster. Intensive Care Med. 2010;36(3):428-443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Swine influenza A (H1N1) infection in two children–Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(15):400-402 [PubMed] [Google Scholar]
- 12.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935-1944 [DOI] [PubMed] [Google Scholar]
- 13.Miller RR, III, Markewitz BA, Rolfs RT, et al. Clinical findings and demographic factors associated with ICU admission in Utah due to 2009 novel influenza A(H1N1) infection. Chest. 2009;137(4):752-758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) Evaluation of rapid influenza diagnostic tests for detection of novel influenza A (H1N1) Virus–United States, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(30):826-829 [PubMed] [Google Scholar]
- 15.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34(5):1297-1310 [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, de Mendonca A, Cantraine F, et al. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Crit Care Med. 1998;26(11):1793-1800 [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204-R212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antonelli M, Levy M, Andrews PJ, et al. Hemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, 27-28 April 2006. Intensive Care Med. 2007;33(4):575-590 [DOI] [PubMed] [Google Scholar]
- 19.Bergin CJ, Castellino RA, Blank N, Berry GJ, Sibley RK, Starnes VA. Acute lung rejection after heart-lung transplantation: correlation of findings on chest radiographs with lung biopsy results. AJR Am J Roentgenol. 1990;155(1):23-27 [DOI] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3, pt 1):818-824 [DOI] [PubMed] [Google Scholar]
- 21.Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33(7):1538-1548 [DOI] [PubMed] [Google Scholar]
- 22.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459-477 [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg AL, Hofer TP, Strachan C, Watts CM, Hayward RA. Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. Ann Intern Med. 2003;138(11):882-890 [DOI] [PubMed] [Google Scholar]
- 24.Denholm JT, Gordon CL, Johnson PD, et al. Hospitalised adult patients with pandemic (H1N1) 2009 influenza in Melbourne, Australia. Med J Aust. 2010;192(2):84-86 [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Hospitalized patients with novel influenza A (H1N1) virus infection–California, April-May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(19):536-541 [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection–Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(17):467-470 [PubMed] [Google Scholar]
- 27.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605-2615 [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Gomez A, Magana-Aquino M, Garcia-Sepulveda C, et al. Severe pneumonia associated with pandemic (H1N1) 2009 outbreak, San Luis Potosi, Mexico. Emerg Infect Dis. 2010;16(1):27-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuhrman C, Bonmarin I, Paty AC, et al. Severe hospitalised 2009 pandemic influenza A(H1N1) cases in France, 1 July-15 November 2009. Euro Surveill. 2010;15(2).pii:19463 [DOI] [PubMed] [Google Scholar]
- 30.Zarychanski R, Stuart TL, Kumar A, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182(3):257-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell A, Rodin R, Kropp R, et al. Risk of severe outcomes among patients admitted to hospital with pandemic (H1N1) influenza. CMAJ. 2010;182(4):349-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.La Ruche G, Tarantola A, Barboza P, Vaillant L, Gueguen J, Gastellu-Etchegorry M, Epidemic Intelligence Team at InVS The 2009 pandemic H1N1 influenza and indigenous populations of the Americas and the Pacific. Euro Surveill. 2009;14(42).pii:19366 [DOI] [PubMed] [Google Scholar]
- 33.Epidemiological task group for overseas French territories of the Pacific* Influenza A(H1N1)2009 in the French Pacific territories: assessment of the epidemic wave during the austral winter. Clin Microbiol Infect 2010;16(4):304-308 [DOI] [PubMed] [Google Scholar]
- 34.Gill JR, Sheng ZM, Ely SF, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010;134(2):235-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scriven J, Mcewen R, Mistry S, et al. Swine flu: a Birmingham experience. Clin Med. 2009;9(6):534-538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaillant L, La Ruche G, Tarantola A, Barboza P, Epidemic Intelligence Team at InVS Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14(33).pii:19309 [DOI] [PubMed] [Google Scholar]
- 37.Louie JK, Acosta M, Jamieson DJ, Honein MA. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med. 2010;362(1):27-35 [DOI] [PubMed] [Google Scholar]
- 38.Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5(3):e9694 http://www.ncbi.nlm.nih.gov/sites/entrez Accessed June 10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention (CDC) Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-10):1-8 [PubMed] [Google Scholar]
- 40.Cao B, Li XW, Mao Y, et al. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N Engl J Med. 2009;361(26):2507-2517 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.